Abstract
Anaplastic thyroid cancer (ATC) is an infrequent thyroid tumor that usually occurs in elderly patients. There is often a history of previous differentiated thyroid cancer suggesting a biological progression. It is clinically characterized by a locally invasive cervical mass of rapid onset. Metastases are found at diagnosis in 50% of patients. Due to its adverse prognosis, a prompt diagnosis is crucial. In patients with unresectable or metastatic disease, multimodal therapy (chemotherapy and external beam radiotherapy) has yielded poor outcomes with 12-month overall survival of less than 20%. Recently, significant progress has been made in understanding the oncogenic pathways of ATC, leading to the identification of BRAF V600E mutations as the driver oncogene in nearly 40% of cases. The combination of the BRAF inhibitor dabrafenib (D) and MEK inhibitor trametinib (T) showed outstanding response rates in BRAF-mutated ATC and is now considered the standard of care in this setting. Recently, it was shown that neoadjuvant use of DT followed by surgery achieved 24-month overall survival rates of 80%. Although these approaches have changed the management of ATC, effective therapies are still needed for patients with BRAF wild-type ATC, and high-quality evidence is lacking for most aspects of this neoplasia. Additionally, in real-world settings, timely access to multidisciplinary care, molecular testing, and targeted therapies continues to be a challenge. Health policies are warranted to ensure specialized treatment for ATC.
The expanding knowledge of ATC´s molecular biology, in addition to the ongoing clinical trials provides hope for the development of further therapeutic options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anaplastic thyroid cancer (ATC) is an infrequent highly aggressive thyroid malignancy composed of undifferentiated thyroid cells, that usually occurs in elderly patients [1]. There is often a history of previous differentiated thyroid cancer (DTC) or long-standing goiter, suggesting a biological progression. Clinically, it is characterized by a cervical mass of rapid onset, with local invasion and compressive symptoms. Metastases are found at diagnosis in nearly 50% of patients [2]. Because of the aggressive behavior of this disease, a prompt diagnosis is crucial to establish therapeutic options.
In selected patients with intrathyroidal disease, complete surgical resection, followed by chemo-radiotherapy may provide long-term survival. Nevertheless, surgery is not feasible in most cases due to local extension. Historically, in patients with unresectable or metastatic disease, multimodal therapy, the combination of different cytotoxic chemotherapy schemes and external beam radiotherapy (EBRT), has yielded poor outcomes, with less than 20% overall survival at 12-months [3].
Recently, significant progress has been made in understanding the oncogenic pathways of ATC, primarily through investigations using next generation sequencing (NGS). This has led to the identification of BRAF V600E mutations as the driver oncogene in nearly 40% of cases. The combination of the BRAF inhibitor dabrafenib (D) and MEK inhibitor trametinib (T) showed significant response rates in BRAF-mutated ATC in a phase 2 trial [4]. Consequently, in 2018, the Food and Drug Administration (FDA) approved this combination for patients with unresectable or metastatic BRAF-mutated ATC. Still, most patients do not achieve complete responses (CR), and ultimately, resistance to BRAF inhibition often develops. Recently, it was shown that neoadjuvant use of DT followed by surgery achieved 24-month overall survival (OS) rates of 80% [5]. This approach represents a radical change in the treatment for this group of patients. Nonetheless, effective therapies are still needed for patients with BRAF wild-type ATC, and high-quality evidence is lacking for most aspects of this neoplasia.
The aim of this review is to discuss the epidemiology, oncogenesis, clinical presentation, and therapy of ATC, focusing on the latest advances and future perspectives.
2 Epidemiology
The worldwide incidence of thyroid cancer is largely driven by an increase in papillary thyroid cancer (PTC), which is the most common histology. Due to its rarity, information on incidence, trends and risk factors for ATC is scarce, and even referral centers may treat a small number of patients. A European survey informed that 69% of 94 institutions within the Endocrine Task Force and the Head and Neck Cancer Group reported less than 5 patients treated per year, and only 14% of these centers had currently ongoing clinical trials [6].
ATC is a rare cancer (occurring in fewer than 15 in 100,000 inhabitants/year) [7]. It is consistently the least common thyroid cancer across different regions, ranging from 1.13 to 4% [8,9,10] In an international study, ATC incidence rates were much lower, and with less geographical variation, than all other thyroid cancer subtypes [11], despite different genetic backgrounds and lifestyles.
In most epidemiological studies, trends for incidence rates of ATC are stable over the years [12,13,14,15]. Moderate but declining rates were observed for ATC in most countries in 2008–2012 [10], which may relate to changes in iodine sufficiency status or improved treatment of DTC. Nevertheless, other studies have found an increase in incidence [16, 17]. Aging of the general population (as most ATC are diagnosed in elderly patients), more prolonged survival of DTC patients with structural disease (leading to dedifferentiation) and more exhaustive inclusion of patients in databases may explain this.
Patients with ATC are diagnosed at a more advanced age than differentiated thyroid carcinoma (DTC). Mean age at diagnosis of ATC is similar across different studies, ranging from 68 to 73 years [16,17,18,19,20]. The age-specific incidence curves for ATC show linear increase [10], with only 2% of patients under 40 years at diagnosis [19]. The female-to-male ratio is 1.4–2.4/1 [9, 16,17,18, 20], which is lower than the proportion generally found in DTC.
Risk factors for ATC remain uncertain, as case/control studies are lacking. A lower level of education, the presence of goiter, and type B blood group were reported as significant risk factors in multivariate logistic regression analysis in a study from Zivaljevic et al. [21]. A meta-analysis including 32 studies found a significantly increased risk for ATC in obese patients [22]. A study from Sicily reported that the incidence of ATC was significantly increased in the provinces closest to the volcanic areas [18]; additional studies on environmental factors are needed to assess their relationship with ATC.
Despite its rarity, ATC is responsible for 19.9–38.5% of thyroid cancer-related deaths [8, 23]. Hazard ratio for disease specific mortality is 12.65 (11.50–13.92), as compared with PTC [24]. Moreover, for patients with ATC, the majority of deaths are due to thyroid cancer and the probability of dying from thyroid cancer during the first year is 75% [24]. The most common specific cause of death in ATC was respiratory insufficiency (35%), followed by airway obstruction (25%) and cerebral cardiovascular-related death (5%) [25].
In a study from Denmark, 1- and 5-year relative survival showed minimal variations in patients treated from 1980 to 1984 and those treated from 2000 to 2014 (12.7 and 9.9% vs. 17.8% and 12.2% respectively) [26]. However, a single-institution analysis of 479 ATC showed significant improvement in survival at 2 years, from 18% in 2000–2013 to 25% in 2014–2016 to 42% in 2017–2019 group (hazard ratio, 0.50) [27], which may be related to the inclusion of rapid access programs, routine molecular testing and neoadjuvant therapy with BRAF inhibitors. However, this approach may not be feasible in many institutions/countries, as technologies for molecular diagnosis and targeted therapies are not readily available.
3 Oncogenesis
In recent years, due to the introduction of NGS, new insights were acquired on the molecular biology of ATC. A stepwise progression process, from DTC to poorly differentiated thyroid cancer (PDTC) to ATC was recognized in a significant percentage of cases. This is in coincidence with the history of a previously resected DTC/PDTC or the coexistence of DTC/PDTC foci in the surgical specimens of 20–60% of ATC patients [19, 28]. In these cases, both DTC and ATC share a common origin (early mutations), but as disease progresses, new molecular alterations (late mutations) accumulate and they can be found exclusively in the ATC component [19, 28].
ATC is a genetically complex disease, with significant inter-patient variability. Individual tumors carry multiple mutations, both in oncogenes and tumor suppressor genes. Tumor mutational burden is significantly higher (median, 5–6 mutations/Mb) [19, 29, 30] than that of DTC (1 mutation/Mb) [31] Nevertheless, contrary to what its clinical aggressiveness may suggest, ATC is not a highly mutated tumor, as the accepted criterion for high tumor mutation burden (> 10 mutations/Mb) is found in only 6% of ATC [29].
Early oncogenic drivers include activating mutations and, less frequently, rearrangements of genes involved in proliferation, survival and differentiation along different signaling pathways, mainly MAPK and PI3K/Akt. Mutually exclusive somatic mutations (mainly BRAF V600E and RAS) are the most frequent drivers of carcinogenesis in DTC, and they are also found in ATC, albeit with a lower frequency. BRAF mutations are found in 36–83% of PTC [32]. In recent series, BRAF mutations were present in 11–82% of ATC [19, 29, 30, 33,34,35,36,37] Besides methodologic variability, these discrepancies may suggest heterogeneity of ATC in different races and ethnicities, highlighting the need for further understanding of the molecular mechanisms leading to tumorigenesis and progression.
RAS mutations, usually NRAS (most frequently exon 2, codon 61), are found in 24–44% of ATC [19, 29, 30, 37], rendering them the second most frequent driver mutation. Compared to BRAF mutated ATC, tumors harboring RAS mutations have a higher frequency of extrathyroidal extension and fewer lymph node metastases [34].
Fusion oncoprotein involving receptor tyrosine kinases, such as NTRK and RET are relevant tumoral drivers, since effective therapies linked to these biomarkers are available. Nevertheless, these alterations are unusual in ATC, and they were each reported in 0-1.5% in different series [19, 30, 33].
NF1 mutations are found in 9–12% of ATC, and they occur in BRAF and RAS wild type ATC [19, 30, 34]. EIF1AX mutations are found in 9–14% of ATC. They were proposed to be mutually exclusive with other alterations, however, a coexistence with RAS mutations was reported in advanced tumors [19, 30].
Gain of secondary aggressive molecular signatures occur when tumors progress from well-differentiated to high grade to ATC. Activation in gene encoding effectors in the PIK3CA-PTEN-AKT-mTOR pathway are more frequently found in ATC than in DTC and PDTC (39–44% vs. 11–20%) [29, 30, 38]. PIK3CA alterations are found in 18% of ATC, and tend to co- occur with BRAF mutations, while PTEN mutations are found in 15% of cases, mainly ATC harboring NF1 mutations [30]. These observations underscore that accumulating mutations along this pathway potentiate progression.
TP53 and TERT promoter mutations are the most common genetic alterations in ATC. Both are consistently rare in indolent DTC, as they are considered late genomic events associated with progression and dedifferentiation. TERT promoter mutations were the most frequent molecular alteration in ATC. They were found in 65–75% of tumors, significantly higher than the frequency found in PDTC (40%) and PTC (9%) [19, 29, 30]. TERT promoter mutations tend to appear in ATC harboring BRAF/RAS mutations. Besides being present in ATC, TERT mutations are also found in advanced DTC and more prominently in widely invasive follicular thyroid cancer. In patients with coexisting ATC and well differentiated components, TERT mutations appear in both histologies. It has been suggested that TERT promoter mutations can anticipate clinical or morphological signs of progression [39]. TERT mutations are associated with shorter OS; prognosis is even more adverse when TERT mutations coexist with BRAF/RAS mutations [19]. Mortality was also higher in patients with PDTC and ATC with concurrent TERT and PIK3CA mutations [38].
Inactivating mutations of TP53 (a tumor suppressor gene involved in the control of cell cycle and apoptosis) can be found in 54–73% of ATC [19, 29, 30, 34]. They are considered a final step in tumor progression, as they are almost never found in DTC. In cases of coexisting DTC and ATC, TP53 mutations are limited to the ATC component. The presence of TP53 mutations was an independent predictor of disease progression, and was associated with reduced progression free survival (PFS) in BRAF mutated ATC treated with BRAF inhibitors [34].
Other genomic alterations found in ATC include mutations of the histone methyltransferase (19–24%), genes encoding components of the chromatin remodeling complex SWI/SNF (18–36%) of ATC, DNA MMR genes 12–22%, B catenin-Wnt pathway CTNNB1NF1 mutation in 10%, and NF2 mutation in 9% [29, 30, 40].
ATC often have aneuploidy [29, 30] showing an abnormal number of chromosomes due to gain and loss, both of whole chromosomes and fragments. Loss of chromosome 1q, 13, and 17 and amplification of chromosomes 2p, 5, 7, 11p and 20 were described in a study by Lu et al in which aneuploidy and genome-wide copy number alteration were found in 67% of 15 ATC samples assessed [41].
