Abstract
Background
Bariatric surgery may be beneficial in mildly obese patients with poorly controlled diabetes. The optimal procedure to achieve diabetes remission is unknown. In 2011, we published the short-term results of a pilot study designed to evaluate the efficacy of diabetic control and the role of duodenal exclusion in mildly obese diabetic patients undergoing laparoscopic sleeve gastrectomy (SG) vs. a laparoscopic single anastomosis (mini-) gastric bypass (SAGB). This study analyzes the 5-year results and evaluates the incretin effect.
Methods
A double-blind randomized trial included 60 participants with a hemoglobin A1c (HbA1c) level higher than 7.5 %, a body mass index (BMI) between 25 and 35 Kg/m2, a C-peptide level ≥1.0 ng/mL, and a diagnosis of type 2 diabetes mellitus (T2DM) for at least 6 months. A SAGB with duodenal exclusion or a SG without duodenal exclusion was performed.
Results
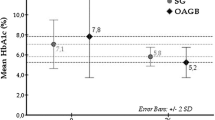
The 5-year results of the primary outcome were as an intention-to-treat analysis for HbA1c ≤6.5 % without glycemic therapy. Assessments of the incretin effect and β cell function were performed at baseline and between 36 and 60 months. The patients were randomly assigned to SAGB (n = 30) and SG (n = 30). At 60 months, 18 participants (60 %; 95 % confidence interval (CI), 42 to 78 %) in the SAGB group and nine participants (30 %; 95 % CI, 13 to 47 %) in the SG group achieved the primary end points (odds ratio (OR), 0.3; 95 % CI, 0.1 to 0.8 %). The participants assigned to the SAGB procedure had a similar percentage of weight loss as the SG patients (22.8 ± 5.9 vs. 20.1 ± 5.3 %; p > 0.05) but achieved a lower level of HbA1c (6.1 ± 0.7 vs. 7.1 ± 1.2 %; p < 0.05) than the SG patients. There was a significant increase in the incretin effect before and after surgery in both groups, but the SAGB group had a higher incretin effect than the SG group at 5 years.
Conclusions
In mildly obese patients with T2DM, SG is effective at improving glycemic control at 5 years, but SAGB was more likely to achieve better glycemic control than SG and had a higher incretin effect compared to SG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is the most effective treatment for morbidly obese patients (body mass index (BMI) >35 Kg/m2) for promoting weight loss and managing obesity-related comorbidities [1–3]. The majority of morbidly obese diabetic patients display a dramatic improvement in their type 2 diabetes mellitus (T2DM), and this group benefits the most from bariatric surgery [4, 5]. For this reason, bariatric surgery has been proposed as a new treatment modality for mildly obese diabetic patients who do not have satisfactory control with current medical treatment [6–10]. It has been hypothesized that changes in gastrointestinal hormone secretions would favor an early improvement of T2DM in gastric bypass surgery, which bypasses the duodenum and upper jejunum (the foregut theory) [11–13]. Elucidating the role of duodenal exclusion in diabetes remission is important for clinicians to understand the mechanism, to choose the appropriate procedures, and to develop novel treatments in the future [14]. This study aims to test the foregut hypothesis. We reported the 1-year results of this study and demonstrated that gastric bypass surgery was more effective than sleeve gastrectomy; we proposed that duodenal exclusion does play a role in the mechanism of diabetes remission [15]. We reported the second year results and studied the post-meal changes in the gut hormones between the two procedures [16]. We also studied insulin secretion and adipocytokines in this group of patients [17]. This article reports the 5-year results of our randomized trial comparing laparoscopic sleeve gastrectomy with gastric bypass surgery for the treatment of mildly obese T2DM patients and evaluates the changes in the incretin effect.
Methods
The study was conducted in the Department of Surgery of Min-Sheng General Hospital, National Taiwan University. The institutional review board (IRB) approved the study, and written informed consent was obtained from each patient.
The interventions included two components: half of the participants had laparoscopic single anastomosis (mini-) gastric bypass (SAGB) and the other half had laparoscopic sleeve gastrectomy (SG). The interventions were provided without charge. Compensation ranged from $50 to $200 for the yearly visit and function studies. The trial was registered with clinicaltrials.gov identifier: NCT00540462.
