Abstract
Parasite alteration of the host (predator) functional response provides a mechanism by which parasites can alter predator–prey population dynamics and stability. We tested the hypothesis that parasitic infection of a crab (Eurypanopeus depressus) by a rhizocephalan barnacle (Loxothylacus panopei) can modify the crab’s functional response to mussel (Brachidontes exustus) prey and investigated behavioral mechanisms behind a potential change in the response. Infection dramatically reduced mussel consumption by crabs across mussel densities, resulting in a decreased attack rate parameter and a nearly eightfold reduction in maximum consumption (i.e. the asymptote, or inverse of the handling time parameter) in a type II functional response model. To test whether increased handling time of infected crabs drove the decrease in maximum consumption rate, we independently measured handling time through observation. Infection had no effect on handling time and thus could not explain the reduction in consumption. Infection did, however, increase the time that it took crabs to begin handling prey after the start of the handling time experiment. Furthermore, crabs harboring relatively larger parasites remained inactive longer before making contact with prey. This behavioral modification likely contributed to the reduced mussel consumption of infected crabs. A field survey revealed that 20 % of crabs inhabiting oyster reefs at the study site (North Inlet estuary, Georgetown, South Carolina, USA) are infected by the barnacle parasite, indicating that parasite infection could have a substantial effect on the population level crab-mussel interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites are increasingly recognized for the important roles they play in natural food webs (Minchella and Scott 1991; Wood et al. 2007; Lafferty et al. 2008). Parasites can make up a substantial portion of food web biomass compared to free-living species (Kuris et al. 2008), and their inclusion in food webs as independent nodes modifies patterns of connectance and food chain length (Lafferty et al. 2008). They can also directly alter the reproductive success and survivorship of their hosts (Minchella and Scott 1991; Marzal et al. 2005), thus mediating host population dynamics and the dynamics of communities.
Parasites can also have indirect effects on food webs by modifying the behavior, physiology, morphology and life-history (i.e. the traits) of their hosts (Holmes and Bethel 1972; Poulin and Thomas 1999; Fitze et al. 2004; Wood et al. 2007; Repetto and Griffen 2012). These effects on hosts can cascade to affect the species interactions that hosts are involved in (Minchella and Scott 1991). For example, trematode parasite infection increases the frequency of conspicuous behaviors exhibited by killifish (Lafferty and Morris 1996). Birds, the final hosts of the trematode parasite, preferentially consume infected killifish due to this behavioral modification (Lafferty and Morris 1996). Similarly, infection by an acanthocephalan parasite changes the color and behavior of amphipods, which in turn increases their susceptibility to predation by stickleback fish, the final host of the parasite (Bakker et al. 1997). Despite an abundance of studies on the effects of parasites on host traits, it remains unclear how trait-mediated effects of parasites can scale up to affect the long-term dynamics and stability of predator–prey populations and food webs (Lafferty et al. 2008).
A likely factor that may link parasite impacts on the individual host to broader impacts on predator–prey or food web dynamics is the predator functional response (Dick et al. 2010; Haddaway et al. 2012). The functional response describes how a predator’s per capita consumption rate of prey changes with the local density of prey (Holling 1959). This response is behavioral, depending on the predator’s rate of attack and the time it takes to handle an individual prey (Jeschke et al. 2002). The functional response is a critical component of population models of species interactions, and the precise shape and parameter values of the functional response are major determinants of short and long-term predator–prey population stability in these models (Murdoch and Oaten 1975; Abrams 2000). For example, a hyperbolic response curve (i.e. type II functional response) typically destabilizes predator–prey dynamics, while a sigmoidal response curve (i.e. type III response), often driven by a refuge from predation at low prey densities (Sih 1987), typically stabilizes interactions (Murdoch and Oaten 1975).
