Abstract
Background
Type 2 diabetes mellitus (T2DM) and class I obesity, which are pandemics of considerable socioeconomic importance, require new treatment modalities due to inadequate control through normal clinical conduct. The aim of the present study was to assess the efficacy and safety of Roux-en-Y gastric bypass (RYGB) in the control of T2DM in patients with a body mass index (BMI) of 30 to 35 kg/m2.
Methods
An observational, retrospective study was carried out at the Universidade Federal de Pernambuco—Brazil. Between 2002 and 2008, 27 patients were submitted to RYGB for the treatment of uncontrolled T2DM, with a mean follow-up period of 20 months. An assessment was performed of the complete resolution of T2DM [HbA1c < 6%/fasting plasma glucose (FPG) < 100 mg/dL/no diabetes medication] and glycemic control. The ethics committee of the university approved the study.
Results
RYGB led to the following results: (1) 23% weight reduction (p < 0.001), BMI stabilized at 25.6 kg/m2 in a mean of 12 months; (2) 46% reduction in glycemia and 27% reduction in HbA1c (p < 0.001); (3) 100% improvement in glycemia and 48% resolution of T2DM; (4) glycemic control was 74% without medication and 93% with medication and five patients required medication in addition to RYGB; (5) mean current FPG is 93 mg/dL and HbA1c is 6%; and (6) there were no severe complications or deaths.
Conclusions
RYGB is a safe and effective option in the treatment of uncompensated T2DM associated to class I obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity in the USA is estimated at more than 30% of the population [1]. Between 2000 and 2030, the prevalence of type 2 diabetes mellitus (T2DM) in the USA is expected to go from 17 to 30 million individuals [2, 3]. Obesity carries a high risk of co-morbidities, including T2DM, which is associated with other metabolic alterations. Chronic hyperglycemia causes organ damage, dysfunction, and failure, especially in the eyes, kidneys, heart, and blood vessels [4]. This condition raises the risk of death due to cardiovascular disease [5].
In this prospective, controlled study, Sjöström et al. [5] showed that bariatric surgery in obese subjects was associated with a reduction in overall mortality, as compared with conventional treatment in contemporaneously matched, obese controls. In the USA, only 37% of patients with T2DM have less than 7% glycosylated hemoglobin (HbA1c) [6, 7]. The surgical treatment of morbid obesity has a low operation mortality (0.1% to 1.1%), with a 78.1% resolution of T2DM and 61.2% loss of excess weight [8, 9]. These are excellent results, but do not include patients with class I obesity [body mass index (BMI) of 30 to 35 kg/m2] and T2DM.
Glycemic control appears to be secondary to gastrointestinal alterations. Two hypotheses have been raised to explain what part of the intestine is responsible for the remission of diabetes. The foregut hypothesis states that food deviated from the duodenum and proximal jejunum avoids the secretion of as-yet unidentified substances that are thought to promote insulin resistance and T2DM [10]. The hindgut hypothesis states that a faster arrival of digested foods to the end of the gastrointestinal tract improves the metabolism of carbohydrates through an increase in the secretion of GLP-1 and other anorexigenic peptides [11, 12].
Based on this, new operations have emerged for the treatment of T2DM. In 2008, De Paula et al. carried out a study involving patients with class I obesity and diabetes. They demonstrated that vertical gastrectomy associated to ileal interposition in the proximal jejunum achieved good results in the remission of diabetes, regardless of duodenal exclusion [13]. In 2009, Ramos et al. operated on nonobese patients with diabetes through the exclusion of only the duodenum and proximal jejunum and achieved a resolution of diabetes in the short term [14]. We believe that the resolution of T2DM has caused the anatomical alterations provoked by surgeries, particularly for the exclusion of the duodeno and jejuno proximal (foregut hypothesis) and/or for the exposition of the ileo to the nutrients (hindgut hypothesis), causing blockade of the Rubinico factor and unchaining the stimulation of GLP-1 [10, 13, 14]. The aim of the present study was to assess the efficacy and safety of Roux-en-Y gastric bypass (RYGB) surgery in the control of T2DM in patients with a BMI of 30 to 35 kg/m2.
Methods
The study was carried out at the General Surgery Unit of the Universidade Federal de Pernambuco—Brazil. Between 2002 and 2008, 27 patients with class I obesity and T2DM were submitted to RYGB. The patients were followed up and the preoperative and postoperative data were compared and analyzed retrospectively. The diagnosis of T2DM and obesity was based on the criteria of the American Diabetes Association (ADA) and the World Health Organization, respectively.
The patients were adults with a BMI ranging from 30 to 35 kg/m2 and with uncontrolled T2DM in treatment with endocrinologists, with diagnosed diabetes for more than 1 year, and taking either oral hypoglycemic drugs and/or insulin. Complete resolution of T2DM was considered when the patient exhibited fasting plasma glucose (FPG) below 100 mg/dL and HbA1c below 6% in the postoperative period without the use of diabetes medication. Glycemic control was considered when the FPG was less than 130 mg/dL and HbA1c was less than 7% without the use of diabetes medication, based on ADA criteria.
