Abstract
Some hosts harbor more parasites than others. Overdispersion of parasitism suggests that coevolution with parasites may be more important to the biology and ecology of certain species. We examined patterns of parasitism and host traits in fished decapod crustaceans, which are economically and ecologically important worldwide. Using a synthesis approach, we determine that host life history, including habitat, longevity, sociality, invasion history, and fisheries involvement, correlate with the number and type of parasite species harbored. Indicator species analysis revealed close relationships between decapods and certain parasite groups, including crabs with rhizocephalans and dinoflagellates; crayfish with mesomycetozoans, oomycetes, branchiobdellids, and fungi; lobsters with copepods and amoebae; and shrimp with viruses. In contrast, Nematomorpha and Nemertea appear to be under-represented and under-studied as parasite groups in decapods. Decapods that are commercially fished, aquacultured, introduced outside their native range, and/or exhibit parental care tend to have higher parasite species richness (PSR). Parasite richness also increases with how well-studied a host group is, which we addressed with a machine learning algorithm that predicts false negative associations. Geographic range is commonly positively correlated with parasite richness, however reliable ranges are not available for most decapod species, highlighting a significant future research need. Identifying patterns such as these increases our broad understanding of decapod disease ecology but also enabled us to develop a series of recommendations on how to focus future research, management, and aquaculture development efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild fisheries and aquaculture support global economies and take advantage of an abundance of protein-rich organisms, including crustaceans (Stentiford et al. 2020; Behringer and Duermit-Moreau 2021). The United Nations Food and Agriculture Organization (FAO) tracks ~ 200 crustacean commercial fisheries and aquaculture that produced 16.5 million metric tons in 2019 (FAO 2021), an increase from 11.7 million metric tons in 2016, which was worth over $50 billion USD (FAO 2018). The capacity for fisheries and aquaculture to produce yield can be limited by the environment and an organism’s biological or ecological requirements, but the most dramatic limits are often imposed by parasitic diseases (Stentiford et al. 2012).
Crustaceans suffer from a wide range of parasitism and disease that can increase mortality, stunt growth, limit recruitment and, ultimately, reduce yield or decrease marketability (Cawthorn 2011; Shields 2011; Lafferty et al. 2015; Bojko and Ovcharenko 2019; Arulmoorthy et al. 2020). Impacts from these parasites can cause great economic damage as well. For example, Hematodinium perezi, a generalist parasite, is estimated to cause losses upward of $1 million USD per year in the US blue crab Callinectes sapidus fishery alone (Stentiford and Shields 2005). Aquaculture losses are easier to assess and are estimated to exceed $6 billion USD per year across all sectors due to disease (Stentiford et al. 2017, 2020). A striking example is the impact of white spot syndrome virus (WSSV), which cost the penaeid shrimp aquaculture industry $8–12 billion USD as of 2012 (Stentiford et al. 2012).
Most animal species host one or more parasites (Price 1980), yet parasitism is not evenly distributed among host groups or individuals (Dritschilo et al. 1975; Nunn et al. 2003; Luis et al. 2013). Some hosts harbor few parasite species, whereas others harbor a diversity of parasites and diseases. Parasites impact behavior, growth, survival, and reproduction (Valenzuela-Sánchez et al. 2021), playing a nuanced role in the evolutionary trade-offs between survival and reproduction (Hochberg et al. 1992). For example, toad populations long exposed to the fungal disease chytridiomycosis breed at smaller sizes than before exposure, suggesting that they breed earlier (Lampo et al. 2022). Valenzuela-Sánchez et al. (2021) show similar patterns in many species of vertebrates exposed to a variety of diseases/parasites, suggesting that coevolution with parasites may be of greater importance to the biology and ecology of some groups over others.
Here, we provide the most thorough synthesis to date of decapod hosts and their parasites, using Linear Discriminant Analysis (LDA) and Indicator Species Analysis to explore correlations and distinctions between host groups, their traits, and their parasites. Our analysis seeks to increase the understanding of disease in decapods and provides information for managers and researchers on where future efforts should focus.
Life history traits of decapod crustaceans
The Food and Agriculture Organization of the United Nations (FAO) curates a list of species in capture fisheries, aquaculture, and ‘of-interest’ to these sectors in their global fishery production database (FAO 2017). Species ‘of-interest’ to the FAO are not commercially captured but may be captured in small artisanal or recreational fisheries. On the FAO list, 915 are decapod crustaceans. We subsampled from this list for decapods with a longevity estimate and at least one known parasite in wild populations (n = 101). Life history data was compiled for each of these species on habitat, sociality, invasion history, and use in commercial fisheries or production.
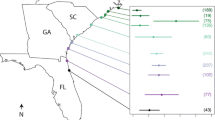
Decapod hosts were grouped taxonomically as ‘crab’ (families: Calappidae, Cancridae, Galatheidae, Geryonidae, Grapsidae, Lithodidae, Majidae, Ocypodidae, Oregoniidae, Portunidae, Xantihdae), ‘crayfish’ (Astacidae, Cambaridae, Parastacidae), ‘lobster’ (Nephropidae, Palinuridae), or ‘shrimp’ (Callianassidae, Crangonidae, Palaemonidae, Pandalidae, Penaeidae, Solenoceridae, Upogebiidae) (FAO 2017). Taxonomic groups were then used in Linear Discriminant Analysis (LDA) using the MASS package in R (Venables and Ripley 2002) on host characteristics, to determine which characteristics best differentiated the groups. While this is not the only suitable approach, LDA is specifically useful as a multivariate approach for determining which set of host characteristics are useful for discriminating between and classifying taxa into groups. Characteristics in the full model included: longevity; log of the number of parasite citations; and binary variables (0/1) for nonmutually exclusive membership to: habitat classes (euryhaline, freshwater, marine); fishery practices (aquaculture, capture, no commercial production); introduced or native; and sociality (gregarious, parental care, reproductive aggregations, solitary). Stepwise model selection using Wilks lambda from the klaR package (Weihs et al. 2005) was used to choose the best model, retaining only the host characteristics that significantly distinguish groups and subsequently removing collinear variables. Each host characteristic was compared between the groups using analysis of variance (ANOVA) for continuous variables and chi-square tests (with Yate’s continuity correction when expected values < 5) on categorical variables (R Core Team 2021). The final LDA model for the taxonomic host groups included the following host characteristics: longevity, parental care, solitary, reproductive aggregations, marine, and non-commercial species (Fig. 1a).
Linear discriminant analysis of decapod crustacean host characteristics. a In taxonomic host groups and b in polythetic agglomerative clusters based on parasite associations. Significant parasite associations based on indicator species analysis are given in legend on right. Arrows depict the direction and strength (i.e. length of arrow) of host life history characteristic associations
Longevity
Age and lifespan are important in the management of fished species and vital for stock assessment (Campana 2001; Sheehy and Prior 2008). Accurately ageing decapod crustaceans has proved difficult historically, in part because hard structures [which are used in ageing molluscs (Schöne et al. 2005), fish (Skurdal et al. 1985), and urchins (Flores et al. 2010)] are shed during molting. Methods used to age crustaceans have varied in their reliability and include captive rearing, mark-recapture, growth models, and lipofuscin pigment analysis (Vogt 2012). More recently, researchers have been working to verify the reliability of bands deposited on the gastric mill to establish chronological age (Kilada and Driscoll 2017; Gnanalingam et al. 2019), and DNA methylation techniques have shown promise as a molecular-based method of aging a wide variety of organisms, including crustaceans (Fairfield et al. 2021). Our dataset includes longevity estimates from peer-reviewed literature that utilizes these methods (Suppl. Table 1).
Longevity is a significant, though weak, differentiator between taxa in the LDA model (Fig. 1a). Lobsters are longer-lived than all other groups, shrimp are shorter-lived, and crab and crayfish are intermediate (F = 29.9, df = 3,97, p < 0.001; Table 1; Suppl. Fig. 1a). Age estimation methods, namely captive rearing, are biased towards shorter-lived species, which is reflected in our dataset with 62.4% of the species under 5 years maximum longevity. Many species of large decapods are thought to live many decades but do not yet have reliable lifespan estimates. In our dataset, there is a 72-fold difference between the shortest-lived species, which live to one year and are all shrimp: Palaemonetes paludosus, Penaeus indicus, Penaeus longistylus, Penaeus vannamei and the longest-lived (72-year lifespan) species, the European lobster Homarus gammarus (Suppl. Table 1).
Longer-lived species often support lucrative fisheries, in part due to indeterminate growth and their resulting large size. The American lobster Homarus americanus is estimated to live upwards of 50 years and is the most valuable fishery in North America (Finch 1994; le Bris et al. 2018). However, because long-lived species also tend to mature later (5–9 years for H. americanus; le Bris et al. 2017) and larger individuals with greater reproductive output are sought (Koopman et al. 2015), they are more susceptible to overfishing (Beamish et al. 2006). The European and American lobster fisheries have both experienced stock declines in recent decades (Pettersen et al. 2009; Howell 2012). Longer-lived animals also tend to be difficult to rear in aquaculture, as it can take many years to reach a marketable size. In contrast, short-lived species are more commonly aquacultured, probably because their faster time to market makes them more profitable, easier to manage, and the economic losses due to disease are less catastrophic on a per outbreak basis. Whiteleg shrimp (P. vannamei) fit this paradigm and have become the most heavily aquacultured crustacean worldwide (FAO 2018).
Evolutionary theory predicts that parasitized individuals can increase their fitness by diverting energy toward reproduction and immunity and away from longevity (Hochberg et al. 1992). This strategy can drive a population or species towards r-selected life history traits, including shorter lifespan, if infection with virulent pathogens is relatively common. This association has been demonstrated in snails (Hochberg et al. 1992) and mammals (Morand and Harvey 2000). Alternatively, long-lived hosts may offer a more stable environment for parasitic infection (Poulin and Morand 2000).
Prediction: If evolution has driven more highly parasitized species toward r-selected life history traits then shorter-lived hosts will have higher PSR.
Alternative prediction: If parasites prefer a stable environment for parasitic infection, then longer-lived hosts will have higher PSR.