The relevance of the improved knowledge of oncogenesis of ATC resides in the potential benefit that at least a subgroup of patients may obtain from receiving commercially available targeted therapies and the inclusion in basket clinical trials. In this regard, current management guidelines from National Comprehensive Cancer Network (NCCN), American Thyroid Association (ATA), American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group, and European Society of Medical Oncology (ESMO) recommend testing for BRAF and other targetable alterations [42,43,44,45].
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells, B cells, dendritic cells, monocytes and natural killer cells. The interaction of PD-1 and PD-L1 results in down regulation of effector T cell response and immune tolerance, leading to the escape of tumoral cells from the immune system, and evidence suggests a role of this pathway in ATC. PDL-1 expression is elevated in tumoral cells in ATC, ranging from 22 to 81%, therefore rendering this tumor as a potential target for therapy with immune checkpoint inhibitors [46,47,48,49,50]. In a German study, PD-L1 tumor proportion score > 50% was found in 42% of ATC [51]; mean PDL-1 expression was significantly higher in ATC than in PDTC and DTC [46, 51, 52]. High expression of PD-1 was found in the inflammatory cells in the tumoral environment and it was significantly associated with worse OS [49]. No association was found between mutation status and PD-L1 expression in the study of Bastman et al. [48].
Tumoral microenvironment includes immune cells, fibroblasts and endothelial cells; this microenvironment has been shown in cell models to influence tumor behavior, progression and response to therapy. Tumor associated macrophages comprise over 50% of ATC infiltrated cells. ATC are extensively infiltrated with type 2 macrophages (which are anti-inflammatory and pro tumoral), forming a network encircling tumor cell; this was not found in DTC [53]. Cell line models of ATC evidenced that co culture with type 2 macrophages facilitates dedifferentiation, proliferation, migration, and invasion in thyroid cancer cells through secretion of WNT1, WNT3 [54] and IGF [55]. Moreover, ATC with abundance of macrophages show a poorer outcome [56]. Targeting the microenvironment could potentially offer therapeutic benefits, however this approach is yet to be examined.
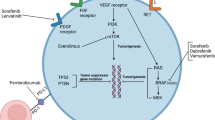
A scheme on the oncogenic pathways of ATC is shown in Fig. 1 [57].
4 Diagnosis
4.1 Clinical features
4.1.1 Disease presentation and symptoms
Most ATC are considered to arise from pre-existing DTC, while some originate de novo from pre-existing multinodular goiters [58,59,60]. Nearly 20–60% of patients with ATCs have a history of DTC or a coexistent thyroid carcinoma (mainly PTC) [19, 28].
Nearly all patients present with a rapid enlargement (within a few days or weeks) of a thyroid mass causing neck pain and tenderness [20, 28, 61]. Most cases show regional spread into the perithyroidal fat and muscle, lymph nodes, larynx, trachea, esophagus, and great vessels of the neck and mediastinum. This may lead to dyspnea (in 35% of cases), dysphagia (30%), hoarseness (25%) and cough with or without hemoptysis (25%). Distant metastases at disease presentation are found in 15 to 50%, being the lungs the most common site (90%) [20, 28]. Other sites of metastases are bone (5–15%), brain (5%), skin, pancreas, liver, kidney, and adrenal glands (less than 5%). These patients often present with constitutional symptoms such as fatigue, anorexia, weight loss, or fever of unknown origin [28].
4.1.2 Physical examination
The thyroid mass is typically firm and fixed to underlying structures on physical examination. The presence of a fluctuant area in a large goiter may indicate tumor necrosis [62]. The skin in the neck can be erythematosus or ulcerated. Retrosternal growth can lead to venous dilatation and superior vena cava syndrome. Lymph node enlargement can be detected in up to 50% of cases [20, 28, 61].
4.2 Imaging
The relevance of diagnostic imaging in patients with suspected ATC relies in the assessment of the extent of disease, determination of resectability criteria, initial therapeutic approach, and response to treatment. Thyroid ultrasonography is a rapidly accessible initial imaging modality for the evaluation of a cervical mass because it is easily available and serves as a valuable tool for percutaneous biopsy. The sonographic appearance of ATC is nonspecific and typically presents as a large, heterogeneous, hypoechoic, solid mass with vascularity [63]. On the other hand, given that patients present a rapidly growing mass with mechanical compression of the aerodigestive tract and/or distant metastases typically at the onset of the disease, cross-sectional imaging is crucial for disease staging and prompt therapeutic decision-making. Therefore, computed tomography (CT) scans of the neck, chest, abdomen and pelvis provide accurate visualization of the extent of the thyroid tumor and enables the detection of tumor invasion into major vessels and the upper aerodigestive tract as well as distant metastases [64]. Typical findings consist of large necrotic masses involving both lobes of the thyroid, with a density similar to or slightly higher than that of skeletal muscle, poorly defined margins, extrathyroidal extension, and prominent calcifications and necrosis with heterogeneous attenuation [64, 65]. Magnetic resonance imaging (MRI) is also valuable for determining the local extent of the disease and identifying distant metastases, especially for excluding brain metastases at diagnosis [43, 66]. If readily accessible, a whole-body PET-CT scan with 18-fluorodeoxyglucose (18FDG) should be conducted as the initial staging image [43]. Typical findings include hypermetabolic tumor activity characterized by intense 18FDG uptake in the primary tumor, as well as in cervical and mediastinal lymph nodes, and distant metastases [67]. A recent study demonstrated that the use of 18FDG PET-CT changed the therapeutic approach in 8 out of 16 patients with ATC at the time of disease diagnosis, as indicated by negative or inconclusive results from other imaging studies [68]. Furthermore, its use can aid in distinguishing post-surgical changes or residual masses following chemoradiotherapy in patients with a history of prior treatment for DTC or multinodular goiter, from specific findings of ATC itself. Besides, a negative PET scan after completed therapy may suggest extended survival [68]. Laryngoscopy is mandatory to adequately assess local extension.
In summary, it is advisable to undergo neck ultrasound, PET/CT with 18FDG, and either a brain MRI or CT for the initial assessment of disease extent. Assuming that PET imaging is likely to complement other cross-sectional imaging modalities, especially when ultrasound, CT, and MRI yield negative or inconclusive results, cross-sectional imaging of the brain, neck, chest, abdomen, and pelvis using CT or MRI can provide adequate staging information when PET/CT scans are not readily available or when delays are expected. In this regard, it is preferable to move forward with the available imaging modalities [43, 68].
Figure 2 displays the main imaging findings observed in patients with ATC.
Illustrative images highlighting clinical and imaging features in ATC
1. Sixty-two-year-old female patient with ATC(1.A, 1.B)Pictures of the patient’s large cervical mass adhered to deep planes with deviation of the midline and extending towards the left side of the neck. Note the prominent skin distension and changes in pigmentation(1.C, 1.D)Axial view of CT scans of the neck shows a 12 × 10 × 8 cm necrotic mass involving both lobes of the thyroid gland, predominantly on the left side, with a density similar of skeletal muscle, marked extrathyroidal extension to local structures, tracheal deviation and infiltration of the prevertebral fascia(1.E)Axial view of CT scans of the lung shows 1 × 0.9 cm dominant nodule (arrow)
2. CT images of a 59-year-old man with 9.2 × 6 cm ATC. Note the heterogeneous, voluminous mass with necrotic areas and gross calcifications infiltrating: (2 A)tracheal lumen, (2B)cervical spine, and(2 C)upper mediastinum
4.3 Biopsy
As previously mentioned, the swiftly advancing and life-threatening nature of ATC requires an expeditious and conclusive diagnosis to ensure prompt intervention. Achieving an accurate diagnosis entails conducting thorough histopathologic and immunohistochemical analyses, in addition to molecular testing especially when contemplating therapeutic approaches [61]. The acquisition of a sufficient tumor sample assumes a crucial role in facilitating this diagnostic precision, and while fine-needle aspiration may yield advantages in specific instances, surgical/core biopsy emerges as the optimal choice, conferring the most substantial benefits [1, 19].
4.3.1 Pathology and immunohistochemistry
ATC is characterized as a highly aggressive tumor consisting of undifferentiated cells that exhibit certain characteristics of epithelial origin upon examination of their morphology and immunohistochemistry (IHC) [69]. ATC samples display diverse and heterogeneous histologic features, including epithelioid and squamous morphology, giant cells, pleomorphic morphology, osteoclast giant cell-rich morphology, and the most prevalent histotype, spindle cell morphology [19, 61]. It is common for ATC to exhibit multiple cytomorphologic features, most frequently the combination of spindle cell and giant cell [19].
In the previous version of the WHO classification [69], squamous cell carcinoma (carcinoma composed almost entirely of squamous cells without a DTC component) was considered a distinct category. However, in the current edition, it is now categorized as a subtype of ATC [57]. The follicular origin of the tumor can be confirmed by examining the immunohistochemical expression of thyroid transcription factor 1 (TTF-1) and PAX-8 [1]. Generally, the prognosis of squamous cell carcinoma appears to be similar to that of ATC, which justifies its inclusion as one of its morphological subtypes [1, 57].
ATCs are characterized by infiltration of adjacent thyroid and surrounding tissues, as well as invasion of lymphatic and blood vessels. All ATCs exhibit a high degree of cellular proliferation which is expressed by high mitotic rates (> 1 mitoses per high-power field) and elevated Ki-67 proliferation indices (typically near 30%, but often higher) [19, 70]. Atypical mitoses are frequently observed. Confluent areas of tumor necrosis are common, particularly visible in resection specimens and large biopsies [61], which can complicate the diagnostic process. On the other hand, the diagnosis of ATC becomes straightforward when a coexistent DTC is identified alongside an undifferentiated component [61]. Therefore, extensive tissue sampling of resection specimens is recommended to increase the chances of detecting the differentiated component, which is often a minority [43].
IHC plays a crucial role in the diagnostic evaluation of ATC. Typically, thyroid-specific proteins like thyroglobulin and thyroid-lineage markers such as TTF-1 are absent in ATC [61, 70], reflecting its undifferentiated nature. However, around 40–60% of ATCs retain focal expression of another thyroid-lineage marker, PAX8, providing essential evidence of follicular cell origin and supporting a diagnosis of ATC within the appropriate morphological context of an undifferentiated high-grade neoplasm [43, 61]. It is worth noting that PAX8 expression is not exclusive to the thyroid and can also occur in the kidney and Müllerian system. Thus, renal cell and ovarian carcinomas should be considered in the differential diagnosis (taking into account that they rarely involve the thyroid gland) [71]. Additionally, some PAX8 antibodies can cross-react with PAX5, a B-cell lineage marker expressed in certain large B-cell lymphomas. Therefore, interpretation of PAX8 IHC should take into account the morphological context and consider a broad panel of IHC markers [43].
IHC for cytokeratins can be helpful in cases where the epithelial nature of the neoplasm is not readily apparent based on morphology, particularly in the spindle cell and giant cell variants. However, the absence of cytokeratin expression does not rule out a diagnosis of ATC [43, 61]. Ki-67 by IHC can be used to evaluate cellular proliferation in cases where assessing proliferation through mitotic activity is challenging, for example in small biopsies.
A panel of common immunohistochemical markers for the assessment of ATC in comparison with other thyroid tumor types is shown in Table 1.
4.4 Molecular testing
Early molecular profiling in ATC aims to identify potential targets that may influence therapeutic approach. Thus, it is imperative to conduct prompt testing for BRAF V600E mutation in order to ensure timely initiation of treatment. This can be accomplished by employing IHC staining on fine needle aspiration or core biopsy samples. The purpose of this expedited testing is to prevent any delays in the administration of treatment [72]. Furthermore, if available, it is advisable to employ NGS to examine the presence of other potentially targetable mutations, encompassing at least, BRAF, TSC1, TSC2, ALK fusion genes, NTRK fusion genes, and RET fusion genes. Such mutations could be addressed ideally through inclusion into a clinical trial or by adherence to standard of care guidelines or a compassionate use program [43]. When available, the outcome of molecular testing is one of the key factors used to decide the first therapy strategy for a patient with ATC, along with staging.