Numerous studies have demonstrated strong effects of parasites on predator–prey interactions (Lafferty 1992; Bernot and Lamberti 2008), yet these studies typically examine predation as a mechanism for parasite transmission between hosts, and rarely consider parasites as interaction modifiers (sensu Wootton 1994) of predator–prey systems in a food web context (but see Wood et al. 2007; Bernot and Lamberti 2008). Indeed, only a few studies (Dick et al. 2010; Haddaway et al. 2012) have examined how parasites affect the consumption rates of their predatory hosts across prey densities, i.e. the predator functional response. Parasite alteration of the host functional response is, therefore, an understudied pathway by which parasites can indirectly alter the dynamics and stability properties of predator–prey populations. Considering the ubiquity of parasites in food webs, and pervasive effects of parasite infection on host behavior (Holmes and Bethel 1972; Poulin and Thomas 1999), parasite modification of host functional responses could be a common trait-mediated indirect interaction in nature.
In the present study, we examined the impact of an invasive rhizocephalan barnacle parasite (Loxothylacus panopei) on the functional response of its host, the flat-backed mud crab (Eurypanopeus depressus) preying on scorched mussels (Brachidontes exustus). Flat-backed mud crabs are important predators of scorched mussels in oyster reefs and other structurally complex habitats along the Gulf and Eastern Coasts of the United States (McDonald 1982; Williams 1984; Lee and Foighil 2004). The parasitic barnacle L. panopei was originally restricted to the Gulf of Mexico, but invaded the East Coast of the United States in 1964, potentially through shipments of oysters that also carried infected crabs from the Gulf of Mexico (Van Engel et al. 1966). Rhizocephalan barnacles such as L. panopei are macroparasites that exclusively infect crustaceans and mainly crabs (Overstreet 1983). Female barnacle cyprid larvae settle on a recently molted crab and produce a system of branching roots throughout the crab’s body cavity (O’Brien and Van Wyk 1985). After this internal phase is complete, a sac-like externa (the parasite’s reproductive body) is extruded under the crab’s abdomen. Male cyprid larvae then settle on and fertilize this externa. Common effects of rhizocephalan barnacles on crab hosts include the inhibition or cessation of growth as well as the castration of both female and male crabs (O’Brien and Van Wyk 1985). Thus, parasite infection precludes reproduction but allows crabs to survive and interact ecologically with conspecifics and other species (Lafferty and Kuris 2009).
We compared the mussel consumption rate of uninfected and infected crabs across mussel densities to examine the hypothesis that parasite infection can alter the predator (host) functional response. We then tested whether increased handling time and/or reaction time (i.e. the time it took crabs to respond to prey) of infected crabs drove the decrease in prey consumption associated with parasite infection. Finally, we surveyed parasite prevalence in the field, providing some insight into the potential population impacts of parasite alteration of the functional response and thus the crab-mussel predator–prey interaction.
Methods
We tested the effects of barnacle (Loxothylaccus panopei) parasite infection on the interaction between the flat-backed mud crab (Eurypanopeus depressus) and its prey, the scorched mussel (Brachidontes exustus). All animals used in experiments were collected from intertidal oyster reefs in tidal creeks throughout North Inlet estuary (33°20′N, 79°10′W), Georgetown, South Carolina, USA. North Inlet is a relatively pristine salt marsh consisting of ocean-dominated tidal creeks with a high average salinity (~34 ppt) and a diurnal tidal cycle (Dame et al. 1986). We ran experiments in the screened-in, outdoor wet laboratory at the adjacent Baruch Marine Field Laboratory. The field survey of parasite prevalence was also conducted in intertidal reefs throughout North Inlet. Experiments and field sampling were conducted from June through August 2012.
Functional response experiment
We first measured the functional responses of uninfected and infected mud crabs (8–13.5 mm carapace width) foraging on the scorched mussel (4–7 mm shell length). Mussels in this size range are abundant in oyster clusters throughout the study site (Toscano and Newsome, personal observations). We identified infected crabs by the presence of parasite externae, indicative of a mature stage of parasite infection (Alvarez et al. 1995). However, we cannot discount the possibility that uninfected crabs were actually in the immature, internal stage of infection. Mussels were offered to crabs in eight densities: 2, 4, 6, 8, 10, 16, 24 and 32 mussels per experimental chamber. Trials were run in a randomized complete block design and each treatment was replicated a total of 12 times (12 blocks). Individual crabs were used once in this experiment.
