Opinion statement
Anaplastic thyroid cancer presents formidable challenges, particularly in cases of recurrence or metastasis. Timely BRAF V600E testing is imperative at diagnosis, initially through immunohistochemistry, followed by comprehensive genomic profiling encompassing genes such as NTRK, RET, ALK, and assessment of tumor mutation burden (TMB). FDA-approved treatment options include dabrafenib and trametinib for patients with BRAF mutations, while those exhibiting high TMB may benefit from pembrolizumab. Further therapeutic decisions hinge upon mutational profile, urgency of response required, airway integrity, and access to targeted therapies There is growing use of immunotherapy for ATC based on published reports of activity, but currently there is no FDA approved agent for ATC. The off-label utilization of “precision medicine” combinations imposes a considerable financial strain, underscoring the necessity for further clinical trials to elucidate promising therapeutic avenues for this orphan disease. There is a pressing need for the development and support of clinical trials investigating genomically driven and immune-based therapies for anaplastic thyroid cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic thyroid cancer (ATC) represents an undifferentiated, highly aggressive and poorly understood form of thyroid malignancy. Despite constituting only 1% of all thyroid cancer cases, it carries a mortality rate approaching 50% [1]. The incidence of ATC is approximately one per million individuals, primarily affecting patients between 60 and 70 years old, with a mean age at diagnosis of 71 years [2, 3]. It is important to highlight that existing literature documents the propensity of anaplastic thyroid cancer (ATC) to originate from well-differentiated thyroid cancer [4,5,6,7,8]. Median survival time for ATC is 9.5 months, with the highest survival duration reaching 14.5 months observed in cases treated with neoadjuvant BRAF-directed therapy followed by bilateral glandular lobes resection with lymph node sparing due to the lack of therapeutic benefit from lymph node resections [2, 9].
Previous research has indicated that younger patients with ATC may exhibit distinct molecular mutation profiles [10]. The one-year survival rate for patients with anaplastic thyroid cancer is less than 20%, highlighting the significant challenges associated with this aggressive form of thyroid cancer. This stands in stark contrast to the markedly higher 5-year survival rates, reaching 98% and 99% for well-differentiated thyroid cancers such as follicular and papillary carcinoma, respectively [11]. Gender disparities in mortality rates have been observed within the context of ATC cases. A comprehensive analysis of the Surveillance, Epidemiology, and End Results (SEER) database spanning 16 years revealed a notable difference in mortality percentages between women and men. Specifically, the mortality percentage for women was approximately 57%, while men constituted approximately 43% of the total deaths attributed to anaplastic thyroid cancer [12]. The potential to improve these concerning survival statistics for ATC may hinge upon unresolved aspects of its pathophysiology.
Oncogenic drivers implicated in ATC have been identified, thus revolutionizing treatment approaches. Whereas traditional chemotherapeutic agents like doxorubicin and newer modalities such as carboplatin and paclitaxel were previously utilized, identification of BRAF V600E mutation now occurs in approximately one-third of ATC patients [10, 13•]. This discovery has resulted in response rates of 56%, significantly controlling ATC progression in certain individuals [10]. Additionally, RET mutation, which represents approximately 6% of poorly differentiated thyroid cancers, may also elicit noteworthy responses, albeit with limited durability [14, 15].
Genetic instability is a hallmark for ATC with a median number of mutations of 6 ± 5 [16]. Interestingly, ATC is the only thyroid malignancy in which age plays no role in TNM staging, despite evidence indicating its crucial role in ATC prognosis [17]. Although surgical resection, radioactive iodide therapy, and hormone-suppressive therapy could result in a good prognosis for most patients with differentiated thyroid cancer, the same is not true for ATC [18]. To date, uncertainty persists among clinicians and researchers regarding the precise incidence, etiology, biology, and management of ATC. The rarity of this malignancy, coupled with its historical perception as an immediate death sentence, has hindered the progress of both clinical trial development and laboratory research. However, recent advancements in cancer biology offer new avenues for comprehending and addressing ATC. It is crucial to investigate the genetic foundations of ATC for the potential to improve diagnostic precision, clarify its association with preceding differentiated thyroid cancer, provide prognostic indications, and notably, advance the creation of personalized treatment approaches tailored to specific mutational patterns.
