Abstract
Background
Most plants have a hyphosphere, the thin zone of soil around extraradical hyphae of arbuscular mycorrhizal (AM) fungi, which extends beyond the rhizosphere. This important interface has critical roles in plant mineral nutrition and water acquisition, biotic and abiotic stress resistance, mineral weathering, the formation of soil macroaggregates and aggregate stabilization, carbon (C) allocation to soils and interaction with soil microbes.
Scope
This review focuses on the hyphosphere of AM fungi and critically appraises the important findings related to the hyphosphere processes, including physical, chemical and biological properties and functions. We highlight ecological functions of AM fungal hyphae, which have profound impacts on global sustainability through biological cycling of nutrients, C sequestration in soil, release of greenhouse gas emissions from soil and the diversity and dynamics of the microbial community in the vicinity of the extraradical hyphae.
Conclusions
As a critical interface between AM fungi and soil, hyphosphere processes and their important ecological functions have begun to be understood and appreciated, and are now known to be implicit in important soil processes. Recent studies provide new insights into this crucial zone and highlight how the hyphosphere might be exploited as a nature-based solution, through understanding of interactions with the microbiome and the impacts on key processes governing resource availability, to increase sustainability of agriculture and minimize its environmental impact. Uncovering hyphosphere chemical and biological processes and their subsequent agricultural, ecological and environmental consequences is a critical research activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well-known that the rhizosphere, defined by Hiltner (1904) as the thin layer of soil surrounding the living roots, is a critical and active zone due to the physical, chemical and biological changes induced by roots (Marschner 1995). Subsequently, a large number of studies about rhizosphere processes have demonstrated that the rhizosphere differs from the bulk soil regarding pH (George et al. 2002), water and redox potential (York et al. 2016), porosity (Koebernick et al. 2017, 2019), concentrations of soluble carbohydrates and nutrients (Lambers et al. 2006, 2009; Lambers 2022), biochemical properties (Zhang et al. 2010), and the structure and functioning of microbial communities (Besset-Manzoni et al. 2018). However, this active zone of soil is not influenced only by plant roots.
Arbuscular mycorrhizal (AM) fungi are obligate biotrophs that colonize more than two thirds of terrestrial plant species to establish a symbiotic relationship (Smith and Read 2008); they have been forming relationship with plants ever since plants first colonized land approximately 450 million years ago (Delaux and Schornack 2021). As pivotal members of the soil microbiome, AM fungi provide multiple services to their host plants, such as mineral nutrition (especially phosphorus uptake), water acquisition, biotic and abiotic stress resistance, and other ecological functions, such as improving ecosystems diversity and productivity (van der Heijden et al. 2015). Importantly, AM fungi provide an alternative and important nutrient acquisition pathway to that of plant roots from soil (Bucher 2007; Smith et al. 2011; Smith and Smith 2011). For example, in many cases, the contribution of the mycorrhizal pathway to total P uptake is greater than that of direct root pathway (Chu et al. 2020; Nagy et al. 2009), and can even completely replace the direct root pathway (Smith et al. 2003, 2004).
When plant roots are colonized by AM fungi, the rhizosphere includes not only roots but also mycelium of AM fungi, whereby mycelial activity significantly changes the rhizosphere properties; moreover, the mycelium extends beyond the rhizosphere, thus influencing soil properties in the zones beyond the reach of the root impacts. It has been demonstrated that colonization of roots with AM fungi influences plant metabolism and causes changes in plant assimilates, which strongly inhibits activity of manganese (Mn)-reducing bacteria, for example, at the interface between soil and the fungi-root symbionts and decreases Mn concentration in plants (Arines et al. 1992; Kothari et al. 1991b; Posta et al. 1994). A large number of researches further demonstrated that the biodiversity, abundance and assembly of microbial community located in the inner root space, rhizosphere and hyphosphere are completely different (Bai et al. 2015; Bulgarelli et al. 2012; Toljander et al. 2007). Moreover, different AM species co-colonizing a single plant root system recruit distinct microbiomes in the hyphosphere (Zhou et al. 2020). Therefore, it is necessary to re-examine the definitions of the soil zones/volumes that are directly influenced by plant roots or AM fungal hyphae or where they interact. The following terms are suggested as a nomenclature for the range of habitats in and around roots (Table 1). The endosphere is defined as inner root tissues (including rhizodermis, cortex and stele) inhabited by microorganisms. Rhizosphere is defined as the thin layer of soil surrounding the living roots not colonized by mycorrhizal fungi. This zone (including the root surface and the adjoining soil) is directly influenced by root exudates. Mycorrhizosphere is defined as a zone of soil surrounding the living roots colonized by mycorrhizal fungi. This zone (including the root and hyphal surface, and the soil adjoining the roots and hyphae) is directly modified by mycorrhizal fungi mediating root exudates and hyphal exudates. The unique feature of the mycorrhizosphere is an overlap of mycorrhized roots and hyphae. The width of mycorrhizosphere was reported to be broader than that of rhizosphere, and the biochemical activities were stronger than those in rhizosphere, at least in terms of phosphorus depletion and pH changes (Li et al. 1991a, c; Joner and Jakobsen 1995; Song et al. 2000). Hyphosphere is defined as a critical and active zone of soil surrounding the living extraradical mycelium of mycorrhizal fungi beyond the rhizosphere and mycorrhizosphere. This zone (only including the hyphal surface and the soil adjacent to the hyphae) is directly influenced by exudates of mycorrhizal fungi (Fig. 1, Table 1). This more precise nomenclature will allow us to highlight the specific effects of AM fungi on the metabolism of plant roots and will be helpful to understanding of the in situ biological, physical and chemical process in the plant root-mycorrhiza-microbe-soil continuum.
Another important consideration is that the diameter of AM hyphae (2–10 μm) is up to two orders of magnitude thinner than that of fine roots (around 0.2 mm or less); hence, the hyphal length density in soil (exceeding 108 m/m3) is much greater than that of roots (ca. 104 m/m3) (Allen 2007; Miller et al. 1995) (Table 2). Moreover, hyphae of AM fungi produce numerous branches (Friese and Allen 1991), thus enlarging the effective root surface area and its contact with the soil (Lanfranco et al. 2018). Compared with the root, morphological advantages of mycelia imply that the hyphosphere, in addition to rhizosphere/mycorrhizosphere, is a critical and active soil zone providing a hotspot of activity in which physical, chemical and biological changes and functions are controlled mainly by the hyphae of AM fungi.
Even though the ecology of the rhizosphere has been investigated for more than 100 years, exploring the nature of hyphosphere interactions has received attention only in the last 30 years. Nevertheless, many ecological processes and functions in the hyphosphere have been characterized using innovative research methodologies (e.g. rhizoboxes, root and AM fungi dual culture, 13C-SIP-DNA/RNA analysis, NanoSIMS, quantum dot labelling, microfluidic chip, etc.). This Marschner review aims to critically evaluate the current knowledge on the processes, ecological functions and importance of the AM hyphosphere.
Hyphosphere processes
Until recently the limitations of research methods meant it was difficult to differentiate the effects of AM fungi and plant roots in the mycorrhizosphere because the fungi and roots grew in the same compartment. In the last 30 years specific techniques have been employed to disentangle the effects in the rhizosphere/mycorrhizosphere and hyphosphere. For example, development of compartmented systems (Kothari et al. 1990; Li et al. 1991a, b, c), using nylon mesh to separate the physical spaces that plant roots and AM fungal hyphae can occupy, has provided new insights into how the extraradical mycelium mediates soil processes. The most compelling findings show that the fungal hyphae, invisible to the naked eye, can cause a range of profound physical, chemical and biological changes to the hyphosphere. Moreover, these changes are of the sufficient magnitude for the hyphosphere to be considered significant within the context of the global carbon (C), nitrogen (N) and phosphorus (P) cycles and their consequent environmental impacts.
Hyphosphere: physical processes
Soil macroaggregate formation and stabilization
Hyphae of AM fungi can promote soil aggregate formation and stabilization by mechanical entanglement or by promoting stickiness (He et al. 2020; Rillig and Mummey 2006) through the production of gelling exudates (Table 2, Fig. 2), independently of the soil organic C (Leifheit et al. 2014; Peng et al. 2013), soil texture and carbonate content (Kohler et al. 2017). In a similar fashion to plant roots, extraradical hyphae of AM fungi serve to enmesh and entangle soil particles, organic materials and small aggregates, facilitating macroaggregate (> 250 μm diameter) formation and enhancing soil structure stabilization (Morris et al. 2019; Rillig et al. 2010). For example, Degens et al. (1996) reported that increases in aggregate stability could be attributed largely to the increased growth of hyphae in a sandy soil. The stabilization of soil aggregates was apparently increased with the increasing proportion of macroaggregates when the hyphae of six different species of the genus Glomus were present in the hyphosphere (Bearden and Petersen 2000). The recent results from a two-compartment system showed that greater hyphal length contributed to the formation and stability of soil water-stable macroaggregates under both well-watered and drought stress conditions (Ji et al. 2019).