Habitat
Salinity tolerance is a major factor in species distribution (de Jonge 1974; Chakraborty et al. 2011) and can impact many aspects of commercial fisheries. Our analysis of FAO fishery data from 2019 indicates that most crustaceans harvested in capture and aquaculture fisheries come from brackish water (38.0%), followed by marine (34.0%), then freshwater (28.0%; FAO 2021). We categorized each species as marine, freshwater, or euryhaline based on the World Register of Marine Species (WoRMS; Horton et al. 2018; Suppl. Table 1). Species that were categorized by WoRMS as ‘brackish’ or under multiple habitats were deemed ‘euryhaline’ here. It is clear that habitat differs among the taxonomic groups (X2 = 86.1, df = 6, p < 0.001; Table 1; Fig. 2a)—e.g. All lobsters are marine, most crayfish are freshwater species, and crab and shrimp are mostly marine with some euryhaline or freshwater species (Fig. 1a).
Prediction: If euryhaline hosts are susceptible to parasites with a variety of salinity tolerances and likely to encounter different parasite species in lower versus higher salinity portions of their range, then euryhaline hosts will have greater PSR.
Sociality
Animals range from colonial/eusocial species to solitary ones, with most falling somewhere in between. Aggregation and social behaviors evolve when “the net benefits of close association with conspecifics exceed the costs” (Silk 2007). Benefits of close association include predator avoidance (Neill and Cullen 1974), reproduction (Stone et al. 1993), and parental care (Thiel 2003), while costs include parasite transmission (Arneberg et al. 1998), resource competition (Korb and Heinze 2016), and agonistic behaviors (Sinclair 1977). Social aggregations are often exploited in capture fisheries, which may select against social behavior by selectively capturing large schools (Guerra et al. 2020).
Four categories of sociality were defined and decapods were categorized based on peer-reviewed literature (Suppl. Table 1). Categories are as follows: (1) ‘gregarious’ species are those known to regularly aggregate; (2) ‘solitary’ species generally reside alone, encountering conspecifics for mating in pairs, not in large groups; (3) species with ‘reproductive aggregations’ form large pods for molting, mating, and/or spawning; and (4) species with ‘parental care’ have aggregations of juveniles with parents (typically the female parent) during early life stages, but other stages are solitary.
Sociality was a strong differentiator in the taxonomic LDA (Fig. 1a), significantly differentiating the taxa (X2 = 75.4, df = 9, p < 0.001; Table 1; Fig. 2c). Shrimp are comprised of species with all sociality types but are largely gregarious. A gregarious nature benefits commercial fisheries using seines and trawls to capture large schools (Tulian 1920; Xiao and Greenwood 1993) and benefits aquaculture in allowing for high stocking densities (Bardera et al. 2019). Lobsters can be solitary or gregarious, often depending on whether they are clawed or spiny, respectively. Gregariousness in the Caribbean spiny lobster, Panulirus argus, is used to the advantage of the fishery. In Florida, traps are baited with live juvenile lobsters that attract legal lobsters using chemosensory cues (Hunt et al. 1986), and elsewhere, lobsters aggregate in casitas for divers to capture. Like clawed lobsters, crabs are largely solitary. The Florida stone crab, Menippe mercenaria, is prized for its large claws, which are the only landed product (Gandy et al. 2016; Duermit et al. 2017). They live a largely solitary life, using these claws in agonistic encounters with conspecifics (Sinclair 1977), only cohabitating for reproduction and mate-guarding (Wilber 1989). Crabs also have the largest proportion of reproductive aggregations, often referred to as “podding” behavior. These pods protect individuals during the vulnerable molting period and subsequent mating (Carlisle 1954). Pods have been utilized by fishers for centuries (Števčić 1971) and Stone et al. (1993) hypothesized that their existence in the economically valuable red king crab, Paralithodes camtschaticus, may have led to overexploitation, resulting in fishery collapse and closure in 1983. Crayfish exhibit more parental care than other decapods. Many decapods incubate eggs on the underside of their abdomen until hatching, but crayfish juveniles often remain with their mother and siblings for weeks to months for further protection, potentially as an adaptation to higher current and different dispersal needs in freshwater habitats (Scholtz and Kawai 2002). Efforts to exploit this trait to increase aquaculture production have not been fruitful (Patoka et al. 2013).
Prediction: If greater interaction among conspecifics increase opportunity for direct transmission of parasites (Anderson and May 1982) then gregarious hosts will have higher PSR than hosts with other social systems.
Introduced species
Invasive and non-native species (INNS) can present great risks to native fauna (Wilcove et al. 1998) and economies (Pimentel et al. 2005; Cook et al. 2007). While most introductions are accidental, they tend to be associated with anthropogenic sources, including fishing and aquaculture. The red claw crayfish, Cherax quadricarinatus, has been introduced to many regions due to its high value in aquaculture. In Mexico, poor management of aquaculture ponds led to the escape and establishment of this species, which is now a significant portion of the catch in the native Macrobrachium americanum fishery (Vega-Villasante et al. 2015). Fishing can also be used as a method of control for invasive species, e.g., blue crab Callinectes sapidus, native to the western hemisphere, invasive and fished in the Mediterranean (Mancinelli et al. 2017).
Using the Global Invasive Species Database Information System on Aquatic Non-Indigenous and Cryptogenic Species, and the European Alien Species Information Network (EASIN) [following methods in (Bojko et al. 2021)] we categorized our decapods as species that have been introduced or species that have not been introduced (Suppl. Table 1). For simplicity, these will be referred to as “introduced” and “native”, respectively, though parasites of introduced hosts are also included from the native ranges. Overall, 34.7% of species in the dataset are introduced and the proportion of introduced species did not differ by taxonomic group (X2 = 3.3, df = 3, p = 0.35; Table 1; Fig. 2b). Introduction was not a significant indicator in the taxonomic LDA (Fig. 1a).
There are two major hypotheses for the diversity of parasites in introduced species. First, the “parasite acquisition hypothesis” sometimes referred to as “parasite spillover and spillback” (Kelly et al. 2009) claims that introduced species have greater parasite diversity, because they can acquire parasites in both their native and introduced ranges and these ranges are likely to have different parasites. The second is an extension of the “enemy release hypothesis” (Colautti et al. 2004), which suggests that a species with many parasites (enemies) in its native range is likely to be introduced without those parasites, allowing for its proliferation and success in the invasive range. This hypothesis suggests that species with many enemies in their native range are more likely to become invasive because of this release. These hypotheses are not mutually exclusive and have been documented to act simultaneously (Sheath et al. 2015).
Prediction: If parasite acquisition and/or enemy release occur then hosts that are introduced will have greater PSR, when accounting for both their native and introduced ranges, than hosts living in their native range only (native).
Aquaculture and commercial fisheries
We categorized species as a part of aquaculture, commercial capture fisheries, both, or neither (“non-commercial”; Suppl. Table 1). Species designated by the FAO as non-commercial are not currently exploited commercially but they are of interest to the FAO, indicating that there may be small artisanal or recreational fisheries, or fisheries and aquaculture are being explored. Overall, 25.7% of species are both cultured and fished: 3.0% in aquaculture only, and 36.6% capture only. We included aquacultured species, but we excluded parasites found only in aquaculture settings, since their wildlife origin or natural evolution is difficult to untangle in parasitological literature.
“Non-commercial” was a moderate differentiator between groups in the taxonomic LDA (Fig. 1a). The crab and lobster taxa are negatively associated with “non-commercial” (i.e. they are commercially exploited). All lobster species in our dataset are exploited in capture fisheries or in both capture and aquaculture. In our dataset, 34.2% of crabs are not exploited in capture or aquaculture.
Prediction: If aquaculture provides many routes for release and introduction of pathogens to wild populations, such as introduction of infected stocks, movement of equipment and fish products, viral and bacterial evolution, and exposure to environmental reservoirs (Murray and Peeler 2005), then wild hosts that are also in aquaculture will have greater PSR.
Alternative prediction: If aquacultured and captured hosts are examined more thoroughly due to their fisheries interest, then these hosts will have greater PSR.
Species sampling intensity
Sampling intensity varies widely between species and, in general, the more a species is studied, the more parasites tend to be found (Poulin 1997). We accounted for sampling intensity via the number of citations on Web of Science for the species name (and common synonyms/previous names) ‘AND pathogen* OR parasite* OR disease*’ in the title or abstract (Suppl. Table 1). In this dataset, 37.6% of species have five or fewer citations mentioning parasite keywords, but two species have > 500: P. vannamei (1,717) and P. monodon (940). These extreme numbers likely reflect that these are the two top aquacultured species worldwide (Thitamadee et al. 2016). Despite this gap, the taxonomic groups are not significantly different in how well-studied they are regarding parasitism (F = 0.77, df = 3,97, p = 0.51; Table 1; Fig. 3a). Sampling intensity was not an important factor in the taxonomic LDA (Fig. 1a).
We examined additional models that accounted for total species sampling intensity (i.e. not necessarily related to disease research). These variables were highly correlated (0.90) and in this case (and all cases hereafter) these models were nearly identical to those that use pathogen search results instead. We chose to include pathogen search results because LDA models that included this term explained slightly more variance than those that included total host species search results.
False negative estimation
Despite the lack of relationship between sampling intensity and the taxonomic groups, there is a positive relationship between sampling intensity and reported PSR (R2 = 0.35; see Supplemental Materials). This pattern suggests that we may be missing many possible parasite-host relationships because they have not been studied, resulting in false negatives in our dataset. To model the probability of given unobserved linkage being a false negative, we predicted the probability of a linkage occurring between a given parasite species infecting one host species using Ensemble Random Forests (ERF; Siders et al. 2020), a machine learning algorithm designed to model rare event data (see detailed methods and results in Supplemental Material). Using the linkages sampled in the literature review, the probability of all parasite-host linkages was predicted assuming the maximum sampling intensity and then the probability of a given linkage being a false negative was calculated using the (Morton et al. 2021) approach. Across the 48 ‘host type’ and ‘pathogen group’ interactions, 11 increased the odds of a parasite-host linkage by more than 50% indicating a high chance of linkages occurring between these host types and pathogen groups (Fig. 4). These false negatives were not further included in analyses but are used as discussion points below to identify and examine areas where under sampling could lead to biases.
