Abstract
Introduction
Obesity in the world has been increasing, and the number of diabetic patients will increase by 114%, and the best treatment results are achieved through surgery. Several techniques have been described; the gastric bypass of an anastomosis (MGB/OAGB) has been gaining popularity for its simplicity and good results. We present a prospective study with this technique in 16 mild obesity patients with type 2 diabetes mellitus or peripheral insulin resistance.
Objective
To evaluate weight loss as well as metabolic changes by measuring fasting glycemia and Hb A1c after 1 year of follow-up.
Material and Methods
Sixteen patients were operated on with the OAGB/MGB technique from September 2014 to January 2016, with some form of metabolic syndrome, whether DM2, RPI, HBP, or dyslipidemia, including patients in the study with a follow-up of at least 12 months.
Results
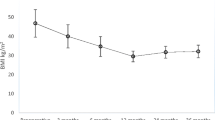
There were 13 cases of female sex and 3 of male, average age of 42.9 years, with an average weight of 87.7 kg and BMI of 32.2 kg/m2. Metabolic values were fasting glycemia of 193.6 ± 52.9 mg/dl and HbA1c of 8.4% ± 1 (preop) and glycemia posop, 78.8 ± 7.6 mg/dl; HbA1c posop, 6.1 ± 0.2; preop weight, 87.7 ± 14 kg (69–116); weight posop, 66.8 ± 10.5 kg (49–90); BMI preop, 32.2 ± 1.8 (30–34.9); BMI posop, 25.4 ± 1.7 (21.7–27.6); percentage of excess weight lost, 87.6 ± 11.8 (70.9–100) % with 100% remission of diabetes.
Conclusion
The results show the benefits of MGB/OAGB in mild obese diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) estimates that between 2000 and 2030, World population will experience a 37% growth, and the number of diabetic patients will increase 114% [1]. However, although many novel pharmaceutical agents have been introduced for the treatment and remission of diabetes, long-term success results of blood glucose control through lifestyle changes and conventional medical therapy are still discouraging [2]. New surgical approaches from surgeons have achieved outstanding developments in bariatric surgery; these techniques are currently the only effective method for long-term treatment of morbid obesity [2, 3], in the case of type 2 diabetes (T2D) in morbid or non-morbid obesity patients [3,4,5,6,7,8], and possibly, in T2D in non-obese patients [9, 10]. Although it is accepted that Roux-en-Y gastric bypass is the gold standard in bariatric surgery, and in the remission of diabetes [3], it may have a high rate of complications when performed by unexperienced surgeons [2]. Therefore, OAGB/MGB is a safe alternative to RYGB, with similar success in weight loss and resolution of metabolic complications, including T2D [11,12,13]. There are few publications about this technique for the surgical treatment of this clinical entity in patients with BMI ≤ 34.9 kg/m2 [14,15,16,17]; this is why we decided to conduct a prospective study using this technique in 16 patients with grade 1 obesity and type 2 diabetes or peripheral insulin resistance. All patients were evaluated in order to assess weight loss and metabolic changes by measuring fasting blood glucose and HbA1c after 1-year follow-up.
Materials and Methods
This is a prospective study performed from September 2014 through January 2016 with a group of 16 patients who evidenced some type of metabolic syndrome: type 2 diabetes mellitus (T2D), peripheral insulin resistance (IR), high blood pressure (HBP), or dyslipidemia, that underwent OAGB/MGB technique surgery. Patients with at least 12-month follow-up were also included in the study. The patients were operated in one institution; Clínica Santa Sofia, Caracas, Venezuela. The series did not include revision cases, whether due to weight gain or other causes and those with gastroesophageal reflux documented through upper digestive endoscopy. Patients with a BMI ≤ 30 kg/m2, previous gastric surgery, major psychiatric disorders, and/or alcohol or drug abuse were likewise, excluded.
Continuous variables were expressed as means, and Wilcoxon test was used for numeric variables.