5 Staging
According to a recent consensus by the American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group [44], every patient with ATC is considered to have advanced disease, even when completely resected or incidentally identified pathologically. This is in agreement with the revised 8th edition American Joint Committee on Cancer TNM classification, in which all ATC are considered stage IV, underscoring their elevated mortality rates. At variance with the previous version, the 8th edition stratifies primary tumors using the same T categories that apply for DTC. Intrathyroidal ATC without lymph node involvement and no distant metastases are considered stage IVa. Primary tumors with gross extrathyroidal extension or those with lymph node metastases are classified as stage IVb. The presence of distant metastases assigns these cases to stage IVc [73].
6 Therapy
As the window of opportunity for implementing treatment in ATC is very brief, facilitating rapid access to clinical care is critical. A multidisciplinary team is mandatory for its management, since virtually every patient will require many therapeutic modalities, according to the stage of the disease and performance status of the patient. The benefits of rapid access to multidisciplinary care were shown in two different studies. In Denmark [14], a fast-track program for head and neck cancer was introduced in 2008. One-year survival significantly improved for patients diagnosed after the program was implemented (44.8 vs. 13.6%). Similarly, after a protocol for rapid access to multidisciplinary care and molecular testing was implemented in the MD Anderson Center in 2014, time from referral to disposition was reduced from 8.7 to 0.5 days [74]. There was an improvement in 12-month OS from 35% before rapid access to 59% after it was implemented [27]; additionally, it allowed for the inclusion of 34% of ATC patients in clinical trials.
ATC was considered an orphan disease up until 2018. Even if multimodal treatment (surgery, chemotherapy and EBRT) appears to be associated with prolonged OS in stage IVa/IVb tumors in retrospective studies, for stage IVc (which represents at least half of the population of ATC), no clear benefit was obtained from aggressive therapy in the majority of the patients. OS did not significantly change over the last two decades for patients treated with conventional therapies [75]. However, activity of BRAF/MEK inhibitors on BRAF mutated ATC has dramatically modified the management of these patients, and their prognosis has significantly improved [5, 27].
Targeted therapies to other molecular alterations and immunotherapy have also shown promising results in some settings (case reports, retrospective studies). Combination of these new agents with classic strategies such as surgery and EBRT may provide better results in terms of survival.
6.1 Best supportive care
Even if there are promising perspectives of therapies, mortality rates remain high in ATC. Delay in diagnosis leading to advanced disease is frequent and molecular testing and/or targeted drugs may not be readily available in many countries. Therefore, in real life practice, ATC still is a lethal disease for the majority of cases. It is recommended that the medical team caring for ATC patients should include palliative care specialists, in order to relieve symptoms related to end-of life situations (pain, dyspnea). Realistic perspectives need to be established and consideration of patient’s preferences regarding whether aggressive treatments will be implemented or not is recommended. In the setting of advanced cancer, therapies such as chemotherapy may decrease quality of life and increase medical costs without improving survival [76]. Likewise, desires of the patient in terms of airway management, feeding, and hospice care need to be discussed early in the course of the disease [77].
Nearly every ATC patient is at risk for airway obstruction. In terms of maintaining a safe airway, elective tracheostomy was in the past considered a palliative option. However, even if tracheostomy may briefly prolong survival, it significantly decreases the quality of life. Elective tracheostomy is not currently recommended. However, this procedure needs to be discussed with the patient and its family, anticipating the event of acute airway distress. Since usually patients with the most locally advanced disease are submitted to tracheostomy, the procedure is expected to be technically demanding, and needs to be performed in the operating room, by an experienced surgeon, under general anesthesia; intubation to secure the airway is mandatory [43, 77, 78]. Bleeding and growth of tumor through the incision are expected complications.
6.2 Surgery
From a practical standpoint, surgical procedures should be discussed after ruling out distant metastasis and carefully assessing the extent of the tumor and its resectability in the unusual cases in which no (or minimal) extrathyroidal extension is found. The goal of surgery is complete macroscopic resection (R0/R1), as debulking procedures (R2) are associated with worse outcomes [79,80,81]. Positive margin status is associated with increased mortality [82].
Complete resection of the disease, followed by adjuvant therapy, is the most likely opportunity for cure in ATC without aerodigestive tract invasion [42, 43]. The ATC Collaborative Research Consortium of Japan retrospectively assessed 547 ATC patients, and those receiving radical surgery had significantly improved cause-specific survival compared with those with no surgery or palliative surgery (HR 0.35; 95% CI 0-28-0.43) [83]. Multimodal treatment (complete surgical resection, associated with adjuvant EBRT/chemoradiotherapy) was significantly related to improved survival in stage IVa and IVb tumors in a Korean retrospective study including 329 ATC patients [84]. A systematic review of 40 studies showed earlier stages tumors have longer survival and that surgery is the mainstay therapy for these patients [85]. This population benefits from an aggressive therapeutic approach, as multimodal therapy probably reduces the risk of death due to local progression of ATC [75]. In a study from Xu et al., patients with OS of 2 years or longer, had a higher frequency of primary resection, as well as clinicopathologic parameters attributed to resectability [86]. The optimal surgical strategy in patients with resectable disease is total thyroidectomy with therapeutic lymph node dissection of the central and/or lateral compartments [84]. An experienced surgical team is mandatory [42, 43, 45].
However, complete surgical resection is usually not feasible, since most ATC patients present with rapidly growing tumors infiltrating the aerodigestive tract and/or vascular structures. In this setting, extensive surgery is not recommended [43], since the morbidity of these procedures is not acceptable in ATC due to its poor prognosis.
Of note, if extrathyroidal extension or lymph node metastases are present, but R0/R1 resection may be anticipated without extensive visceral/vascular resection, surgical resection is recommended as well [84]. Performance status, comorbidities and preferences of the patient should be considered.
In the setting of distant disease, surgical resection of the primary tumor, when feasible, also needs to be considered, in order to avoid airway and digestive tract obstruction. In this regard, a study of 54 patients with metastatic disease, surgical resection was associated with improved survival in multivariate analysis, probably due to better local control, as airway obstruction was significantly lower in the group with resection [25]. Similarly, a retrospective analysis of the SEER database from 1998 to 2015, including 433 stage IVc ATC found that those submitted to total thyroidectomy had better OS vs. those with no/unknown surgery (HR = 0.616) [87].
6.3 EBRT
Morbidity in ATC is, in most cases, related to locoregional complications (aerodigestive tract or vascular invasion). Therefore, improving local control is extremely relevant.
No randomized studies have been performed regarding the use of curative intent EBRT. Nevertheless, retrospective evidence consistently indicates that patients with R0/R1 resection benefit from post-surgical EBRT.
The pooled results from a systematic review and meta-analysis including 17 retrospective studies showed that post-surgical EBRT reduced the risk of death vs. surgery alone (HR 0.556, 95% CI 0.149–0.737) [88]. This analysis included patients submitted to any type of surgery except biopsy, therefore, R0/R1/R2 were included. In four of the studies included, results were analyzed according to stage, survival was improved for stage IVa and IVb, but not for IVc. A retrospective analysis of the National Cancer Data Base including 496 nonmetastatic ATC which underwent surgery with curative intent, found that the 375 patients who received adjuvant EBRT had a significantly longer survival (12.3 vs. 9.1 months) [89].
Post-operative EBRT needs to be started rapidly after patients recover from R0/R1 surgery, ideally after 2–3 weeks, and no later than 6 weeks. Intensity-modulated radiation therapy (IMRT) is currently the standard of care, as it allows for effective radiation dose to tumoral areas while decreasing toxicity to adjacent normal tissues [42, 43, 45]. Most frequent adverse events (AEs) of EBRT are mucositis and dysphagia (occurring in 13–32% of patients). Weight loss and gastrostomy tube placement (in up to 34% of patients) may be necessary. However, this may reflect disease progression rather than AEs of EBRT. A higher percentage of patients with ATC (17% vs. 2.2% for DTC) do not complete EBRT treatment, most frequently due to death or transition to hospice care [90].
High dose radiation (50–70 Gy) is recommended after surgery with the aim of maximizing locoregional control, as higher doses are associated with improved survival [42, 43]. In a retrospective study of 32 years including 104 patients, a dose of radiation ≥ 60 cGy was significantly associated with decreased risk of local progression and longer OS (10.6 vs. 3.6 months [91]. A retrospective study of 62 patients found a radiation dose 50 cGy or higher was a predictive factor of survival [92]. Standard fractionation schemes are currently recommended [42, 43]. Inclusion of II-IV and VI neck areas in the target area is recommended, as well as mediastinum and V level [93].
In the palliative setting, for patients with R2 or unresectable disease, EBRT may also improve local control [43].
6.4 Chemotherapy
Several cytotoxic chemotherapy regimens have been used in ATC, including doxorubicin, cisplatin and taxanes (paclitaxel/docetaxel), either alone or in combination. No direct comparison has been made on the efficacy of each drug.
When treating patients with EBRT after R0/R1 surgery for stage IVa/IVb ATC, there is retrospective evidence that adding a systemic agent, mainly as a radiosensitizer, improves both survival and locoregional control. This approach is recommended for patients with appropriate performance status, due to the significant toxicities reported [43].
In the study from Saeed et al., for completely resected nonmetastatic ATC treated with post operative EBRT, concurrent chemotherapy was an independent predictor for better OS (HR 0.6, 95% CI 0.5-0.8) [89]. In a study of 104 all-stage ATC patients, by Fan et al., multimodal therapy significantly improved locoregional free survival (one year local PFS 85.9% vs. 54.1% for those treated with other modalities) [91].
An analysis of the SEER database including 491 ATC patients (2004–2015) found a statistically significant improvement in OS in cases treated with chemoradiotherapy vs. EBRT alone (2 vs. 4 months). This benefit was observed whether patients were submitted or not to surgery, and in patients with local or metastatic disease [94]. Coincidently, an analysis of the National Cancer Data Base (2004–2013) of 858 ATC found that those treated with chemotherapy and EBRT (67%) vs. EBRT (33%) had a significantly decreased risk of death (HR 0.66, 95% CI 0.57–0.77, P < .001). Median OS was 2.6 vs. 4.7 months; 1-year OS was 14.3% vs. 25.5%; this was maintained in a propensity score cohort. The benefit was limited to patients receiving a dose 30 Gy or higher, and with non-metastatic disease [95].
As patients treated with multimodal therapies experience improved local control, treatment failure occurs mainly in distant sites (up to 74%) [96]. The major cause of death in this population is due to distant metastases, as opposed to those not treated (or treated only with surgery or EBRT), in which airway complications are the main cause for mortality [97]. Taken together, these data suggest both that ATC should be approached as a systemic disease, and that aggressive multimodal therapy enhances locoregional PFS.
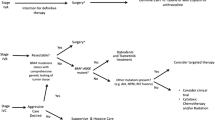
In patients with unresectable/metastatic disease with no targetable alterations and good performance status who wish aggressive therapy, the combination of EBRT and cytotoxic chemotherapy is the preferred approach (Fig. 3). However, the response rates are low, and the benefit, if achieved, is usually transient. Early initiation of “bridging” chemotherapy may be a useful approach until results of genomic interrogation (allowing for the use of targeted therapies) are available [43].
6.5 Targeted therapies
6.5.1 BRAF/MEK inhibitors
BRAFV600E mutation is the most frequently observed actionable genetic alteration in ATC, occurring in 20–50% of cases [98]. However, this percentage is higher when PTC is present combined with ATC in the pathological sample [96]. In 2017, the open label Rare Oncology Agnostic Research (ROAR) basket trial showed that the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) had significant clinical effects on the ATC cohort with BRAF V600E mutation [99]. Out of 16 patients, the overall response rate (ORR) was 69%, including one CR, and the one-year OS rate was 80% [99]. In an updated analysis of this trial, at the time of data cut-off (about six years) the ORR reached 56%, with three patients archiving a CR, and the 12-month duration of response was 50% [4]. Median PFS and OS were 6.7 and 14.5 months, respectively [4]. Real world data was provided in a multicentric UK study including 16 ATC patients. In this investigation, the median PFS was 4.7 months, and the OS was 6.9 months [100]. The Argentinian experience using DT in ATC patients was recently published, including 5 patients from four different hospitals [101]. The best responses were CR in 40%, PR in 40% and stable disease in 20%. The median duration of best responses was 20 weeks (95% CI 15.7–24.3) and overall mortality was 60% at 20 weeks of follow-up [101]. Finally, a phase 2 pilot study (NCT04238624) is being performed to evaluate the efficacy of the addition of cemiplimab, an anti-PD-1 antibody, to the treatment of subjects with ATC who are no longer responding to DT [102].