Accumulated local effects of the host type and pathogen group interactions measuring the change in the ensemble random forests predicted linkage probability with the circle indicating the median value and the segment indicating the 90% confidence interval. Also shown is the observed prevalence of host type and pathogen group links from the 31,412 possible links
Parasite species richness in fished decapods
Parasites and their characteristics were compiled by searching Web of Science and Google Scholar for each decapod species of interest ‘AND pathogen* OR parasite* OR virus’ (year-range: 1864–2020). Commensal symbionts were excluded, as were parasites that were not identified to the genus level (excluding viruses, which were identified to the family level). Parasites were classified by their higher taxonomy (Acanthocephala, Amoebae, Apicomplexa, Bacteria, Branchiobdellida, Cestoda, Ciliophora, Copepoda, Dinoflagellate, Fungi, Haplosporidia, Isopoda, Hirudinea, Mesomycetozoa, Microsporidia, Nematoda, Nematomorpha, Nemertea, Oomycete, Paramixida, Rhizocephala, Trematoda, or Virus). Relationships between parasite and host were categorized as natural (i.e. detected in wild populations), cultured (i.e. only seen in aquaculture setting), or both natural and cultured. For this analysis, passaged parasites (i.e. pathogenicity only known from laboratory inoculation) and those found exclusively in aquaculture were excluded from the dataset. Decapods host 311 known parasites and a total of 550 parasite-host associations have been recorded (Fig. 5a). The groups accounting for the most parasite-host relationships are protozoans (Amoeba, Apicomplexa, Ciliophora, Dinoflagellate, Haplosporidia, Mesomycetozoa, Oomycete, Paramixida) (18%) and viruses (Birnavirdiae, Bunyavirales, Cruliviridae, Dicistroviridae, Mininucleoviridae, Nidovirales, Nimaviridae, Nodaviridae, Nudiviridae, Parvoviridae, Picornaviridae, Reoviridae, Rhabdoviridae, Tombusviridae, Totiviridae) (17%). Variation in the number of parasites per host species (PSR) was analyzed using generalized linear mixed effect models (GLMM) with a negative binomial fit for count data in the package glmmTMB (Brooks et al. 2017). Differences between groups were then analyzed with Analysis of Variance using the car package (Fox 2011).
The relative number of parasite taxonomic groups in wild populations of decapod crustaceans: a all groups combined and b by host group. Several taxa are grouped here that are kept separate for analyses: Annelida (Brachiobdella, Hirudinea), Crustacea (Rhizocephala, Isopoda), Platyhelminth (Cestoda, Trematoda), and Protozoan (Amoeba, Apicomplexa, Ciliophora, Dinoflagellate, Haplosporidia, Mesomycetozoa, Oomycete, Paramixida)
Taxonomic host groups have distinct parasite assemblages (PERMANOVA: F = 6.3, R2 = 0.16, p < 0.001; Fig. 5b), except crab and lobster (F = 1.2, R2 = 0.03, p = 0.31). Indicator species analysis, which combines a parasite’s host fidelity and relative abundance and predicts the probability of finding a higher indicator value in another taxon (Dufrene and Legendre 1997), revealed eight parasite taxa strongly associated with host taxa (Fig. 1a). Rhizocephalans and dinoflagellates were indicators of the crab group (indicator value = d = 0.33, p = 0.03 and d = 0.29, p = 0.04, respectively); the crayfish group was indicated by mesomycetozoeans (d = 0.76, p = 0.001), oomycetes (d = 0.51, p = 0.003), branchiobdellids (d = 0.51, p = 0.001) and fungi (d = 0.25, p = 0.03); and indicators of the lobsters were copepods (d = 0.33, p = 0.01) and amoebae (d = 0.18, p = 0.04). There were no statistically significant parasite indicators for shrimp (but see Sect. “Shrimp parasites”). Overall, crayfish had a significantly greater reported PSR than shrimp (Fig. 6a; F = 10.6, df = 3, p = 0.014) but other pairwise differences were not significant.
Crab parasites
Rhizocephalans are parasitic barnacles that can infect decapods, molluscs, or can be free-living (Høeg 1995; Boyko and Williams 2009). In our dataset they are exclusive to crabs (Anomura and Brachyura), with brachyurans accounting for two-thirds of all known hosts (Shields et al. 2015). Female rhizocephalans infect their host with an endoparasitic internal phase before extruding a virgin externa through the host’s abdomen, which attracts males for reproduction (Høeg 1995). These parasites castrate male and female crabs (O’Brien and Wan Wyk 1985) often altering host behavior (Toscano et al. 2014). Rhizocephalans affect crab marketability and recruitment, often causing issues for fisheries (Lafferty et al. 2015).
Dinoflagellate parasites are represented by Hematodinium spp. in our dataset, though this is not the only dinoflagellate parasite of crustaceans (Stentiford and Shields 2005). These parasites appear to be generalists among brachyurans but can also infect wild Norway lobster Nephrops norvegicus (Field and Appleton 1995), cultured ridgetail prawn Exopalaemon carinicauda (Xu et al. 2010) and giant tiger prawn P. monodon (Wang et al. 2017), and amphipods (Messick and Shields 2000). Hematodinium sp. proliferates in the hemolymph, leading to a milky appearance, which is often accompanied by hyperpigmentation of the exoskeleton, similar to a cooked animal (Small 2012). Other clinical signs have led to a range of syndromes, notably Bitter Crab Disease (BCD) in important capture fisheries including snow and tanner crabs Chionoecetes spp., the velvet crab Necora puber, and the king crabs Paralithodes camtschaticus and P. platypus (Meyers et al. 1987; Wilhelm and Mialhe 1996; Ryazanova 2008). Infection can also proliferate in aquaculture systems and transmit between susceptible species in polyculture systems (Xu et al. 2010; Wang et al. 2017). Economic impacts from this parasite are difficult to calculate but have ranged from $250,000 USD to nearly $10 million USD for various fisheries (Stentiford and Shields 2005).
From the ERF, we estimated that there are two groups of crab parasites that are underrepresented in our dataset: Nematomorpha and Nemertea. Nemerteans are quite prevalent as egg predators and gill parasites in crabs and lobsters but are understudied despite the potential for massive brood mortality and associated population and evolutionary consequences (Kuris and Wickham 1987). Wickham (1979) estimated that epidemic levels of the nemertean Carcinonemertes errans on the Dungeness crab Cancer magister was causing direct mortality of 55% of eggs produced and could lead to local fishery collapse.
Nematomorpha are seemingly a much rarer, though underreported, parasite of decapods, with only five instances in our dataset, two of which are in crab hosts. They are represented by a single genus Nectonema, a marine horsehair worm also infecting shrimp, lobsters, and isopods (Schmidt-Rhaesa et al. 2013; Kakui et al. 2021). Effects of these parasites on host behavior and physiology are unclear, some studies report reduced gonad size and fecundity, particularly in freshwater Nematomorpha, (summarized in Stevens 2022) though this parasite group is not well-studied.
Crayfish parasites
Branchiobdellids are obligate symbionts of crayfish and can be commensal (Longshaw 2011), mutualistic (Brown et al. 2002), or parasitic (Hobbs Jr et al. 1967). This group can elicit a host immune response in particularly heavy infestations (Alderman and Polglase 1988), have been implicated in mortality (Hubault 1935), and can transfer to sympatric crab species (Gelder et al. 2001). As obligate symbionts of crayfish, it seems likely that these groups experience some degree of coevolution; however, branchiobdellid taxonomy remains in flux (Skelton et al. 2013). While a linkage between crayfish and Branchiobdellids was common, as Annelids, this group also represents a linkage with a high change in probability from the ERF, thus there are likely false negative associations that are missed in our dataset. These false negatives may be due to branchiobdellids’ common classification as crayfish commensals, which were removed from our analysis.
Mesomycetozoeans are represented by the Psorospermium genus (Order Ichthyophonida) in our dataset and are only noted as parasites of crayfish. This genus infects wild and aquacultured crayfish, causing significant mortalities (Cerenius et al. 1991). Mesomycetozoans are a relatively new taxonomic designation (Glockling et al. 2013) and their relationship with non-crayfish decapods as commensals versus parasites is unclear (McDermott 2011).
Fungi are a more concerning parasite of decapods, particularly the genus Fusarium, which causes black gill disease (Souheil et al. 1999; Mahmoud 2019). Proliferation of this filamentous fungus in the gills leads to melanization, impacting osmoregulatory ability and causing mortality that can have grave economic impacts to fisheries and aquaculture (Souheil et al. 1999). Another concerning fungal parasite is Batrachochytrium dendrobatidis, which is implicated in the worldwide decline of amphibian species (Cheng et al. 2011). Crayfish are also susceptible to this “chytrid fungus” and may even be a reservoir and vector of the disease to amphibians (McMahon et al. 2013).
Aphanomyces astaci (oomyete; crayfish plague) has devastated crayfish in Europe, where it was introduced by invasive North American crayfish in the nineteenth century (Alderman 1996) and has since become one of the best-studied invertebrate parasites (Svoboda et al. 2017). Whereas infected European crayfish experience rapid mortality, North American crayfish are generally latent carriers of the disease suggesting that it evolved with crayfish there (Svoboda et al. 2017). Despite devastating impacts, North American crayfish are still being introduced to Europe for aquaculture purposes (Svoboda et al. 2017).
Aside from Annelids, represented largely by Branchiobdellids, the other largely underreported parasite group in crayfish, according to the ERF, is Nematomorpha. This is an intriguing correlation because there are no linkages in our dataset and we were not able to find any reports in the literature for Nematomorpha parasitizing crayfish, though crayfish predation on these worms has been documented (Cochran et al. 1999). Nematomorpha are divided into two groups—the gordiids or “freshwater horsehair worms” that infect millipeds and insects and the nectonematids or “marine horsehair worms” that infect decapods and a few other crustacean groups (Bolek et al. 2015). Thus, there may be a disconnect between host-parasite evolution and environmental needs that limits the ability of nematomorphs to parasitize freshwater crayfish, which would highlight a weakness in our false negative analysis.