All patients were included in the protocol after signing informed consent. The Bioethics Committee of the institution approved the project.
We performed the technique described by doctors Carbajo and Garcia-Caballero [18], as a modification of the Mini-Gastric Bypass (MGB) described by Rutdlege [19] to avoid biliary reflux. Using six ports: 3 of 12 mm and 3 of 5 mm, with an arrangement (Fig. 1), very similar to the one used in Roux-en-Y, as follows: The surgeon and the camera assistant to the right of the patient, the assistant to the left and in front of the surgeon, and the monitor placed on the upper left end of the patient. Using four blue loads of a 60-mm stapler, a gastric reservoir was made beginning with a first shot into the angular incisure. Thereafter, the Greater Omentum was sectioned searching for the fixed loop in the Angle of Treitz, measuring 150 cm of small bowel and at that distance, one anastomosis was performed, from the intestinal loop to the pouch which had been previously suspended from the end-point of the second vertical shot, to make the anti-reflux mechanism with a 2–0 silk running suture, and then a latero-lateral gastrojejunostomy was performed using a blue load of 30-mm stapler. The orifice was closed in two layers of running 2–0 polyglactin suture, and intraoperative air leak test was performed. A Blake® 19 Fr drain was left in place for 48 h.
Routine antimicrobial with Ampicillin-Sulbactam was used. Antithrombotic prophylaxis was used only in those cases with history of deep venous thrombosis, pulmonary embolism, antiphospholipid syndrome, and with severe venous insufficiency of the lower limbs. All patients began oral tolerance with clear liquids after 36 h and were discharged once tolerance was adequate and after the Blake® drain was withdrawn.
Patients were monitored weekly during the first month, monthly the first term, and quarterly up to the first year; from then on, they were evaluated every 6 months.
The following scheme was used to evaluate the results in terms of metabolic control:
Remission—no medication/FPG < 110 mg/dl, HbA1c < 6.5%
Partial Remission—no medication/FPG < 126 mg/dl, HbA1c < 7%
Improvement—less medication/FPG < 126 mg/dl, HbA1c < baseline
No response—same or worse than baseline
Results
Sixteen patients underwent surgery: 13 (81.2%) women and 3 (18.8%) men.
Mean Age, 42.9 ± 11.6 years (17–58) (Table 1).
Hospitalization time, 2 days (Table 2).
Mean surgical time, 70 min (Table 2).
BMI between 30 and 34.9 kg/m2; mean weight was 87.7 kg; BMI was 32.2 kg/m2.
Preoperative metabolic values were fasting blood glucose 193.6 mg/dl and HbA1c 8.4% (Table 1).
Weight loss results were as follows:
Weight preop 87.7 ± 14 kg (69–116)/Weight postop 66.8 ± 10.5 Kg (49–90) (Table 3).
BMI preop 32.2 ± 1.8 (30–34.9) kg/m2/BMI postop 25.4 ± 1.7 (21.7–27.6) kg/m2 (Table 3).
Percentage of excess weight loss (%EWL), 87.6 ± 11.8 (70.9–100) (Table 3).
Metabolic parameters evaluated were as follows:
Fasting blood glucose: Preop, 193.6 ± 52.9 mg/dl/Postop, 78.8 ± 7.6 mg/dl (Table 3).
HbA1c: Preop, 8.4 ± 1%/Postop, 6.1 ± 0.2% (Table 3).
Comorbidities: T2D, 9; Remission, 100%.
IRP, 4 resolutions: All cases.
HBP, 3: Two cases without medication and one with lower dosage.
Mixed dyslipidemia: 4 resolutions (Table 3).
There is no morbidity nor mortality.
Discussion
The gastric bypass—whether Roux-en-Y or one anastomosis techniques—is without a doubt an excellent procedure to improve or resolve type 2 diabetes mellitus in patients with grade 2 obesity or with more severe disease [3, 11, 12, 20].