Patient Eligibility and Enrollment
Between September 2007 and June 2008, 60 patients participating in the study were randomized to receive SAGB (n = 30) or SG (n = 30). The patients were recruited through mass media advertisements, contact with professional groups, and a practice-based database. The patients were included if they were 30 to 60 years old, had been receiving treatment for T2DM for at least 6 months before recruitment, had a hemoglobin A1c (HbA1c) level higher than 7.5 %, and had a serum C-peptide level higher than 1.0 ng/mL. The participants had a BMI of 25.1 to 34.9 Kg/m2 and were willing to accept randomization to either surgical group and follow the full treatment.
The randomization assignment was double-blinded until 1 month after surgery. The randomization schedule used permuted blocks for every ten patients. The investigators, data collectors, and outcome adjudicators were blinded to aggregate outcomes until the final patient completed the yearly follow-up.
Surgical Technique
The surgical team performed both types of surgical procedures and had broad experience with both techniques. For SAGB, a simplified “laparoscopic single anastomosis (mini-) gastric bypass” was performed as previously described [18, 19]. Briefly, through a standard 5-port laparoscopic technique, a long-sleeved gastric tube was created by the EndoGIA stapler (Tyco, United States Surgical Corporation, Norwalk, CT, USA) approximately 2.0-cm wide along the lesser curvature from the antrum to the angle of His. A Billroth II type loop single gastroenterostomy was created with the small bowel about 120 cm distal to the ligament of Treitz, using the EndoGIA stapler.
SG, the other procedure had been performed at our center since 2005 and is described elsewhere [20]. In brief, a vertical gastrectomy was performed by resecting the greater curvature from the distal antrum (4 cm proximal to the pylorus) to the angle of His including the complete fundus, using a 28 Fr size gastroendoscopy as a calibration tube. The remnant stomach tube was approximately 2-cm wide along the lesser curvature. The resected portion of the stomach was extracted from the extended periumbilical trocar site. A running absorbable seromuscular suture was applied to the staple line with calibration tube in the side to prevent hemorrhage and leakage.
Follow-Up and Medical Management
All the patients attended monthly postoperative visits for the first 3 months, then every 3 months for the first year, and yearly thereafter. All the patients were given routine tests for nutritional deficiencies, and multivitamins and supplements were prescribed accordingly. All patients were followed by the same endocrinologist (K. Chong) and referred back to their primary physician for continued management of their conditions.
Outcomes
The primary outcome was considered successful if patients achieved an HbA1c ≤6.5 % without glycemic agents at the 60-month visit. Secondary outcome measures included the percentage of weight loss and resolution of the metabolic syndrome defined by the National Cholesterol Education Program Adult Treatment Panel III criteria [21]. Any adverse events and revision surgeries were recorded.
Measurement and Data Collection
Collected data included the height, weight, blood pressure, pulse rate, medications used, and adverse events. Laboratory measurement included a complete blood cell count, blood levels of HbA1c, fasting lipid profile, hepatic panel, ferritin, iron, calcium, parathyroid hormone, fasting glucose, insulin, and C-peptide concentrations. Change in the indirect measures of insulin resistance using the homeostasis model assessments (HOMA) was measured [22].
Incretin Effect
The patients were studied at 2 days before surgery and again after surgery for more than 56 months. The incretin effect was defined as the difference in insulin secretion (area under curve (AUC)) in response to an oral glucose tolerance test (OGTT) and to an isoglycemic intravenous glucose test (IsoG IVGT). The OGTT and the IsoG IVGT were administered on separate days.
The patients first underwent a 3-h OGTT. For this test, the patients consumed 50 g of glucose (in a total volume of 300 mL). Blood samples were collected 15 min prior to the oral glucose load; immediately before the load; and at 15, 30, 60, 90, 120, 150, and 180 min afterwards to measure blood glucose, C-peptide, and insulin levels. For the IsoG IVGT, glucose (sterile 50 % dextrose solution in water) was infused intravenously over 3 h using an infusion pump. A blood sample was collected every 5 min using a contralateral antecubital intravenous catheter, and the glucose level was measured from this sample at the patient’s bedside. The glucose infusion rate was adjusted to match blood glucose concentrations obtained at each time point for the same patient during the earlier 3-h OGTT.
The differences in the B cell responses (insulin AUC) to the oral and isoglycemic IV glucose stimuli represented the incretin effect. The action of the incretin factor is expressed as a percentage of the physiological response to oral glucose using the following formula: incretin effect = Insulin AUC (OGTT) − Insulin AUC (isoG IVGTs) / Insulin AUC (OGTT) × 100 %.