We ran functional response experiments in plastic chambers (15 cm length × 13 cm width × 7.6 cm height) containing oyster shells to simulate the structure of natural oyster reef habitat. Each chamber received five cleaned and dried oyster shells (7–10 cm shell length) to provide a relatively consistent substrate for mussels to attach to. The necessary number of mussels for a given treatment was evenly distributed over the shell throughout each chamber. Experimental chambers were then placed in a larger cylindrical flow-through seawater tank (97 cm diameter × 41 cm depth, water depth: 25 cm) and mussels were allowed to attach to oyster shells over a 12 h period. Crabs were starved for a 24 h period before placement in the chambers to standardize hunger levels. After starvation, crabs were allowed to forage for a 13 h period overnight, generally from 1900 to 0800 h. Chambers received a constant flow of unfiltered sea water from North Inlet throughout this period. After 13 h, the number of remaining mussels was recorded. All dead mussels showed signs of being preyed upon by crabs (cracked shells).
Functional response models were fit separately to uninfected and infected crabs, allowing us to examine the effects of parasite infection on the functional response. First, to determine the type of functional response (i.e. type I, II or III), we used polynomial logistic regression on the proportion of prey consumed as a function of prey density (Juliano 2001). For both uninfected and infected crabs, the first order term in this regression was significantly negative (i.e. declining proportion consumed at very low prey densities), indicative of a type II functional response (Juliano 2001). Because prey were depleted over the 24 h that crabs foraged and not replaced, a Rogers type II functional response model that accounts for prey depletion was fit separately to uninfected and infected crabs (Rogers 1972):
where N e is the number of prey eaten, N 0 is the initial prey density, α is attack rate, T h is handling time, P is the number of predator individuals (set to 1), and T is the experimental duration (set to 13 h). Equation 1 is a recursive function of Ne, and so we used the Lambert W function to implement the model (see Bolker 2008 for details):
where W is the Lambert W function and all other parameters are the same as in Eq. 1. This functional response model was fit to prey consumption data using maximum likelihood estimation with binomial errors in the statistical software R (package “bblme”).
Handling and reaction time experiment
During the functional response experiment, we noticed that the maximum consumption rate of infected crabs was substantially lower than that of uninfected crabs. In traditional functional response models, maximum consumption rate is equivalent to the inverse of handling time, or the time it takes to capture, subdue and consume an individual prey (Juliano 2001). These models assume that predators forage continuously (Tully et al. 2005; Jeschke et al. 2002), and in such a situation, predators are only limited by handling time at high prey densities. Thus, the reduced maximum consumption rate of infected crabs suggested an increase in the handling time of infected crabs. To test this, we observed and compared the handling time of uninfected and infected crabs independently of the functional response experiment.
Crabs and mussels used in this experiment fell within the same size ranges used in the functional response experiment. We recorded the carapace width of each crab and length of each mussel before trials and crabs were starved for 24 h to standardize hunger levels. The handling time of crabs was observed in cylindrical glass containers (6 cm diameter × 5 cm height) with one crab and one mussel per container. Ten crabs were observed at once and the experiment was conducted over multiple nights. Handling time was observed at night (generally from 1900 to 2400 h) under a red light to minimize disturbance to crabs (Griffen et al. 2012). Crabs were allowed to acclimate for 5 min before exposure to a mussel. Once a mussel was introduced, we recorded the time it took for a crab to make contact with the mussel (reaction time), as well as the time it took to completely consume the mussel after the first contact (handling time). Crabs were given 1 h to begin handling mussels before the experiment was terminated. Crabs that did not begin handling mussels during this time were excluded from the analysis. The reaction time of uninfected and infected crabs was measured and compared to test whether a longer period of inactivity before reacting to mussel prey contributed to the decreased consumption of infected crabs.
After this experiment, we removed and weighed the parasite externae of infected crabs to test whether the externa mass relative to the crab’s body mass influenced the handling or reaction time of infected crabs. This could be expected if the size of the externa is indicative of the level of parasite infection, or acts as a physical impediment to crab handling of mussels. We compared the dry weight of the externa to the dry weight of the remaining crab body to determine relative parasite mass. Both the removed externae and crab bodies were dried in an oven at 60 °C for 72 h before measurement of dry weight.