The most common AEs with the combination of DT in ATC were fatigue (33–40% of cases), fever (20–47%), nausea (33–60%) anorexia (33–80%), and anemia (25–36%) [4, 99, 101]. Grade 3/4 AEs were reported in 58% of patients in the six-year clinical trial, most commonly anemia (19%), pneumonia (19%) and hyponatremia (17%) [4]. In 17% of cases, the AEs lead to permanent discontinuation of treatment [4].
On May 4, 2018, the FDA approved the combination of DT for the treatment of patients with locally advanced or metastatic ATC with BRAF V600E mutation and with no satisfactory locoregional treatment options.
On the other hand, a phase 2 basket trial assessed the efficacy of vemurafenib (another BRAF inhibitor) in BRAF V600E mutation–positive non-melanoma cancers. This study included seven ATC patients (5.7%) and vemurafenib showed an ORR of 29%, with two patients achieving CR and partial response (PR), respectively [103]. The combination of vemurafenib and cobimetinib (MEK inhibitor) is being studied in an ongoing clinical trial on BRAF positive thyroid cancers, including ATC (NCT05768178) [104].
The development of resistance to BRAF inhibitors can occur through secondary mutations in the MAPK pathway or activation of the PI3K/Akt/mTOR pathway or Hgf/Met [105, 106]. Since this event has scarcely been reported in thyroid cancer patients, data is insufficient to make clinical recommendations at this point. Potential strategies to combat BRAF resistance could include employing selective inhibitors for KRASG12C, PI3K, ERK or combining immunotherapy with BRAF inhibitors [105].
6.5.2 Other targeted therapies
NTRK, RET and ALK fusions can be found in less than 2–3% of ATC patients [19]. However, finding one of these alterations might greatly impact the patient prognosis given the high response rates reported in different basket trials [107,108,109].
In 2022, a study that pooled data from three phase I/II clinical trials of larotrectinib included 7 patients (24%) with ATC. For the ATC cohort, the ORR was 29% and the DOR was 50% at 12 months [107]. Additionally, in one phase I trial of solid tumors with NTRK fusion in children, none of the two ATC patients receiving larotrectinib progressed after seven months of treatment [108]. Larotrectinib and entrectinib (another NTRK inhibitor) have been granted FDA approval for treatment of any solid tumor bearing an NTRK fusion mutation. Entrectinib received a conditional marketing authorization for the treatment of adults with NTRK fusion-positive solid tumors in the European Union in July 2020 [61].
Considering RET inhibitors, the phase I-II clinical research (LIBRETTO-001) which comprised 143 MTC patients and 19 previously treated RET fusion-positive TC patients (including 2 ATC patients), evaluated for the efficacy of selpercatinib [109]. A favorable response of one of these ATC patients was later reported, showing a PR ongoing for more than 19 months [109]. Selpercatinib, as well as pralsetinib (another RET inhibitor) have been approved by FDA for any solid tumor harboring an RET fusion or mutation.
Regarding ALK inhibition, a lasting response to crizotinib has been reported in a single patient with an ALK-rearranged ATC [110].
In patients with NTRK, RET or ALK fusion mutations, with stage IVB or IVC disease, specific inhibitors can be considered, preferably in a clinical trial setting [43].
Inhibitors targeting the PI3K/Akt/mTOR pathway have shown disappointing results [111,112,113]. The efficacy of everolimus was assessed in a multicentric phase 2 clinical trial in locally advanced or metastatic thyroid patients with all histologic subtypes, including 6 ATC. [111]. The ATC cohort showed a PFS of 10.4 months with only one patient with PR [111]. Other studies have shown limited or no therapeutic benefits for everolimus in ATC [112,113,114].
6.5.3 Antiangiogenic tyrosine kinase inhibitors
In a meta-analysis aimed to assess the efficacy of lenvatinib in ATC patients, which included 10 clinical trials, the PR was 15%, SD 42% and disease control rate 63% [115]. A phase-II, international multicenter, open-label clinical trial included in this meta-analysis, enrolled 34 patients with ATC to receive lenvatinib 24 mg once-daily [116]. The study was halted for futility since the minimum ORR threshold of 15% was not met upon interim analysis [116]. On the other hand, a recent Japanese study (HOPE) assessed the effectiveness of lenvatinib in unresectable ATC patients with an estimated one-year OS rate of 11.9%, an objective remission rate of 11.9%, and a clinical benefit rate of 33.3%, with a low number of responders to the drug but a more sustained response [117]. On this basis, lenvatinib was approved for use in Japan. However, considering that ATC commonly invades the trachea, esophagus and great vessels, antiangiogenic therapy has risk of bleeding and fistula and therefore is not recommended by other international guidelines [43].
In coincidence with the results found with lenvatinib, other tyrosine kinase inhibitors such as sorafenib, imatinib, gefinitnib, and pazopanib used as a single agent have not shown promising results [118,119,120,121]. Nevertheless, lenvatinib can synergistically enhance the antineoplastic effects when combined with anti-PD1/PD-L1 agents, vinorelbine, and paclitaxel [122,123,124]. These findings suggest that the combination of lenvatinib with other antineoplastic drugs may be more effective than using single agents for patients with ATC.
A summary of the protocols and suggested doses of target agents for ATC is provided in Table 2.
6.6 Neoadjuvant therapy in ATC
It is well known that the outcomes of patients with ATC which are completely resected are improved compared to those unresectable cases [125,126,127].
The first reported series on neoadjuvant therapy with DT in six patients with BRAF V600E- mutated ATC was presented by Wang et al. [128]. The median duration of neoadjuvant DT therapy was 3.6 months (range 1.6 to 12 months) and 4 of 6 patients were alive without evidence of disease at the time of manuscript publication (median follow-up from DT initiation, 15 months). All six patients also received chemoradiation within two or three months after surgery and DT was initiated immediately after patients’ recovery from EBRT. Additionally, these authors hypothesized that patients can rapidly escape from the blockage of the BRAF-MEK pathway and consistently, checkpoint inhibitors such as pembrolizumab may prolong disease-free survival when added to DT. In this series, all patients in whom pembrolizumab was added remained alive and disease-free [128].
This study showed for the first time that ATC patients could undergo complete surgical resection without requiring tracheostomy or radical resection, even in cases of re-operation. Interestingly, a high rate of pathological response was observed, with a significant reduction in the viability of the ATC component in all resected specimens, and well-differentiated components of PTC were found, instead. These findings emphasize the considerable intratumor heterogeneity and clonal evolution of ATC.
Almost one year later, the first Latin American experience with neoadjuvant therapy with DT in a 62 y.o patient with a diagnosis of ATC was published. After 13 weeks of neoadjuvant DT treatment, an R1 surgical resection was possible to be performed. It was subsequently followed by EBRT but DT withdrawal due to lack of supply by the patient´s HMO determined a complete regrowth of the tumor to the original size and the patient died 8 months after initial diagnosis [129]. This may show gross differences in the management of advanced thyroid cancer patients according to regional realities [130].
Following their initial experience, investigators from the MD Anderson Cancer Center published one study that might completely change the initial approach of BRAF V600E-mutated ATC patients [5]. Although retrospective, this study analyzed the outcomes of 57 stage IVb and IVc ATC patients who were treated with BRAF-directed therapy for at least one month. BRAF-directed therapy was defined as treatment with a BRAF and MEK inhibitor with or without checkpoint inhibitor immunotherapy. BRAF inhibitors included the use of vemurafenib, dabrafenib, and encorafenib. MEK inhibitors included the prescription of cobimetinib, trametinib, and binimetinib. Immunotherapy was defined as any treatment with a PD-1 or PD-L1 checkpoint inhibitor.
Around 70% of patients (n = 32) treated with BRAF / MEK inhibitors received surgical treatment. One-year OS for those patients who had neoadjuvant therapy followed by surgery was 94% (95% CI, 85–100%). Patients who received upfront surgery before BRAF-directed therapy (n = 12) had one year OS of 74% (95% CI, 49-97%) compared to the last group which did not receive neoadjuvant therapy (n = 12), which had one year OS of only 38% (95%CI 62.6 to 100%).
This study showed for the first time a differential prognosis for ATC patients who received initial surgical treatment for the original tumor, whether upfront or after BRAF / MEK inhibition. Ongoing clinical trials (NCT04675710 and NCT03181100) will help to clarify the roles of immunotherapy, surgery, and radiation therapy as adjuvant therapies for these patients [5].
An anecdotal case report was published after the use of an NTRK inhibitor, entrectinib, in one patient with ATC harboring an ETV6-NTRK3 fusion, showing a decrease of the initial tumor of around 40%, which was then amenable for surgical treatment. Chemoradiation was then indicated. The patient was alive 15 months after diagnosis [131].
Neoadjuvant therapy could change the outcomes of patients with a diagnosis of ATC, given that most of the deaths occurring in these patients are related to the locally advanced disease in the neck. Future prospective clinical trials will help to elucidate this.
6.7 Immunotherapy
Immunotherapy may be a promising treatment option, mainly in patients without a BRAF mutation, in light of the high expression of PD-1/PD-L1 in anaplastic thyroid carcinoma [49]. Several studies have shown that targeting these pathogenic components by immune checkpoint inhibitors, alone or in combination with other treatments, is associated with durable clinical benefit and prolonged PFS [132,133,134,135,136].
Pembrolizumab (an anti PD-1 antibody) was granted agnostic approval by FDA in 2017 for patients with unresectable or metastatic, microsatellite instability-high or mismatch repair-deficient solid tumors that have progressed previous treatment and do not have satisfactory alternative therapies [137]. The use of pembrolizumab or nivolumab, in 13 patients with locally advanced or metastatic unresectable ATC was recently reviewed in a retrospective case study [138]. Only two patients in the research experienced a PR, while three patients continued to have stable disease, according to the study’s objective response rate of 16%. One-year survival rate was 38%, and the median OS was 4.4 months. Notably, despite a median follow-up of 13.5 months among patients who experienced clinical benefit, the median OS had not been met, and one patient was still alive at 29 months. In terms of safety, 46% of patients experienced immune-related AEs, with two patients (15%) experiencing grade 3 or higher AEs [138].
Spartalizumab, a PD-1 inhibitor, was studied in a phase I/II clinical study for patients with locally advanced and/or metastatic ATC [134]. The results of Phase II of the study revealed an ORR of 19% (including 7% CR) was attained with 42 patients recruited. The median PFS and OS was 1.7 months and 5.9 months, respectively, and a one-year survival rate of 40%. All patients who experienced a CR were BRAF wild-type. The results of the biomarker study showed that 70% of patients were PD-L1 positive (1% positive in tumor cells), and that this positivity was associated with statistically significant differences in ORR as well as patients who had baseline CD8 expression levels of 1% showed a significantly higher ORR [134].
The initial investigation into the combined therapy of immune checkpoint inhibitors and multikinase inhibitors was conducted through the Anaplastic Thyroid Carcinoma Lenvatinib Pembrolizumab (ATLEP) trial [133]. This retrospective study aimed to evaluate the effects of combining lenvatinib and pembrolizumab in six patients with metastatic BRAFV600E wild-type ATC who exhibited high PD-L1 expression (tumor proportion score [TPS] 1–90%), following the failure of chemotherapy. Four out of six patients achieved complete remission and 1/6 experienced stable disease, while one patient had progressive disease. The median PFS and OS were 16.5 months and 18.5 months, respectively [133]. A subsequent study, known as the prospective ATLEP trial, involved a larger cohort of 27 patients meeting the specified criteria prospectively followed-up. The findings of the study were consistent with the previous research, demonstrating a 51.9% PR rate and a 44.4% stable disease rate over the course of two years of treatment. The median PFS was 9.5 months, while the median OS was 10.25 months. Notably, a quarter of the patients survived for more than two years [139]. These encouraging results showed that lenvatinib and pembrolizumab appear to be safe and effective for treating ATC patients, as evidenced by the high response rates and sustained remissions.