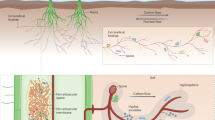
The AM hyphae-mediated physical, chemical and biological changes and nutrient cycling in the hyphosphere. The lines with different colors on the left depict physical, chemical and biological processes at the hyphae-soil interface. Physical processes (red line) include soil aggregate formation and stabilization, physical weathering, acquisition, utilization and redistribution of soil water. Chemical processes (blue line) involve metal cation adsorption, nutrient depletion, acidification and oxidation–reduction. Biological processes (green line) include exudates, enzymes, small peptides and microorganisms. The cylinder in the centre depicts the hyphae and contains information on the internal carbon, nitrogen and phosphorus cycling mediated by transporters, permeases and metabolic pathways in the extraradical hyphae. The four zones on the right depict carbon, nitrogen and phosphorus cycling and their subsequent environmental consequences in the hyphosphere mediated by exudates of extraradical hyphae. These mycelial exudates contain low-molecular-weight compounds, such as glucose, fructose, oligosaccharides and amino acids, and they could serve as a C sources substrate or signalling molecules to stimulate growth and activity of AM fungi-associated bacteria. These bacteria are involved in decomposing organic matter through decomposing enzymes, and consequently release CO2. The N cycle in the hyphosphere is primarily controlled by microorganisms (saprotrophic fungi, bacteria, protists, etc.), and they are involved in transformation of inorganic and organic N forms by different pathways. The AM fungi-associated bacteria stimulated by mycelial exudates secrete phosphatase and organic acid anions to mobilize sparingly soluble inorganic and organic P, and consequently promote plant P uptake
Some compounds (e.g. glomalin or glomalin-related soil proteins (GRSP), mucilage, polysaccharides, etc.) released by extraradical mycelia of AM fungi are involved in soil aggregate formation and stabilization (Rillig 2004; Rillig and Mummey 2006; Rillig et al. 2001; Singh et al. 2013) (Fig. 2). Although these compounds are not uniquely produced by AM fungi, they are regarded as “glue” tightly binding to soil particles and play a dominant role in converting clay mineral to structured aggregations. For example, exudation of glomalin (a specific immunoreactive glycoprotein secreted into the soil by hyphae and spores of AM fungi, Wright and Upadhyaya 1998) or GRSP correlated positively with soil aggregate stability in natural systems. A recent review suggested that glomalin is a 60 kDa heat shock protein (HSP60), both AM fungi and other soil organisms can produce HSP60 (Irving et al. 2021). Path model analysis showed that the easily extractable GRSP and total GRSP exhibited significant positive effects on mean weight diameter of water-stable aggregates (Ji et al. 2019). Additionally, total GRSP content and the proportion of soil water-stable macroaggregates > 5 mm in diameter were significantly increased in an experimental hyphal compartment under both well-watered and drought stress conditions (Ji et al. 2019). In brief, GRSP can improve aggregate formation and enhance soil stability by binding to soil particles.
Mineral weathering by AM hyphae
AM fungal hyphae can play an active role in parent mineral weathering (Koele et al. 2014; Quirk et al. 2012; Thorley et al. 2015; Verbruggen et al. 2021) (Table 2, Fig. 2). Fossil evidence for biotite etching and weathering by AM fungal hyphae in the Miocene (5–23 Mya) was found (Sanz-Montero and Rodríguez-Aranda 2012). Moreover, laboratory experiments have also demonstrated that inoculation with AM fungi accelerated biotite weathering and formation of low-potassium clay minerals (Arocena et al. 2012).
The mechanisms of mineral weathering by AM hyphae are diverse (Verbruggen et al. 2021). On the one hand, they can trace the contours of particle surfaces or strongly adhere to mineral surfaces and exert forces using high turgor pressures, subsequently entering pores and fissures in minerals (Thorley et al. 2015) and acting as efficient sinks for ions released by the mineral weathering (Quirk et al. 2015). On the other hand, AM hyphae may also form weathering trenches in silicate mineral surfaces buried in proximity to roots of trees (Quirk et al. 2012, 2014). However, hyphal tunnelling appears to be slow and contributes only modestly to weathering, accounting for around 2% of total feldspar weathering in a temperate coniferous forest (Smits et al. 2005). Additionally, GRSP secreted by extraradical mycelia of AM fungi (Wright and Upadhyaya 1998) may play important roles in mineral weathering by increasing the amount of soil aggregates, or by enhancing biological and chemical weathering of small soil particles.
AM fungal mycelium mediated redistribution of soil water
Arbuscular mycorrhizal fungi are well known for improving water acquisition and utilization by plants, enhancing drought tolerance of plants through extension of root growth and greater hyphal water absorption rate (Allen 2007). They therefore play a major role in water redistribution processes in the soil–plant system (Table 2, Fig. 2). The external hyphae can also directly absorb and transport water to their host plants from the soil (and even from the bulk soil beyond the influence of roots by extension of the extraradical mycelium) via penetration into soil pores and enlargement of the absorptive surface area (Allen 2007; Faber et al. 1991; Querejeta 2017), although contribution from extraradical mycelium to plant water metabolism is relatively smaller than roots (Hallett et al. 2009; Khalvati et al. 2005; Püschel et al. 2020; Ruiz-Lozano and Azcon 1995). Using mycorrhiza-resistant mutants and wild type plants, Bitterlich et al. (2018) found that arbuscular mycorrhiza improved hydraulic conductivity of the substrate (with root excluded) in the range of plant-relevant water availabilities.
Hyphosphere: chemical processes
Precipitation-dissolution and adsorption–desorption
In neutral or alkaline soils, P ions precipitate as calcium phosphates, whereas Fe and Al have a high capacity to combine with P ions under acidic conditions. The dissolution of these types of P minerals depends on the soil pH and anion exchange reactions. Decreased soil pH is associated with increased solubility of calcium phosphates, whereas soil alkalinization results in Fe–P and Al-P solubilization (Hinsinger 2001). Mycorrhizal hyphae are known to change the pH of the hyphosphere and thus influence P availability. By contrast, there is no convincing evidence to demonstrate that AM fungi have a capacity to mobilize sparingly soluble phosphates by releasing organic ligands or anions to compete with P ions for adsorption on the soil exchange complex.
Arbuscular mycorrhizal hyphae are implicated in detoxification of metals and have strong metal adsorption capacity relative to other microorganisms (Table 2, Fig. 2). The competition between Cd2+, Ca2+ and Zn2+ ions for adsorption sites on AM hyphae seems to favour Cd2+ over Ca2+ and Zn2+ (Joner et al. 2000). This may be due to the presence of cysteine ligands in AM fungi, forming a class of "metallothionein" binding substances. In addition to these organic ligands, metals were also adsorbed to inorganic binding sites in the cell walls of AM fungal hyphae. Some free amino acids and carboxyl, hydroxyl, phosphate and sulfhydryl groups in mycelium cell walls are all negatively charged and can adsorb metal cations, thus preventing them from entering the fungal cells (Meier et al. 2012). Another possibility is that the mycelia of AM fungi immobilize Cd and consequently reduce Cd concentrations and accumulation in shoots (Luo et al. 2017; Rask et al. 2019). In this way, AM fungi substantially reduce the harmful effects of Cd for human health. Additionally, the glomalin was incorporated in the cell wall of AM mycelia and spores. Upon release of glomalin into soil, it formed GRSP (Rillig 2004). The content of GRSP in soil was significantly positively correlated with the concentration of metals (Vodnik et al. 2008). In metal-contaminated soils, GRSP can adsorb metals and decrease their availability. This has implications for the increasing use of poor-grade rock-phosphates as fertilizer, which tend to have relatively high metal contamination. By promoting crops colonized with mycorrhizal hyphae, there is opportunity to increase the utilization of the remaining sources of rock phosphate and extending the timespan of global phosphate reserves.
Chemical weathering of parent material
Most minerals are dissolved in acidic microenvironments, and acidification is one of mechanisms underpinning mineral weathering by AM fungi and leads to the replacement of cations with protons on the mineral surface. Arbuscular mycorrhizal hyphae can release protons (Wang et al. 2013), influencing mineral weathering. Alkali cations such as Na+, K+ and Ca2+ on rock surfaces can be released via exchange with protons (Table 2).