Lobster parasites
Crabs and lobsters were not distinct in their parasite assemblages (Fig. 1a), though parasitic copepods and amoeba were indicators of lobsters because of their relative prevalence (40%). Parasitic copepods (Choniosphaera sp. and Nicothoe astaci) were identified in crabs and lobsters, respectively, in our dataset. In crabs, these copepods are egg predators and considered parasitic, because they live within the clutch and mimic the appearance of eggs (Gotto 2004). Choniosphaera sp. can have a significant impact on fecundity, suggesting negative impacts to fishery recruitment, if sufficient prevalence and burden is reached (Shields and Wood 1993). In lobsters, N. astaci is a gill parasite that feeds on host hemolymph, can have high burden and up to 100% prevalence in populations (Wootton et al. 2011), and impedes host respiratory function (Davies et al. 2015). Impacts of N. astaci on lobster fisheries are not well understood, but there is evidence that adverse effects increase with stress (Gibson 1961; ICES 2007).
Consequences of amoebic infections in crustacean fisheries are better documented. Neoparamoeba sp. infect crab and lobster haemal spaces and connective tissue, circulating through the hemolymph in terminal infections causing tissue damage and lethargy (Johnson 1977) and infect invasive, co-habiting, Carcinus maenas (Bojko et al. 2018). Infection can spread rapidly in blue crab Callinectes sapidus shedding facilities (Shields 2012) and is implicated in the mass mortality of American lobster H. americanus in Long Island Sound in 1999–2000 (Mullen et al. 2004). This epidemic played a role in a local fishery collapse, where H. americanus catch was reduced by 90–99% (Mullen et al. 2004; Shields 2012).
Lobsters have more parasite groups with high probability of false negative than the other host taxa, possibly due to relatively low observed prevalence of parasite groups (Fig. 4). The parasite groups likely underrepresented in our dataset include Acanthocephala, Bacteria, Fungi, Nematoda, Nematomorpha, and Nemertea.
Shrimp parasites
Shrimp account for 40.6% of hosts in our dataset and 33.6% of decapod host-parasite associations. This is low given the general perception that shrimp harbor many parasites relative to other crustaceans. Lafferty et al. (2015) showed that 61.5% of crustacean diseases of economic consequence impact shrimp. However, aquaculture is the source of much of the economic losses and our dataset only included parasites that have been found in wild decapod populations. It is believed that most aquaculture parasites are sourced from wild stocks (McVicar 1997; Kurath and Winton 2011), that they impact stressed animals in crowded monoculture ponds (Kent 2000), and that most of these diseases are subsequently exported from farms to wild populations (Lafferty et al. 2015). Infectious hypodermal and hematopoietic virus (IHHNV) is an important example of disease spillover from aquaculture to wild shrimp populations (Lightner et al. 1992). IHHNV was introduced to the Gulf of California in 1987 via a shipment of infected P. vannamei and spread through regional farms of Penaeus stylirostris (Lightner et al. 1992). By 1990, IHHNV was found in wild fisheries of P. stylirostris, which had declined by 50% and remained dampened for a decade (Morales-Covarrubias et al. 1999).
For these reasons, many of the viruses that cause issues in aquaculture (e.g. white spot syndrome virus, yellow-head virus, Taura syndrome virus, IHHNV) have been included in our data, and shrimp are shown to account for 57.1% of the host-virus relationships. Viruses were strong indicators of the shrimp taxon (d = 0.24); however, this was not significant in our model (p = 0.20), because viruses were also important, though weak, indicators of the other taxa (crab d = 0.07, crayfish d = 0.08, lobster d = 0.05).
For shrimp, the parasite group most likely to be underrepresented is the Nematomorpha, discussed in more detail in Sects. “Crab parasites” and “Crayfish parasites”.
Parasite species richness and host traits
To examine how host traits influence PSR, we first grouped hosts using polythetic agglomerative hierarchical clustering (Sneath and Sokal 1973) according to a Bray–Curtis distance matrix of parasite associations (hereafter ‘agglomerative clustering’). This method is blind to host characteristics allowing us to later examine how parasite species and host characteristics are correlated. The distance matrix was calculated on parasite counts within each host species, where parasites were grouped taxonomically. The clustering method yielded four host groups with distinct parasite assemblages (PERMANOVA: F = 16.6, R2 = 0.34, p < 0.001; Fig. 1b) and n = 8, 48, 17, and 28 host species, respectively. These groups were not equivalent to the four taxonomic host groups.
Eleven parasite taxa were associated with the groups as indicator species: nemerteans were indicators of Group 1 (d = 0.86, p = 0.001); viruses (d = 0.52, p = 0.002), apicomplexans (d = 0.31, p = 0.008), dinoflagellates (d = 0.20, p = 0.007), and cestodes (d = 0.25, p = 0.02) were indicators of Group 2; mesomycetozoans (d = 0.76, p = 0.001), oomycetes (d = 0.59, p = 0.001), branchiobdellids (d = 0.52, p = 0.001), bacteria (d = 0.37, p = 0.006), and fungi (d = 0.25, p = 0.015) were indicators of Group 3; and isopods (d = 0.57, p = 0.001) were indicators of Group 4. Groups 1 and 2 had significantly greater PSR (Fig. 6b; F = 21.0, df = 3, p < 0.001) relative to Group 4.
Host clusters were then analyzed with an LDA using host characteristics to determine which characteristics best differentiated the groups. Characteristics in the full model included longevity, log of number of parasite citations, and “dummy” variables (0/1) for habitat, fishery, introduced/native, and sociality. Stepwise model selection using Wilks lambda was used to choose the best model, retaining only the host characteristics that significantly distinguished the clusters and removing collinear variables. The final LDA model for the host clusters included the following host characteristics: freshwater, parental care, solitary, and log of number of pathogen-related citations (Fig. 1b; Table 2).
Parasites relative to host longevity
Longevity was not a strong predictor of parasite assemblages in taxonomic or agglomerative clustering groups (Fig. 1). Despite lobsters being longer-lived, shrimp shorter-lived, and crabs and crayfish intermediate (Suppl. Fig. 1a), the taxonomic LDA showed that longevity is a weak differentiator of parasite assemblage in these taxa (Fig. 1a). This result is reinforced in the agglomerative cluster LDA, where longevity was not included in the final model and did not differ among the groups (F = 0.74, df = 3,97, p = 0.53; Suppl. Fig. 1b). Additionally, the association was random between longevity and reported PSR (R2 = − 0.007, F = 0.27, df = 1,99, p = 0.60). This result does not support our prediction that shorter-lived hosts will have higher PSR, which could be due to interactive effects of parasitized hosts evolving r-selected life history traits and parasites finding more stable environments in long-lived hosts. Our result may also be due to the bias towards shorter-lived species in our dataset and the overall difficulty in aging crustaceans.
Parasites and host habitat
In the taxonomic LDA there was a positive association between the marine habitat and lobster hosts, indicated by both copepod and amoebae parasites; and crab hosts, indicated by rhizocephalans and dinoflagellates (Fig. 1a). These results indicate that copepods, amoebae, rhizocephalans, and dinoflagellates are more likely to be found in marine hosts than elsewhere. Prevalence of rhizocephalan infections vary with salinity in estuarine crabs (Blakeslee et al. 2021) and larvae are unable to survive at low salinities (Reisser and Forward 1991). The dinoflagellate Hematodinium sp. is also found to infect hosts more commonly at higher salinities and, while it can survive within hosts at low salinity, cannot proliferate and transfer between hosts (Messick and Shields 2000; Coffey et al. 2012). Logically, there was a negative association between the marine habitat and crayfish hosts, which were indicated by mesomycetozoans, oomycetes, branchiodellids, and fungi (Fig. 1a), meaning these parasite groups are less likely to be found in marine habitats. This result was supported by the agglomerative clustering LDA (Fig. 1b; Fig. 7a) where there was a strong positive association between freshwater habitats and Group 3, which was indicated by mesomycetozoans, oomycetes, branchiobdellids, bacteria, and fungi, and a negative association between freshwater and Group 4, which was indicated by parasitic isopods (Fig. 1b), which are rarer in freshwater environments. Branchiobdellids, in particular, have only been observed in freshwater environments and from freshwater hosts (Govedich et al. 2010).
There was no significant difference in reported PSR by host habitat (F = 3.1, df = 2, p = 0.21; Fig. 8a), despite our prediction that euryhaline hosts would have the highest parasite species diversity because they may encounter parasite species with a variety of salinity tolerances. The blue crab C. sapidus provides an excellent case study as a euryhaline host with high PSR, since it is a highly efficient osmoregulator, allowing it to move freely between freshwater and saltwater, most notably during mating and spawning. Many C. sapidus parasites seem to be infectious in only high or low salinity water (Tindle et al. 2004; Coffey et al. 2012). Avoidance behavior may have acted as a selection pressure for the blue crab’s catadromous migrations (Behringer et al. 2018). Callinectes sapidus also provides an example for greater PSR in marine hosts because more parasitic species have been described in marine versus freshwater blue crabs (Shields and Overstreet 2007; Walters et al. 2023). If parasite diversity increases with geographic range, the vastness of the ocean would suggest that there should be higher parasite diversity in marine hosts. However, in fish, freshwater hosts have greater PSR than their marine counterparts (Poulin 2016). Greater diversification of species in freshwater may be due to greater habitat heterogeneity or isolation in freshwater (e.g., lakes) that leads to parasite or host speciation (Wiens 2015).
A comparison of parasite species richness in decapod crustacean hosts by host characteristics: a habitat, b sociality, c introduced species, and d fishery exploitation. Lowercase letters above boxes indicate significant differences. Abbreviated outliers: Cambarus bartonii, Paranephrops planifrons, Procambarus alleni, Austropotamobius torrentium, Macrobrachium nipponense
Parasites and host sociality
In the taxonomic LDA (Fig. 1a) there was a strong positive association between parental care and crayfish, which were indicated by mesomycetozoans, oomycetes, branchiobdellids, and fungi; suggesting that these parasite groups are more commonly found in hosts exhibiting parental care, with parasites possibly benefitting from vertical transmission. Crabs were associated with solitary lifestyles and are indicated by rhizocephalan barnacles and dinoflagellates. These parasite groups have complex lifecycles with free-living stages. The infectious stage is not passed directly between hosts of the same species and may not benefit from their host’s social behaviors. There was a negative association between shrimp and solitary lifestyle and reproductive aggregations, meaning shrimp do not commonly have these social structures. Shrimp are pseudo-indicated by viruses (see Sect. “Shrimp parasites”), which typically do not survive well outside of a host and therefore benefit from high densities of gregarious hosts for direct transmission.