Based on these results, the most important institutions that oversee the performance of bariatric and metabolic surgery in the world—ASMBS [21], IFSO [22], IDF [23], SECO [24]—have decided to reproduce these results through prospective studies in patients with mild obesity with T2D. In this sense, many works have already been published using RYGB technique in patients with BMI < 35 kg/m2. This surgical technique has evidenced good results and is proven to be safe in patients with mild obesity [5,6,7,8, 25, 28]. All four institutions agree that it is also effective in patients with poorly controlled T2D with high HbA1c > 7% or very high fasting blood glucose levels [21,22,23,24].
The mean age of our patients was 42 years, a younger age to those referred in similar RYGB series: Huang [25] 47 years old, Boza [7] 48, Shah [5] 45, and De Sa [6] 50; however, older than the ages in the studies of Cohen [26] 34 and Lee [27] 39 and notably younger than the García Caballero [15] series with MGB/OAGB that reported a mean age of 63 years. This is of great significance considering that young adult age is a positive predictive factor [10, 25, 27, 28] . We have a predominance of 81% women as in the other study series [6, 25,26,27,28] unlike Garcia Caballero [15] which reports 23%. Regarding the BMI, we have a mean of 32 kg/m2 similar to the RYGB series [6, 7, 25, 26, 28] and Lee [27] with MGB/OAGB. Results reported in the Asian series behave differently since diabetes appears in lower BMI values [29]; reporting BMI’s of 25 kg/m2 a year after surgery similar to a number of cross-sectional Asian studies [6,7,8, 26, 27]: Sha [5] 23 kg/m2, Huang [25] 23.7, and Lee [28] 23.1.
These results were achieved with a gastric pouch of approximately 50 cc, very similar in size to those described by De Sa [6] and Carbajo [12] and other series with smaller size pouches [7, 25, 27].
Our surgical time was 70 min, similar to Navarrete [30] with 69.01 min, who reported a significant difference with RYGB operative time, longer than Rudtlege [19], 36.9 min. and Kular [31], 50 min, but shorter when compared to the studies of Carbajo [12] and Noun [32]. Hospitalization time was similar for both techniques; therefore, the recovery of intestinal transit is similar [30].
It is necessary to highlight that early and late morbidity was 0%, much lower than those reported from the best series. We recently published about the complications of OAGB/MGB in a 100 case prospective series [33], reporting three cases of bleeding as immediate complication, two intraabdominal and one intraluminal; Kular [31] reported 0.6%, Noun [32] 1.7%, Musella [34] 3.4% and Wang [35] 1.7% (as a major bleeding anastomotic complication), with no leakage. In relation to leaks Rutledge [19] reported 1.08%, Carbajo [18] 1.9% and Wang [35] 0.7%. We consider this is due to both improvements in surgical technique and the small size of the series, which makes it a very safe technique.
Given the values of preoperative metabolic data, our patients would seem to have a less severe disease given the preop values of fasting blood glucose and HbA1: 193.6 ± 52.9 mg/dl and 8.4 ± 1%. These results are comparable with those from other series studies. A study by García Caballero [15] with 13 non-obese patients which underwent OAGB/MGB showed values of blood glucose 203 mg/dl and HbA1c 8.3%; and a study by Kim [17] with ten non-obese patients with blood glucose of 222 mg/dl and HbA1c of 9.7%.
It is important to highlight a series [36] from India which included more cases—128 patients that underwent OAGB/MGB—82 mildly obese women, with preoperative BMI of 33.4 ± 3.3 kg/m2, T2D patients with mean on-set age of the disease of 6.5 years, with elevated values of HbA1c of 10.7 ± 1.5% (8.4 ± 1%), and blood glucose over 200 mg/dl.
This study reports good results; however, it has low remission values when compared to our study. After 1 year, they registered 64% remission in the first 24 patients. When compared with this small sample, the value is somewhat lower than ours probably due to the remission criteria: HbA1c < 6% versus our 6.5%.