We used linear mixed models (LMM) to test the fixed effects of parasite infection and the crab:mussel size ratio on handling and reaction time. Data were normally distributed, justifying use of a Gaussian distribution. We also used LMM to test the fixed effects of the crab:mussel size ratio and the parasite:crab mass ratio on the handling and reaction time of infected crabs. The day of observation was modeled as a random factor in all models to control for pseudoreplication. We fit models with and without fixed factors while retaining the random factor (day of observation) and compared models using Akaike’s information criterion (AIC) to determine whether additional factors improved the fit despite increased model complexity.
Field survey
We surveyed the prevalence of the barnacle parasite in flat-backed mud crabs in the field. This survey allowed us to determine the overall rate of parasite infection in crabs, as well as some intrinsic factors associated with infection. We sampled crabs in haphazardly placed quadrats (0.25 m2) in intertidal oyster reefs throughout North Inlet. Nine oyster reefs were sampled in total and six quadrat samples were taken from each reef (a total of 54 quadrat samples). All quadrat samples were taken from the upper intertidal sections of reefs. Within each quadrat, all E. depressus were removed by hand and their carapace width, sex, and infection status were recorded. We recorded the presence of crabs below 5 mm, but were unable to accurately measure the carapace width or discern the sex of these crabs.
We used a generalized linear mixed model (GLMM) with a binomial error distribution to test the effect of crab sex on the probability of parasite infection. A binomial error distribution was used to model binary (presence-absence) data. Crab sex was modeled as a fixed factor and quadrat was modeled as a random factor, with individual infection status (uninfected or infected) as the response variable. We used a non-parametric Kolmogorov–Smirnov test to test for a difference in the size distribution of uninfected and infected crabs. All statistical analyses were conducted in R version 2.15.2 (R Development Core Team).
Results
Functional response
Parasite infection dramatically reduced the mussel consumption rate of crabs across mussel prey densities (Fig. 1). In a type II functional response model fit to infected crabs, this reduction in mussel consumption was manifested as a ~30 % decrease in the attack rate parameter and a nearly eightfold increase in the handling time parameter compared to uninfected crabs.
Effect of barnacle parasite (Loxothylaccus panopei) infection on the functional response of a mud crab predator (Eurypanopeus depressus) foraging on mussel (Brachidontes exustus) prey. Points indicate mean consumption ±1 SE of uninfected (white points; n = 96) and infected crabs (black points; n = 96). Lines depict functional response model (Eq. 1) fits to mussel consumption data of uninfected (dotted line) and infected (black line) crabs. Mussels were offered to crabs in eight densities (2, 4, 6, 8, 10, 16, 24, and 32 mussels per chamber) and crabs were allowed to forage for 13 h
Handling time
The predator–prey size ratio was an important predictor of individual crab handling time measured through observation, while parasite infection (i.e. uninfected or infected) had little effect on handling time (Fig. 2). Handling time was best explained with a model containing the crab:mussel size ratio as a fixed effect (weight = 0.923); the crab:mussel size ratio reduced handling time (estimate ±1 SE = −0.106 ± 0.016; Fig. 2) in this model. This model was substantially better than the model containing both the crab:mussel size ratio and parasite infection as fixed effects (ΔAIC = 4.96, weight = 0.077), as well as the model containing only parasite infection as a fixed effect (ΔAIC = 32.34, weight = 0.00).
In infected crabs, the predator–prey size ratio was again an important predictor of handling time. The linear model containing this fixed factor (weight = 0.504, crab:mussel size ratio estimate ±1 SE = −0.122 ± 0.029) performed similarly to the model containing both the crab:mussel size ratio and the parasite:crab mass ratio as fixed factors (ΔAIC = 0.10, weight = 0.481, crab:mussel size ratio estimate ±1 SE = −0.121 ± 0.030, parasite:crab mass ratio estimate ±1 SE = −0.251 ± 1.293), and substantially better than the model containing just the parasite:crab mass ratio as a fixed factor (ΔAIC = 7.01, weight = 0.015).
Reaction time
The reaction time of crabs (i.e. latency in responding to mussel prey) was best predicted by a model containing parasite infection as a fixed effect (weight = 0.808, parasite infection estimate ±1 SE = 0.050 ± 0.029; Fig. 3); this model was substantially better than both the model containing the crab:mussel size ratio as a fixed effect (ΔAIC = 3.11, weight = 0.171) and the model containing both these factors as fixed effects (ΔAIC = 7.26, weight = 0.021). Furthermore, though excluded from the analysis, 23 infected crabs never began handling mussels over the duration of the experiment (1 h) compared to 13 uninfected crabs.