Statistical Design and Analysis
The 5-year results of the primary outcomes were as an intention-to-treat analysis for HbA1c ≤6.5 % without glycemic therapy. For participants who did not have data for the metabolic syndrome at 60 months, the methods of multiple imputation were based on all prior data [23]. Logistic regressions were used to compare proportions of success in the two groups (SAS PROC MI and SAS PROCMIANALYZE, SAS 9.2, SAS institute Inc.).
Estimates of the sample size were based on the following assumptions: a two-sided significance level of P < 0.05 and a standard superiority trial design, 95 % power, and an alternative hypothesis of success rates of 80 % success rate in the gastric bypass group vs. 40 % in the sleeve gastrectomy group. These estimates were derived from our previous study [2]. This alternative hypothesis resulted in a sample size estimate of 56 participants (28 in each group).
Multiple imputations were used to address the issue of missing outcome data [23] for participants missing the 5-year visit with PROC MI in SAS. The following baseline covariates were used: age, sex, HbA1c, low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol, triglycerides, systolic and diastolic blood pressures, blood glucose, weight, and waist circumference. Information regarding the crossover status was used, but no other post-crossover information was used. Analysis of the imputed data set was based on a logistic regression carried out using PROC MIANALYZE in SAS. The graphs indicate the means and the 95 % confidence intervals (CIs).
Results
Participant Characteristics
The methodology of recruitment and screening is summarized in Fig. 1. A total of 219 candidates for the study were screened to attain 60 randomized patients. The patient characteristics at baseline are summarized in Table 1. The participants had T2DM for an average of 6.4 years (95 % CI, 4.2–8.5 years) at enrollment. The mean (SD) BMI was 30.6 Kg/m2 (25.1–34.7), the mean age was 45 years (34–58), and the mean HbA1c was 10.0 % (7.5–15.0). The baseline characteristics were similar across the randomized groups except a slight difference in high-density lipoprotein and C-peptide. Before surgery, all the patients (100 %) had oral medications, 18.3 % of them had insulin usage and 55 % of the patients took lipid-lowering or antihypertensive medication; no difference between the SAGB and SG group was observed.
Twelve (20 %) of the 60 enrolled participants were lost to follow-up at 5 years; this included six patients from each group. There were four crossovers: four participants randomized to the SG group underwent revision Roux-en-Y gastric bypass because of persistent diabetes in three and intractable acid reflux esophagitis in one.
Treatment Effects
At 5 years, 18 participants (60 %; 95 % CI, 42–78 %) in the SAGB group and nine participants (30 %, 95 % CI, 13–47 %) in the SG group achieved the primary end points (odds ratio (OR), 0.3; 95 % CI, 0.1–0.8; Table 2). The mean reduction of HbA1c was 2.8 in the SG group and 3.9 in the SAGB group 5 years after surgery. There was an approximately 30 % discrepancy between the groups (Fig. 2)
At 5 years after surgery, both groups had a marked reduction of body weight and improvement of other associated metabolic disorders including reduction of waist size, blood pressure, insulin, C-peptide, and blood lipids (Table 2). The weight loss was similar between the groups through the 5 years (Fig. 2). The mean (SD) weight loss after SAGB was 22.8 % (5.9 %) of the starting weight at 5 years without a significant difference when compared to the 20.1 % (5.3) reduction after SG. SAGB achieved a significantly lower mean BMI than SG (23.3 (2.0) vs. 25.1 Kg/m2 (2.4)] at 5 years after surgery. SAGB also had significantly lower levels of blood lipids and blood pressure than SG (Table 2). Twenty-four patients (80 %) in SG still had metabolic syndrome compared with only eight patients (26.7 %) after SAGB (OR, 4.1; 95 % CI, 1.5–11.5). SAGB had lower levels of albumin and iron than SG at 5 years.
At 5 years after surgery, 39.6 % of them (16.7 % in SAGB and 62.5 % in SG, p = 0.002) had used oral medication and 4.2 % (0 % in SAGB and 8.3 % in SG, p = 0.150) had insulin usage. In addition, significantly lower percentage of patients were still receiving lipid-lowering and antihypertensive medication in the SAGB group than in SG group (8.3 and 16.7 vs 45.8 and 45.8 %, p = 0.005 and 0.037) (Table 2).