Methods and patients
A literature review was conducted using the search term “Anaplastic thyroid cancer” to screen for clinical trials in PubMed and Medline from 2012 to May 2022. A total of 30 articles were gathered, with duplicates and irrelevant studies subsequently removed. The abstracts of publications were individually screened to collect evidence regarding genetic mutations and their associated treatment outcomes, genetic mapping, and current therapeutic standards for anaplastic thyroid cancer. Eventually, 16 articles were included in the review. The use of a broad search term aimed to capture a diverse range of articles containing information on the genetic landscape of ATC that might not have been obtained using more specific criteria. Additionally, a literature review was performed using the same search terms for case controls, cohorts, reviews, ex-vivo and in-vitro studies, clinicaltrials.gov, and case reports.
Genomic mutations and implications on treatment outcomes
Etiology and risk factors
As suggested by a number of studies and case reports, ATC biopsies often yield well-differentiated thyroid cancer in addition to anaplastic cells with some findings suggesting transformation from well-differentiated thyroid cancer to anaplastic thyroid cancer [4,5,6,7,8].
During our search in the literature, we have found that the most important risk factor for ATC is a history of thyroid cancer (especially well-differentiated thyroid cancer) [19]. Also, it is reported that about 20-70% of all ATC cases had preexistent well-differentiated thyroid cancer [4, 20,21,22]. Smoking, alcohol intake, and professional exposure to chemicals were not found to be correlated with the pathogenesis of ATC but history of radiation exposure was debated [19, 23].
Clinical presentation and sample collection
Patients with ATC commonly manifest with either a pre-existing neck mass previously deemed non-interventional or with a sudden, rapid enlargement of a neck mass. In either scenario, accompanying symptoms may include alterations in voice, swallowing function, compromised airway integrity, and the onset of neck pain. Subsequently, patients seek evaluation from specialized healthcare providers such as endocrine surgeons or otolaryngologists for comprehensive assessment and management. Notably, a significant proportion of these patients may reside at considerable distances from specialized centers of excellence with primary focus on endocrine oncology.
After harvesting samples, mainly through surgical excision, histopathological and genomic sequencing take place. Most of the samples collected are either formalin-fixed, paraffin-embedded (FFPE) tissue, or fresh frozen tissue (FFT) [24]. Core needle biopsy is recognized as a minimally invasive and reliable procedure, providing a histological sample that maintains its cytologic features. It may be considered particularly useful in cases where surgical intervention is not feasible for the patient [25] Additionally, there is increasing attention on liquid biopsy for thyroid malignancies, involving the detection of circulating cell-free DNA. This approach has shown promising outcomes in the early detection of thyroid malignancies and exhibits high concordance rates with tissue sequencing, typically conducted on surgically excised tissue [26, 27]. Fine needle aspiration (FNA) biopsy may be used initially for analyzing thyroid nodules, but the low cellularity yielded often leads to uncertainty about the diagnosis of aggressive thyroid cancer. Samples used for ATC genomic analysis are both thyroid cancer–derived cell lines and patient-derived. Thyroid cancer–derived cell lines is significantly smaller compared with other common tumor types and has been studied in the literature [28].
Current diagnostic and management at our center
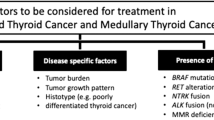
Anaplastic thyroid cancer presents challenges at all stages of diagnosis, treatment, age and medical comorbidity. Guidelines are available from the American Thyroid Association, National Comprehensive Cancer Network and American Society of Clinical Oncology (among others) that can guide patients and physicians in optimal treatment of this rare disease. In this review we focus specifically on genomic and immune therapy treatments for anaplastic thyroid cancer, however a close and speedy collaboration between all members of the thyroid cancer treatment team is essential. We recommend that patients be seen or connected with high volume centers with significant experience in diagnosing and treating anaplastic thyroid cancer. These centers are also locations where clinical trials are available for patients, which would allow us to strengthen the evidence base that underpins many recommendations.
In many cases of anaplastic thyroid cancer that present to our center, there is often a rapid onset of symptoms around the neck, typically dysphagia or airway insecurity. Patients may or may not have had previous neck biopsies that yielded non diagnostic results. If a patient has a rapidly enlarging neck mass with a thyroid malignancy, the exact level of differentiation (well/poorly/undifferentiated) almost does not matter. As long as other possible malignancies such as squamous or hematologic are sufficiently ruled out our first step is to check a BRAF V600E immunohistochemistry stain. Clinically the patient is seen by surgeons, endocrinologists, medical and radiation oncologists as an emergency case. The priority is recognizing the severity of the diagnosis and expediting workup with extra emphasis on airway security.