Effect of the barnacle parasite on reaction time (i.e. the time it took to begin handling mussels after the start of the handling time experiment) of mud crabs. Main graph depicts the influence of the parasite:crab size ratio on the reaction time of infected crabs (n = 55). Inset graph depicts the influence of parasite infection on the reaction time of uninfected (white points; n = 53) and infected (black points; n = 55) crabs. Reaction time was measured through observation of predator–prey interactions
In infected crabs, reaction time was best predicted by a linear model containing the parasite:crab mass ratio as a fixed factor (weight = 0.966). This model performed substantially better than the model containing both the parasite:crab mass ratio and the crab:mussel size ratio as fixed factors (ΔAIC = 7.16, weight = 0.027), and the model containing only the crab:mussel size ratio as a fixed factor (ΔAIC = 9.84, weight = 0.007). In the best-fit model, infected crabs with relatively larger parasites took longer to begin handling mussels (parasite:crab mass ratio estimate ±1 SE = 2.590 ± 1.615; Fig. 3).
Field survey
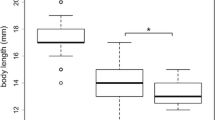
Nearly 20 % of crabs sampled in North Inlet estuary were infected by the barnacle parasite (86 out of 446 crabs). Crab sex had a significant effect (GLMM: estimate ±1 SE = −0.991 ± 0.299, z = −3.319, p < 0.001) on the probability of infection. Specifically, the sex ratio of infected crabs was heavily skewed towards females (Fig. 4). The size distribution of infected crabs also differed from that of uninfected crabs (KS-test: D = 0.486, p < 0.001). The smallest infected crab found was 7.2 mm carapace width, while uninfected crabs below 7 mm carapace width were abundant (Fig. 4).
Discussion
We found that barnacle parasite infection drastically reduced the magnitude of the crab functional response to mussel prey. The most striking effect of infection was a nearly eightfold decrease in the maximum consumption rate (i.e. the inverse of the handling time model parameter) of infected crabs. Two other studies (Dick et al. 2010; Haddaway et al. 2012) have tested the effects of parasite infection on the host functional response to prey. Dick et al. (2010) found that acanthocephalan parasite infection actually increased the functional response of an amphipod host foraging on isopods, though the mechanism behind this effect was not investigated. Specifically, infection increased attack rate and handling time parameters in a type II functional response model (Dick et al. 2010). Similar to our study, Haddaway et al. (2012) found that microsporidian parasite infection of crayfish foraging on amphipods reduced attack rate and handling time parameters in a type II functional response model, though neither of these studies found as strong an effect of infection on host prey consumption as in the present study.
We identified infected crabs by the presence of parasite externae and, therefore, failed to detect whether uninfected crabs were actually in the immature, internal stage of parasite infection. Furthermore, we could not find evidence of the internal portion of the parasite in our dissections of these “uninfected” crabs, and thus could not determine the prevalence of this stage of infection. The internal stage of infection lasts approximately 30 days on average in a different species of Xanthid crab, Rhithropanopeus harrisii (Walker et al. 1992; Alvarez et al. 1995). If crabs in the immature stage of infection exhibited reduced prey consumption similar to crabs in the mature stage of infection, our inclusion of these crabs as “uninfected” would reduce the magnitude of the negative effect of infection on the functional response. Our results, therefore, represent a conservative estimate of the effects of parasite infection on crab consumption rates. However, rhizocephalan parasite effects on the grooming and burrowing behavior of a Portunid crab (Charybdis longicollis) were only observed in crabs harboring externae (Innocenti et al. 1998).