Oral Glucose Tolerance Test and Insulin Secretion
The AUC of glucose during the OGTT significantly improved in both groups without difference at 5 years after surgery, SAGB 2,232 ± 1,300 uIU.min/mL and SG 2,399 ± 1,118 uIU.min/mL. Insulin resistance measured by the HOMA index was dramatically reduced after bariatric surgery without differences between the groups, 10.6 (23.2) after SAGB and 9.1 (9.6) after SG groups, and it decreased to 0.9 (0.6) and 1.1 (0.5), respectively, at 5 years.
Incretin Effect
Data in both groups were combined to look at the incretin response to intravenous (IV) glucose and the isoglycemic glucose tolerance tests. In the patients before bariatric surgery, the insulin responses to oral glucose were not significantly higher than the responses to IV glucose, indicating a blunted incretin effect with a preoperative level of 19.8 ± 21.9 % (Fig. 3). After bariatric surgery, the incretin effect increased dramatically in both groups at 3 months and persisted up to 5 years with a mean effect of 58.7 ± 21.4 %. The incretin effect on C-peptide also increased significantly after metabolic surgery (from 12.0 ± 17.2 % at baseline to 38.2 ± 14.8 % at 5 years after surgery). When evaluated separately, the SAGB group had a significantly higher mean (SD) incretin effect than the SG at 5 years in insulin (65.2 % (17.2 %) vs. 46.8 % (24.5 %); difference 18.4 %, 95 % CI −33.1 to −3.8) and in C-peptide (44.2 % (11.6 %) vs. 27.1 % (14.4 %); difference 17.1 %, 95 % CI −26.3 to −7.9] (Fig. 4).
Adverse Events and Crossover
In total, five (10.4 %) patients had major adverse events possibly related to diabetes during the follow-up. One patient in the SAGB group died at 54 months after surgery due to acute myocardial infarction. Another patient had acute myocardial ischemia but only required stent treatment. One patient in the SG group developed end stage kidney disease and received regular hemodialysis 2 years after surgery. One patient in the SG group developed a minor stroke with left hemiparesis 2 years after surgery. One patient in the SAGB group received a left lower leg amputation after a traffic accident at 4 years after surgery. One patient (4.2 %) had marginal ulcer in SAGB. There were two patients (8.4 %) in both SAGB and SG group still required proton pump inhibitor treatment at 5-year after surgery.
Five (10.4 %) patients required revision operations. One patient in the SAGB group was converted to Roux-en-Y gastric bypass (RYGB) because of intractable bile reflux esophagitis at 4 years after surgery. Four patients in the SG group had undergone crossover to RYGB. One patient underwent conversion from SG to RYGB after 2 years because of aggravation of her diabetes. Her initial HbA1c was 9.1 %. Two years after the SG, she had a 12.8 % weight loss and her HbA1c dropped to 8.4 % then increased to 9.1 %. After revision to gastric bypass, her weight loss increased to 22 % and her HbA1c decreased to 6.9 %. Another patient received a revision bypass surgery at 3 years after SG. Her initial HbA1c was 8.3 % but it increased to 9.5 % at 3 years with a weight loss of 12.1 %. After the revision surgery, her weight loss increased slightly to 12.9 % and her HbA1c was 7.9 %. One patient received a revision bypass after 1 year because of inadequate weight loss. Her weight loss was 17.8 % with a BMI dropped from 34 to 28 and her HbA1c decreased from 9.6 to 6.9 %. After the revision surgery, her BMI decreased to 27 Kg/m2 and her weight loss increased to 22 %, but her HbA1c remained at 6.8 %. The last patient received a revision RYGB surgery at 4 years after SG because of intractable acid reflux esophagitis. He had a 28.3 % weight loss before the revision surgery and his HbA1c decreased from 7.7 to 6.5 %. After the revision surgery, his weight loss increased to 34.2 % and his HbA1c dropped to 6.2 %.