Anaplastic thyroid cancer is one where typically proceeding directly to debulking surgery or radiation is to be avoided. We tend to use drug therapy first, typically genomically driven +/- immune therapy, or paclitaxel with carboplatin if genomic information is pending but treatment must be started. This can be delivered for 1-3 weeks while pathologic testing, patient stabilization and a definitive treatment plan is established. After initial staging imaging, which includes brain imaging, we typically proceed with radiation or surgery if the disease is localized without distant metastatic disease. In patients who present with locally advanced unresectable or metastatic disease the following approaches based on genomic abnormalities and immune therapies are implemented.
Genomic mutations
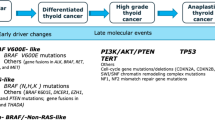
Differentiated thyroid cancers can evolve to more aggressive forms, poorly differentiated (PDTC) and ATC, by acquiring additional genetic alterations which deregulate key pathways. SWI/SNF (SWItch/Sucrose Non-Fermentable) disruption appears to lock thyroid cells into a de-differentiated state that is no longer reversible by blocking the MAPK pathway. This suggests that SWI/SNF mutations may be markers of resistance to redifferentiation strategies [15]. Also, mutations in the promoter of telomerase reverse transcriptase (TERT) were observed 73% of ATC, and their occurrence correlated with aggressive behavior and the propensity for metastasis [15].. There are specific combinations of genetic defects in ATC within the PI3K pathway showing distinct trends alongside mutations that activate the MAPK pathway. Oncogenic mutations in PIK3CA tend to aggregate within tumors already carrying the BRAFV600E mutation, whereas losses in PTEN frequently occur alongside mutations in RAS and NF1 [29]. However, no pathognomonic mutation or histopathologic finding is sufficient in isolation to confirm a diagnosis of ATC.
There is often a requirement of expert pathologic input to confirm the diagnosis. From a histopathological diagnostic perspective, ATC is an undifferentiated carcinoma that may present with a myriad of histological growth patterns. The diagnostic challenge in small biopsies is that a concomitant well-differentiated thyroid carcinoma may not be sampled. As such, the differential could include other locoregional or metastatic processes. From a morphological perspective they can show cells that are architecturally epithelioid, spindle, giant cell, pleomorphic, rhabdoid, angiomatoid, or squamous. They may even show metaplastic chondroid or osteoid material, which may additionally confound the pathological interpretation [30]. Immunohistochemical studies can be challenging to interpret as well. 75% of ATC may show some degree of cytokeratin positivity, however, thyroid lineage marker expression is variable: thyroglobulin is only expressed by a minority of these tumors, TTF1 is often only focally expression, and about half of the cases express PAX8. Finally, presentations can either be pure ATC, ATC with a well-differentiated component (papillary or follicular), or ATC with a poorly differentiated component [4, 31]. In cases of de novo presentation of ATC, it is unclear whether there was an intermediate step of differentiated thyroid cancer that has since been obscured by the ATC component. In clinical practice there is no discernible difference in genomic profile obtained of ATC only vs ATC that also has a differentiated component.
In published analyses as well as clinical practice, BRAF (a proto-oncogene) or TP53 (a tumor suppressor gene) mutations are the most frequent mutations found in the genetic landscaping of ATC (Table 1), with clinically actionable genomic mutations bolded [4, 5, 31]. Also, other frameshift deletions and novel anaplastic gene fusions are common for this type of cancer [30].
Targeted therapy approaches for recurrent/metastatic ATC
The wide availability of genomic testing and its incorporation into clinical practice has led to a change in clinical trial approaches over the last decade. While there is still a need for therapy options regardless of mutation status, newer clinical trials are including a genomic testing requirement and a biomarker testing component, at least for BRAF status [32••, 33].