In the preoperative period, the group had T2DM for an average of 8.8 ± 6.7 years; mean age was 50.3 ± 8.3 years; mean height was 1.63 ± 0.09 m; and 20 patients were women (74%). Mean weight was 89.3 ± 12.1 kg and mean BMI was 33.5 ± 1.5 kg/m2, with an average of 22.9 kg above the ideal weight. Mean FPG was 176 ± 46 mg/dL and mean HbA1c was 8.3 ± 2.0%. All patients made use of some medication (1.7 drugs per individual) for T2DM and no patient had controlled glycemia. Six patients (22%) made use of an average of 72 IU of insulin daily and 26 patients (96%) took oral hypoglycemic drugs, whether or not combined to insulin.
Surgery was performed by laparotomy in 17 cases (63%) and by videolaparoscopy on 10 patients (37%). The surgical technique was a gastric pouch with a volume of about 50 mL, without ring and with a 150-cm alimentary limb. The biliopancreatic limb was 100 cm long. Mean postoperative follow up was 20 months, ranging from 4 to 86 months.
For the statistical analysis, the Student’s t test and Fisher’s exact test were used, with the level of significance set at p < 0.05. The study received approval from the ethics committee of the university, and all subjects gave written informed consent.
Results
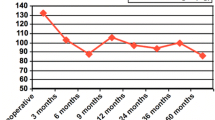
In the postoperative follow-up, there was a 23% reduction in mean weight and BMI (Table 1). The loss of excess weight was 94.5%, with a reduction of 20.8 kg, and the lowest BMI reached was 20.3 kg/m2. In the first year, the BMI reached 25.8 kg/m2, with 24.3 kg/m2 in the second year, and 26.5 kg/m2 thereafter. Weight loss was more accentuated in the first 6 months (19.6%) and then remained stable (Fig. 1). Previous BMI, duration of T2DM, or type of medication had no affect on this reduction (Fig. 2).
There was a 46% reduction in FPG and a 27% reduction in HbA1c (Table 1). Twenty patients (74%) no longer needed anti-diabetes medication. Remission of T2DM occurred in 13 patients (48%) and glycemic control (ADA criteria) without medication was achieved in 20 patients (74%). All patients exhibited improvement in glycemia levels in comparison to the preoperative period (Table 2). Only two patients did not exhibit normal glycemia following surgery, but achieved lower laboratory levels and required low doses of diabetes medication.
Diabetes remission and glycemic control were not affected by the type of medication used in the preoperative period or previous weight. However, patients with less than 7 years since the diagnosis of diabetes achieved better glycemic control (p = 0.03), and for resolution, no difference was found, but the p value was low enough to suggest a trend (p = 0.08). No patient exhibited clinical signs of malnutrition and all had normal serum albumin. There were no serious complications or deaths. There were four cases of seroma of the operation wound, two cases of superficial infection of the surgical site, and one case of stenosis of the gastrojejunal anastomosis, which was treated by endoscopic dilation (Table 3).
Discussion
The present study demonstrates that RYGB in patients with type 2 diabetes and class I obesity promotes glycemic control and weight control at levels similar to the population without diabetes. Bariatric surgery leads to a 56% to 61% loss of excess weight, with a 33% reduction from initial weight values and an absolute loss of approximately 40 kg among patients with morbid obesity [8, 9]. The percentage of weight loss and excess weight loss stemming from surgery on patients with mild obesity is not yet determined, as there are no significant studies on operated patients with this weight profile. It is reasonable to expect a normalization of weight following RYGB, with the complete elimination of excess weight, but the BMI is not expected to drop below 20 kg/m2. In the present study, the ideal BMI (25 kg/m2) was reached and no patient dropped below 20 kg/m2. There was weight loss of 21 kg and 23% of the initial weight. This demonstrates the safety of RYGB on this population of patients regarding nutritional issues.
Surgery is clearly superior to clinical treatment with regard to weight control. In a meta-analysis assessing clinically treated obese patients with diabetes, only 22% managed to lose more than 5% of their initial weight, losing an average of only 4.5 kg [15]. Studies on class I obese patients with diabetes having undergone surgery have demonstrated more accentuated weight loss in the first year, remaining stable over time due to the anatomic alterations in the restriction and absorption of nutrients [13, 14, 16, 17]. O’Brien et al. demonstrated that surgical treatment using laparoscopic adjustable gastric banding was statistically significantly more effective than nonsurgical therapy in reducing weight, resolving the metabolic syndrome, and improving quality of life during a 24-month treatment program [18].
The metabolic alterations following RYGB, with glycemic control, appear to be a consequence of the reorganization of the gastrointestinal anatomy and not merely due to the reduction in calorie intake and weight loss. This hypothesis is based on the rapid resolution of diabetes in the postoperative period, when weight loss has not yet occurred, despite the reduced calorie intake. This was also seen in an experimental study in which only the intestinal transit was altered and not food intake or even the stomach [10]. RYGB excludes the duodenum and proximal jejunum, triggering changes in the entero-insular axis, which may explain the anti-diabetogenic state stemming from the surgery [10, 11, 15].