Discussion
The current evidence suggests that bariatric surgery is associated with better short to intermediate weight loss and glycemic control outcomes than non-surgical therapy in patients with diabetes and a BMI <35 Kg/m2 [24–26]. Until now, the data on the long-term control from randomized clinical trials were insufficient [27, 28]. This study provides the first evidence from a randomized trial showing persistent improved glucose outcomes as measured by the HbA1c up to 5 years after metabolic surgery in previously poorly controlled T2DM patients with a BMI <35 Kg/m2. Of the patients with poorly controlled T2DM and a BMI of 25.1–34.9 Kg/m2 who underwent bariatric surgery, the mean HbA1c decreased from 10.0 to 6.6 %, and 45 % of the patients achieved the primary goal of an HbA1c <6.5 % at 5 years after surgery. Five (10.4 %) patients developed diabetes-related serious adverse events, and there was one (2.1 %) death in 5 years, but this incidence is lower than an expected 35 % incidence of serious events in 10 years from an observational study in Asia [29]. We reported a significant reduction of UK Prospective Diabetes Study 10-year cardiovascular risk up to 40 % in this group of patients [17]. This study supports an increased usage of gastric bypass surgery in the treatment of non-severely obese (BMI <35 Kg/m2) T2DM who are poorly controlled with their current treatments.
To our knowledge, this is the first trial comparing different metabolic procedures to treat T2DM in mildly obese subjects. Several researchers have reported that sleeve gastrectomy had a similar efficacy with gastric bypass in the treatment of morbidly obese patients with T2DM [30–32]. In non-morbidly obese patients, we found that the overall glycemic control was improved more in the SAGB group than in the SG group since the first year [15] and persistent up to 5-year follow-up in this study. In addition, more patients in the SG group still required medication for hypoglycemic, lipid-lowering, and antihypertension than in the SAGB group. These findings were similar to the Schauer et al. study [25]. In the Schauer et al. study, the diabetes remission rate was similar between RYGB and SG groups, but 30 % of the SG patients were on glycemic agents but none in RYGB patients were on these agents. A recent meta-analysis also supported the superior of gastric bypass over sleeve gastrectomy for T2DM treatment [33].
The patients who received SAGB achieved a lower HbA1c and had a lower total cholesterol, triglyceride, and diastolic blood pressure than the patients who received SG. Gastric bypass surgery had a special advantage in treating the metabolic syndrome compared with sleeve gastrectomy. In our previous study, bariatric surgery had a 95.6 % cure rate of the metabolic syndrome in morbidly obese patients, but no difference was observed between the gastric bypass and the restrictive type procedure [2]. The remission rate of the metabolic syndrome after bariatric surgery was only 47 % and was significantly different between SAGB and SG groups in this study. These findings highlight the difference of the nature of metabolic syndrome and diabetes between morbidly obese and non-morbidly or mildly obese patients.
The underlying mechanism for the superiority of SAGB than SG on diabetes remission is intriguing. The most well-known important mechanism is a rapid decrease of insulin resistance after bariatric surgery. The effect of the bariatric surgery-induced insulin resistance decrease has been evaluated in a large number of studies. Calorie restriction and subsequent weight loss contribute to the majority of the effect. In this study, SAGB and SG had a similar effect of a 20 % weight loss and a significant reduction of HOMA-IR without differences between the two methods at 5 years. Other mechanisms may be important in the differing glycemic control between SAGB and SG.
One possible mechanism is the bypass of the hormonally active foregut. We investigated this theory by designing this study comparing the duodenal exclusion SAGB surgery with a control group of SG without duodenal bypass. This study demonstrated a better glycemic control with SAGB than SG but did not observe a significant role of duodenal exclusion. No dramatic improvement in the glycemic control was found by adding duodenal exclusion to the SG in this study. The possible duodenal factor related to diabetes remission remained unknown, but we demonstrated an eliminated post-meal response of cholecystokinin in the SAGB subjects comparing with the SG subjects in our previous report [16]. An associated advantage of SAGB is the better control of blood lipids and blood pressures, which is believed to be related to the duodenal exclusion and the malabsorption effect. More elaborate studies to elucidate the role of duodenal exclusion in the mechanism of T2DM remission after gastric bypass are needed.
Another possible mechanisms involved is the rapid recovery of the incretin effect after bariatric surgery [34]. A reduced or absent incretin effect has been demonstrated in patients with type 2 diabetes [35] and is considered a consequence rather than a cause of diabetes [36]. In the present study, we found that SAGB and SG can rapidly augment the blunt incretin effect, and this effect persists up to 5 years. We demonstrate that SAGB had a significantly better incretin effect than SG at longer follow-up. The improvement of the incretin effect can be explained by the increase of incretin, glucagon-like-peptide-1 (GLP-1). We investigated the gut hormone changes of the same group at 2 years after surgery and found a robust increase in insulin secretion and elevated post-meal GLP-1 responses in the SAGB and SG groups, but the SAGB patients were found to have a slightly higher GLP-1 response than the SG patients, which corroborates with the current finding [16]. Other studies also had similar findings [37–40]. The difference in the incretin effect did not correspond with the overall insulin secretion measured by the AUC method. The SG and SAGB groups had the same amount of insulin secretion, but the SAGB group had a higher early peak of insulin secretion [16]. This early insulin response effect may contribute to the improved incretin effect found in this study.