Older ATC clinical trials do not mention the exact mutation prevalent in the population under investigation. However, studies that did genomic testing yielded promising results (Table 2). Subbiah et al. investigated the effects of dabrafenib plus trametinib in BRAF V600-mutated ATC (Fig. 1). This regimen proven to be the most effective with 69% of patients achieving partial or complete response to treamtment [34]. on the other hand, sorafenib-treated achieved very little response rate (0-10%) with no significant improvement in overall survival [35, 36].. Sherman et al. investigated the efficacy of sorafenib in combination with temsirolimus in two BRAF V600-negative patients and one patient (50%) showed a major response to therapy [37]. Nonetheless, more studies are needed to investigate the combination of sorafenib and temsirolimus which may potentially have synergistic effect Another report showed that combination of radiation and paclitaxel plus sorafenib has significant anticancer activity leading to reduction in the tumor size and an increase in survival in the ATC xenograft model [38]. The RTOG 0912 trial was the largest clinical trial that studied the addition of pazopanib to radiotherapy plus paclitaxel. The study found that no significant improvement in overall survival was recorded in the pazopanib group. However, the study only recruited 71 patients despite being called the largest clinical trial studying anaplastic thyroid cancer which reflects the difficulty researchers find to study such rare disease [13•].
Targets for immune therapy and small molecules in anaplastic thyroid cancer (Figure created with BioRender.com).
Other ongoing ATC clinical trials include the use of abemaciclib [33], a CDK 4/6 inhibitor that has shown significant promise in preclinical data and animal models [39]. The high rate of cellular growth, intact Rb, CDKN2A/B abnormalities and not elevated p16 levels seen in patient samples suggest that there may be a role for CDK4/6 inhibitors in recurrent/metastatic ATC.
Immune therapy in recurrent/metastatic ATC
The immune therapy revolution of the last decade has transformed treatment options and dramatically improved dozens of cancer types, from melanoma to triple negative breast cancer. Immune therapy has been tested in numerous clinical trials of ATC with inconsistent results but is still regularly used. A cohort study spanning approximately 20 years examined patients diagnosed with anaplastic thyroid carcinoma (ATC) undergoing diverse treatment modalities across varying disease stages. The study findings indicate that the incorporation of immune therapy alongside targeted therapy correlated with improvement in overall survival duration [9]. Commonly used biomarkers correlating with enhanced or diminished response of immune checkpoint inhibitors include microsatellite stable or unstable, PD-L1 score as determined by assorted assays on tumor cells and effector white blood cells, tumor mutation burden (TMB) and genomic markers such as STK11, KEAP1 ARID1A. A high combined positive score (CPS) for PD-L1 expression is not reliably associated with higher responses, as can be found in other tumors [40]. Median TMB also tends to be lower than 10 mutations per megabase, and microsatellite status tends to be stable. This means the activity of drugs such as spartalizumab [41]was limited and did not lead to FDA-approval of anti-PD1 therapies. Patients with a PD-L1 score greater than or equal to 50 had a response rate of 35%, while those with 0 PD-L1 expression had no responses seen. The numbers in this trial were small, so it is important not to draw too many conclusions about anti-PD1 therapy based on this trial.
BRAF wild type ATC tends to be the most difficult to treat ATC. Anti PD-1 therapy is used more regularly in clinical practice. Dierks et al. documented the outcomes of six metastatic ATC patients lacking BRAF V600E mutations who received Lenvatinib in combination with Pembrolizumab. These patients achieved a progression-free survival (PFS) of 16.5 months [42]. Subsequently, a phase II (ATLEP) trial in 35 patients without the BRAF V600E mutation revealed a median PFS of 9.5 months and an overall survival (OS) of 10.25 months [43]. Another factor contributing to the increased utilization of anti-PD-1 therapy in clinical settings is not necessarily rooted in an overwhelming confidence in its efficacy. Rather, it often stems from the limited viability of alternative treatment modalities, such as conventional chemotherapy, which can induce excessive toxicity in this patient cohort. Moreover, targeted therapies demonstrate inefficacy in the absence of identifiable genomic drivers. Anti-PD-1 therapies, characterized by their comparatively lower incidence of adverse effects, occasionally yield notable and enduring responses in patients with anaplastic thyroid cancer. In addition, the failure of further development of spartalizumab, which is no longer available, and the lack of data using other anti-PD1 therapies means that oncologists often must make this decision without a strong backing from the scientific literature.
There is also a promising role for anti-PD1 therapies in BRAF V600E mutated ATC. While BRAF mutation status does not appear to enhance or diminish response to anti-PD1 therapy, early reports suggest that the addition of pembrolizumab to may result in superior response rates and have been tried as a neoadjuvant approach [44]. Zafereo and colleagues have been testing this approach in a prospective clinical trial NCT04675710, which may further define the role of combination targeted and immune therapy-dabrafenib, trametinib and pembrolizumab (DTP).