Other mechanisms by which AM fungi stimulate weathering are chelation reactions. Some organic acid anions chelate metal ions (Fe3+, Al3+, Cu2+, Zn2+, Mn2+, etc.), significantly increasing the mobility of these elements (Landeweert et al. 2001). Arbuscular mycorrhizal hyphae can exude sugars and organic acid anions (Bharadwaj et al. 2012; Luthfiana et al. 2021; Toljander et al. 2007) that can act as chelators to destabilize mineral surfaces, increasing weathering potential. Moreover, the turnover of organic compounds, hyphae and bacteria associated with them increases respiration on mineral surfaces, which further stimulates silicate weathering (Verbruggen et al. 2021).
pH variation
It is generally recognized that acidification is one of the important mechanisms of mobilization of sparingly soluble phosphates in the rhizosphere (Andersson et al. 2015, 2016; Ding et al. 2011) or hyphosphere (Li et al. 1991b; Wang et al. 2013) in neutral-to-alkaline soils (Table 2, Fig. 2). Many studies have reported that the imbalanced uptake of anions and cations by plant roots or AM mycelia induce variation of pH in the rhizosphere (Andersson et al. 2016) or hyphosphere (Wang et al. 2013). Reduction of soil pH (by 0.2–0.5 units) was detected at both the root-soil and the AM hyphae-soil interfaces when the soil was supplied with NH4+ under axenic conditions (Bago et al. 1996; Ding et al. 2011; Li et al. 1991b; Villegas and Fortin 2001, 2002; Villegas et al. 1996; Wang et al. 2013). The fungi prefer ammonium to nitrate because of the greater costs of metabolic energy associated with absorption and assimilation of nitrate compared to ammonium (Hawkins et al. 2000). Acidification in the hyphosphere induced by NH4+ uptake enhanced mobilization of sparingly soluble inorganic phosphates through releasing phosphate ions combined with Ca2+ or mineralization of organic phosphates by increasing phosphatase activity in calcareous soils (Li et al. 1991b; Wang et al. 2013).
Redox reactions
For some metal elements with polyvalent states, such as Mn and Fe, their availability in soil usually depends on a soil oxidation–reduction potential and protons and electron-carrying reducing agents excreted by plant roots and microorganisms (Table 2, Fig. 2). The reduced forms of these elements are available to plants (Marschner 1988). Generally, oxidation reactions are mostly biological, but Mn reduction may be either biological or chemical in nature (Rengel 2000).
Depletion of oxygen in the growing medium changes the redox potential, with Mn and Fe serving as alternative electron acceptors for microbial respiration and are transformed into reduced ionic forms. This process increases the solubility and availability of Mn and Fe, but their availability is a complex variable that depends on plant genotypes, soil chemistry and microbial activity (Rengel 2015). For instance, an increase in the number of Mn-oxidizing bacteria in the wheat mycorrhizosphere affected quantity of exchangeable Mn rather than available Mn (Arines et al. 1992). Similarly, Nogueira et al. (2004) reported that stimulation of Mn-oxidizing bacteria and suppression of Mn-reducing bacteria in the rhizosphere of mycorrhizal (Claroideoglomus etunicatum or Glomus macrocarpum) soybean plants may have contributed to the lower Mn concentration in these plants. However, Nogueira et al. (2007) found the Mn-reducing bacteria were stimulated in the rhizosphere of mycorrhizal soybean plants, but Mn-oxidizing bacteria were diminished, suggesting exudation of carbohydrates from mycorrhizal roots may have been responsible for such an opposite effect. For the hyphosphere, AM fungi were found to decrease the number of Mn-reducing bacteria in the hyphal compartment (Kothari et al. 1991b; Posta et al. 1994), thereby indirectly diminishing the reducing potential and availability of Mn in the mycorrhizosphere or hyphosphere. This was thought to be the main cause of AM-colonized plants having relatively low Mn concentration (Arines et al. 1989).
The capacity of Fe-reducing bacterium Klebsiella pneumoniae strain L17 to enhance Fe(III) reduction in the mycorrhizosphere was up-regulated by exogenous application of labile C added as a pulsed input under the dual inoculation of Fe-reducing bacterium and arbuscular mycorrhizal fungus Funneliformis mosseae (Ding et al. 2014), suggesting that organic molecules can shuttle electrons from organisms to solid phase Fe(III) minerals and therefore affect Fe(III) reduction rates. Hence, the release of Fe–P was closely linked to the reduction of Fe oxide by Fe-reducing bacteria.
Hyphosphere: biochemical and biological processes
Metabolites in AM hyphal exudates
Symbioses between plants and AM fungi are based predominantly on an exchange of plant C for soil P (Genre et al. 2020; Smith and Read 2008). Isotopic tracing studies showed that AM fungi utilize 5–20% of the net C fixed by plants (Jakobsen and Rosendahl 1990; Pearson and Jakobsen 1993), and the flows of C to extraradical mycelia via roots have been determined by several studies. Two-compartment microcosms were employed to trace the C fluxes from host plant to the extraradical mycelial network of AM fungus in situ using pulse labelling by 13CO2. This demonstrated for the first time the C flux from plants to the AM mycelium-associated phosphate-solubilizing bacteria (Kaiser et al. 2015; Zhou et al. 2020), increasing the δ13C of hyphosphere soil from -22.1‰ to + 176.6‰ (Wang et al. 2016). Therefore, exudation of carbohydrates by AM fungal extraradical mycelia may be an important source of energy for microorganisms associated with hyphae (Table 2, Fig. 2).
By combining the mycorrhizal root organ culture in the split Petri dish system and proton nuclear magnetic resonance spectrometry, low-molecular-weight compounds, such as formate, acetate, glucose and oligosaccharides, were detected in mycelial exudates (Toljander et al. 2007). Similarly, exudates of Rhizoglomus irregulare extraradical mycelium contained carbohydrates, amino acids and unidentified compounds, and all these C sources could serve as a substrate to stimulate growth of AM fungi-associated bacteria (Bharadwaj et al. 2012; Luthfiana et al. 2021). In addition, fructose exuded by the extraradical hyphae of Rhizophagus irregularis may be a key signalling molecule (Zhang et al. 2018a). Even though these studies provide evidence that the metabolites detected in hyphal exudates have a positive effect on growth of mycelium-associated bacteria, the full suite of chemical forms and functions of hyphal exudates is poorly understood due to technical difficulties in collecting and quantifying the hyphal exudates (Table 3).
Activity of microorganisms
Studies on microorganisms in the hyphosphere began with microscopic inspection and revealed a number of important findings. First, soil bacteria, such as Paenibacillus brasilensis, Pseudomonas fluorescens and Bacillus cereus, were shown to be attached to AM hyphae (Artursson and Jansson 2003; Battini et al. 2016; Scheublin et al. 2010; Toljander et al. 2006) and spores (Levy et al. 2003; Roesti et al. 2005), and formed biofilms on the surface of AM hyphae (Lecomte et al. 2011). Moreover, a recent study showed that bacteria could move in a thick water film formed around extraradical hyphae of AM fungi, which suggests that AM hyphae can act as a “highway” for bacteria to move along to reach organic P patches, therefore enhancing utilization of these discrete sources of organic P (Jiang et al. 2021) (Fig. 3c, d). Second, the hyphae-associated bacteria, called mycorrhiza helper bacteria (MHB), have been shown to stimulate mycelial growth, spore germination and mycorrhization (Artursson et al. 2006; Frey-Klett et al. 2007; Xavier and Germida 2003). Third, hyphae-associated bacteria have also been shown to inhibit growth and activity of extraradical mycelium (Leigh et al. 2011; Svenningsen et al. 2018). In return, AM hyphae or hyphal exudates were demonstrated to stimulate bacterial growth and activity (Filion et al. 1999; Mansfeld-Giese et al. 2002; Toljander et al. 2007), and alter the bacterial community structure (Nuccio et al. 2013; Scheublin et al. 2010; Toljander et al. 2007). There is a clear role for AM hyphae in influencing bacterial abundance and function in the hyphosphere, but the underlying mechanisms and ecological functions of AM fungi-bacteria associations are not well understood. Most of what is known has been derived from axenic experiments, which may not correctly reflect the actual interactions in the environment and their effects on plant and/or soil biological processes. Therefore, studies on AM fungal hyphae-associated bacteria and their function and interactions in soil are critical for understanding responses of agricultural and natural systems to environmental change and are likely to become a hot research topic in the future (Table 3).