The underlying mechanism of diabetes remission after SAGB and SG is still unclear, but weight loss is definitely a deciding factor for diabetes remission after bariatric/metabolic surgery. In previous studies, weight loss was the most important independent predictor of diabetes remission after surgery [4, 24, 41, 42] even in normal weight gastric cancer patients [43]. In this study, the weight loss was a little higher in SAGB group although statistically no significance, but the SAGB patients achieved a significantly lower BMI than the SG patients at 5 years. Patients who received a crossover bypass surgery following SG were interesting to observe. No dramatic improvement in the glycemic control can be expected by adding duodenal exclusion to the SG, and the improvement of glycemic control was always associated with some degree of weight loss. No matter how intriguing the mechanism of diabetes remission after bariatric/metabolic surgery may be, weight loss is still the cornerstone of diabetes remission.
The gastric bypass surgery had a better glycemic control for type 2 diabetes, but we need to be careful of the disadvantage of long-term micronutrient malnutrition after gastric bypass. In this study, the SAGB patients had significantly lower iron and calcium levels than the SG patients at 5 years. These side effects may cause long-term malnutrition complications, such as chronic iron deficiency anemia and osteoporosis. Purely restrictive type procedure, such as laparoscopic adjustable banding, may be considered in patients with newly onset diabetes [44], and SG procedures may be considered in patients with good islet cell preservation and high C-peptide levels [45].
The strengths of this study include the randomized design, the long-term follow-up, and the intention-to-treat comparison. A relatively high level of participant follow-up was obtained. The weaknesses include the relatively small sample size and the use of estimated end points for serious adverse events outcome. A long-term follow-up for more than 10 years is indicated.
In summary, both laparoscopic gastric bypass and sleeve gastrectomy are effective metabolic surgeries for the remission of non-morbidly obese diabetic patients with inadequate control by current medical treatment. SAGB had better glycemic, blood lipid, and blood pressure control than SG. The superior effect of SAGB over SG on T2DM remission might be attributed to a higher incretin effect.
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA : J Am Med Assoc. 2004;292(14):1724–37. doi:10.1001/jama.292.14.1724.
Lee WJ, Huang MT, Wang W, et al. Effects of obesity surgery on the metabolic syndrome. Arch Surg. 2004;139(10):1088–92. doi:10.1001/archsurg.139.10.1088.
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi:10.1056/NEJMoa035622.
Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi:10.1056/NEJMoa066254.
Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA : J Am Med Assoc. 2012;307(1):56–65. doi:10.1001/jama.2011.1914.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. doi:10.1016/j.amjmed.2008.09.041.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med : J B Diabet Assoc. 2011;28(6):628–42.
Lee WJ, Wang W. Bariatric surgery: Asia-Pacific perspective. Obes Surg. 2005;15(6):751–7. doi:10.1381/0960892054222614.
Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI > 35 and <35 kg/m2. J Gastrointest Surg : Off J Soc Surg Aliment Tract. 2008;12(5):945–52. doi:10.1007/s11605-007-0319-4.
Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251(3):399–405.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9. doi:10.1097/01.sla.0000224726.61448.1b.
Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11. doi:10.1097/01.sla.0000102989.54824.fc.
Zervos EE, Agle SC, Warren AJ, et al. Amelioration of insulin requirement in patients undergoing duodenal bypass for reasons other than obesity implicates foregut factors in the pathophysiology of type II diabetes. J Am Coll Surg. 2010;210(5):564–72. doi:10.1016/j.jamcollsurg.2009.12.025.
Schouten R, Rijs CS, Bouvy ND, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251(2):236–43. doi:10.1097/SLA.0b013e3181bdfbff.
Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146(2):143–8. doi:10.1001/archsurg.2010.326.
Lee WJ, Chen CY, Chong K, et al. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis : Off J Am Soc Bariatric Surg. 2011;7(6):683–90. doi:10.1016/j.soard.2011.07.009.
Chen CY, Lee WJ, Asakawa A, et al. Insulin secretion and interleukin-1beta dependent mechanisms in human diabetes remission after metabolic surgery. Curr Med Chem. 2013;20(18):2374–88.