A similar approach was taken by Lorch and colleagues using combined anti-CTLA4 (ipilimumab) and anti-PD1 (nivolumab) in a trial that showed promising results [45]. In 10 patients with ATC 3/10 patients were reported as having a profound response that persisted for many months. While toxicity of dual immune checkpoint therapy is often a concern, the preliminary report suggested that it was well tolerated in the ATC population. Further details are expected from the publication.
Conclusion
Advances in the treatment of recurrent or metastatic anaplastic thyroid cancer mean have resuled in patients achieving remission, longer progression-free survival, and improved overall survival with even modest cure rates. The classical default recommendation of hospice has been replaced by a plethora of clinical trials and active research into this aggressive disease. Investment of resources into a very uncommon disease and advocacy by patients have resulted in improved outcomes, though overall, the diagnosis often is followed by a poor prognosis. Molecular testing is paramount with the hope of identifying a BRAF V600E mutation while there is still an intact airway. In this scenario, patients may be treated with FDA approved dabrafenib and trametinib (DT) and can sometimes result in extraordinary results. In patients who subsequently progress on DT, or lack an oncogenic driver, treatment options are fewer. Adding pembrolizumab to DT can be done for those who have progressed or attempting the combination of lenvatinib and pembrolizumab [46]. Occasionally these can result in dramatic responses. Finally immune therapy either as monotherapy or combined with targeted therapies or other immune therapies is given to ATC patients. In this scenario, treatment options remain poor and highlight the urgency for more clinical trials in ATC.
Summary of diagnostic and therapeutic approach for recurrent/metastatic ATC
-
1.
Anaplastic thyroid cancer (ATC) is a rare, aggressive form of thyroid malignancy with a mortality rate approaching 50%, primarily affecting older individuals.
-
2.
Genetic instability is a hallmark of ATC, with identified mutations like BRAF V600E and RET potentially guiding treatment approaches
-
3.
Immunohistochemistry and DNA next generation testing (preferred) should be performed immediately after a diagnosis of ATC is made.
-
4.
Determination of BRAF V600E mutated or wild type status is urgent and essential, as dabrafenib and trametinib are FDA-approved options that often result in rapid responses.
-
5.
Determination of tumor mutation burden, which if greater than 10 mutations/Megabase can be treated with pembrolizumab as an tumor agnostic FDA-approved treatment option.
-
6.
Identification of less common but clinically actionable tumor agnostic genomic abnormalities such as NTRK, RET with FDA-approved treatment options.
-
7.
There is growing body of evidence supporting Immune therapy, particularly in BRAF wild-type ATC.
-
8.
When clinical trials are not available, in select cases under expert guidance, a genomic profile identifying oncogenic drivers can help select off-label drugs given as monotherapy or combinations.
-
9.
Advances in treatment options have improved outcomes, but overall prognosis remains poor, emphasizing the need for continued research and personalized approaches.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–48. https://doi.org/10.1001/jama.2017.2719.
Lin B, Ma H, Ma M, et al. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am J Transl Res. 2019;11(9):5888–96.
Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–5. https://doi.org/10.1002/cncr.20936.
Duan H, Li Y, Hu P, et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology. 2019;75(6):890–9. https://doi.org/10.1111/his.13942.
Kunstman JW, Juhlin CC, Goh G, et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet. 2015;24(8):2318–29. https://doi.org/10.1093/hmg/ddu749.
Skwiersky S, Hevroni G, Singh G, et al. Concurrent anaplastic and papillary thyroid carcinomas: a case report. Am J Med Case Rep. 2020;8(7):202–5.
Shahi S, Bhandari TR, Pantha T, Gautam D. A rare coexistence of papillary carcinoma and anaplastic carcinoma of thyroid in multinodular goitre: case report and literature review. Ann Med Surg. 2020;56:161–4. https://doi.org/10.1016/j.amsu.2020.06.024.
Khairy G. Anaplastic transformation of differentiated thyroid carcinoma. Int J Health Sci. 2009;3(1):93–6.
Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol. 2020;6(9):1397–404. https://doi.org/10.1001/jamaoncol.2020.3362.
Khan SA, Ci B, Xie Y, et al. Unique mutation patterns in anaplastic thyroid cancer identified by comprehensive genomic profiling. Head Neck. 2019;41(6):1928–34. https://doi.org/10.1002/hed.25634.
Survival Rates for Thyroid Cancer. https://www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 30 May 2024.