The agglomerative clustering LDA (Fig. 1b; Fig. 7c) revealed a positive association between parental care and Group 3, indicated by mesomycetozoans, oomycetes, branchiobdellids, bacteria, and fungi, reinforcing the crayfish associations in the taxonomic LDA. There were negative associations between solitary lifestyle and Group 2, indicated by viruses, apicomplexans, dinoflagellates, and cestodes, and Group 4, indicated by isopods. This result suggests that solitary lifestyle hinders virus, apicomplexan, dinoflagellate, cestode, and isopod parasite groups.
Hosts with parental care have significantly greater reported PSR than gregarious hosts (F = 11.9, df = 3, p = 0.008; Fig. 8b) but neither are significantly different from solitary hosts or those with reproductive aggregations. Gregarious hosts would seem to have a greater opportunity to transmit parasites among conspecifics, which presents an opportunity for parasite diversification, and yet our data indicate that they have reduced PSR. This pattern may be a result of evolved parasite avoidance behaviors, as exemplified by the gregarious Caribbean spiny lobster P. argus (Behringer et al. 2018), which aggregate with conspecifics using chemical cues (Anderson and Behringer 2013) but are also able to detect and avoid conspecifics infected with the pathogenic Panulirus argus virus 1 (PaV1) (Behringer et al. 2006; Subramaniam et al. 2020). Alternately, species with parental care would not benefit from avoiding a diseased parent or sibling, because they would be susceptible to much higher predation rates. This type of association could be utilized by directly transmitted parasites resulting in a diversity of parasites in this group.
Parasites in introduced hosts
Introduced species were not a significant discriminator in either the taxonomic LDA or agglomerative clustering LDA (Fig. 1; Fig. 7b), suggesting that being an INNS is not an important factor in the type of parasites that a decapod acquires. However, introduced species have significantly higher reported PSR (F = 18.1, df = 1, p < 0.001; Fig. 8c) than native species. For introduced species, parasites were added to our dataset regardless of whether they were found in the native or introduced range and perhaps due to the range extent of introduction, the number of parasites encountered may increase. Thus, it is likely that both “parasite acquisition” in the invasive range and “enemy release” from many parasites in the native range are probably explanations for greater PSR in introduced species.
A further consideration in invasion biology is the potential spread of introduced parasites (spillover), which may also have the potential to infect animal/plant culture systems, fisheries, and native wildlife (Roy et al. 2017). Out of the updated list of crustacean invaders (n = 323), it has been estimated that only 31.2% have at least one known symbiont, totaling 391 known symbionts (genus level or better) across the group (Bojko et al. 2021). Knowledge of such associations highlights the importance for informed legislation to limit their spread and impact (Foster et al. 2021).
The European shore crab, Carcinus maenas, is a destructive invader with 42 identified parasites in our dataset, though some estimates are higher (Bojko et al. 2018) and more are discovered regularly (Bojko et al. 2019; Subramaniam et al. 2020). This species exemplifies both enemy release and parasite acquisition hypotheses, having 65 documented symbionts in its native range, 13 in both native and invasive range, and two species found in its invasive range only (Bojko et al. 2021). Release from the multitude of symbionts found only in its native range may have contributed to the shore crab’s success as an invader – it has invaded coastal habitats worldwide (Torchin et al. 2001). Shore crabs have also acquired parasites, a microsporidian and a nemertean egg predator (Torchin et al. 1996; Bojko et al. 2017), in their North American invasive range—so far increasing the symbiont tally by two with many more portions of their invasive range remaining unscreened. The symbionts in both native and introduced ranges represent co-invasions with the crab and several are capable of spillover to infect alternative hosts, such as salmon and American lobster, within the invasive range (Bojko et al. 2018).
Parasites in aquaculture and commercial fisheries
In the taxonomic LDA there was a negative association between the non-commercial category and lobsters, meaning that lobsters and their parasites in our dataset are most predominant in capture fisheries (Fig. 1a). The amoebic parasite Neoparamoeba pemaquidensis of H. americanus provides a pertinent example of lobster parasitism affecting commercial capture fisheries. Paramoebiasis was implicated in a devastating fishery collapse in Long Island Sound in 1999 (Mullen et al. 2004, 2005). Between 1999 and 2002 the fishery was declared a commercial fishery failure by United States Congress and decreased in value from $40 to $7 million USD annually (Seara et al. 2022). It has yet to recover.
Decapods in both capture fisheries and aquaculture have greater reported PSR (F = 16.5, df = 3, p < 0.001; Fig. 8d) than decapods not targeted by fisheries or aquaculture. This pattern likely relates to greater study efforts on fished hosts and greater interest and funding for determining causes of mortality. But parasites are also known to have interactive effects in fished ecosystems (Wood et al. 2010; Wood and Lafferty 2015). In general, PSR is reduced in areas where fishing occurs likely due to reduced host density, selective removal of the largest individual hosts, which tend to carry more parasites, and reduced food web complexity (Wood et al. 2014). The conditions of aquaculture are known to promote viral and bacterial evolution. Vibrio parahaemolyticus is a common bacterial component of aquatic systems that acquired pathogenicity in aquaculture systems (Lee et al. 2015).
Parasitism and host sampling intensity
Host sampling intensity was not a significant factor in either taxonomic or agglomerative clustering LDAs, suggesting that this is not a strong differentiator of the groups and the types of parasites associated with them (Fig. 1). In the agglomerative clustering groups, Group 2 had significantly more citations than Group 1 and Group 4 (F = 6.9, df = 2,97, p = 0.0003; Fig. 3b). There was also a significant positive relationship between the number of citations and reported PSR (R2 = 0.35, F = 55.9, df = 1,99, p < 0.001; Suppl. Fig. 2).
There are many concerning ecological and economic implications of host-parasite relationships in crustaceans. The more we know about these relationships, the better we can identify and predict potential issues and guide aquaculture/fisheries management and choices. The study of parasites and disease should take a cue from the study of invasive species, where many government and research organizations are conducting “horizon scanning”, a systematic process to identify the risk associated with potential invasive species to a particular region (Therriault et al. 2008; Roy et al. 2014). Host species in Groups 1 and 4 offer a promising starting point for future studies because they are less well-studied than species in other groups.
Conclusions
Here we have presented the first synthesis of parasites in wild decapod crustaceans, with particular emphasis on fished and aquacultured species. Our analyses aimed to highlight patterns in available data as well as important data gaps. These patterns should not be interpreted as causational but rather correlations that deserve further examination. Below are our conclusions and recommendations.
-
Most parasites were protozoan (18%) or viral (17%).
-
Indicator species analysis revealed that crabs are indicated by rhizocephalans and dinoflagellates; crayfish are indicated by mesomycetozoans, oomycetes, branchiobdellids, and fungi; lobsters are indicated by copepods and amobae; shrimp were pseudo-indicated by viruses.
-
Decapod species that are fished, aquacultured, introduced, exhibit parental care, or live in freshwater tend to have higher parasite species diversity.
-
A solitary lifestyle hinders virus, apicomplexan, dinoflagellate, cestode, and isopod parasite groups and may be an ‘avoidance behavior’.
-
Longevity was not a good predictor of parasite diversity or specific host-parasite relationships, but our dataset was biased to shorter-lived species, because accurately ageing crustaceans is difficult.
-
Parasite diversity increased with how well-studied a host is. We recommend increased parasite screening in decapods and suggest this could be modeled after the horizon scanning approach used in invasion science.
-
Similarly, we recommend addressing data gaps with methods aimed at highlighting biases such as the ERF and false negative analyses used here. These modeling approaches can be used to identify potential screening candidates, which can then be used to refine the model.
-
The exclusion of geographic range data from these analyses is a significant caveat to our conclusions. Lack of reliable range data is an issue for nearly all marine organisms and collecting such information should be a focus of future research and collaboration. New technologies and techniques in remote sensing and ROVs, as well as curated databases to house this information could improve research in this area.
Data availability
The data used to create this manuscript are available in Figshare with the identifier: https://doi.org/https://doi.org/10.6084/m9.figshare.25513399.