Lee WJ, Yu PJ, Wang W, et al. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005;242(1):20–8.
Wang W, Wei PL, Lee YC, et al. Short-term results of laparoscopic mini-gastric bypass. Obes Surg. 2005;15(5):648–54. doi:10.1381/0960892053923752.
Ser KH, Lee WJ, Lee YC, et al. Experience in laparoscopic sleeve gastrectomy for morbidly obese Taiwanese: staple-line reinforcement is important for preventing leakage. Surg Endosc. 2010;24(9):2253–9. doi:10.1007/s00464-010-0945-x.
Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA : J Am Med Assoc. 2001;285(19):2486–97.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–98.
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA : J Am Med Assoc. 2013;309(21):2240–9. doi:10.1001/jama.2013.5835.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 0(0):null. doi:10.1056/NEJMoa1401329.
Lee WJ, Chong K, Chen CY, et al. Diabetes remission and insulin secretion after gastric bypass in patients with body mass index <35 kg/m2. Obes Surg. 2011;21(7):889–95. doi:10.1007/s11695-011-0401-6.
Lebovitz HE. Metabolic surgery for type 2 diabetes with BMI <35 kg/m(2): an endocrinologist’s perspective. Obes Surg. 2013;23(6):800–8. doi:10.1007/s11695-013-0907-1.
Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA : J Am Med Assoc. 2013;309(21):2250–61. doi:10.1001/jama.2013.4851.
Yang X, So WY, Kong AP, et al. Development and validation of a total coronary heart disease risk score in type 2 diabetes mellitus. Am J Cardiol. 2008;101(5):596–601. doi:10.1016/j.amjcard.2007.10.019.
Jimenez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9. doi:10.1097/SLA.0b013e318262ee6b.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7. doi:10.1097/SLA.0b013e318156f012.
Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258(5):690–4. doi:10.1097/SLA.0b013e3182a67426.
Yang X, Yang G, Wang W, et al. A meta-analysis: to compare the clinical results between gastric bypass and sleeve gastrectomy for the obese patients. Obes Surg. 2013;23(7):1001–10. doi:10.1007/s11695-013-0938-7.
Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709–16. doi:10.2337/dc06-1549.
Doar JW, Wilde CE, Thompson ME, et al. Influence of treatment with diet alone on oral glucose-tolerance test and plasma sugar and insulin levels in patients with maturity-onset diabetes mellitus. Lancet. 1975;1(7919):1263–6.
Kelley DE, Wing R, Buonocore C, et al. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77(5):1287–93. doi:10.1210/jcem.77.5.8077323.
Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes. 2010;34(3):462–71. doi:10.1038/ijo.2009.254.
Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–95. doi:10.1038/ijo.2009.79.
Valderas JP, Irribarra V, Rubio L, et al. Effects of sleeve gastrectomy and medical treatment for obesity on glucagon-like peptide 1 levels and glucose homeostasis in non-diabetic subjects. Obes Surg. 2011;21(7):902–9. doi:10.1007/s11695-011-0375-4.
Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24(2):241–52. doi:10.1007/s11695-013-1066-0.
Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36(1):20–6. doi:10.2337/dc12-0779.
Dixon JB, Hur KY, Lee WJ, et al. Gastric bypass in type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diabet Med : J B Diabet Assoc. 2013;30(4):e127–34. doi:10.1111/dme.12107.
Kim JW, Cheong JH, Hyung WJ, et al. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol : WJG. 2012;18(1):49–54. doi:10.3748/wjg.v18.i1.49.
Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA : J Am Med Assoc. 2008;299(3):316–23. doi:10.1001/jama.299.3.316.
Lee WJ, Ser KH, Chong K, et al. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147(5):664–9. doi:10.1016/j.surg.2009.10.059.
Acknowledgments
This work was supported by the research grants from Min Sheng General Hospital, Taiwan.
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration clinicaltrials.gov identifier: NCT00540462
Rights and permissions
About this article
Cite this article
Lee, WJ., Chong, K., Lin, YH. et al. Laparoscopic Sleeve Gastrectomy Versus Single Anastomosis (Mini-) Gastric Bypass for the Treatment of Type 2 Diabetes Mellitus: 5-Year Results of a Randomized Trial and Study of Incretin Effect. OBES SURG 24, 1552–1562 (2014). https://doi.org/10.1007/s11695-014-1344-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1344-5