Patel S, Pappoppula L, Guddati AK, Annamaraju P. Analysis of Race and Gender Disparities in Incidence-Based Mortality in Patients Diagnosed with Thyroid Cancer from 2000 to 2016. Int J Gen Med. 2020;13:1589–94. https://doi.org/10.2147/IJGM.S280986.
• Sherman EJ, Harris J, Bible KC, et al. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): a randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. 2023;24(2):175–86. https://doi.org/10.1016/S1470-2045(22)00763-X. This reference is of importance as this is the largest clinical trial for anaplastic thyroid cancer which explored the addition of pazopanib to the combination of paclitaxel and radiotherapy. Although it showed pazopanib to be a safe addition to the combination, there is no statistically significant improvement of survival in the intervention group versus placebo.
Subbiah V, Wolf J, Konda B, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23(10):1261–73. https://doi.org/10.1016/S1470-2045(22)00541-1.
Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–66. https://doi.org/10.1172/JCI85271.
Volante M, Lam AK, Papotti M, Tallini G. Molecular pathology of poorly differentiated and anaplastic thyroid cancer: what do pathologists need to know? Endocr Pathol. 2021;32(1):63–76. https://doi.org/10.1007/s12022-021-09665-2.
Kong N, Xu Q, Zhang Z, Cui A, Tan S, Bai N. Age influences the prognosis of anaplastic thyroid cancer patients. Front Endocrinol. 2021;12. https://www.frontiersin.org/article/10.3389/fendo.2021.704596.
Jin S, Borkhuu O, Bao W, Yang YT. Signaling pathways in thyroid cancer and their therapeutic implications. J Clin Med Res. 2016;8(4):284–96. https://doi.org/10.14740/jocmr2480w.
Zivaljevic V, Slijepcevic N, Paunovic I, et al. Risk factors for anaplastic thyroid cancer. Int J Endocrinol. 2014;2014:815070. https://doi.org/10.1155/2014/815070.
McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–34. https://doi.org/10.1067/msy.2001.118266.
Spires JR, Schwartz MR, Miller RH. Anaplastic thyroid carcinoma. Association with differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. 1988;114(1):40–4. https://doi.org/10.1001/archotol.1988.01860130044012.
Wallin G, Bäckdahl M, Tallroth-Ekman E, Lundell G, Auer G, Löwhagen T. Co-existent anaplastic and well differentiated thyroid carcinomas: a nuclear DNA study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 1989;15(1):43–8.
Molinaro E, Romei C, Biagini A, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13(11):644–60. https://doi.org/10.1038/nrendo.2017.76.
Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019;29(3):311–21. https://doi.org/10.1089/thy.2018.0509.
Matrone A, De Napoli L, Torregrossa L, et al. Core needle biopsy can early and precisely identify large thyroid masses. Front Oncol. 2022;12:854755. https://doi.org/10.3389/fonc.2022.854755.
Qin Y, Wang JR, Wang Y, et al. Clinical utility of circulating cell-free dna mutations in anaplastic thyroid carcinoma. Thyroid. 2021;31(8):1235–43. https://doi.org/10.1089/thy.2020.0296.
Marotta V, Cennamo M, La Civita E, Vitale M, Terracciano D. Cell-Free DNA analysis within the challenges of thyroid cancer management. Cancers. 2022;14(21):5370. https://doi.org/10.3390/cancers14215370.
Landa I, Pozdeyev N, Korch C, et al. Comprehensive genetic characterization of human thyroid cancer cell lines: a validated panel for preclinical studies. Clin Cancer Res. 2019;25(10):3141–51. https://doi.org/10.1158/1078-0432.CCR-18-2953.
Franco AT, Malaguarnera R, Refetoff S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci. 2011;108(4):1615–20. https://doi.org/10.1073/pnas.1015557108.
Kasaian K, Wiseman SM, Walker BA, et al. The genomic and transcriptomic landscape of anaplastic thyroid cancer: implications for therapy. BMC Cancer. 2015;15(1):984. https://doi.org/10.1186/s12885-015-1955-9.
Rashid M, Agarwal A, Pradhan R, et al. Genetic alterations in anaplastic thyroid carcinoma. Indian J Endocrinol Metab. 2019;23(4):480–5. https://doi.org/10.4103/ijem.IJEM_321_19.