Finally, drawing from pre-clinical data indicating the potential effectiveness of combined cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) blockade in highly aggressive radioiodine-refractory thyroid cancer, a recent study conducted at a single institution investigated the responses to ipilimumab and nivolumab in a specific subgroup of ATC patients [140]. The study reported that out of the 10 patients included, three demonstrated PR, resulting in a response rate of 30% (95% CI: 7-65%). Importantly, two of these patients remained without definitive evidence of disease at 26 and 13 months following the initiation of treatment [140].
In conclusion, the characteristics of the tumor microenvironment, tumor mutational burden, and microsatellite instability in ATC provide a compelling rationale for employing immune checkpoint inhibitors as a treatment strategy. However, clinical trials assessing the efficacy of immunotherapy as a standalone approach in ATC have yielded disappointing results, so as previously noted, the immunomodulatory properties of lenvatinib offer a mechanistic rationale for combining it with anti PD-1 agents which promote its use.
Table 3 show the studies evaluating immunotherapy in ATC.
6.8 Therapeutic algorithms
Based on case series and clinical experience, the ATA and NCCN guidelines for the management of patients with ATC propose that the selection of initial therapy should be determined by the stage of the disease and the results of molecular testing, if accessible [42, 43].
Complete surgical resection followed by combination chemotherapy and radiation (grade 2 C) is the recommended course of action for patients with stage IVa and IVb with a resectable tumor, irrespective of BRAF mutation status. If possible, with low morbidity, total thyroidectomy is recommended over lobectomy (grade 2 C). ATC and DTC frequently coexist, and complete thyroidectomy makes it easier to treat DTC later on. However, a more aggressive surgical technique comprises thyroidectomy with broad margins of neighboring soft tissue on the side of the tumor in rare cases of intrathyroidal ATC without a coexistent DTC. Doxorubicin, taxanes, or cisplatin are available as choices for chemotherapy.
In turn, DT treatment (Grade 2 C) is the recommended therapy for patients with irresectable stage IVb or IVc cancer and the presence of the BRAFV600E mutation. This is followed by a resectability assessment. Surgical resection is done if there is a favorable response to DT and the tumor is judged resectable, as previously mentioned. Chemoradiation is then administered for stage IVB disease, while DT is restarted for stage IVc disease. If there is evidence or disease stability or improvement, DT may be maintained if the condition is still unresecable. Alternative management include chemoradiation, enrollment in clinical trials, or best supportive care in case of unfavorable outcomes.
For BRAF wild type ATC, the extent of surgery in stage IVb depends on how much the surrounding soft tissues are involved. Options for treatment include en-bloc resection, lobectomy with broad margins of neighboring soft tissue, and complete thyroidectomy. In these cases, chemorradiation is reserved for patients with complete surgical resection.
In contrast, patients without a BRAF V600E mutation who have unresectable stage IVb or IVc disease have a range of treatment options, including targeted therapy in the case of another targetable mutation is found, supportive care, chemoradiation, and clinical trials. Prioritizing symptom relief in instances where advanced disease lacks a targetable mutation is essential. Treatment should focus on securing the airway, ensuring access to nutritional support and joining in clinical trials if performance status is acceptable.
Figure 3 depicts a conceptual framework for the practical implementation of genetic profiling as a therapeutic guidance.
7 Developing therapies
Although significant progress has been made in the treatment of ATC in recent years, there is a growing need for innovative therapeutic strategies that can maximize efficacy in terms of survival and minimize adverse effects. Several targeted agents alone or in combination with other drugs/therapies are currently being investigated in anaplastic thyroid cancer. A summary of some relevant ongoing clinical trials for the treatment of ATC is provided in Table 4.
8 Disparities in the management of ATC
Managing ATC patients requires swift access to specialized health care and high-complexity diagnostic and therapeutic protocols. As a result of the elevated costs of these procedures and disparities in coverage of different health care systems, significant inequities arise. Lower socioeconomic status adversely impacts outcomes of cancer in general and this a is also the case for ATC patients [141].
Importantly, cancer patients from low and middle income countries usually face multifactorial disadvantages (such as socio-cultural issues and weak health care systems) [142]. Genomic interrogation, targeted therapies and/or clinical trials are not widely available in many regions of the world [6, 130]. Prognosis for ATC remains poor for most patients in this setting.
9 Conclusions
Due to its rarity and rapidly progressive course, managing ATC remains a challenge. Recent studies have shown a dramatically improved prognosis for BRAF-mutated ATC. However, ATC is a heterogeneous disease, and therapeutic solutions are elusive for cases with BRAF wild-type mutations. Furthermore, in real-world scenarios, timely access to multidisciplinary care, molecular testing, and targeted therapies may be limited.
Increasing comprehension of ATC´s molecular biology will hopefully lead to the development of novel targeted therapies. While implementing prospective studies can be challenging, there is a growing number of ongoing clinical trials. As a result, what was once an inevitably life-threatening disease without treatment options has, in some instances, transitioned into a serious but potentially manageable condition.
Abbreviations
- ATA:
-
American Thyroid Association
- ATC:
-
Anaplastic thyroid carcinoma
- CR:
-
Complete response
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DT:
-
Dabrafenib + trametinib
- DTC:
-
Differentiated thyroid carcinoma
- EBRT:
-
External beam radiation
- ESMO:
-
European society of medical oncology
- FDA:
-
Food and Drug Administration
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- ICI:
-
Immune checkpoint inhibitors
- IHC:
-
Immunohistochemistry
- NCCN:
-
National Comprehensive Cancer Network
- NGS:
-
Next generation sequencing
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PD-1:
-
Cell death protein 1
- PD-L1:
-
Programmed cell death ligand 1
- PDTC:
-
Poorly differentiated thyroid cancer
- PFS:
-
Progression free survival
- PR:
-
Partial response
- PTC:
-
Papillary thyroid carcinoma
- RR:
-
Relative risk
- TMB:
-
Tumor mutation burden
- WHO:
-
World Health Organization
References
Basolo F, Macerola E, Poma AM, Torregrossa L. The 5th edition of WHO classification of tumors of endocrine organs: changes in the diagnosis of follicular-derived thyroid carcinoma. Endocrine. 2023;80:470–6. https://doi.org/10.1007/s12020-023-03336-4.
Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Cancer. 2005;103(7):1330–5. https://doi.org/10.1002/cncr.20936.
Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol. 2010;22:486–97.
Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski CC, Cabanillas ME, Boran A, Ilankumaran P, Burgess P, Romero Salas T, Keam B. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol. 2022; 33(4): 406 – 15. https://doi.org/10.1016/j.annonc.2021.12.014.
Zhao X, Wang JR, Dadu R, Busaidy N, Xu L, Learned K, Chasen N, Vu T, Maniakas A, Eguia A, Diersing J, Gross N, Goepfert R, Lai S, Hofmann M, Ferrarotto R, Lu C, Gunn G, Spiotto M, Subbiah V, Williams M, Cabanillas M, Zafereo M. Surgery after BRAF-directed therapy is associated with improved survival in BRAF V600E mutant anaplastic thyroid cancer: a single-center retrospective cohort study. Thyroid. 2023;33(4):484–91. https://doi.org/10.1089/thy.2022.0504.
Locati L, Colombo E, Dedecjus M, de la Fouchardiere C, Sents W, Bongiovanni M, Netea-Maier R. Current picture of anaplastic thyroid cancer patients’ care and meetable needs: A survey of 94 Institutions from the EORTC Endocrine and Head and Neck Cancer Groups. Eur J Cancer. 2023; 180: 146 – 54. Doi: 0.1016/j.ejca.2022.12.002.
United States of America, National Institutes of Health. National Cancer Institute. : Dictionary of Cancer Terms: “rare cancer”. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/rare-cancer. Accessed July 13, 2023.
Lim H, Devesa S, Sosa JA, Check D, Kitahara C. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2013;317(13):1338–48. https://doi.org/10.1001/jama.2017.2719.
Dal Maso L, Tavilla A, Pacini F, Serraino D, van Dijk B, Chirlaque M, Capocaccia R, Larrañaga N, Colonna N, Agius M, Ardanaz D, ́-Casadevall ER, Kowalska J, Virdone A, Mallone S, Amash S, De Angelis H. R, and the EUROCARE-5 Working Group Survival of 86,690 patients with thyroid cancer: A population-based study in 29 European countries from EUROCARE-5. Eur J Cancer 77. 2017; 77:140 – 52. https://doi.org/10.1016/j.ejca.2017.02.023.
Da Mota Borges A, Ferreira J, Koifman S, Koifman RJ. Thyroid cancer in Brazil: a descriptive study of cases held on hospital-based cancer registries, 2000–2016. Epidemiol Serv. Saude, Brasília. 2020; 29(4): e2019503. Doi: 0.5123/s1679-49742020000400012.
Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, Dal Maso L. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021;9:225–34. https://doi.org/10.1016/S2213-8587(21)00027-9.
Pereira M, Williams V, Hallanger Johnson J, Valderrabano P. Thyroid Cancer Incidence Trends in the United States: Association with Changes in Professional Guidelines’ recommendations. 2020. Thyroid; 2020; 30(8):1132-40. https://doi.org/10.1089/thy.2019.0415.
Lin B, MaH, Ma M, Zhang Z, Sun Z, Hsieh IY, Okenwa O, Guan H, Li J, Lv W. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am J Transl Res. 2019;11(9):5888–96. https://doi.org/10.2147/OTT.S107913.
Hvilsom GB, Londero SC, Hahn CH, Schytte S, Pedersend HB, Christiansen P, Kiss KLarsen SR, Jespersen ML, Lelkaitisi G, Godballea C. Anaplastic thyroid carcinoma in Denmark 1996–2012: a national prospective study of 219 patients. Cancer Epidemiol. 2018;53:65–71. https://doi.org/10.1016/j.canep.2018.01.011.
Locati L, Cavalieri S, Dal Maso L, Busco S, Anderson L, Botta L, Bento MJ, Carulla M, Chirlaque López MD, Fusco M, Guevara M, Innos K, Johannesen TB, Micallef R, Minicozzi P, Panato C, Petrova D, Rubio-Casadevall J, Smailyte G, Vitale MF, Trama A. The RARECAREnet Working Group. Rare thyroid malignancies in Europe: data from the information network on rare cancers in Europe (RARECAREnet). Oral Oncol. 2020;108:104766.
Janz TA, Neskey DM, Nguyen SA, Lentsch EJ. Is the incidence of anaplastic thyroid cancer increasing: a population based epidemiology study. World J Otorhinolaryngol Head Neck Surg. 2018;5(1):34–40. https://doi.org/10.1016/j.wjorl.2018.05.006.
de Ridder M, van Dijkum EN, Engelsman A, Kapiteijn E, Klümpen HJ, Rasch CRN Anaplastic thyroid carcinoma: a nationwide cohort study on incidence, treatment and survival in the Netherlands over 3 decades. Eur J Endocrinol. 2020;183(2):203–9. https://doi.org/10.1530/EJE-20-0080.
Tavarelli M, Malandrino P, Vigneri P, Richiusa P, Maniglia A, Violi M, Sapuppo G, Vella V, Dardanoni G, Vigneri R, Pellegriti G. Anaplastic thyroid cancer in Sicily: the role of environmental characteristics. Front Endocrinol (Lausanne). 2017;20(8):277. https://doi.org/10.3389/fendo.2017.00277.
Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, Tuttle RM, Sherman E, Gill AJ, Ghossein R. Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid. 2020;30(10):1505–17. https://doi.org/10.1089/thy.2020.0086.
Wendler J, Kroiss M, Gast K, Kreissl M, Allelein S, Lichtenauer U, Blaser R, Spitzweg C, Fassnacht M, Schott M, Führer D, Tiedje V. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: results of a multicenter study in Germany. Eur J Endocrinol. 2016;175(6):521–9. https://doi.org/10.1530/EJE-16-0574.