References
Alderman DJ (1996) Geographical spread of bacterial and fungal diseases of crustaceans. Rev Sci Tech 15:603–632. https://doi.org/10.20506/rst.15.2.943
Alderman DJ, Polglase JL (1988) Pathogens, parasites and commensals. In: Holdich DM, Lowery RS (eds) Freshwater crayfish: biology, management and exploitation. Croom Helm, London, pp 167–212
Anderson JR, Behringer DC (2013) Spatial dynamics in the social lobster Panulirus argus in response to diseased conspecifics. Mar Ecol Prog Ser 474:191–200. https://doi.org/10.3354/meps10091
Anderson RM, May RM (1982) Coevolution of hosts and parasites. Parasitology 85:411. https://doi.org/10.1017/S0031182000055360
Arneberg P, Skorping A, Grenfell B, Read AF (1998) Host densities as determinants of abundance in parasite communities. Proc R Soc Lond B 265:1283–1289
Arulmoorthy MP, Anandajothi E, Vasudevan S, Suresh E (2020) Major viral diseases in culturable penaeid shrimps: a review. Aquacult Int 28:1939–1967
Bardera G, Usman N, Owen M et al (2019) The importance of behaviour in improving the production of shrimp in aquaculture. Rev Aquac 11:1104–1132. https://doi.org/10.1111/RAQ.12282
Beamish RJ, McFarlane GA, Benson A (2006) Longevity overfishing. Prog Oceanogr 68:289–302. https://doi.org/10.1016/j.pocean.2006.02.005
Behringer DC, Duermit-Moreau E (2021) Crustaceans, One Health and the changing ocean. J Invertebr Pathol. https://doi.org/10.1016/j.jip.2020.107500
Behringer DC, Butler MJ, Shields JD (2006) Avoidance of disease by social lobsters. Nature 441:421. https://doi.org/10.1038/441421
Behringer DC, Karvonen A, Bojko J (2018) Parasite avoidance behaviours in aquatic environments. Philos Trans R Soc Lond B Biol Sci 373:20170202. https://doi.org/10.1098/rstb.2017.0202
Blakeslee AMH, Pochtar DL, Fowler AE et al (2021) Invasion of the body snatchers: the role of parasite introduction in host distribution and response to salinity in invaded estuaries. Proc R Soc B: Biol Sci. https://doi.org/10.1098/rspb.2021.0703
Bojko J, Ovcharenko M (2019) Pathogens and other symbionts of the Amphipoda: taxonomic diversity and pathological significance. Dis Aquat Organ 136:3–36
Bojko J, Clark F, Bass D et al (2017) Parahepatospora carcini n. gen., n. sp., a parasite of invasive Carcinus maenas with intermediate features of sporogony between the Enterocytozoon clade and other microsporidia. J Invertebr Pathol 143:124–134. https://doi.org/10.1016/j.jip.2016.12.006
Bojko J, Stebbing PD, Dunn AM et al (2018) Green crab Carcinus maenas symbiont profiles along a North Atlantic invasion route. Dis Aquat Organ 128:147–168. https://doi.org/10.3354/dao03216
Bojko J, Subramaniam K, Waltzek TB et al (2019) Genomic and developmental characterisation of a novel bunyavirus infecting the crustacean Carcinus maenas. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-49260-4
Bojko J, Burgess AL, Baker AG, Orr CH (2021) Invasive non-native crustacean symbionts: diversity and impact. J Invertebr Pathol 186:107482. https://doi.org/10.1016/j.jip.2020.107482
Bolek MG, Schmidt-Rhaesa A, De Villalobos LC, Hanelt B (2015) Phylum Nematomorpha. In: Thorp J, Rogers DC (eds) Thorp and Covich’s freshwater invertebrates: ecology and general biology, 4th edn. Academic Press, pp 303–326
Boyko CB, Williams JD (2009) Crustacean parasites as phylogenetic indicators in decapod evolution. In: Martin JW, Crandall KA, Felder DL (eds) Decapod crustacean phylogenetics, Crustacean. CRC Press, Boca Raton, pp 197–220
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal 9:378–400. https://doi.org/10.32614/rj-2017-066
Brown BL, Creed RP, Dobson WE (2002) Branchiobdellid annelids and their crayfish hosts: Are they engaged in a cleaning symbiosis? Oecologia 132:250–255. https://doi.org/10.1007/s00442-002-0961-1
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol 59:197–242. https://doi.org/10.1111/J.1095-8649.2001.TB00127.X
Carlisle DB (1954) On the hormonal inhibition of moulting in decapod crustacea. J Mar Biol Assoc UK 33:61–63. https://doi.org/10.1017/S0025315400003477
Cawthorn RJ (2011) Diseases of American lobsters (Homarus americanus): a review. J Invertebr Pathol 106:71–78. https://doi.org/10.1016/j.jip.2010.09.010
Cerenius L, Henttonen P, Lindqvist OV, Söderhäll K (1991) The crayfish pathogen Psorospermium haeckeli activates the prophenoloxidase activating system of freshwater crayfish in vitro. Aquaculture 99:225–233. https://doi.org/10.1016/0044-8486(91)90243-Z
Chakraborty P, Acharyya T, Raghunadh Babu PV, Bandyopadhyay D (2011) Impact of salinity and pH on phytoplankton communities in a tropical freshwater system: an investigation with pigment analysis by HPLC. J Environ Monit 13:614–620. https://doi.org/10.1039/c0em00333f
Cheng TL, Rovito SM, Wake DB, Vredenburg VT (2011) Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A 108:9502–9507. https://doi.org/10.1073/pnas.1105538108
Cochran PA, Kinziger AP, Poly WJ (1999) Predation on horsehair worms (phylum nematomorpha). J Freshw Ecol 14:211–218. https://doi.org/10.1080/02705060.1999.9663672
Coffey AH, Li C, Shields JD (2012) The effect of salinity on experimental infections of a Hematodinium sp. in blue crabs, Callinectes sapidus. J Parasitol 98:536–542. https://doi.org/10.1645/GE-2971.1
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733. https://doi.org/10.1111/j.1461-0248.2004.00616.x
Cook DC, Thomas MB, Cunningham SA et al (2007) Predicting the economic impact of an invasive species on an ecosystem service. Ecol Appl 17:1832–1840. https://doi.org/10.1890/06-1632.1
Davies CE, Vogan CL, Rowley AF (2015) Effect of the copepod parasite Nicothoë astaci on haemolymph chemistry of the European lobster Homarus gammarus. Dis Aquat Organ 113:169–175. https://doi.org/10.3354/dao02814
de Jonge VN (1974) Classification of brackish coastal inland waters. Hydrobiol Bull. https://doi.org/10.1007/BF02254903
Dritschilo W, Cornell H, Nafus D, O’Connor B (1975) Insular biogeography: of mice and mites. Science 190:467–469
Duermit E, Shervette V, Whitaker JD et al (2017) A field assessment of claw removal impacts on the movement and survival of stone crabs Menippe spp. Fish Res 193:43–50. https://doi.org/10.1016/j.fishres.2017.03.019
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Fairfield EA, Richardson DS, Daniels CL et al (2021) Ageing European lobsters (Homarus gammarus) using DNA methylation of evolutionarily conserved ribosomal DNA. Evol Appl 14:2305–2318. https://doi.org/10.1111/eva.13296
FAO (2017) Fishery and Aquaculture Statistics. Global production by production source 1950–2015 (FishstatJ)
FAO (2018) The State of World Fisheries and Aquaculture 2018—Meeting the sustainable development goals. Rome
FAO (2021) Fishery and aquaculture statistics. Global production by production source 1950–2019 (FishstatJ)
Field RH, Appleton PL (1995) A Hematodinium-like dinoflagellate infection of the Norway lobster Nephrops norvegicus: observations on pathology and progression of infection. Dis Aquat Organ 22:115–128. https://doi.org/10.3354/dao022115
Finch CE (1994) Longevity, senescence, and the genome. University of Chicago Press
Flores L, Ernst B, Parma AM (2010) Growth pattern of the sea urchin, Loxechinus albus (Molina, 1782) in southern Chile: evaluation of growth models. Mar Biol 157:967–977. https://doi.org/10.1007/s00227-009-1377-9
Foster R, Peeler E, Bojko J et al (2021) Pathogens co-transported with invasive non-native aquatic species: implications for risk analysis and legislation. NeoBiota 69:79–102. https://doi.org/10.3897/neobiota.69.71358
Fox J WS (2011) Car, R package
Gandy R, Crowley C, Chagaris D, Crawford C (2016) The effect of temperature on release mortality of declawed Menippe mercenaria in the Florida stone crab fishery. Bull Mar Sci 92:1–15. https://doi.org/10.5343/bms.2015.1036
Gelder SR, Carter HC, Lausier DN (2001) Distribution of crayfish worms or branchiobdellidans (annelida: clitellata) in New England. Northeast Nat (steuben) 8:79–92. https://doi.org/10.1656/1092-6194(2001)008[0079:DOCWOB]2.0.CO;2
Gibson FA (1961) Gaffkaemia in stored lobsters. ICES Shellfish Committee C 58
Glockling SL, Marshall WL, Gleason FH (2013) Phylogenetic interpretations and ecological potentials of the Mesomycetozoea (Ichthyosporea). Fungal Ecol 6:237–247. https://doi.org/10.1016/j.funeco.2013.03.005
Gnanalingam G, Butler MJ, Matthews TR et al (2019) Directly ageing the Caribbean spiny lobster, Panulirus argus with validated band counts from gastric mill ossicles. ICES J Mar Sci 76:442–451. https://doi.org/10.1093/icesjms/fsy177
Gotto V (2004) Commensal and parasitic copepods associated with marine invertebrates. In: Crothers JH, Hayward PJ (eds) Synopses of the British fauna, 2nd edn. Field Studies Council, p 352
Govedich FR, Bain BA, Moser WE, et al (2010) Annelida (Clitellata): Oligochaeta, Branchiobdellida, Hirudinida, and Acanthobdellida, Third Edit. Elsevier Ltd
Guerra AS, Kao AB, McCauley DJ, Berdahl AM (2020) Fisheries-induced selection against schooling behaviour in marine fishes: FIE and schooling behaviour. Proc R Soc B: Biol Sci. https://doi.org/10.1098/rspb.2020.1752
Hobbs HH Jr, Holt PC, Walton M (1967) The crayfishes and their epizootic ostracod and branchiobdellid associates of the Mountain Lake, Virginia, region. Proc United States Natl Mus 123:84. https://doi.org/10.4039/Ent3436-2
Hochberg ME, Michalakis Y, De Meeus T (1992) Parasitism as a constraint on the rate of life-history evolution. J Evol Biol 5:491–504. https://doi.org/10.1046/j.1420-9101.1992.5030491.x
Høeg JT (1995) The biology and life cycle of the rhizocephala (cirripedia). J Mar Biol Assoc UK 75:517–550. https://doi.org/10.1017/S0025315400038996
Horton T, Kroh A, Ahyong S, et al (2018) World Register of Marine Species (WoRMS)
Howell P (2012) The status of the southern New England lobster stock. J Shellfish Res 31:573–579. https://doi.org/10.2983/035.031.0217