•• Khan SA. Phase II trial of pembrolizumab in metastatic or locally advanced anaplastic/ undifferentiated thyroid cancer. clinicaltrials.gov; 2022. Accessed 31 Dec. 2022. https://clinicaltrials.gov/study/NCT02688608. This reference is of outstanding importance as it is a comprehensive genomic profiling of 90 ATC specimens. It showed that clinically relevant genomic alteration in ATC specimens varies by patient age which provides insight into individualized medicine for targeted therapy in ATC patients.
Khanna V, Miles C, Sundaram V, et al. Abemaciclib in metastatic or locally advanced anaplastic thyroid cancer. J Clin Oncol. 2022;40(16_suppl):TPS6112. https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS6112.
Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600–mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7–13. https://doi.org/10.1200/JCO.2017.73.6785.
Ito Y, Onoda N, Ito K, et al. Sorafenib in Japanese patients with locally advanced or metastatic medullary thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid. 2017;27(9):1142–8. https://doi.org/10.1089/thy.2016.0621.
Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid Off J Am Thyroid Assoc. 2013;23(5):600–4. https://doi.org/10.1089/thy.2012.0103.
Sherman EJ, Dunn L, Ho AL, et al. Phase II study evaluating the combination of Sorafenib and Temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer. 2017;123(21):4114–21. https://doi.org/10.1002/cncr.30861.
Kim SY, Kim SM, Chang H, Chang HS, Park CS, Lee YS. Synergistic anticancer activity of sorafenib, paclitaxel, and radiation therapy on anaplastic thyroid cancer in vitro and in vivo. Head Neck. 2020;42(12):3678–84. https://doi.org/10.1002/hed.26431.
Pita JM, Raspé E, Coulonval K, et al. CDK4 phosphorylation status and rational use for combining CDK4/6 and BRAF/MEK inhibition in advanced thyroid carcinomas. Front Endocrinol. 2023;14 Accessed 31 Oct. 2023. https://www.frontiersin.org/articles/10.3389/fendo.2023.1247542.
Surucu A, Hou T, Kuhar M, Durm G, Mesa H. Comparison of PD-L1 IHC 22C3 PharmDx Combined Positive Score (CPS) in primary versus metastatic nodal squamous cell carcinomas of the head and neck: is there a significant difference? Appl Immunohistochem Mol Morphol. 2023;31(8):550. https://doi.org/10.1097/PAI.0000000000001140.
Capdevila J, Wirth LJ, Ernst T, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(23):2620–7. https://doi.org/10.1200/JCO.19.02727.
Dierks C, Seufert J, Aumann K, et al. Combination of lenvatinib and pembrolizumab is an effective treatment option for anaplastic and poorly differentiated thyroid carcinoma. Thyroid Off J Am Thyroid Assoc. 2021;31(7):1076–85. https://doi.org/10.1089/thy.2020.0322.
Dierks C, Ruf J, Seufert J, et al. 1646MO phase II ATLEP trial: final results for lenvatinib/pembrolizumab in metastasized anaplastic and poorly differentiated thyroid carcinoma. Ann Oncol. 2022;33:S1295. https://doi.org/10.1016/j.annonc.2022.07.1726.
Cabanillas ME, Ferrarotto R, Garden AS, et al. Neoadjuvant BRAF- and immune-directed therapy for anaplastic thyroid carcinoma. Thyroid Off J Am Thyroid Assoc. 2018;28(7):945–51. https://doi.org/10.1089/thy.2018.0060.
Sehgal K. A Phase 2 study of nivolumab plus ipilimumab in RAI refractory, aggressive thyroid cancer with exploratory cohorts in medullary and anaplastic thyroid cancer. clinicaltrials.gov; 2022. https://clinicaltrials.gov/study/NCT03246958.
Iyer PC, Dadu R, Gule-Monroe M, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer. 2018;6(1):68. https://doi.org/10.1186/s40425-018-0378-y.
Acknowledgements
None
Ethics declarations
Conflict of interest
Saad Khan: reports receiving honoraria for consulting/advisory board work from Foundation Medicine, Eisai, Coherus, EMD Serono, Kineta, Roche Pakistan.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdalla, A.S., Rahman, M. & Khan, S.A. Promising Therapeutic Targets for Recurrent/Metastatic Anaplastic Thyroid Cancer. Curr. Treat. Options in Oncol. 25, 869–884 (2024). https://doi.org/10.1007/s11864-024-01219-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-024-01219-y