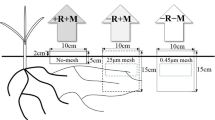
The mesh-based microcosm for investigating AM hyphae-mediated ecological functions in the hyphosphere. (a) The in-growth tubes (10 cm in diameter, 6 cm in length), sealed with 30 μm mesh (permitting AM fungal hyphae but not roots to grow into) or 0.45 μm membrane (excluding both AM fungal hyphae and roots) at the two ends, were buried near the roots (in the layer 20–30 cm deep and 15 cm away from a maize plant) in the field to study the hyphosphere interaction effects (Zhang et al. 2018b). (b) Spatial separation of soil zones for root and hyphal growth and soil zones. The chamber has five compartments, a central one for root growth (including mycorrhizal structures) separated from the two adjacent ones by 30 μm mesh. The bulk soil compartments are separated from the hyphal compartments by 0.45 μm mesh. (c, d) Schematic diagram describing the underground ‘hyphae highway’ formed by mycorrhizal network of AM fungi. The phosphate-solubilizing bacteria can quickly migrate toward an organic P patch along the hyphae highway (Jiang et al. 2021; Sun et al. 2021)
Biological cycling of elements in the hyphosphere
Nutrient cycling
Nitrogen
The extraradical hyphae of AM fungi can effectively acquire nitrate (NO3−), ammonium (NH4+) and amino acids (AAs) from soil (Bonfante and Genre 2010; Jin et al. 2012). It has been found that N uptake and transport in AM symbiosis is offset by a C flux from the plant, a similar reward mechanism to that described for P and C exchange (Fig. 2). In detail, various N sources are absorbed and transformed into arginine (Arg) by extraradical hyphae. Ammonium, nitrate and amino acid transporters and permeases in the extraradical hyphae of AM fungi participate in N absorption. The glutamine synthetase-glutamate synthase pathway (GS-GOGAT) is involved in the urea cycle and Arg biosynthesis. Arginine is then transferred from extraradical to intraradical hyphae combined with Poly P and catabolized through a urea cycle in the intraradical hyphae, releasing NH3/NH4+ into arbuscules. Because of the acidic environment in the periarbuscular space, the NH4+ ion is deprotonated prior to its transport across the plant membrane via the ammonia channels and released in its uncharged NH3 form into the plant cytoplasm (Govindarajulu et al. 2005; Jin et al. 2005, 2012).
The N cycle in soil is primarily controlled by bacteria and archaea (Rozmoš et al. 2022). Even though AM hyphae predominantly take up inorganic N as either NH4+ or NO3− (Hodge and Storer 2015; Tanaka and Yano 2005), they also have the capacity to acquire N from organic sources such as glutamate and glycine (Hawkins et al. 2000). In the presence of oxygen, NH4+ can be oxidized to NO3− through the action of ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA), whereas in the absence of oxygen, NO3− can be used by many microbes as a respiratory electron acceptor in the process known as dissimilatory nitrate reduction to NH4+ during denitrification (Canfield et al. 2010; Kuypers et al. 2018). It seems that extraradical hyphae of AM fungi could potentially compete for NH4+ and NO3− with other microorganisms involved in nitrification and denitrification (Bollmann et al. 2002). Therefore, the content of available NH4+ and NO3− in the hyphosphere is decreased; nitrification and/or denitrification may also be impaired with consequent reductions in NOx emissions from soil (Table 2, Fig. 2).
Accumulating evidence indicates that extraradical mycelia of AM fungi can obtain substantial amounts of N from decomposing organic matter and increase N capture for host plants (Hodge et al. 2001; Hodge and Fitter 2010; Leigh et al. 2009, 2011). This phenomenon likely relies on the other microorganisms (saprotrophic fungi, bacteria, protists, etc.) influenced by AM fungal hyphae (Bukovská et al. 2018; Hestrin et al. 2019; Rozmoš et al. 2022) because AM fungi have no known saprotrophic capacity (Smith and Read 2008). Studies in soil have shown that plant N uptake from a litter patch was significantly increased when AM fungi accessed the patch compartment, and 15 N analysis of the plant material indicated that 2–4% of plant N originated from litter decomposed by bacteria associated with extraradical AM hyphae (Herman et al. 2012; Leigh et al. 2011). Recent in vivo experiments with 15 N isotope tracing provided direct evidence that organic N utilization by an AM fungus is mediated by a soil bacterium Paenibacillus sp. and a protist Polysphondylium pallidum (Rozmoš et al. 2022). Further research is therefore needed to understand the mechanisms and pathways of organic N cycling in the hyphosphere (Table 3).
Free-living diazotrophs, colonizing the surface of extraradical hyphae of AM fungi, can fix N2 from the atmosphere (Shi et al. 2021), suggesting specific bacteria interacting with AM hyphae could improve the N status of host plants grown on a low-N soil. Results from a recent study featuring root- and AMF-exclusion plots around leguminous Faidherbia albida trees in smallholder fields using the 15 N natural abundance technique showed that one-third of tree-derived N in maize leaves was attributed to AMF-mediated N uptake from beyond the maize rooting zone (Dierks et al. 2022). Therefore, AM fungi quantitatively contribute to the crop uptake of legume-derived N and enhance agroecosystem functioning, which is indispensable in attaining sustainable agroecosystems.
Phosphorus
Extraradical mycelia enlarge the absorptive area of plant roots and profoundly influence biochemical properties of the hyphosphere, resulting in improved availability of the poorly mobile soil P and increased plant P uptake (Fig. 2, Fig. 3b). In addition to hyphal extension, the external hyphae of AM fungi also exude protons to mobilize sparingly soluble inorganic P by acidification of the alkaline hyphosphere soil (Li et al. 1991b; Wang et al. 2013; Yao et al. 2001). However, for the hydrolysis of organic phosphates, it must be catalyzed in the presence of phosphatases to release free orthophosphate. Previous studies using either an in situ histochemical method (van Aarle et al. 2002; Feng et al. 2002), a split-dish in vitro carrot-mycorrhiza system (Koide and Kabir 2000; Sato et al. 2015, 2019) or a compartmented pot culture (Joner and Jakobsen 1995; Joner and Johansen 2000) were conducted to demonstrate that extraradical hyphae of AM fungi could release phosphatase, moreover, transcriptome analyses suggested that AM fungi had the potential to secrete phosphatases (Liu et al. 2013; Tisserant et al. 2012), but there is still no strong evidence that AM hyphae have capacity to secrete phosphatase. Therefore, organic P mineralization, particularly of inositol phosphates (the predominant form of organic P in soils), in the AM fungal hyphosphere is likely to be driven by microbially derived phosphatases and phytases.
Recently, several important findings regarding interactions between AM fungi and phosphate-solubilizing bacteria (PSB) to promote mineralization of soil P in the hyphosphere have been reported. For example, the AM fungus Rhizophagus irregularis was shown to interact with a phosphate-solubilizing bacterium Pseudomonas alcaligenes in the hyphosphere, increasing hyphosphere soil phosphatase activity and enhancing Na-phytate mineralization (Zhang et al. 2014). The photosynthates from 13CO2-labelled maize were translocated to hyphosphere PSB via the extraradical AM hyphae, and extraradical mycelium-associated PSB enhanced mineralization and turnover of soil organic P in the hyphosphere (Wang et al. 2016).
The cooperation between AM fungi and PSB was influenced by the initial P availability and C:P ratio in the hyphosphere soil (Zhang et al. 2016). Fructose, one component of hyphal exudates, was shown to be not only a C source, but also a signal molecule to trigger mineralization of organic P mediated by a single PSB Rahnella aquatilis (Zhang et al. 2018a). In a rhizobox study in greenhouse, fructose was found to change the bacterial community by increasing the relative abundance of specific taxa (Zhang et al. 2020). However, the finding of various PSB on mycorrhizal hyphae in vitro (Taktek et al. 2015) suggest that a wide range of microorganisms found in the rhizosphere and hyphosphere may have similar function. A recent study in the field demonstrated that AM fungi and their hyphae-associated microbiome play a role in promoting mineralization of soil organic P in the hyphosphere (Zhang et al. 2018b) (Fig. 3a).
Co-inoculations with AM fungus Rhizoglomus irregulare strain QS69 and PSB belonging to the genus Pseudomonas in a two-compartment experimental system showed the potential for mobilization of inorganic and organic phosphate along with other plant growth-promoting traits (Sharma et al. 2020). Moreover, addition of fructose to the soil of hyphal compartment in a greenhouse set-up was shown to (i) act as an energy source to stimulate bacterial phosphatase activity by altering soil bacterial community structure and (ii) promote organic P mineralization, thus enhancing the utilization of P accumulated in organic forms in soils (Zhang et al. 2020). Interestingly, the extraradical hyphae may facilitate the transport of bacteria to soil nutrient patches. A phosphate-solubilizing bacterium was found to move in a water film along the AM fungal hyphae, being nourished by hyphal exudates on its way towards the phytate patch, where it extended the functional capacity of the fungus to utilize this otherwise inaccessible P source (Jansa and Hodge 2021; Jiang et al. 2021).
The interaction between AM fungi and other (non-PSB) bacteria to promote P nutrient turnover and plant uptake has also been reported. For example, extraradical hyphae of Funneliformis mosseae and Fe-reducing bacteria Klebsiella pneumoniae L17 in the hyphal chamber showed a competitive interaction in low-P soil, and a complementary, or possibly a C-dependent synergistic, function at high P availability (Zhang and Ding 2018). It is likely that P released from Ferralsols by Fe-reducing bacteria may be enhanced by additions of exogenous C.