We believe that RYGB is the ideal surgery for diabetic individuals without clinical control. The probable advantages are (1) the technique is known worldwide, which can be performed by well trained surgeons and taught in medical residency programs, (2) low indices of collateral effect compared with other experimental surgeries such as bypass jejuno-ileal or duodeno-jejenual or ileal interposition, (3) the results are already known, and (4) the treatment is known with good resolution for eventual complications.
RYGB unites a single surgery to two theories for the control of glycemia and resolution of diabetes. The anatomic alterations of proximal jejunum exclusion (foregut hypothesis) or earlier arrival of nutrients to the ileum (hindgut hypothesis) determined an average FPG of 94 mg/dL and HbA1c of 6% in these 27 patients. Surgery for diabetes leads to normal glycemia levels [13, 14, 17]. These results are better among patients treated with medications, such as metformin [2].
The rigorous criteria for diabetes remission in the present study (absence of medication/HbA1c < 6%/GJ < 100 mg/dL), found in individuals without diabetes or glucose intolerant, can be considered a real resolution of this disease, providing it continues over time. This has been occurring over 20 months of follow-up in the present study. RYGB benefited all patients, obtaining better glycemic control. T2DM was controlled by 74% without medication and by 92% with small doses of medication (ADA parameters). Surgeries for diabetes achieve similar levels. However, some techniques are new and there is as-yet no information on possible long-term complications [9, 13, 17, 19].
De Paula et al. report that patients with a shorter duration of T2DM achieve better surgical results, possibly due to a pancreatic reserve that can be stimulated by the incretins stemming from the surgical alterations [13]. This association was also found in the present study. Previous weight and type of hypoglycemia medication do not appear to affect the results of glycemic control and diabetes resolution. Patients with a BMI between 30 and 35 kg/m2 have better glycemic control in comparison to nonobese individuals [13]. Weight loss does not affect glycemic resolution or control [13].
The reduction in the use of medication found in this study (as well as by other authors) provides an important long-term economy, minimizes the complications stemming from glycemia alterations, and reduces the side effects of the chronic use of insulin, such as an aggravation of central obesity and weight gain [8, 9, 13, 14, 16, 17]. As a significant portion of patients with diabetes and class I obesity have uncontrolled glycemia, this small, uncontrolled, retrospective series suggests that RYGB may be a safe therapy for the treatment of type 2 diabetes in this patient correlation. Further respective randomized trials will be required to confirm this hypothesis.
References
National Center for Health Statistics NHANES IV Report. http://www.cdc.gov/nchs/product/pubs/pubd/hestats/obes/obese99.htm2002. Accessed 9 Mar 2009.
AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Endocr Pract. 2007;13 Suppl 1:1–68.
World Health Organization. Prevalence of diabetes in the WHO region of the Americas. Disponível em: http://www.who.int/diabetes/facts/world_figures/em/index3.html. Accessed 7 Fev 2009.
American Diabetes Association. Standards of medical care en diabetes (position statements). Diab Care. 2009;32 Suppl 1:S13–61.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Parnes BL, Main DS, Dickinson LM, et al. Clinical decisions regarding HbA1c results in primary care: a report from CaReNet and HPRN. Diab Care. 2004;27(1):13–6.
Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diab Care. 2004;27(1):17–20.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery, a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. JAMA. 2009;122:248–56.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9.
Cummings DE, Overduin J, Foster-Schubert KE, et al. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis. 2007;3:109–15.
Cummings DE, Overduin J, Foster-schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89(6):2608–15.
DePaula AL, Macedo ALV, Rassi N, et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc. 2008;22(3):706–16.
Ramos AC, Galvão Neto MP, Souza YM, et al. Laparoscopic duodenal–jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI < 30 kg/m2 (LBMI). Obes Surg. 2009;19(3):307–12.
Norris SL, Zhang X, Avenell A, et al. Efficacy of pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Arch Intern Med. 2004;164(12):1395–404.
Cohen R, Pinheiro JS, Correa JL, et al. Laparoscopic Roux-en-Y gastric bypass for BMI 35 kg/m2: a tailored approach. Surg Obes. 2006;2:401–4.
Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BM1 > 35 and BM1 < 35 kg/m2. J Gastrointest Surg. 2008;12:945–52.
O’Brien PE, Dixon JB, Playfair J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program. Med Inter. 2006;144:625–33.
Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes. A randomized controlled trial. JAMA. 2008;299(3):316–23.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sa, V.C.T., Ferraz, A.A., Campos, J.M. et al. Gastric Bypass in the Treatment of Type 2 Diabetes in Patients with a BMI of 30 to 35 kg/m2 . OBES SURG 21, 283–287 (2011). https://doi.org/10.1007/s11695-010-0318-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0318-5