Zivaljevic V, Slijepcevic N, Paunovic I, Diklik A, Kalezic N, Marinkovic J, Zivic R, Vekic B, Sipetic S. Risk factors for anaplastic thyroid cancer. Int J Endocrinol. 2014; 815070. https://doi.org/10.1155/2014/815070.
Ma J, Huang M, Wang L, Ye W, Tong Y, Wang H. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monit. 2015;21:283–91. https://doi.org/10.12659/MSM.892035.
Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, Ito K, Ito K, Tanaka S. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84:4043–9.
Du B, Wang F, Wu L, Wang Z, Zhang D, Huang Z, Gao L, Li Y, Liang C, Li P, Yao R. Cause-specific mortality after diagnosis of thyroid cancer: a large population-based study. Endocrine. 2021;72(1):179–89. https://doi.org/10.1007/s12020-020-02445-8.
Yamazaki H, Sugino K, Katoh R, Masudo K, Matsuzu K, Kitagawa W, Nagahama M, Rino Y, Ito K. Impact of Local Control on Clinical Course in Stage IVC anaplastic thyroid carcinoma. World J Surg. 2022;46(12):3034–42. https://doi.org/10.1007/s00268-022-06739-y.
Mirian C, Grønhøj C, Jensen DH, Jakobsen KK, Karnov K, Jensen JS, Hahn CH, Kitmoller TA, Bentzen J, von Buchwald C. Trends in thyroid cancer: retrospective analysis of incidence and survival in Denmark 1980–2014. Cancer Epidemiol. 2018;55:81–7. https://doi.org/10.1089/thy.2018.0067.
Maniakas A, Dadu R, Busaidy N, Wang J, Ferrarotto R, Lu C, Williams M, Gunn GB, Hofmann MC, Cote G, Sperling J, Gross N, Sturgis E, Goepfert R, Lai S, Cabanillas ME, Zafereo M. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000–2019. JAMA Oncol. 2020;6(9):1397–404. https://doi.org/10.1001/jamaoncol.2020.3362.
Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011;2011:542358. https://doi.org/10.1155/2011/542358.
Pozdeyev N, Gay L, Sokol E, Hartmaier R, Deaver K, Davis S, French J, Vanden Borre P, LaBarbera D, Tan A, Schweppe R, Fishbein L, Ross J. Haugen B1, Bowles D. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018;24(13):3059–68. https://doi.org/10.1158/1078-0432.CCR-18-0373.
Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf J, Shah R, Dogan S, Ricarte-Filho J, Krishnamoorthy G, Xu B, Schultz N, Berger M, Sander C, Taylor B, Ghossein R, Ganly I, Fagin J. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–66. https://doi.org/10.1172/JCI85271.
Agrawal N, Cancer Genome Atlas Research Consortium. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell. 2014; 159 (3): 676 – 90. https://doi.org/10.1016/j.cell.2014.09.050.
Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simoes M. Endocrine tumours: genetic predictors of thyroid cancer outcome. Eur J Endocrinol. 2016;174:R117–26. https://doi.org/10.1530/EJE-15-0605.
Yoo SK, Song YS, Lee EK, Hwang J, Kim HH, Jung G, Kim YA, Kim SJ, Cho SW, Won JK, Chung EJ, Shin JY, Lee KE, Kim JI, Park YJ, Seo JS. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun. 2019;10:2764.
Wang JR, Montierth M, Xu L, Goswami M, Zhao X, Cote G, Wang W, Iyer P, Dadu R, Busaidy NL, Lai SY, Gross ND, Ferrarotto R, Lu C, Gunn GB, Williams MD, Routbort M, Zafereo ME, Cabanillas ME. Impact of somatic mutations on survival outcomes in patients with anaplastic thyroid carcinoma. JCO Precis Oncol. 2022;6:e2100504.
Tiedje V, Ting S, Herold T, Synoracki S, Latteyer S, Moeller L, Zwanziger D, Stuschke M, Fuehrer D, Werner Schmid K. NGS based identification of mutational hotspots for targeted therapy in anaplastic thyroid carcinoma. Oncotarget. 2017;8(26):42613–20. https://doi.org/10.18632/oncotarget.17300.
Zhang L, Ren Z, Su Z, Liu Y, Yang T, Cao M, Jiang Y, Tang Y, Chen H, Zhang W, Gong R, Wei T, Peng Y, Liu B, Zhang W, Yang L, Hu Y, Li Z, Zhu J, Xu H, Shu Y, Luo H. Novel recurrent altered genes in chinese patients with anaplastic thyroid cancer. J Clin Endocrinol Metab. 2021;106(4):988–98. https://doi.org/10.1210/clinem/dgab014.
Kunstman J, Christofer Juhlin C, Goh G, Brown T, Stenman A, Healy J, Rubinstein J, Choi M, Kiss N, Nelson-Williams C, Mane S, Rimm D, Prasad M, Höög A, Zedenius J, Larsson C, Korah R, Lifton R, Carling T. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet. 2015;24(8):2318–29. https://doi.org/10.1093/hmg/ddu749.
Duan H, Li Y, Hu P, Gao J, Ying J, Xu W, Zhao D, Wang Z, Ye J, Lizaso A, He Y, Wu H, Liang Z. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology. 2019;75(6):890–9. https://doi.org/10.1111/his.13942.
Volante M, Lam AK, Papotti M, Tallini G. Molecular Pathology of poorly differentiated and anaplastic thyroid Cancer: what do pathologists need to know? Endocr Pathol. 2021;32(1):63–76. https://doi.org/10.1007/s12022-021-09665-2.
Qiao PP, Tian KS, Han LT, Ma B, Shen CK, Zhao RY, Zhang Y, Wei WJ, Chen XP. Correlation of mismatch repair deficiency with clinicopathological features and programmed death-ligand 1 expression in thyroid carcinoma. Endocrine. 2022;76(3):660–70. https://doi.org/10.1007/s12020-022-03031-w.
Lu L, Wang JR, Henderson YC, Bai S, Yang J, Hu M, Shiau CK, Pan T, Yan Y, Tran TM, Li J, Kieser R, Zhao X, Wang J, Nurieva R, Williams MD, Cabanillas ME, Dadu R, Busaidy NL, Zafereo M, Navin N, Lai SY, Gao R. Anaplastic transformation in thyroid cancer revealed by single-cell transcriptomics. J Clin Invest. 2023;133(11):e169653. https://doi.org/10.1172/JCI169653.
NCCN clinical practice guidelines in oncology. Thyroid carcinoma (version 2.23-May 18,2023). In: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed 3, Jul 2023.
Bible K, Kebebew E, Brierley J, Brito J, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov Y, Randolph G, Rosenthal MS, Sawka A, Shah M, Shaha A, Smallridge R, Wong-Clark C. 2021 american thyroid Association Guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2021;31(3):337–86. https://doi.org/10.1089/thy.2020.0944.
Shonka D, Ho A, Chintakuntlawar A, Geiger J, Park J, Seetharamu N, Ryder M, Shin J, Lori J, Wirth, Ahmed A, Brose M, Jasim S, Bible K, Cabanillas M, Davies L, William C, Faquin, Ghossein R, Gopal R, Miyauchi A, Nikiforov Y, Ringel M, Dabekaussen K, Dias-Santagata D, Robinson B, Sherman E, Peter M, Sadow S Jr, Tuttle B, Zafereo RM, Randolph M. Genomic testing in thyroid 2022 American Head and Neck Society endocrine surgery section and international thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: defining advanced thyroid cancer and its targeted treatment. Head Neck. 2022;44(6):1277–300. https://doi.org/10.1002/hed.27025.
Filetti S, Durante C, Hartl D, Leboulleux S, Locati L, Newbold K, Papotti M, Berruti A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856–83. https://doi.org/10.1093/annonc/mdz400.
Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim JS, Kim JH, Choe JH, Shin JH, Hahn SY, Oh YL, Chung JH. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer. 2017;24(2):97–106. https://doi.org/10.1530/ERC-16-0421.
Zwaenepoel K, Jacobs J, De Meulenaere A, Silence K, Smits E, Siozopoulou V, Hauben E, Rolfo C, Rottey S, Pauwels P. CD70 and PD-L1 in anaplastic thyroid cancer - promising targets for immunotherapy. Histopathology. 2017;71(3):357–65. https://doi.org/10.1111/his.13230.
Bastman JJ, Serracino HS, Zhu Y, Koenig MR, Mateescu V, Sams SB, Davies KD, Raeburn CD, McIntyre RC Jr, Haugen BR, French JD. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2016;101(7):2863–73. https://doi.org/10.1210/jc.2015-4227.
Chintakuntlawar AV, Rumilla KM, Smith CY, Jenkins SM, Foote RL, Kasperbauer JL, Morris JC, Ryder M, Alsidawi S, Hilger C, Bible KC. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. 2017;102(6):1943–50. https://doi.org/10.1210/jc.2016-3756.
Cantara S, Bertelli E, Occhini R, Regoli M, Brilli L, Pacini F, Castagna MG, Toti P. Blockade of the programmed death ligand 1 (PD-L1) as potential therapy for anaplastic thyroid cancer. Endocrine. 2019;64(1):122–9. https://doi.org/10.1007/s12020-019-01865-5.
Adam P, Kircher S, Sbiera I, Koehler VF, Berg E, Knösel T, Sandner B, Fenske WK, Bläker H, Smaxwil C, Zielke A, Sipos B, Allelein S, Schott M, Dierks C, Spitzweg C, Fassnacht M, Kroiss M. FGF-Receptors and PD-L1 in anaplastic and poorly differentiated thyroid Cancer: evaluation of the Preclinical Rationale. Front Endocrinol (Lausanne). 2021;12:712107. https://doi.org/10.3389/fendo.2021.712107.
Cameselle-García S, Abdulkader-Sande S, Sánchez-Ares M, Rodríguez-Carnero G, Garcia-Gómez J, Gude-Sampedro F, Abdulkader-Nallib I, Cameselle-Teijeiro JM. PD-L1 expression and immune cells in anaplastic carcinoma and poorly differentiated carcinoma of the human thyroid gland: a retrospective study. Oncol Lett. 2021;22(1):553. https://doi.org/10.3892/ol.2021.12814.
Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M, Dupuy C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS ONE. 2011;6(7):e22567. https://doi.org/10.1371/journal.pone.0022567.
Lv J, Feng ZP, Chen FK, Liu C, Jia L, Liu PJ, Yang CZ, Hou F, Deng ZY. M2-like tumor-associated macrophages-secreted Wnt1 and Wnt3a promotes dedifferentiation and metastasis via activating β-catenin pathway in thyroid cancer. Mol Carcinog. 2021;60(1):25–37. https://doi.org/10.1002/mc.23268.
Lv J, Liu C, Chen FK, Feng ZP, Jia L, Liu PJ, Yang ZX, Hou F, Deng ZY. M2like tumourassociated macrophagesecreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol Med Rep. 2021;24(2):604. https://doi.org/10.3892/mmr.2021.12249.
Luo Y, Yang YC, Ma B, Xu WB, Liao T, Wang Y. Integrated analysis of novel macrophage related signature in anaplastic thyroid cancer. Endocrine. 2022;78(3):517–30. https://doi.org/10.1007/s12020-022-03179-5.
Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33(1):27–63. https://doi.org/10.1007/s12022-022-09707-3.
Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6(4):292–306. https://doi.org/10.1038/nrc1836.
Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103(11):2261–8.
Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16(1):17–44. https://doi.org/10.1677/ERC-08-0154.
Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, Deschamps F, Lamartina L, Lacroix L, Dupuy C, Baudin E, Do Cao C, Hadoux J. Anaplastic thyroid carcinoma: an update. Cancers (Basel). 2022;14(4):1061. https://doi.org/10.3390/cancers14041061.
Aldinger KA, Samaan NA, Ibanez M, Hill CS Jr. Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41(6):2267–75. https://doi.org/10.1002/1097-0142(197806)41:6%3C2267:. aid-cncr2820410627 > 3.0.co;2–7.
Hahn SY, Shin JH. Description and comparison of the Sonographic characteristics of poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. J Ultrasound Med. 2016;35(9):1873–9. https://doi.org/10.7863/ultra.15.09058.