Micronutrients
Arbuscular mycorrhizal fungi also play a crucial role in micronutrient acquisition by plants. The uptake of Cu and Zn by the external hyphae (in hyphal compartments) may account for about 53–62% of the total Cu uptake by white clover and 25% of the total Zn uptake by maize (Kothari et al. 1991a; Li et al. 1991c). Subsequently, Jansa et al. (2003) quantified the contribution of the mycorrhizal pathway for uptake of Zn by maize plants inoculated with the AM fungus Glomus intraradices (syn. Rhizophagus irregularis) using the proportion of labelled Zn taken up. More recently, Watts-Williams et al. (2015) used 65Zn to quantify the total amount of Zn and the relative contribution of Zn delivered via the mycorrhizal pathway of uptake in shoots of tomato plants inoculated with R. irregularis. Results showed that the highest relative contribution of the mycorrhizal pathway to Zn uptake was up to 24% in the low Zn concentration treatments. In more recent studies the mycorrhizal pathway of Zn accumulation in the edible portion of wheat and barley contributed up to 24% of total above-ground Zn in wheat, and up to 13% in barley. The greatest contribution by the mycorrhizal pathway was observed in barley at the lowest Zn addition, and in wheat at the highest one (Coccina et al. 2019). It is likely that the contribution by the mycorrhizal pathway to plant Zn uptake is highly dependent on the host plant species and soil Zn availability.
Mycorrhizal inoculation had a significant influence on the concentration of 59Fe in shoots of sorghum plants, suggesting extraradical AM hyphae can mobilize Fe and promote both its uptake from the hyphal compartment and translocation to the host plant (Caris et al. 1998). By contrast, a decrease in Mn uptake by mycorrhizal plants could be due to decreased uptake and transport of Mn in the external hyphae or a decline in Mn availability due to decreased abundance of Mn-reducing bacteria (Kothari et al. 1991b).
Elements such as Zn and Fe are also essential micronutrients for humans, and studies indicate that AM fungi play a positive role on improving Zn and Fe concentrations in edible parts for a variety of crops (Ercoli et al. 2017; Lehmann et al. 2014). The role of AM fungi in food security therefore goes beyond just allowing more sustainable production of crops, but also by impacting the nutritional quality of food, promoting nutritional security by improving micronutrient nutrition and health for humans through the food chain.
Cycling of C between plants and hyphosphere
As obligate biotrophs, the AM fungi are an important sink for organic C fixed by the plant through photosynthesis, with a portion of that C transferred by the AM fungus into the soil (Genre et al. 2020) (Fig. 2). In addition, AM hyphae can contribute to priming of soil organic matter mineralization (Paterson et al. 2016). Apart from stimulating litter decomposition, mycorrhizae can also stabilize litter C by reducing the abundance and activity of other soil microbes (Leifheit et al. 2015; Verbruggen et al. 2016). Nevertheless, AM hyphae may have a quick turnover, potentially acting as a rapid pathway for returning C to the atmosphere (Staddon et al. 2003). The AM hyphae not only accelerate decomposition of complex organic materials like grass litter, leading to enhanced CO2 emission, but also contribute to additional C respired back to the atmosphere (Cheng et al. 2012); this net emission of CO2 was likely due to increased C fixation by plants and transport of this C to the soil via AM fungi (Kowalchuk 2012).
Nitrous oxide emissions
Nitrous oxide (N2O), a potent greenhouse gas, is a product of a disrupted N cycle caused by incomplete denitrification in soils. Nitrification and denitrification of inorganic N (NH4+ and NO3−) feed the N cycle to produce N2O, and this process is driven by nitrifying and denitrifying bacteria (Hino et al. 2010). Recent meta-analysis has concluded that AM fungi can significantly decrease soil N2O emission (Shen and Zhu 2021) (Fig. 2). For example, a microcosm experiment demonstrated that AM fungi suppressed N2O emissions in sandy but not clay soils amended with rice straw (Zhai et al. 2021). The intense competition for inorganic N between AM fungi and nitrifying/denitrifying bacteria could decrease the availability of N substrates for N2O producers and consequently lead to a reduction in N2O emissions. In fact, AM fungi have been demonstrated to reduce N2O emissions from soil through increasing the number of copies of nosZ gene that encodes nitrous oxide reductase, the enzyme that reduces N2O to N2 (Bender et al. 2014, 2015; Kuypers et al. 2018).
Recent studies with compartmented microcosms have demonstrated that extraradical AM hyphae also decrease N2O emissions from soil. For example, Storer et al. (2018) found that production of N2O from organic patches decreased due to diminished nitrification rates in the presence of AM hyphae. Gui et al. (2021) found that extraradical AM hyphae reduced N2O emission by decreasing the abundance of key genes responsible for denitrification (nirK and nosZ).
Bacterial community composition
There is evidence in the literature that root- or hyphae-associated bacteria utilize root or hyphal exudates as easily available C and energy sources for fast growth and reproduction, contributing to an increase in the number of bacteria in the rhizosphere and hyphosphere (Toljander et al. 2007; Zhang et al. 2016) (Fig. 2). In vitro, the addition of exudates produced by AM extraradical mycelia to a bacterial community extracted from soil not only stimulated bacterial growth and activity, but also changed community composition (Toljander et al. 2007). Similarly, in a chamber microcosm in situ under both greenhouse and field conditions, the presence of AM extraradical hyphae altered the soil bacterial community (Herman et al. 2012; Nuccio et al. 2013; Wang et al. 2019; Zhang et al. 2018b). However, when only extraradical AM hyphae had access to an organic patch in a compartmented microcosm, there was a limited impact on bacterial community structure due to bacterial competition for resource (Leigh et al. 2011).
Because hyphal exudates are found in smaller quantities than root exudates (Qin et al. 2022), the influence of extraradical hyphae on bacterial community in the hyphosphere is possibly less than that of roots in the rhizosphere. Qin et al. (2022) compared the differences in P-mobilizing bacterial community between rhizosphere and hyphosphere using compartmented rhizoboxes. The mycorrhizal regulation was greater in the mycorrhizosphere than the hyphosphere, and the P-mobilizing bacterial community in the hyphosphere was not the subset of that in the mycorrhizosphere. Likewise, results of alkaline phosphatase genes (phoD) and pyrroloquinoline quinone biosynthesis gene (pqqC) expression indicated that the P-mobilizing bacterial community in the rhizosphere differed from that in the hyphosphere. Consequently, the importance of bacterial community in the hyphosphere for promoting the P uptake of host plants should be investigated further.
Many bacterial phyla, such as Proteobacteria, Actinobacteria, Firmicutes, Gemmatimonadetes, Bacteroidetes, Chloroflexi, Acidobacteria, Cyanobacteria, Planctomycetes and Fusobacteria, have been identified in the hyphosphere. The hyphal effect on soil bacterial community resulted in the enrichment of some bacterial phyla such as Proteobacteria (Emmett et al. 2021). These enriched bacteria played a positive role as mycorrhiza helper bacteria (Frey-Klett et al. 2007). Interestingly, the abundance of bacteria harbouring genes that encode enzymes involved in organic P mineralization [alkaline phosphatase (phoD) and β-propeller phytase (BPP)] increased due to the presence of extraradical AM hyphae (Wang et al. 2019; Zhang et al. 2018b).
The bacterial community composition and function were also influenced by interaction with some abiotic factors such as P forms (Wang et al. 2019) and concentrations of phytate (Zhang et al. 2018b) and fructose (Zhang et al. 2020) in the hyphosphere. Some biotic factors also influenced the hyphosphere bacterial community significantly. Using a split-root culture system and simultaneous inoculation with two different AM fungal species, Zhou et al. (2020) found that different AM fungi co-colonizing a single plant root system recruited distinct microbiomes. Emmett et al. (2021) also found that the host plant species strongly influenced the hyphosphere bacterial community. However, the mechanisms involved in the recruitment of these distinct bacterial communities by different AM fungal species and host plants are still unknown. In addition, how the interaction between AM fungi and bacterial community assemblage influenced the growth of AM fungi and eventually the host plant growth and development also warrants further studies (Table 3). Moreover, some AM fungi also harbour endobacteria that have important influence on the functions of AM fungi, which merits further research (Salvioli et al. 2016).
Future research perspectives
Although the AM hyphae are not visible to the naked eye, the biophysical, biochemical and biological reactions driven by AM fungi make the fungi-soil interface a unique critical zone affecting ecosystem functions that have global consequences. It has become clear in recent years that AM fungi, which form one of two nutrient acquiring pathways of plant in soil, not only influence the nutrient uptake efficiency of the mycorrhizal plants, but also have significant impacts on C balance and greenhouse gas emissions. However, many questions still remain about the full impact of this microscale interface harbouring highly complex ecological processes. We propose several key areas for further research (Table 3).
First, we should integrate better the research on effects of mycorrhizosphere, rhizosphere (influenced by non-AM colonized roots) and hyphosphere in mycorrhizal plants. How plants balance different strategies in these ‘spheres’ to acquire resources based on the optimized C economy is a fundamental question in biology and ecology. Therefore, an integrated approach to quantify the trade-off between physical, chemical and biological processes in both the direct and mycorrhizal uptake pathways is needed in further studies.