Likewise, this same series is comparable to ours in age and BMI parameters: 41.6 years and 33.4 kg/m2, although with a lower percentage of women 64% vs 81.2%. It is interesting that as a series from an Asian country, patients are not considered within the criteria of moderate-risk obesity, but as low-risk obesity [37], when it is well known that high-risk obesity has a higher chance of better outcomes.
In any case, they reach a mean of HbA1c of 6.2% a year, the same as Lee’s [38] prospective comparative series study with sleeve gastrectomy: 6.1%, with a BMI mean of 30.6 kg/m2.
However, all these series present preoperative data with greater metabolic instability, contrasting with results reported by Navarrete in moderate-risk obese patients that underwent RYGB [28] with blood glucose of 120 mg/dl and HbA1c 7.6%, but similar to Sha’s [5] 10.1%.
It seems that preoperative metabolic instability is not a predictive factor for improvement [5], as are—at least in Western countries—both a BMI < 30 kg/m2 or older age patients [9, 10].
Conclusion
Results show the benefits of MGB/OAGB in mild obese diabetic or metabolic syndrome patients.
While BMI is suitable for classifying the degree of obesity, it does not seem suitable to select T2D candidates for metabolic surgery. The clinical status of T2D is important for the selection of eligible candidates for metabolic surgery in addition to the current BMI criteria for bariatric surgery. Although several concerns need to be addressed, metabolic surgery for patients with low body mass index is increasingly approaching the mainstream of diabetes treatment.
References
Wild S, Relic G, Green SR, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–52.
Adams TD, Grass RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–76.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
De Paula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index < 35 kg/m2. Surg Endosc. 2008;22:706–16.
Shah SS, Todkar JS, Shah PS, et al. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index < 35 kg/m2. Surg Obes Relat Dis. 2010;6:332–8.
De Sa VC, Ferraz AA, Campos J, et al. Gastric bypass in the treatment of type 2 diabetes in patients with a BMI of 30 to 35 kg/m2. Obes Surg. 2011;21:283–7.
Boza C, Muñoz R, Salinas J, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in non-severely obese patients. Obes Surg. 2011;21:1330–6.
Fried M, Ribaric G, Buchwald JN, et al. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI < 35 kg/m2: an integrative review of early studies. Obes Surg. 2010;20:776–90.
Navarrete SA, Leyba JL, Llopis SN. Laparoscopic sleeve gastrectomy with duodenojejunal bypass for the treatment of type 2 diabetes in non-obese patients: technique and preliminary results. Obes Surg. 2011;21(5):663–7.
Scopinaro N, Adami G, Papadia F, et al. The effects of biliopancreatic diversion on type 2 diabetes mellitus with mild obesity (BMI 30–35 Kg/m2) and simply overweight (BMI 25–30 Kg/m2): a prospective controlled study. Obes Surg. 2011;21(7):880–8.
Rutledge R, Walsh T. Continued excellent results with the mini-gastric bypass: six-year study in 2.410 patients. Obes Surg. 2005;15(9):1304–8.
Carbajo M, Luque-de-León E, Jiménez J, et al. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg. 2017;27(5):1153–67.
Lee WJ, Lin YH. Single-anastomosis gastric bypass (SAGB): appraisal of clinical evidence. Obes Surg. 2014;24:1749–56.
Garcia-Caballero M, Reyes-Ortiz A, Garcia M, et al. Changes of body composition in patients with BMI 23-50 after tailored one anastomosis gastric bypass (BAGUA): influence of diabetes and metabolic syndrome. Obes Surg. 2014;24:2040–7.
Garcia-Caballero M, Valle M, Martinez-Moreno JM, et al. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with one anastomosis gastric bypass. Nutr Hosp. 2012;27:623–31.
Garcia-Caballero M, Martínez-Moreno JM, Tovar JA, et al. Improvement of C-peptide zero BMI 24–34 diabetic patients after tailored one anastomosis gastric bypass (BAGUA). Nutr Hosp. 2013;28(Supl. 2):35–46.
Kim MJ, Hur KY. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J Surg. 2011;35:631–6.