Takashima S, Morimoto S, Ikezoe J, Takai S, Kobayashi T, Koyama H, Nishiyama K, Kozuka T. CT evaluation of anaplastic thyroid carcinoma. AJR Am J Roentgenol. 1990;154(5):1079–85. https://doi.org/10.2214/ajr.154.5.2108546.
Lee JW, Yoon DY, Choi CS, Chang SK, Yun EJ, Seo YL, Rho YS, Cho SJ, Kim KH. Anaplastic thyroid carcinoma: computed tomographic differentiation from other thyroid masses. Acta Radiol. 2008;49(3):321–7. https://doi.org/10.1080/02841850701813120.
Miyakoshi A, Dalley RW, Anzai Y. Magnetic resonance imaging of thyroid cancer. Top Magn Reson Imaging. 2007;18(4):293–302. https://doi.org/10.1097/RMR.0b013e318572b76.
Poisson T, Deandreis D, Leboulleux S, Bidault F, Bonniaud G, Baillot S, Aupérin A, Al Ghuzlan A, Travagli JP, Lumbroso J, Baudin E, Schlumberger M. 18F-fluorodeoxyglucose positron emission tomography and computed tomography in anaplastic thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37(12):2277–85. https://doi.org/10.1007/s00259-010-1570-6. Epub 2010 Aug 6.
Bogsrud TV, Karantanis D, Nathan MA, Mullan BP, Wiseman GA, Kasperbauer JL, Reading CC, Hay ID, Lowe VJ. 18F-FDG PET in the management of patients with anaplastic thyroid carcinoma. Thyroid. 2008;18(7):713–9. https://doi.org/10.1089/thy.2007.0350.
El-Naggar AK, Baloch ZW, Eng C, Evans HL, Fagin JA, Faquin WC, Fellegara C, Franssila KO, Giuffrida D, Katoh R, Kebebew E, Kondo T, Matias-Guiu X, Nikiforov YE, Papotti M, Smallridge R, Sugitani I, Tallini G, Wakely PE, Westra WH, Wick MR, Williams MD. Anaplastic thyroid carcinoma. In: Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO Classification of tumours of endocrine organs.4t edition. 2017, pp.104-6.
Deeken-Draisey A, Yang GY, Gao J, Alexiev BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol. 2018;82:140–8. https://doi.org/10.1016/j.humpath.2018.07.027.
Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, Chirieac LR, Lis R, Loda M, Hornick JL, Drapkin R, Hirsch MS. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35(6):816–26. https://doi.org/10.1097/PAS.0b013e318216c112.
Smith AL, Williams MD, Stewart J, Wang WL, Krishnamurthy S, Cabanillas ME, Roy-Chowdhuri S. Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma. Cancer Cytopathol. 2018;126(6):406–13. https://doi.org/10.1002/cncy.21992.
Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 8th ed. Hoboken, NJ, USA: John Wiley & Sons; 2017.
Cabanillas M, Williams M, Gunn GB, Weitzman S, Burke L, Busaidy N, Ying A, Yiin Y, Lu C, Lai S. Facilitating anaplastic thyroid cancer specialized treatment: a model for improving access to multidisciplinary care for patients with anaplastic thyroid cancer.Head Neck. 2017; 39(7):1291–5. https://doi.org/10.1002/hed.24784.
Wächter S, Vorländer C, Schabram J, Mintziras I, Fülber I, Manoharan J, Holzer K, Bartsch DK, Maurer E. Eur Arch Otorhinolaryngol. 2020;277(5):1507–14. https://doi.org/10.1007/s00405-020-05853-8.
Paiva CE, Preto DD, de Lima C, Paiva BSR. To treat or not to treat? Dilemmas when deciding on antineoplastic treatment in patients with far advanced cancers. Cancer Control. 2023;30:10732748231176639. https://doi.org/10.1177/10732748231176639.
Moyer K, Marcadis A, Shaha A. Airway management, symptom relief, and best supportive care in anaplastic thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2020;28(2):74–8.
Shaha AR, Ferlito A, Owern RP, Silver CE, Rodrigo JP, Haigetnz M Jr, Mendenhall WM, Rinaldo A, Smallridge RC. Eur Arch Otorhinolaryngol. 2013;270(10):2579–83. https://doi.org/10.1007/s00405-013-2556-3.
Brignardello E, Gallo M, Baldi I, Palestrini N, Piovesan A, Grossi E, Ciccone G, Boccuzzi G. 2007 anaplastic thyroid carcinoma: clinical outcome of 30 consecutive patients referred to a single institution in the past 5 years. Eur J Endocrinol. 2007;156(4):425–30. https://doi.org/10.1530/EJE-06-0677.
Brignardello E, Palestini N, Felicetti F, Castiglione A, Piovesan A, Gallo M, Freddi M, Ricardi U, Gasparri G, Ciccone G, Arvat E, Boccuzzi G. Early surgery and survival of patients with anaplastic thyroid carcinoma: analysis of a case series referred to a single institution between 1999 and 2012. Thyroid. 2014;24(11):1600–6. https://doi.org/10.1089/thy.2014.0004.
McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–34. https://doi.org/10.1067/msy.2001.118266.
Goffredo P, Thomas SM, Adam MA, Sosa JA, Roman SA. Impact of timeliness of resection and thyroidectomy margin status on survival for patients with anaplastic thyroid cancer: an analysis of 335 cases. Ann Surg Oncol. 2015;22(13):4166–74. https://doi.org/10.1245/s10434-015-4742-6.
Sugitani I, Onoda N, Ito K, Suzuki S. Management of anaplastic thyroid carcinoma: the fruits from the ATC Research Consortium of Japan. J Nippon Med Sch. 2018;85:18–27. https://doi.org/10.1272/jnms.2018_85-3.
Baek SK, Lee MC, Hah JH, Ahn SH, Son YI, Rho YS, Chung PS, Lee YS, Koo BS, Jung KY, Lee BJ. Role of surgery in the management of anaplastic thyroid carcinoma: korean nationwide multicenter study of 329 patients with anaplastic thyroid carcinoma, 2000 to 2012. Head Neck. 2016;39(1):133–9. https://doi.org/10.1002/hed.24559.
Hu S, Helman S, Hanly E, Likhterov I. The role of surgery in anaplastic thyroid cancer: a systematic review. Am J Otolaryngol. 2017;38(3):337–50. https://doi.org/10.1016/j.amjoto.2017.02.005.
Xu B, Zhang L, Setoodeh R, Mohanty AS, Landa I, Balzer B, Tiedje V, Ganly I, Dogan S, Fagin JA, Ghossein R. Prolonged survival of anaplastic thyroid carcinoma is associated with resectability, low tumor-infiltrating neutrophils/myeloid-derived suppressor cells, and low peripheral neutrophil-to-lymphocyte ratio. Endocrine. 2022;76(3):612–19. https://doi.org/10.1007/s12020-022-03008-9.
Song T, Chen L, Zhang H, Lu Y, Yu K, Zhan W, Fang M. Multimodal treatment based on thyroidectomy improves survival in patients with metastatic anaplastic thyroid carcinoma: a SEER analysis from 1998 to 2015. Gland Surg. 2020;9(5):1205–13. https://doi.org/10.21037/gs-20-503.
Kwon J, Kim B, Jung HW, Besic N, Sugitani I, Wu HG. The prognostic impacts of postoperative radiotherapy in the patients with resected anaplastic thyroid carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2016;59:34–45. https://doi.org/10.1016/j.ejca.2016.02.015.
Saeed N, Kelly J, Deshpande H, Bhatia A, Burtness B, Judson B, Mehra S, Edwards H, Yarbrough W, Peter P, Holt E, Decker R, Husai Z, Park H. Adjuvant external beam radiotherapy for surgically resected, nonmetastatic anaplastic thyroid cancer. Head Neck. 2020;1–14. https://doi.org/10.1002/hed.26086.
Goodsell K, ERmer J, Amjad W, Swisher-McClure S, Wachtel H. External beam radiotherapy for thyroid cancer: patients, complications, and survival. Am J Surg. 2023;225(6):994–9. https://doi.org/10.1016/j.amjsurg.2023.01.009.
Fan D, Ma J, Bell A, Groen A, Olsen K, Lok B, Leeman J, Anderson E, Riaz N, McBride S, Ganly I, Shaha A, Sherman E, Tsai CJ, Kang JK, Lee N. Outcomes of multimodal therapy in a large series of patients with anaplastic thyroid cancer. Cancer. 2020;126(2):444–52. https://doi.org/10.1002/cncr.32548.
Jacob J, Vordermakr D, Lorenz K, Medenwald D. Prognostic factors in radiotherapy of anaplastic thyroid carcinoma: a single center study over 31 years. Radiat Oncol. 2023;18(1):71. https://doi.org/10.1186/s13014-023-02249-w.
Gao R, Foote R, Garces Y, Ma D, Neben-Witticha M, Toutman D, Patel S, Ko S, McGee L, Bible K, Chintakuntlawar A, Ryder M, Morris J, Van Abel K, Rivera M, Abraha F, Lester S. Outcomes and patterns of recurrence for anaplastic thyroid cancer treated with comprehensive chemoradiotherapy. Pract Radiat Oncol. 2022;12(2):113–9. https://doi.org/10.1016/j.prro.2021.10.006.
Zhou W, Yue Y, Zhang X. Radiotherapy plus chemotherapy leads to prolonged survival in patients with anaplastic thyroid cancer compared with radiotherapy alone regardless of surgical resection and distant metastasis: a retrospective population study. Front Endocrinol (Lausanne). 2021;12:748023. https://doi.org/10.3389/fendo.2021.748023.
Tian S, Switchenko JM, Fei T, Press RH, Abugideri M, Saba NF, Owonikoko TK, Chen AY, Beitler JJ, Curran WJ, Gillespie TW, Higgins KA. .Survival advantage of chemoradiotherapy in anaplastic thyroid carcinoma: propensity score matched analysis with multiple subgroups. Head Neck. 2020;42(4):678–87. https://doi.org/10.1002/hed.26042.
Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, Gunn GB, Lu C, Ferrarotto R, Lai SY, Cabanillas ME. Thyroid. 2017;27(5):672–81. https://doi.org/10.1089/thy.2016.0395.
Park J, Jung HA, Shim JH, Park WY, Kim TH, Lee SH, Kim SW, Ahn MJ, Park K, Chung JH. Multimodal treatments and outcomes for anaplastic thyroid cancer before and after tyrosine kinase inhibitor therapy: a real-world experience. Eur J Endocrinol. 2021;184(6):837–45. https://doi.org/10.1530/EJE-20-1482.
Guerra A, Di Crescenzo V, Garzi A, Cinelli M, Carlomagno C, Tonacchera M, Zeppa P, Vitale M. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(Suppl 2):44. https://doi.org/10.1186/1471-2482-13-S2-S44.
Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7–13. https://doi.org/10.1200/JCO.2017.73.6785.
Lorimer C, Cheng L, Chandler R, Garcez K, Gill V, Graham K, Grant W, Sardo Infirri S, Wadsley J, Wall L, Webber N, Wong KH, Newbold K. Dabrafenib and trametinib therapy for advanced anaplastic thyroid cancer - real-world outcomes from UK centres. Clin Oncol (R Coll Radiol). 2023;35(1):e60–6. https://doi.org/10.1016/j.clon.2022.10.017.
Bueno F, Smulever A, Califano I, Guerra J, Del Grecco A, Carrera JM, Giglio R, Rizzo M, Lingua A, Voogd A, Negueruela MDC, Abelleira E, Pitoia F. Dabrafenib plus trametinib treatment in patients with anaplastic thyroid carcinoma: an argentinian experience. Endocrine. 2023;80(1):134–41. https://doi.org/10.1007/s12020-022-03295-2.
Study of Cemiplimab Combined With Dabrafenib and Trametinib in People With. Anaplastic Thyroid Cancer. https://www.clinicaltrials.gov/study/NCT04238624. Accessed August 1, 2023.
Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373(8):726 – 36. https://doi.org/10.1056/NEJMoa1502309. Erratum in: N Engl J Med. 2018;379(16):1585.
https://www.clinicaltrials.gov/study/NCT05768178. Accessed August 1, 2023.