Second, a single plant root system is usually colonized by diverse AM species simultaneously, forming common mycorrhizal networks with overlapping hyphospheres in the plant-soil continuum in nature. Different AM fungal species showed diverse capacity to influence hyphosphere functions (such as mobilizing sparingly soluble mineral nutrients or promoting soil aggregate formation) through recruiting distinct microbiome (Zhou et al. 2020). Despite this substantial ecological footprint, we know little about some fundamental aspects of the biology, ecology and agricultural significance of the hyphal exudates driving hyphospheric biological interactions, ecological functions and their subsequent environmental consequences. We need to shed more light on:
-
(i)
The molecular mechanisms through which the fungi and biotic/abiotic environmental factors interact;
-
(ii)
How the composition of AM hyphal exudates responds to soil conditions and environmental variation;
-
(iii)
How the hyphosphere microbiome plays a role of an extended genome and impacts AM performance; and
-
(iv)
How and to what extent the fungi-microbiome-plant interactions govern the cycling of key resources in plant-soil system and therefore the environmental impact of ecosystem functions.
Third, it is widely accepted that the Glomeromycota fungi are crucial for crop production, fruit quality, plant health and soil fertility, but we know almost nothing about the biomass and nutrient demand of this agriculturally crucial taxon. It is essential and urgent to develop new methodologies to quantify the dynamics of biomass of the fungi and the content of C, N, P, etc. in their biomass. Such information will help design improved fertilizer management regimes to feed not only the crop but the fungi first.
Finally, one of the most prominent methodology innovations in the last four decades of studying mycorrhizal hyphospheric effects is the mesh-based compartmented rhizobox. By using meshes with pore diameter of 30 µm or 0.45 µm, this approach has successfully separated the zones that are influenced by mycorrhizal roots or only by mycorrhizal hyphae. In vitro culture of AM fungi together with roots or whole plant is another prominent approach that provides a way to test the effects of hyphal exudates on nutrient mobilization or microbiome in the hyphosphere. New in situ analysis methods need to be developed that can facilitate sampling, measuring or imaging at the nano- to micrometer scale. For example, integration of microfluid chip system, nanoSIMS, nanoDESI and single cell transcriptomic methods may uncover new mechanisms governing the interactions between AM hyphae and hyphosphere microbiota.
We think that the application of modern scientific techniques to these key knowledge gaps regarding the function of the hyphosphere will underpin a better understanding of the role of AM fungi in solving some of the most pressing issues that the human race currently faces, such as agricultural sustainability, climate change mitigation and adaptation, and reversing biodiversity loss.
References
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J 6:291–297
Andersson KO, Tighe MK, Guppy CN, Milham PJ, McLaren TI (2015) Incremental acidification reveals phosphorus release dynamics in alkaline vertic soils. Geoderma 259–260:35–44
Andersson KO, Tighe MK, Guppy CN, Milham PJ, McLaren TI (2016) The release of phosphorus in alkaline vertic soils as influenced by pH and by anion and cation sinks. Geoderma 264:17–27
Arines J, Porto ME, Vilarifio A (1992) Effect of manganese on vesicular-arbuscular mycorrhizal development in red clover plants and on soil Mn-oxidizing bacteria. Mycorrhiza 1:127–131
Arines J, Vilarino A, Sainz M (1989) Effect of different inocula of vesicular-arbuscular mycorrhizal fungi on manganese content and concentration in red clover (Trifolium pratense L.) plants. New Phytol 112:215–219
Arocena JM, Velde B, Robertson SJ (2012) Weathering of biotite in the presence of arbuscular mycorrhizae in selected agricultural crops. Appl Clay Sci 64:12–17
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8:1–10
Artursson V, Jansson JK (2003) Use of bromodeoxyuridine immunocapture to identify active bacteria associated with arbuscular mycorrhizal hyphae. Appl Environ Microbiol 69:6208–6215
Bago B, Vierheilig H, Piche Y, Azcon-Aguilar C (1996) Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol 133:273–280
Bai Y, Müller DB, Srinivas G, Garrido-Oter R, Potthoff E, Rott M, Dombrowski N, Münch PC, Spaepen S, Remus-Emsermann M, Hüttel B, McHardy AC, Vorholt JA, Schulze-Lefert P (2015) Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528:364–369
Battini F, Cristani C, Giovannetti M, Agnolucci M (2016) Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol Res 183:68–79
Bearden BN, Petersen L (2000) Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of a vertisol. Plant Soil 218:173–183
Bender SF, Conen F, Van der Heijden MG (2015) Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol Biochem 80:283–292
Bender SF, Plantenga F, Neftel A, Jocher M, Oberholzer HR, Kohl L, Giles M, Daniell TJ, Van der Heijden MG (2014) Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J 8:1336–1345
Besset-Manzoni Y, Rieusset L, Joly P, Comte G, Prigent-Combaret C (2018) Exploiting rhizosphere microbial cooperation for developing sustainable agriculture strategies. Environ Sci Pollut Res 25:29953–29970
Bharadwaj DP, Alström S, Lundquist PO (2012) Interactions among Glomus irregulare, arbuscular mycorrhizal spore-associated bacteria, and plant pathogens under in vitro conditions. Mycorrhiza 22:437–447
Bitterlich M, Franken P, Graefe J (2018) Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Front Plant Sci 9:301
Bollmann A, Bar-Gilissen M-J, Laanbroek HJ (2002) Growth at low ammonium concentrations and starvation response as potential factors involved in niche differentiation among ammonia-oxidizing bacteria. Appl Environ Microbiol 68:4751–4757
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun 1:48
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173:11–26
Bukovská P, Bonkowski M, Konvalinková T, Beskid O, Hujslová M, Püschel D, Řezáčová V, Gutiérrez-Núñez MS, Gryndler M, Jansa J (2018) Utilization of organic nitrogen by arbuscular mycorrhizal fungi–is there a specific role for protists and ammonia oxidizers? Mycorrhiza 28:269–283
Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of Earth’s nitrogen cycle. Science 330:192–196
Caris C, Hördt W, Hawkins H-J, Römheld V, George E (1998) Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza 8:35–39
Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
Chu Q, Zhang L, Zhou J, Yuan L, Chen F, Zhang F, Feng G, Rengel Z (2020) Soil plant-available phosphorus levels and maize genotypes determine the phosphorus acquisition efficiency and contribution of mycorrhizal pathway. Plant Soil 449:357–371
Coccina A, Cavagnaro TR, Pellegrino E, Ercoli L, McLaughlin MJ, Watts-Williams SJ (2019) The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant Biol 19:133
Cui M, Caldwell MM (1996) Facilitation of plant phosphate acquisition by arbuscular mycorrhizas from enriched soil patches. II Hyphae exploiting root-free soil. New Phytologist 133:461–467
Degens BP, Spading GP, Abbott LK (1996) Increasing the length of hyphae in a sandy soil increases the amount of water-stable aggregates. Appl Soil Ecol 3:149–159
Delaux PM, Schornack S (2021) Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 371:eaba6605
Dierks J, Blaser-Hart WJ, Gamper HA, Six J (2022) Mycorrhizal fungi-mediated uptake of tree-derived nitrogen by maize in smallholder farms. Nat Sustain 5:64–70
Ding X, Fu L, Liu C, Chen F, Hoffland E, Shen J, Zhang F, Feng G (2011) Positive feedback between acidification and organic phosphate mineralization in the rhizosphere of maize (Zea mays L.). Plant Soil 349:13–24
Ding X, Zhang S, Wang R, Liao X, Li S (2014) Exogenous labile C application enhances Fe-P utilization for mycorrhizal plants through iron-reducing bacteria in subtropical soil. J Soil Sci Plant Nutr 14:803–817
Egerton-Warburton LM, Querejeta JI, Allen MF (2008) Efflux of hydraulically lifted water from mycorrhizal fungal hyphae during imposed drought. Plant Signal Behav 3:68–71
Emmett BD, Lévesque-Tremblay V, Harrison MJ (2021) Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J 15:2276–2288
Ercoli L, Schüßler A, Arduini I, Pellegrino E (2017) Strong increase of durum wheat iron and zinc content by field-inoculation with arbuscular mycorrhizal fungi at different soil nitrogen availabilities. Plant Soil 419:153–167
Faber BA, Zasoski RJ, Munn DN, Hackel K (1991) A method for measuring hyphal nutrient and water uptake in mycorrhizal plants. Can J Bot 69:87–94
Feng G, Su Y, Li X, Wang H, Zhang F, Tang C, Rengel Z (2002) Histochemical visualization of phosphatase released by arbuscular mycorrhizal fungi in soil. J Plant Nutr 25:969–980
Filion M, St-Arnaud M, Fortin JA (1999) Direct interaction between the arbuscular mycorrhizal fungus Glomus intraradices and different rhizosphere microorganisms. New Phytol 141:525–533