Hubault E (1935) Une épizootie sur Potamobius pallipes Lereb. Ann Parasitol Hum Comp 13:109–112
Hunt JH, Lyons WG, Kennedy FS Jr (1986) Effects of exposure and confinement on spiny lobsters, Panulirus argus, used as attractants in the Florida trap fishery. Fishery Bulletin (US) 84:69–76
ICES (2007) Report on the working gorup of pathology and diseases of marine organisms (WGPDMO). Tenerife, Spain
Johnson PT (1977) Paramoebiasis in the blue crab, Callinectes sapidus. J Invertebr Pathol 29:308–320
Kakui K, Fukuchi J, Shimada D (2021) First report of marine horsehair worms (Nematomorpha: Nectonema) parasitic in isopod crustaceans. Parasitol Res 120:2357–2362. https://doi.org/10.1007/s00436-021-07213-9
Kelly DW, Paterson RA, Townsend CR et al (2009) Parasite spillback: a neglected concept in invasion ecology? Ecology 90:2047–2056. https://doi.org/10.1890/08-1085.1
Kent ML (2000) Marine netpen farming leads to infections with some unusual parasites. Int J Parasitol 30:321–326. https://doi.org/10.1016/S0020-7519(00)00018-7
Kilada R, Driscoll JG (2017) Age determination in crustaceans: a review. Hydrobiologia 799:21–36. https://doi.org/10.1007/s10750-017-3233-0
Koopman HN, Westgate AJ, Siders ZA (2015) Declining fecundity and factors affecting embryo quality in the american lobster (Homarus americanus) from the Bay of Fundy. Can J Fish Aquat Sci 72:352–363. https://doi.org/10.1139/cjfas-2014-0277
Korb J, Heinze J (2016) Major hurdles for the evolution of sociality. Annu Rev Entomol 61:297–316. https://doi.org/10.1146/annurev-ento-010715-023711
Kurath G, Winton J (2011) Complex dynamics at the interface between wild and domestic viruses of finfish. Curr Opin Virol 1:73–80. https://doi.org/10.1016/j.coviro.2011.05.010
Kuris AM, Wickham DE (1987) Effect of nemertean egg predators on crustaceans. Bull Mar Sci 41:151–164
Lafferty KD, Harvell CD, Conrad JM et al (2015) Infectious diseases affect marine fisheries and aquaculture economics. Ann Rev Mar Sci 7:471–496. https://doi.org/10.1146/annurev-marine-010814-015646
Lampo M, Señaris C, Ballestas O (2022) Smaller size of harlequin toads long exposed to the lethal fungal disease chytridiomycosis. Biotropica. https://doi.org/10.1111/btp.13220
le Bris A, Pershing AJ, Gaudette J et al (2017) Multi-scale quantification of the effects of temperature on size at maturity in the American lobster (Homarus americanus). Fish Res 186:397–406. https://doi.org/10.1016/J.FISHRES.2016.09.008
le Bris A, Mills KE, Wahle RA et al (2018) Climate vulnerability and resilience in the most valuable North American fishery. Proc Natl Acad Sci U S A 115:1831–1836. https://doi.org/10.1073/pnas.1711122115
Lightner DV, Williams RR, Bell TA, et al (1992) A collection of case histories documenting the introduction and spread of the virus disease IHHN in penaeid shrimp culture facilities in Northwestern Mexico. Introductions and Transfers of Aquatic Species Selected Papers from a Symposium Held in Halifax, Nova Scotia, 12–13 June 1990, pp 9–105
Longshaw M (2011) Diseases of crayfish: a review. J Invertebr Pathol 106:54–70. https://doi.org/10.1016/j.jip.2010.09.013
Luis AD, Hayman DTS, O’Shea TJ et al (2013) A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc R Soc B: Biol Sci 280:20122753–20122753. https://doi.org/10.1098/rspb.2012.2753
Mahmoud MM (2019) Fusarium solani infection of red swamp crayfish (Procambarus Clarkii). Assiut Vet Med J 65:50–59. https://doi.org/10.21608/avmj.2019.168745
Mancinelli G, Chainho P, Cilenti L et al (2017) The Atlantic blue crab Callinectes sapidus in southern European coastal waters: distribution, impact and prospective invasion management strategies. Mar Pollut Bull 119:5–11. https://doi.org/10.1016/j.marpolbul.2017.02.050
McDermott JJ (2011) Parasites of shore crabs in the genus Hemigrapsus (Decapoda: Brachyura: Varunidae) and their status in crabs geographically displaced: a review. J Nat Hist 45:2419–2441. https://doi.org/10.1080/00222933.2011.596636
McMahon TA, Brannelly LA, Chatfield MWH et al (2013) Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proc Natl Acad Sci U S A 110:210–215. https://doi.org/10.1073/pnas.1200592110
McVicar AH (1997) Disease and parasite implications of the coexistence of wild and cultured Atlantic salmon populations. ICES J Mar Sci 54:1093–1103. https://doi.org/10.1016/s1054-3139(97)80014-8
Messick GA, Shields JD (2000) Epizootiology of the parasitic dinoflagellate Hematodinium sp. in the American blue crab Callinectes sapidus. Dis Aquat Organ 43:139–152. https://doi.org/10.3354/dao043139
Meyers T, Koeneman T, Botelho C, Short S (1987) Bitter crab disease: a fatal dinoflagellate infection and marketing problem for Alaskan Tanner crabs Chionoecetes bairdi. Dis Aquat Organ 3:195–216. https://doi.org/10.3354/dao003195
Morales-Covarrubias MS, Nunan LM, Lightner DV et al (1999) Prevalence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in wild adult blue shrimp Penaeus stylirostris from the Northern Gulf of California, Mexico. J Aquat Anim Health 11:296–301. https://doi.org/10.1577/1548-8667(1999)011<0023:PAGDOI>2.0.CO;2
Morand S, Harvey PH (2000) Mammalian metabolism, longevity and parasite species richness. Proc R Soc B: Biol Sci 267:1999–2003. https://doi.org/10.1098/rspb.2000.1241
Morton DN, Antonino CY, Broughton FJ et al (2021) A food web including parasites for kelp forests of the Santa Barbara Channel, California. Sci Data 8:1–14. https://doi.org/10.1038/s41597-021-00880-4
Mullen TE, Russell S, Tucker MT et al (2004) Paramoebiasis associated with mass mortality of American lobster Homarus americanus in Long Island Sound, USA. J Aquat Anim Health 16:29–38. https://doi.org/10.1577/H02-045.1
Mullen TE, Nevis KR, O’Kelly CJ et al (2005) Nuclear small-subunit ribosomal RNA gene-based characterization, molecular phylogeny and PCR detection of the neoparamoeba from Western Long Island Sound Lobster. J Shellfish Res 24:719–731. https://doi.org/10.2983/0730-8000(2005)24[719:NSRRGC]2.0.CO;2
Murray AG, Peeler EJ (2005) A framework for understanding the potential for emerging diseases in aquaculture. Prev Vet Med 67:223–235. https://doi.org/10.1016/j.prevetmed.2004.10.012
Neill S, Cullen JM (1974) Experiments on whether schooling by their prey affects the hunting behaviour of cephalopods and fish predators. J Zool 172:549–569
Nunn CL, Altizer S, Jones KE, Sechrest W (2003) Comparative tests of parasite species richness in primates. Am Nat 162:597–614. https://doi.org/10.1086/378721
O’Brien J, Wan Wyk P (1985) Effects of crustacean parasitic castrators (epicaridean isopods and rhizocephalan barnacles) on growth of crustacean hosts. In: Wenner AM (ed) Crustacean issues, factors in adult growth, vol 3. Balkema Press, Rotterdam, pp 191–218
Patoka J, Petryl M, Kalous L (2013) Growth of juvenile red swamp crayfish (Procambarus clarkii)(Decapoda: Cambaridae) reared in groups consisting of either sibling and non sibling individuals. Acta Societatis Zoologicae Bohemicae 77:67–71
Pettersen AR, Moland E, Olsen EM, Knutsen JA (2009) Lobster reserves in coastal Skagerrak—an integrated analysis of the implementation process. Integr Coast Zone Manag. https://doi.org/10.1002/9781444316285.ch14
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288. https://doi.org/10.1016/j.ecolecon.2004.10.002
Poulin R (1997) Species richness of parasite assemblages: evolution and patterns. Annu Rev Ecol Syst 28:341–358
Poulin R (2016) Greater diversification of freshwater than marine parasites of fish. Int J Parasitol 46:275–279. https://doi.org/10.1016/j.ijpara.2015.12.002
Poulin R, Morand S (2000) The diversity of parasites. Q R Biol 75:277–293
Price PW (1980) Evolutionary biology of parasites, Vol. 15. Princeton University Press
Reisser CE, Forward RB (1991) Effect of salinity on osmoregulation and survival of a rhizocephalan parasite, Loxothylacus panopaei, and its crab host, Rhithropanopeus harrisii. Estuaries 14:102–106. https://doi.org/10.2307/1351987
Roy HE, Peyton J, Aldridge DC et al (2014) Horizon scanning for invasive alien species with the potential to threaten biodiversity in Great Britain. Glob Chang Biol 20:3859–3871. https://doi.org/10.1111/gcb.12603
Roy HE, Hesketh H, Purse BV et al (2017) Alien pathogens on the horizon: opportunities for predicting their threat to wildlife. Conserv Lett 10:476–483. https://doi.org/10.1111/conl.12297
Ryazanova TV (2008) Bitter crab syndrome in two species of king crabs from the Sea of Okhotsk. Russ J Mar Biol 34:411–414. https://doi.org/10.1134/S1063074008060102
Schmidt-Rhaesa A, Pohle G, Gaudette J, Burdett-Coutts V (2013) Lobster (Homarus americanus), a new host for marine horsehair worms (Nectonema agile, Nematomorpha). J Mar Biol Assoc UK 93:631–633. https://doi.org/10.1017/S0025315412000719
Scholtz G, Kawai T (2002) Aspects of embryonic and postembryonic development of the Japanese freshwater crayfish Cambaroides japonicus (Crustacea, Decapoda) including a hypothesis on the evolution of maternal care in the Astacida. Acta Zoologica 83:203–212. https://doi.org/10.1046/j.1463-6395.2002.00113.x
Schöne BR, Fiebig J, Pfeiffer M et al (2005) Climate records from a bivalved Methuselah (Arctica islandica, Mollusca; Iceland). Palaeogeogr Palaeoclimatol Palaeoecol 228:130–148. https://doi.org/10.1016/j.palaeo.2005.03.049
Seara T, Owens A, Pollnac R et al (2022) Lessons learned from a natural resource disaster: the long-term impacts of the Long Island Sound lobster die-off on individuals and communities. Mar Policy 136:104943. https://doi.org/10.1016/j.marpol.2021.104943
Sheath DJ, Williams CF, Reading AJ, Robert Britton J (2015) Parasites of non-native freshwater fishes introduced into england and wales suggest enemy release and parasite acquisition. Biol Invasions 17:2235–2246. https://doi.org/10.1007/s10530-015-0857-8