We further tested the possibility that increased handling time of infected crabs drove the reduction in consumption by comparing the mussel handling time of uninfected and infected crabs independently of the functional response experiment. Parasite infection had no effect on handling time measured through observation and, therefore, could not explain the reduction in consumption. Infected crabs did, however, show some signs of increased latency in reacting to mussel prey in the handling time experiment. Specifically, infection increased the time that it took crabs to begin handling prey after the start of the experiment, and infected crabs with larger parasites took longer to make contact with mussel prey than crabs with relatively smaller parasites. This finding is consistent with behavioral observations indicating that infected crabs spend less time active and more time hiding compared to uninfected crabs (Newsome, personal observations). A similar reduction in activity concomitant with rhizocephalan barnacle infection has been observed in shore crabs (Carcinus maenas) (Mouritsen and Jensen 2006). Typical functional response models, including the type II model used here, do not incorporate such non-foraging activities (Jeschke et al. 2002) and instead assume that a predator’s time is divided between searching for prey and handling prey, though this assumption is rarely tested (Abrams 1990; Tully et al. 2005). Thus, while the type II functional response model described consumption data well in the present study, it did not explain the underlying mechanism behind the reduction in mussel consumption of infected crabs, and we think this reduced consumption is due in part to reduced foraging activity of infected crabs (i.e. violation of the assumption of constant foraging activity).
Several non-mutually exclusive explanations exist for the effects of infection on crab behavior and mussel consumption revealed in the present study. First, parasite infection may reduce the crab’s energy demands. Previous work has shown that infection by rhizocephalan barnacles can lead to the reduction or cessation of crab somatic growth, potentially due to the reallocation of energy to the parasite (O’Brien and Van Wyk 1985). Thus, it is possible that this reduced need for energy for growth could reduce crab foraging effort if the parasite cost is less than the savings of reduced growth. Second, rhizocephalan roots can invade all organs and tissues of the host and the number of rootlets increases over the course of the infection (Bortolini and Alvarez 2008). Therefore, the internal portion of the parasite could compete for space with other internal organs such as the crab’s gut, potentially reducing space for food storage before or during digestion. Our dissections of infected crabs, however, revealed no clear effects of crowding, and so we think this explanation is unlikely. Third, parasite infection could reduce the crab host’s digestive capabilities, thereby increasing digestion time and reducing foraging effort and prey consumption (see also Wood et al. 2007). Rhizocephalan parasite infection of blue crabs (Callinectes sapidus) damages the crab hepatopancreas and causes significant loss of hepatopancreas tissue (Bortolini and Alvarez 2008). This organ serves multiple functions in digestion, including the secretion of digestive enzymes and absorption of nutrients, and therefore, any damage to the hepatopancreas could reduce the rate of digestion. Lastly, parasite infection could impair the neurosensory capabilities of crabs, altering their behavior and foraging ability.
Potential effects of parasite infection on crab-mussel population dynamics
Twenty percent of crabs sampled harbored parasite externae, indicative of the mature stage of parasitic infection (Alvarez et al. 1995). Again, our survey did not examine the immature, internal stage of parasite infection and, therefore, likely underestimated actual parasite prevalence. Our survey also revealed that the sex ratio of infected crabs was heavily skewed towards females. This is potentially due to the parasitic feminization of male crabs, a common effect of rhizocephalan barnacles on crab hosts (O’Brien and Van Wyk 1985). Furthermore, in accordance with another study (Alvarez et al. 1995), we found that infected crabs were intermediate in body size compared to uninfected crabs (i.e. few crabs <7 and >15 mm carapace width). This is likely due to the reduced growth rate or cessation of growth in infected crabs (O’Brien and van Wyk 1985).
Considering the prevalence of parasite infection in flat-backed mud crabs and strong effects of infection on the crab functional response, the parasitic barnacle could have substantial effects on the long-term dynamics of the crab-mussel interaction. Rhizocephalan barnacles castrate their crab hosts, thus precluding reproduction by infected individuals (O’Brien and Van Wyk 1985). Therefore, reduced consumption by infected crabs cannot directly feedback to affect the population dynamics of crabs (i.e. the numerical response) as modeled in a typical Lotka-Volterra predator–prey framework. However, reduced mussel consumption of infected crabs could provide some predation refuge for mussels, allowing mussel density to increase and indirectly enhancing the population growth of uninfected crabs. These predictions are speculative and require further study in the field. Interestingly, the larvae of the parasitic barnacle L. panopei cannot tolerate low salinity (Reisser and Forward 1991). Therefore, spatial and temporal variation in salinity could mediate barnacle parasite effects on crab-mussel dynamics.