Frey-Klett P, Garbaye J, Tarkka M (2007) The mycorrhiza helper bacteria revisited. New Phytol 176:22–36
Friese CF, Allen MF (1991) The spread of VA mycorrhizal fungal hyphae in the soil: Inoculum types and external hyphal architecture. Mycologia 83:409–418
Gai J, Gao W, Liu L, Chen Q, Feng G, Zhang J, Christie P, Li X (2015) Infectivity and community composition of arbuscular mycorrhizal fungi from different soil depths in intensively managed agricultural ecosystems. J Soils Sediments 15:1200–1211
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649–660
George TS, Gregory PJ, Wood M, Read D, Buresh RJ (2002) Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biol Biochem 34:1487–1497
González-Chávez MC, Carrillo-González R, Wright SF, Nichols KA (2004) The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130:317–323
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gui H, Gao Y, Wang Z, Shi L, Yan K, Xu J (2021) Arbuscular mycorrhizal fungi potentially regulate N2O emissions from agricultural soils via altered expression of denitrification genes. Sci Total Environ 774:145133
Hallett PD, Feeney DS, Bengough AG, Rillig MC, Scrimgeour CM, Young IM (2009) Disentangling the impact of AM fungi versus roots on soil structure and water transport. Plant Soil 314:183–196
Hawkins HJ, George E (2001) Reduced 15N-nitrogen transport through arbuscular mycorrhizal hyphae to Triticum aestivum L. supplied with ammonium vs. nitrate nutrition. Ann Bot 87:303–311
Hawkins HJ, Johansen A, George E (2000) Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226:275–285
He J, Chi G, Zou Y, Shu B, Wu Q, Srivastava AK, Kuča K (2020) Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl Soil Ecol 154:103592
Herman DJ, Firestone MK, Nuccio E, Hodge A (2012) Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–247
Hestrin R, Hammer EC, Mueller CW, Lehmann J (2019) Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun Biol 2:233
Hiltner L (1904) Über neuere Ehrfahrungen und Problem auf dem Gebiet der Bodenbakteriologie unter besonderer Berücksichtigung der Grundüngung und Brache. Arbeiten Der Deutsche Landwirtschaftliche Gesellschaft 98:59–78
Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y (2010) Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330:1666–1670
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci 107:13754–13759
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19
Irving TB, Alptekin B, Kleven B, Ane J-M (2021) A critical review of 25 years of glomalin research: a better mechanical understanding and robust quantification techniques are required. New Phytol 232:1572–1581
Jakobsen I, Murmann LM, Rosendahl S (2021) Hormetic responses in arbuscular mycorrhizal fungi. Soil Biol Biochem 159:108299
Jakobsen I, Rosendahl L (1990) Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol 115:77–83
Jansa J, Hodge A (2021) Swimming, gliding, or hyphal riding? On microbial migration along the arbuscular mycorrhizal hyphal highway and functional consequences thereof. New Phytol 230:14–16
Jansa J, Mozafar A, Frossard E (2003) Long-distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maize. Agronomie 23:481–488
Ji L, Tan W, Chen X (2019) Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res 185:1–8
Jiang F, Zhang L, Zhou J, George TS, Feng G (2021) Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol 230:304–315
Jin H, Liu J, Liu J, Huang X (2012) Forms of nitrogen uptake, translocation, and transfer via arbuscular mycorrhizal fungi: A review. Sci China Life Sci 55:474–482
Jin H, Pfeffer PE, Douds DD, Piotrowski E, Lammers PJ, Shachar-Hill Y (2005) The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol 168:687–696
Johansen A, Jakobsen I, Jensen ES (1992) Hyphal transport of 15N-labelled nitrogen by a vesicular-arbuscular mycorrhizal fungus and its effect on depletion of inorganic soil N. New Phytol 122:281–288
Johansen A, Jakobsen I, Jensen ES (1994) Hyphal N transport by a vesicular-arbuscular mycorrhizal fungus associated with cucumber grown at three nitrogen levels. Plant Soil 160:1–9
Joner EJ, Briones R, Leyval C (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 226:227–234
Joner EJ, Jakobsen I (1995) Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol Biochem 27:1153–1159
Joner EJ, Johansen A (2000) Phosphatase activity of external hyphae of two arbuscular mycorrhizal fungi. Mycol Res 104:81–86
Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, Solaiman ZM, Murphy DV (2015) Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol 205:1537–1551
Khalvati MA, Hu Y, Mozafar A, Schmidhalter U (2005) Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol 7:706–712
Koebernick N, Daly KR, Keyes SD, Bengough AG, Brown LK, Cooper LJ, George TS, Hallett PD, Naveed M, Raffan A, Roose T (2019) Imaging microstructure of the barley rhizosphere: particle packing and root hair influences. New Phytol 221:1878–1889
Koebernick N, Daly KR, Keyes SD, George TS, Brown LK, Raffan A, Cooper LJ, Naveed M, Bengough AG, Sinclair I, Hallett PD, Roose T (2017) High-resolution synchrotron imaging shows that root hairs influence rhizosphere soil structure formation. New Phytol 216:124–135
Koele N, Dickie IA, Blum JD, Gleason JD, Graaf L (2014) Ecological significance of mineral weathering in ectomycorrhizal and arbuscular mycorrhizal ecosystems from a field-based comparison. Soil Biol Biochem 69:63–70
Kohler J, Roldán A, Caravaca MCF (2017) Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil 410:273–281
Koide RT, Kabir Z (2000) Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol 148:511–517
Kothari SK, Marschner H, Romheld V (1990) Direct and indirect effects of VA mycorrhizal fungi and rhizosphere microorganisms on acquisition of mineral nutrients by maize (Zea mays L.) in a calcareous soil. New Phytol 116:637–645
Kothari SK, Marschner H, Romheld V (1991a) Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant Soil 131:177–185
Kothari SK, Marschner H, Romheld V (1991b) Effect of a vesicular-arbuscular mycorrhizal fungus and rhizosphere micro-organisms on manganese reduction in the rhizosphere and manganese concentrations in maize (Zea mays L.). New Phytol 117:649–655
Kowalchuk GA (2012) Bad news for soil carbon sequestration? Science 337:1049–1050
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276
Kuzyakov Y, Razavi BS (2019) Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol Biochem 135:343–360
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73:17–42
Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321:83–115
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Landeweert R, Hoffland E, Finlay RD, Kuyper WT, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Lanfranco L, Fiorilli V, Gutjahr C (2018) Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol 220:1031–1046
Lecomte J, St-Arnaud M, Hijri M (2011) Isolation and identification of soil bacteria growing at the expense of arbuscular mycorrhizal fungi. FEMS Microbiol Lett 317:43–51
Lehmann A, Veresoglou SD, Leifheit EF, Rillig MC (2014) Arbuscular mycorrhizal influence on zinc nutrition in crop plants-A meta-analysis. Soil Biol Biochem 69:123–131
Leifheit EF, Verbruggen E, Rillig MC (2015) Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol Biochem 81:323–328
Leifheit EF, Veresoglou SD, Lehmann A, Morris EK, Rillig MC (2014) Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—a meta-analysis. Plant Soil 374:523–537
Leigh J, Fitter AH, Hodge A (2011) Growth and symbiotic effectiveness of an arbuscular mycorrhizal fungus in organic matter in competition with soil bacteria. FEMS Microbiol Ecol 76:428–438
Leigh J, Hodge A, Fitter AH (2009) Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207
Levy A, Chang BJ, Abbott LK, Kuo J, Harnett G, Inglis TJJ (2003) Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl Environ Microbiol 69:6250–6256
Li X, George E, Marschner H (1991a) Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 136:41–48
Li X, George E, Marschner H (1991b) Phosphorus depletion and pH decrease at the root-soil and hyphae-soil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytol 119:397–404
Li X, Marschner H, George E (1991c) Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136:49–57
Liu Q, Parsons AJ, Xue H, Jones CS, Rasmussen S (2013) Functional characterisation and transcript analysis of an alkaline phosphatase from the arbuscular mycorrhizal fungus Funneliformis mosseae. Fungal Genet Biol 54:52–59
Luo N, Li X, Chen AY, Zhang LJ, Zhao HM, Xiang L, Cai QY, Mo CH, Wong MH, Li H (2017) Does arbuscular mycorrhizal fungus affect cadmium uptake and chemical forms in rice at different growth stages? Sci Total Environ 599–600:1564–1572
Luthfiana N, Inamura N, Tantriani ST, Saito K, Oikawa A, Chen W, Tawaraya K (2021) Metabolite profiling of the hyphal exudates of Rhizophagus clarus and Rhizophagus irregularis under phosphorus deficiency. Mycorrhiza 31:403–412
Mansfeld-Giese K, Larsen J, Bodker L (2002) Bacterial populations associated with mycelium of the arbuscular mycorrhizal fungus Glomus intraradices. FEMS Microbiol Ecol 41:133–140