Cabanillas ME, Dadu R, Iyer P, Wanland KB, Busaidy NL, Ying A, Gule-Monroe M, Wang JR, Zafereo M, Hofmann MC. Acquired secondary RAS mutation in BRAFV600E-mutated thyroid cancer patients treated with BRAF inhibitors. Thyroid. 2020;30(9):1288–96. https://doi.org/10.1089/thy.2019.0514.
Knauf JA, Luckett KA, Chen KY, Voza F, Socci ND, Ghossein R, Fagin JA. Hgf/Met activation mediates resistance to BRAF inhibition in murine anaplastic thyroid cancers. J Clin Invest. 2018;128(9):4086–97. https://doi.org/10.1172/JCI120966.
Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, Bauman J, Casanova M, Krishnamurthy A, Kummar S, Leyvraz S, Oh DY, Park K, Sohal D, Sherman E, Norenberg R, Silvertown JD, Brega N, Hong DS, Cabanillas ME. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol. 2022;186(6):631–43. https://doi.org/10.1530/EJE-21-1259.
Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, Nagasubramanian R, Davis JL, Rudzinski E, Feraco AM, Tuch BB, Ebata KT, Reynolds M, Smith S, Cruickshank S, Cox MC, Pappo AS, Hawkins DS. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705–714. doi: 10.1016/S1470-2045(18)30119-0. Epub 2018 Mar 29. Erratum in: Lancet Oncol. 2018;19(5):e229.
Dias-Santagata D, Lennerz JK, Sadow PM, Frazier RP, Govinda Raju S, Henry D, Chung T, Kherani J, Rothenberg SM, Wirth LJ. Response to RET-Specific therapy in RET Fusion-Positive anaplastic thyroid carcinoma. Thyroid. 2020;30(9):1384–9. https://doi.org/10.1089/thy.2019.0477.
Godbert Y, Henriques de Figueiredo B, Bonichon F, Chibon F, Hostein I, Pérot G, Dupin C, Daubech A, Belleannée G, Gros A, Italiano A, Soubeyran I. Remarkable response to Crizotinib in Woman with anaplastic lymphoma kinase-rearranged anaplastic thyroid carcinoma. J Clin Oncol. 2015;33(20):e84–7. https://doi.org/10.1200/JCO.2013.49.6596.
Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, Park CS, Nam KH, Kang SW, Kim MK, et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol. 2013;24:3089–94. https://doi.org/10.1093/annonc/mdt379.
Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJM, Gelderblom H, Roozen ICFM, Bos M, Corver WE, van Wezel T, et al. Everolimus in patients with Advanced Follicular-Derived thyroid Cancer: results of a phase II clinical trial. J Clin Endocrinol Metab. 2017;102:698–707. https://doi.org/10.1210/jc.2016-2525.
Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, Haddad RI, Kraft S, Cabanillas ME, Rabinowits G, O’Neill A, Limaye SA, Alexander EK, Moore FD Jr, Misiwkeiwicz K, Thomas T, Nehs M, Marqusee E, Lee SL, Jänne PA, Lorch JH. Genomic correlates of response to Everolimus in Aggressive Radioiodine-refractory thyroid Cancer: a phase II study. Clin Cancer Res. 2018;24(7):1546–53. https://doi.org/10.1158/1078-0432.CCR-17-2297.
Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, Gray N, Barletta JA, Guo Y, Swanson SJ, Ruan DT, Hanna GJ, Haddad RI, Getz G, Kwiatkowski DJ, Carter SL, Sabatini DM, Jänne PA, Garraway LA, Lorch JH. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371(15):1426–33. https://doi.org/10.1056/NEJMoa1403352.
Huang D, Zhang J, Zheng X, Gao M. Efficacy and safety of lenvatinib in anaplastic thyroid carcinoma: a meta-analysis. Front Endocrinol (Lausanne). 2022;13:920857. https://doi.org/10.3389/fendo.2022.920857.
Wirth LJ, Brose MS, Sherman EJ, Licitra L, Schlumberger M, Sherman SI, Bible KC, Robinson B, Rodien P, Godbert Y, De La Fouchardiere C, Newbold K, Nutting C, Misir S, Xie R, Almonte A, Ye W, Cabanillas ME. Open-label, single-arm, multicenter, phase II trial of lenvatinib for the treatment of patients with anaplastic thyroid cancer. J Clin Oncol. 2021;39(21):2359–66. https://doi.org/10.1200/JCO.20.03093.
Higashiyama T, Sugino K, Hara H, Ito KI, Nakashima N, Onoda N, Tori M, Katoh H, Kiyota N, Ota I, Suganuma N, Hibi Y, Nemoto T, Takahashi S, Yane K, Ioji T, Kojima S, Kaneda H, Sugitani I, Tahara M. Phase II study of the efficacy and safety of lenvatinib for anaplastic thyroid cancer (HOPE). Eur J Cancer. 2022;173:210–8. https://doi.org/10.1016/j.ejca.2022.06.044.
Savvides P, Nagaiah G, Lavertu P, Fu P, Wright JJ, Chapman R, Wasman J, Dowlati A, Remick SC. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–4. https://doi.org/10.1089/thy.2012.0103.
Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, Worden FP. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20:975–80. https://doi.org/10.1089/thy.2010.0057.
Pennell NA, Daniels GH, Haddad RI, Ross DS, Evans T, Wirth LJ, Fidias PH, Temel JS, Gurubhagavatula S, Heist RS, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18:317–23.
Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, Karlin NJ, Traynor AM, Kumar P, Goh BC, Lim WT, Bossou AR, Isham CR, Webster KP, Kukla AK, Bieber C, Burton JK, Harris P, Erlichman C. Mayo Phase 2 Consortium; Mayo Clinic Endocrine Malignances Disease oriented Group. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97(9):3179–84. https://doi.org/10.1210/jc.2012-1520.
Gunda V, Gigliotti B, Ashry T, Ndishabandi D, McCarthy M, Zhou Z, Amin S, Lee KE, Stork T, Wirth L, et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int J Cancer. 2019;144:2266–78. https://doi.org/10.1002/ijc.32041.
Di Desidero T, Orlandi P, Gentile D, Banchi M, Alì G, Kusmic C, Armanetti P, Cayme GJ, Menichetti L, Fontanini G, Francia G, Bocci G. Pharmacological effects of vinorelbine in combination with lenvatinib in anaplastic thyroid cancer. Pharmacol Res. 2020;158:104920. https://doi.org/10.1016/j.phrs.2020.104920.
Jing C, Gao Z, Wang R, Yang Z, Shi B, Hou P. Lenvatinib enhances the antitumor effects of paclitaxel in anaplastic thyroid cancer. Am J Cancer Res. 2017;7(4):903–12.
Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, Duh QY, Clark OH. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91(12):2335–42.
Pierie JP, Muzikansky A, Gaz RD, Faquin WC, Ott MJ. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol. 2002;9(1):57–64. https://doi.org/10.1245/aso.2002.9.1.57.
Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. Multimodality treatment for anaplastic thyroid carcinoma–treatment outcome in 75 patients. Radiother Oncol. 2009;92(1):100–4. https://doi.org/10.1016/j.radonc.2009.02.016.
Wang JR, Zafereo ME, Dadu R, Ferrarotto R, Busaidy NL, Lu C, Ahmed S, Gule-Monroe MK, Williams MD, Sturgis EM, Goepfert RP, Gross ND, Lai SY, Gunn GB, Phan J, Rosenthal DI, Fuller CD, Morrison WH, Iyer P, Cabanillas ME. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma. Thyroid. 2019;29(8):1036–43. https://doi.org/10.1089/thy.2019.0133.
Smulever A, Barrio Lower Daniele S, Damiano G, Pitoia F. Re: complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma by Wang. Thyroid. 2020;30(8):1224–25. https://doi.org/10.1089/thy.2020.0251.
Pitoia F, Scheffel RS, Califano I, Gauna A, Tala H, Vaisman F, Gonzalez AR, Hoff AO, Maia AL. Management of radioiodine refractory differentiated thyroid cancer: the latin american perspective. Rev Endocr Metab Disord. 2023;29. https://doi.org/10.1007/s11154-023-09818-0.
Damásio I, Simões-Pereira J, Donato S, Horta M, Cavaco BM, Rito M, Gomes P, Leite V. Entrectinib in the neoadjuvant setting of anaplastic thyroid cancer: a case report. Eur Thyroid J. 2022;12(1):e220179. https://doi.org/10.1530/ETJ-22-0179.
Boudin L, Morvan JB, Thariat J, Métivier D, Marcy PY, Delarbre D. Rationale efficacy and safety evidence of Lenvatinib and Pembrolizumab Association in anaplastic thyroid carcinoma. Curr Oncol. 2022;29(10):7718–31. https://doi.org/10.3390/curroncol29100610.
Dierks C, Seufert J, Ruf J, et al. 1915P the lenvatinib/pembrolizumab combination induces long lasting and complete responses in patients with metastatic anaplastic or poorly differentiated thyroid carcinoma: results from a retrospective study and first results from the prospective phase II ATLEP trial. Ann Oncol. 2020;31:1085.
Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PA, Fasolo A, Führer D, Hütter-Krönke ML, Forde PM, Wrona A, Santoro A, Sadow PM, Szpakowski S, Wu H, Bostel G, Faris J, Cameron S, Varga A, Taylor M. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2020;38(23):2620–7. https://doi.org/10.1200/JCO.19.02727.
Lorch JH, Barletta JA, Nehs M, et al. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). J Clin Oncol. 2020;38:6513.
Cabanillas ME, Dadu R, Ferrarotto R, et al. Atezolizumab combinations with targeted therapy for anaplastic thyroid carcinoma (ATC). J Clin Oncol. 2020;38:6514.
FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication., May 2017. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. Accessed July 3, 2023.
Hatashima A, Archambeau B, Armbruster H, Xu M, Shah M, Konda B, Lott Limbach A, Sukrithan V. An evaluation of clinical efficacy of Immune Checkpoint inhibitors for patients with anaplastic thyroid carcinoma. Thyroid. 2022;32(8):926–36. https://doi.org/10.1089/thy.2022.0073.
Dierks C, Ruf J, Seufert J, Kreissl M, Klein C, Spitzweg C, Kroiss M, Thomusch O, Lorenz K, Zielke A, Miething C. 1646MO phase II ATLEP trial: final results for lenvatinib/pembrolizumab in metastasized anaplastic and poorly differentiated thyroid carcinoma. Ann Oncol. 2022;33:1295.
Lorch JH, Barletta JA, Nehs M, Uppaluri R, Alexander EK, Haddad RI, Hanna GJ, Margalit DN, Tishler RB, Schoenfeld JD, Goguen LA, Jabiev A, Sorensen MJ, Ahmadi S, Marqusee E, Kim MI, Stanizzi D, Harris E, Kacew A, Barbie DA. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). J Clin Oncol 2020 38:15_suppl, 6513–3.
Roche AM, Fedewa SA, Shi LL, Chen AY, Ansley M. Treatment and survival vary by race/ethnicity in patients with anaplastic thyroid cancer. Cancer. 2018;124(8):1780–90. https://doi.org/10.1002/cncr.31252.
dos-Santos-Silva I, Gupta S, Orem J, Shulman LN. Global disparities in access to cancer care. Commun Med. 2022;2:31. https://doi.org/10.1038/s43856-022-00097-5.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search and data analysis was made by all authors. IC, AS, FJ, and FP wrote the main manuscript text. FJ and AS prepared Tables 1, 2, 3 and 4. IC and AS prepared Figs. 1, 2 and 3. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Fabián Pitoia: principal investigator in multicenter studies for Bayer, Exelixis, Novartis. Speaker for Bayer. Inés Califano: investigator in multicenter studies for Novartis. Speaker for Bayer, Biotoscana/Knightt. Anabella Smulever and Fernando Jerkovich have no conflicts of interest relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Califano, I., Smulever, A., Jerkovich, F. et al. Advances in the management of anaplastic thyroid carcinoma: transforming a life-threatening condition into a potentially treatable disease. Rev Endocr Metab Disord 25, 123–147 (2024). https://doi.org/10.1007/s11154-023-09833-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09833-1