Carbajo M, Garcia-Caballero M, Toledano M, et al. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg. 2005;15:398–404.
Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11:276–80.
Schauer P, Kashyap S, Wolski K, et al. Bariatric surgery vs. intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76.
ASMBS Clinical Issues Committee. Bariatric surgery in class Iobesity (BMI 30–35 kg/m2). Surg Obes Relat Dis. 2013;9:e1–e10.
Bucetto L, Dixon J, De Luca M, et al. Bariatric surgery in class I obesity, a position statement from the International Federation for the Surgery of Obesity and Metabolic Disrorders (IFSO). Obes Surg. 2014;24(4):487–519.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med. 2011 Jun;28(6):628–42.
Rubioa M, Monereoa S, Lecubea A, et al. Posicionamiento de las sociedades SEEN-SECO-SEEDO-SED sobre la cirugía metabólica en la diabetes mellitus tipo-2 Joint. Position Statement of the SEEN-SECO-SEEDO-SED Societies on metabolic surgery for type 2 diabetes mellitus. Endocrinol Nutr. 2013;60(10):547–8.
Huang C, Shabbir A, Lo CH, et al. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25-35. Obes Surg. 2011;21:1344–9.
Cohen R, Pinheiro JS, Correa JL, et al. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis. 2006;2:401–4.
Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic minigastric bypass for type 2 diabetes mellitus: comparison of BMI > 35 and < 35 kg/m2. J Gastrointest Surg. 2008;12:945–52.
Navarrete S, Leyba JL, Navarrete LLS, et al. Bypass gástrico en Y de Roux para el tratamiento de pacientes con diabetes mellitus tipo II con IMC de 30 a 35 kg/m2. Nutr Hosp. 2012;27(4):1160–5.
Lee WJ, Wang W. Bariatric surgery: Asia-pacific perspective. Obes Surg. 2005;15:751–7.
Navarrete S, Leyba JL, Li SN, et al. Results of the comparative study of 200 cases: one anastomosis gastric bypass vs Roux-en y- gastric bypass. Obes Surg. 2018;28(9):2597–602.
Kular K, Manchanda NY, Rutledge R. A 6-year experience with 1054 mini-gastric bypasses—first study from Indian subcontinent. Obes Surg. 2014;24:1430–5.
Noun R, Skaff J, Riachi E, et al. One thousand consecutive minigastric bypass: short- and long-term outcome. Obes Surg. 2012;22:697–703.
Navarrete S, Leyba JL, Navarrete LLS, et al. Complications of the one anastomosis gastric bypass/minigastric bypass. Myths and realities. BMI. 2018;8.3.3:2344–54.
Musella M, Susa A, Greco F, et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014;28(1):156–63.
Wang W, Wei P, Lee Y. Short-term results of laparoscopic mini-gastric bypass. Obes Surg. 2005;15:648–54.
Kular KS, Manchanda N. Cheema GKSeven years of mini-gastric bypass in type II diabetes patients with a body mass index <35 kg/m(2). Obes Surg. 2016;26(7):1457–62.
Kasama K, Mui W, Lee WJ, et al. IFSO-APC consensus statements 2011. Obes Surg. 2012;22(5):677–84.
Lee WJ, Chong K, Lin YH, et al. Laparoscopic sleeve gastrectomy versus single anastomosis (mini-) gastric bypass for the treatment of type 2 diabetes mellitus: 5-year results of a randomized trial and study of incretin effect. Obes Surg. 2014;24:1552–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Approval from the local ethics committee and informed consent from all patients were obtained. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Navarrete Aulestia, S., Leyba, J.L., Navarrete Llopis, S. et al. One Anastomosis Gastric Bypass/Minigastric Bypass in Patients with BMI < 35 kg/m2 and Type 2 Diabetes Mellitus: Preliminary Report. OBES SURG 29, 3987–3991 (2019). https://doi.org/10.1007/s11695-019-04071-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04071-4