Sheehy MRJ, Prior AE (2008) Progress on an old question for stock assessment of the edible crab Cancer pagurus. Mar Ecol Prog Ser 353:191–202. https://doi.org/10.3354/meps07229
Shields JD (2011) Diseases of spiny lobsters: a review. J Invertebr Pathol 106:79–91. https://doi.org/10.1016/j.jip.2010.09.015
Shields JD (2012) The impact of pathogens on exploited populations of decapod crustaceans. J Invertebr Pathol 110:211–224. https://doi.org/10.1016/j.jip.2012.03.011
Shields JD, Wood FEI (1993) Impact of parasites on the reproduction and fecundity of the blue sand crab Portunus pelagicus from Moreton Bay, Australia. Mar Ecol Prog Ser 92:159–170. https://doi.org/10.3354/meps092159
Shields JD, Williams JD, Boyko CB (2015) Parasites and diseases of Brachyura. Brill, Leiden, pp 639–774
Shields JD, Overstreet RM (2007) Diseases, parasites, and other symbionts. In: The blue crab Callinectes sapidus. Maryland Sea Grant
Siders ZA, Ducharme-Barth ND, Carvalho F et al (2020) Ensemble random forests as a tool for modeling rare occurrences. Endanger Species Res 43:183–197. https://doi.org/10.3354/esr01060
Silk JB (2007) The adaptive value of sociality in mammalian groups. Philos Trans R Soc B: Biol Sci 362:539–559
Sinclair MEE (1977) Agonistic behavior of the stone crab, Menippe mercenaria (Say). Anim Behav 25:193–207
Skelton J, Farrell KJ, Creed RP et al (2013) Servants, scoundrels, and hitchhikers: current understanding of the complex interactions between crayfish and their ectosymbiotic worms (Branchiobdellida). Freshw Sci 32:1345–1357. https://doi.org/10.1899/12-198.1
Skurdal J, Vøllestad LA, Qvenild T (1985) Comparison of scales and otoliths for age determination of whitefish Coregnous lavaretus. Fish Res 3:237–243. https://doi.org/10.1016/0165-7836(85)90024-4
Small HJ (2012) Advances in our understanding of the global diversity and distribution of Hematodinium spp.—Significant pathogens of commercially exploited crustaceans. J Invertebr Pathol 110:234–246. https://doi.org/10.1016/j.jip.2012.03.012
Sneath PHA, Sokal RR (1973) Numerical taxonomy. In: The principles and practice of numerical classification. Freeman, San Francisco
Souheil H, Vey A, Thuet P, Trilles JP (1999) Pathogenic and toxic effects of Fusarium oxysporum (Schlecht.) on survival and osmoregulatory capacity of Penaeus japonicus (Bate). Aquaculture 178:209–224. https://doi.org/10.1016/S0044-8486(99)00138-6
Stentiford GD, Shields JD (2005) A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis Aquat Organ 66:47–70. https://doi.org/10.3354/dao066047
Stentiford GD, Neil DM, Peeler EJ et al (2012) Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J Invertebr Pathol 110:141–157
Stentiford GD, Sritunyalucksana K, Flegel TW, Williams BAP, Withyachumnarnkul B, Itsathitphaisarn O, Bass D (2017) New paradigms to help solve the global aquaculture disease crisis. PLoS Pathog 13:1–6. https://doi.org/10.1371/journal.ppat.1006160
Stentiford GD, Bateman IJ, Hinchliffe SJ et al (2020) Sustainable aquaculture through the One Health lens. Nat Food 1:468–474. https://doi.org/10.1038/s43016-020-0127-5
Števčić Z (1971) Laboratory observations on the aggregations of the spiny spider crab (Maja squinado Herbst). Anim Behav 19:18–25. https://doi.org/10.1016/S0003-3472(71)80130-6
Stevens SA (2022) Behavioural, anatomical and physiological examinations of crab host-parasite interactions. 1–142
Stone RP, O’Clair CE, Shirley TC (1993) Aggregating behavior of ovigerous female red king crab, Paralithodes camtschaticus, in Auke Bay, Alaska. Can J Fish Aquat Sci 50:750–758. https://doi.org/10.1139/f93-086
Subramaniam K, Behringer DC, Bojko J et al (2020) A new family of DNA viruses causing disease in crustaceans from diverse aquatic biomes. Mbio 11:1–14. https://doi.org/10.1128/mBio.02938-19
Svoboda J, Mrugała A, Kozubíková-Balcarová E, Petrusek A (2017) Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: a review. J Fish Dis 40:127–140. https://doi.org/10.1111/jfd.12472
te Lee C, Chen IT, Yang YT et al (2015) The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci U S A 112:E5445. https://doi.org/10.1073/pnas.1517100112
Team RC (2021) R: a language and environment for statistical computing
Therriault TW, Herborg L, Locke A, Mckindsey CW (2008) Risk assessment for European green crab (Carcinus maenas) in Canadian Waters
Thiel M (2003) Extended parental care in crustaceans-an update Cuidado parental extendido en crustáceos-conocimiento actual. Rev Chil Hist Nat 76:205–218
Thitamadee S, Prachumwat A, Srisala J et al (2016) Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 452:69–87. https://doi.org/10.1016/j.aquaculture.2015.10.028
Tindle S, Boone E, O’Brien J, Boettcher A (2004) Effects of salinity on larval stages of the rhizocephalan barnacle Loxothylacus texanus: survival and metamorphosis in response to the host, Callinectes sapidus. J Exp Mar Biol Ecol 302:165–176. https://doi.org/10.1016/j.jembe.2003.10.012
Torchin ME, Lafferty KD, Kuris AM (1996) Infestation of an introduced host, the European green crab, Carcinus maenas, by a symbiotic nemertean egg predator, Carcinonemertes epialti. J Parasitol 82:449–453
Torchin EM, Lafferty DK, Kuris MA (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345
Toscano BJ, Newsome B, Griffen BD (2014) Parasite modification of predator functional response. Oecologia 175:345–352. https://doi.org/10.1007/s00442-014-2905-y
Tulian EA (1920) Louisiana—Greatest in the production of shrimp—Penaeus aetiferus. Trans Am Fish Soc 49:115–124. https://doi.org/10.1577/1548-8659(1919)49
Valenzuela-Sánchez A, Wilber MQ, Canessa S et al (2021) Why disease ecology needs life-history theory: a host perspective. Ecol Lett 24:876–890. https://doi.org/10.1111/ele.13681
Vega-Villasante F, Ávalos-Aguilar JJ, Nolasco-Soria H et al (2015) Wild populations of the invasive Australian red claw Crayfish Cherax quadricarinatus (Crustacea, Decapoda) near the northern coast of Jalisco, Mexico: a new fishing and profitable resource. Lat Am J Aquat Res 43:781–785. https://doi.org/10.3856/vol43-issue4-fulltext-17
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vogt G (2012) Ageing and longevity in the Decapoda (Crustacea): a review. Zool Anz 251:1–25. https://doi.org/10.1016/j.jcz.2011.05.003
Walters EA, Bojko J, Crowley CE et al (2023) Salinity and temperature affect the symbiont profile and host condition of Florida USA blue crabs Callinectes sapidus. J Invertebr Pathol 198:107930. https://doi.org/10.1016/j.jip.2023.107930
Wang JF, Li M, Xiao J et al (2017) Hematodinium spp. Infections in wild and cultured populations of marine crustaceans along the coast of China. Dis Aquat Organ 124:181–191. https://doi.org/10.3354/dao03119
Weihs C, Ligges U, Luebke K, Raabe N (2005) klaR analyzing German business cycles. In: Baier D, Decker R, Schmidt-Thieme L (eds) Data analysis and decision support. Springer-Verlag, Berlin, pp 335–343
Wickham DE (1979) Predation by the nemertean Carcinonemertes errans on eggs of the Dungeness crab Cancer magister. Mar Biol 55:45–53. https://doi.org/10.1007/BF00391716
Wiens JJ (2015) Faster diversification on land than sea helps explain global biodiversity patterns among habitats and animal phyla. Ecol Lett 18:1234–1241. https://doi.org/10.1111/ele.12503
Wilber DH (1989) The influence of sexual selection and predation on the mating and postcopulatory guarding behavior of stone crabs (Xanthidae, Menippe). Behav Ecol Sociobiol 24:445–451. https://doi.org/10.1007/BF00293274
Wilcove DS, Rothstein D, Dubow J et al (1998) Quantifying threats to imperiled species in the United States: assessing the relative importance of habitat destruction, alien species, pollution, overexploitation, and disease. Bioscience 48:607–615. https://doi.org/10.2307/1313420
Wilhelm G, Mialhe E (1996) Dinoflagellate infection associated with the decline of Necora puber crab populations in France. Dis Aquat Organ 26:213–219. https://doi.org/10.3354/dao026213
Wood CL, Lafferty KD (2015) How have fisheries affected parasite communities? Parasitology 142:134–144. https://doi.org/10.1017/S003118201400002X
Wood CL, Lafferty KD, Micheli F (2010) Fishing out marine parasites? Impacts of fishing on rates of parasitism in the ocean. Ecol Lett 13:761–775. https://doi.org/10.1111/j.1461-0248.2010.01467.x
Wood CL, Sandin SA, Zgliczynski B et al (2014) Fishing drives declines in fish parasite diversity and has variable effects on parasite abundance. Ecology 95:1929–1946
Wootton EC, Pope EC, Vogan CL et al (2011) Morphology and pathology of the ectoparasitic copepod, Nicothoë astaci ('lobster louse’) in the European lobster, Homarus gammarus. Parasitology 138:1285–1295. https://doi.org/10.1017/S003118201100093X
Xiao Y, Greenwood JG (1993) The biology of Acetes (Crustacea: Sergestidae). In: Ansell AD, Gibson RN, Barnes M (eds) Oceanography and marine biology: an annual reviews. UCL Press, London, UK, pp 256–444
Xu W, Xie J, Shi H, Li C (2010) Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture 300:25–31. https://doi.org/10.1016/j.aquaculture.2009.12.024
Acknowledgements
EDM was supported by a University of Florida Preeminent Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
EDM and DCB—conceived of the study. EDM, JB, and NCS—compiled data. ZAS—performed false negative analysis, while EDM—performed remaining data analyses and figure development. All authors contributed to the development of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duermit-Moreau, E., Bojko, J., Siders, Z.A. et al. Decapod fisheries and parasite species richness: an exploration of host traits and parasitic influence. Rev Fish Biol Fisheries 34, 935–958 (2024). https://doi.org/10.1007/s11160-024-09860-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-024-09860-4