In sum, parasite modification of host behavior and physiology is widespread (Poulin and Thomas 1999), and these trait changes likely influence host functional responses (Dick et al. 2010; Haddaway et al. 2012; the present study), and, therefore, the dynamics of predator–prey populations and energy flow in food webs. More broadly, parasite modification of the host functional response is part of a general class of trait-mediated indirect interactions driven by parasite alteration of host traits. This class of interactions includes parasite modification of host ecosystem engineering (Thomas et al. 1999), as well as parasite mediation of interspecific competition involving hosts (Park 1948; Schall 1992). Future studies of such parasite-mediated interactions could benefit by measuring the curvature of responses and trade-offs as we have done here, as opposed to two-level experiments (e.g. absence vs. presence, or “low” vs. “high”). Such multi-level experiments allow long-term, population-level prediction in ecological models (Bolker et al. 2003), and will, therefore, enhance our understanding of the key functional roles of parasites in community and food web ecology (Lafferty et al. 2008).
References
Abrams PA (1990) The effects of adaptive behavior on the type-2 functional response. Ecology 71:877–885
Abrams PA (2000) The evolution of predator-prey interactions: theory and evidence. Annu Rev Ecol Syst 31:79–105
Alvarez F, Hines AH, Reaka-Kudla ML (1995) The effects of parasitism by the barnacle Loxothylacus panopaei (Gissler) (Cirripedia: Rhizocephala) on growth and survival of the host crab Rhithropanopeus harrisii (Gould) (Brachyura: Xanthidae). J Exp Mar Biol Ecol 192:221–232
Bakker TCM, Mazzi D, Zala S (1997) Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology 78:1098–1104
Bernot RJ, Lamberti GA (2008) Indirect effects of a parasite on a benthic community: an experiment with trematodes, snails and periphyton. Freshw Biol 53:322–329
Bolker BM (2008) Ecological models and data in R. Princeton University Press, Princeton
Bolker B, Holyoak M, Křivan V, Rowe L, Schmitz O (2003) Connecting theoretical and empirical studies of trait-mediated interactions. Ecology 84:1101–1114
Bortolini JL, Alvarez F (2008) Hepatopancreas alteration of the blue crab Callinectes sapidus by the rhizocephalan barnacle Loxothylacus texanus. J Invertebr Pathol 99:354–356
Dame R, Chrzanowski T, Bildstein K, Kjerfve B, McKellar H, Nelson D, Spurrier J, Stancyk S, Stevenson H, Vernberg F, Zingmark R (1986) The outwelling hypothesis and North Inlet, South Carolina. Mar Ecol Progr Ser 33:217–229
Dick JT, Armstrong M, Clarke HC, Farnsworth KD, Hatcher MJ, Ennis M, Kelly A, Dunn AM (2010) Parasitism may enhance rather than reduce the predatory impact of an invader. Biol Lett 6:636–638
Fitze PS, Tschirren B, Richner H (2004) Life history and fitness consequences of ectoparasites. J Anim Ecol 73:216–226
Griffen BD, Toscano BJ, Gatto J (2012) The role of individual behavior type in mediating indirect interactions. Ecology 93:1935–1943
Haddaway NR, Wilcox RH, Heptonstall RE, Griffiths HM, Mortimer RJ, Christmas M, Dunn AM (2012) Predatory functional response and prey choice identify predation differences between native/invasive and parasitised/unparasitised crayfish. PLoS ONE 7:e32229
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Holmes JC, Bethel WM (1972) Modification of intermediate host behaviour by parasites. Zool J Linn Soc 51:123–149
Innocenti G, Vannini M, Galil B (1998) Notes on the behaviour of the portunid crab Charybdis longicollis Leene parasitized by the rhizocephalan Heterosaccus dollfusi Boschma. J Nat Hist 32:1577–1585
Jeschke JM, Kopp M, Tollrian R (2002) Predator functional responses: discriminating between handling and digesting prey. Ecol Monogr 72:95–112
Juliano SA (2001) Nonlinear curve fitting. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, Oxford, pp 178–196
Kuris AM et al (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518
Lafferty KD (1992) Foraging on prey that are modified by parasites. Am Nat 140:854–867
Lafferty KD, Kuris AM (2009) Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol 25:564–572
Lafferty KD, Morris AK (1996) Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77:1390–1397
Lafferty KD, Allesina S, Arim M, Briggs CJ, De Leo G, Dobson AP, Dunne JA, Johnson PT, Kuris AM, Marcogliese DJ (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546
Lee T, Foighil D (2004) Hidden Floridian biodiversity: mitochondrial and nuclear gene trees reveal four cryptic species within the scorched mussel, Brachidontes exustus, species complex. Mol Ecol 13:3527–3542
Marzal A, De Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
McDonald J (1982) Divergent life history patterns in the co-occurring intertidal crabs Panopeus herbstii and Eurypanopeus depressus (Crustacea: Brachyura: Xanthidae). Mar Ecol Progr Ser 8:173–180
Minchella DJ, Scott ME (1991) Parasitism: a cryptic determinant of animal community structure. Trends Ecol Evol 6:250–254
Mouritsen KN, Jensen T (2006) The effect of Sacculina carcini infections on the fouling, burying behaviour and condition of the shore crab, Carcinus maenas. Mar Biol Res 2:270–275
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
O’Brien J, Van Wyk P (1985) Effects of crustacean parasitic castrators (epicaridean isopods and rhizocephalan barnacles) on growth of crustacean hosts. In: Wenner AM (ed) Crustacean issues, factors in adult growth, vol 3. Balkema Press, Rotterdam, pp 191–218
Overstreet RM (1983) Metazoan symbionts of crustaceans. In: Provenzano AJ (ed) The biology of crustacea, pathobiology, vol 6. Academic Press, New York, pp 155–250
Park T (1948) Experimental studies of interspecies competition. I. competition between populations of the flour beetles, Tribolium confusum Duval and Tribolium castaneum Herbst. Ecol Monogr 18:265–308
Poulin R, Thomas F (1999) Phenotypic variability induced by parasites: extent and evolutionary implications. Parasitol Today 15:28–32
Reisser CE, Forward RB (1991) Effect of salinity on osmoregulation and survival of a rhizocephalan parasite, Loxothylacus panopaei, and its crab host, Rhithropanopeus harrisii. Estuaries 14:102–106
Repetto M, Griffen BD (2012) Physiological consequences of parasite infection in the burrowing mud shrimp, Upogebia pugettensis, a widespread ecosystem engineer. Mar Freshw Res 63:60–67
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383
Schall JJ (1992) Parasite-mediated competition in Anolis lizards. Oecologia 92:58–64
Sih A (1987) Prey refuges and predator prey stability. Theor Popul Biol 31:1–12
Thomas F, Poulin R, de Meeüs T, Guégan JF, Renaud F (1999) Parasites and ecosystem engineering: what roles could they play? Oikos 84:167–171
Tully T, Cassey P, Ferriere R (2005) Functional response: rigorous estimation and sensitivity to genetic variation in prey. Oikos 111:479–487
Van Engel WA, Dillon WA, Zwerner D, Eldridge D (1966) Loxothylacus panopaei (Cirripedia, Sacculinidae) an introduced parasite on a xanthid crab in Chesapeake Bay, USA. Crustaceana 10:110–112
Walker G, Clare A, Rittschof D, Mensching D (1992) Aspects of the life-cycle of Loxothylacus panopaei (Gissler), a sacculinid parasite of the mud crab Rhithropanopeus harrisii (Gould): a laboratory study. J Exp Mar Biol Ecol 157:181–193
Williams AB (1984) Shrimps, lobsters, and crabs of the Atlantic coast of the Eastern United States, Maine to Florida. Smithsonian Institution Press, Washington, DC
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AM (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104:9335–9339
Wootton JT (1994) The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst 25:443–466
Acknowledgments
Funding was provided by an NSF Graduate Research Fellowship under Grant No. DGE-0929297, by NSF Grant No. OCE-1129166, and by a Magellan grant for undergraduate research by the University of South Carolina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pete Peterson.
Rights and permissions
About this article
Cite this article
Toscano, B.J., Newsome, B. & Griffen, B.D. Parasite modification of predator functional response. Oecologia 175, 345–352 (2014). https://doi.org/10.1007/s00442-014-2905-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2905-y