Marschner H (1988) Mechanisms of manganese acquisition by roots from soils In: RD Graham, RJ Hannam, NC Uren (eds) Manganese in Soils and Plants. Kluwer Academic Publishers, The Netherlands
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Meier S, Borie F, Bolan N, Cornejo P (2012) Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit Rev Environ Sci Technol 42:741–775
Miller RM, Reinhardt DR, Jastrow JD (1995) External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103:17–23
Morris EK, Morris DJP, Vogt S, Gleber S-C, Bigalke M, Wilcke W, Rillig MC (2019) Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J 13:1639–1646
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181:950–959
Nogueira MA, Magalhães GC, Cardoso EJBN (2004) Manganese toxicity in mycorrhizal and phosphorus-fertilized soybean plants. J Plant Nutr 27:141–156
Nogueira MA, Nehls U, Hampp R, Poralla K, Cardoso EJBN (2007) Mycorrhiza and soil bacteria influence extractable iron and manganese in soil and uptake by soybean. Plant Soil 298:273–284
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–1881
Oehl F, Sieverding E, Ineichen K, Ris E-A, Boller T, Wiemken A (2005) Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol 165:273–283
Paterson E, Sim A, Davidson J, Daniell TJ (2016) Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation. Plant Soil 408:243–254
Pearson JN, Jakobsen I (1993) Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol 124:481–488
Peng S, Guo T, Liu G (2013) The effects of arbuscular mycorrhizal hyphal networks on soil aggregations of purple soil in southwest China. Soil Biol Biochem 57:411–417
Posta K, Marschner H, Romheld V (1994) Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. Mycorrhiza 5:119–124
Püschel D, Bitterlich M, Rydlová J, Jansa J (2020) Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: a Gordian knot of roots and hyphae. Mycorrhiza 30:299–313
Qin Y, Zhang W, Feng Z, Feng G, Zhu H, Yao Q (2022) Arbuscular mycorrhizal fungus differentially regulates P mobilizing bacterial community and abundance in rhizosphere and hyphosphere. Appl Soil Ecol 170:104294
Querejeta JI (2017) Soil water retention and availability as mediated by mycorrhizal symbiosis: consequences for individual plants, communities, and ecosystems. In: Johnson NC, Gehring CA, Jansa J (eds) Mycorrhizal Mediation of Soil: Fertility, Structure, and Carbon Storage. Elsevier Inc., Amsterdam, Netherlands, pp 299–317
Quirk J, Andrews MY, Leake JR, Banwart SA, Beerling DJ (2014) Ectomycorrhizal fungi and past high CO2 atmospheres enhance mineral weathering through increased below-ground carbon-energy fluxes. Biol Let 10:20140375
Quirk J, Beerling DJ, Banwart SA, Kakonyi G, Romero-Gonzalez ME, Leake JR (2012) Evolution of trees and mycorrhizal fungi intensifies silicate mineral weathering. Biol Let 8:1006–1011
Quirk J, Leake JR, Johnson DA, Taylor LL, Saccone L, Beerling DJ (2015) Constraining the role of early land plants in Palaeozoic weathering and global cooling. Proc Biol Sci 282:20151115
Rask KA, Johansen JL, Kjøller R, Ekelund F (2019) Differences in arbuscular mycorrhizal colonisation influence cadmium uptake in plants. Environ Exp Bot 162:223–229
Rengel Z (2015) Availability of Mn, Zn and Fe in the rhizosphere. J Soil Sci Plant Nutr 15:397–409
Rengel Z (2000) Uptake and transport of manganese in plants. In: Sigel A, Sigel H (eds) Metal Ions in Biological Systems. Marcel Dekker, New York
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop Pasture 60:124–143
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363
Rillig MC, Mardatin NF, Leifheit EF, Antunes PM (2010) Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol Biochem 42:1189–1191
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53
Rillig MC, Wright SF, Eviner VT (2001) The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant Soil 238:325–333
Roesti D, Ineichen K, Braissant O, Redecker D, Wiemken A, Aragno M (2005) Bacteria associated with spores of the arbuscular mycorrhizal fungi Glomus geosporum and Glomus constrictum. Appl Environ Microbiol 71:6673–6679
Rozmoš M, Bukovská P, Hršelová H, Kotianová M, Dudáš M, Gančarčíková K, Jansa J (2022) Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. ISME J 16:676–685
Ruiz-Lozano JM, Azcon R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95:472–478
Salvioli A, Ghignone S, Novero M, Navazio L, Venice F, Bagnaresi P, Bonfante P (2016) Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J 10:130–144
Sanz-Montero ME, Rodríguez-Aranda JP (2012) Endomycorrhizae in Miocene paleosols: Implications in biotite weathering and accumulation of dolomite in plant roots (SW Madrid Basin, Spain). Palaeogeogr Palaeoclimatol Palaeoecol 333–334:121–130
Sato T, Ezawa T, Cheng W, Tawaraya K (2015) Release of acid phosphatase from extraradical hyphae of arbuscular mycorrhizal fungus Rhizophagusclarus. Soil Sci Plant Nutr 61:269–274
Sato T, Hachiya S, Inamura N, Ezawa T, Cheng W, Tawaraya K (2019) Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza 29:599–605
Scheublin TR, Sanders IR, Keel C, van der Meer JR (2010) Characterisation of microbial communities colonising the hyphal surfaces of arbuscular mycorrhizal fungi. ISME J 4:752–763
Sharma S, Compant S, Ballhausen M-B, Ruppel S, Franken P (2020) The interaction between Rhizoglomus irregulare and hyphae attached phosphate solubilizing bacteria increases plant biomass of Solanum lycopersicum. Microbiol Res 240:126556
Shen Y, Zhu B (2021) Arbuscular mycorrhizal fungi reduce soil nitrous oxide emission. Geoderma 402:115179
Shi J, Zhang L, Jiang F, Wang X, Feng G (2021) Dual functions of bacteria colonized on am fungal hyphae-fixing N2 and solubilizing phosphate. Acta Pedol Sin 58:1289–1298
Singh PK, Singh M, Tripathi BN (2013) Glomalin: an arbuscular mycorrhizal fungal soil protein. Protoplasma 250:663–669
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057
Smith SE, Read D (2008) Mycorrhizal Symbiosis. Third Edition edn. Academic Press, London, UK
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
Smits MM, Hoffland E, Jongmans AG, van Breemen N (2005) Contribution of mineral tunneling to total feldspar weathering. Geoderma 125:59–69
Song Y, Li X, Feng G, Zhang F, Peter C (2000) Rapid assessment of acid phosphatase activity in the mycorrhizosphere and in arbuscular mycorrhizal fungal hyphae. Chin Sci Bull 45:1187–1190
Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH (2003) Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science 300:1138–1140
Storer K, Coggan A, Ineson P, Hodge A (2018) Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol 220:1285–1295
Sun N, Jiang F, Zhang L, Feng G (2021) Hyphal exudates of an arbuscular mycorrhizal fungus Rhizophagus irregularis induce phosphate-solubilizing bacterium Rahnella aquatilis to swim towards its hyphae. Chin Sci Bull 66:4157–4168
Svenningsen NB, Watts-Williams SJ, Joner EJ, Battini F, Efthymiou A, Cruz-Paredes C, Nybroe O, Jakobsen I (2018) Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J 12:1296–1307
Taktek S, Trépanier M, Servin PM, St-Arnaud M, Yves PJ, Fortin J-A, Antoun H (2015) Trapping of phosphate solubilizing bacteria on hyphae of the arbuscular mycorrhizal fungus Rhizophagus irregularis DAOM 197198. Soil Biol Biochem 90:1–9
Tanaka Y, Yano K (2005) Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant, Cell Environ 28:1247–1254
Thorley RMS, Taylor LL, Banwart SA, Leake JR, Beerling DJ (2015) The role of forest trees and their mycorrhizal fungi in carbonate rock weathering and its significance for global carbon cycling. Plant Cell Environ 38:1947–1961
Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A, Croll D, Silva CD, Gomez SK, Koul R, Ferrol N, Fiorilli V, Formey D, Franken P, Helber N, Hijri M, Lanfranco L, Lindquist E, Liu Y, Malbreil M, Morin E, Poulain J, Shapiro H, Tuinen D, Waschke A, Azcón-Aguilar C, Bécard G, Bonfante P, Harrison MJ, Küster H, Lammers P, Paszkowski U, Requena N, Rensing SA, Roux C, Sanders IR, Shachar-Hill Y, Tuskan G, Young JPW, Gianinazzi-Pearson V, Martin F (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytologist 193:755–769
Tobar R, Azcon R, Barea JM (1994) Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol 126:119–122
Toljander JF, Artursson V, Paul LR, Jansson JK, Finlay RD (2006) Attachment of different soil bacteria to arbuscular mycorrhizal fungal extraradical hyphae is determined by hyphal vitality and fungal species. FEMS Microbiol Lett 254:34–40
Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD (2007) Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol Ecol 61:295–304
Toussaint JP, St-Arnaud M, Charest C (2004) Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can J Microbiol 50:251–260
van Aarle IM, Rouhier H, Saito M (2002) Phosphatase activities of arbuscular mycorrhizal intraradical and extraradical mycelium, and their relation to phosphorus availability. Mycol Res 106:1224–1229
van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423
Verbruggen E, Jansa J, Hammer EC, Rillig MC (2016) Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil. J Ecol 104:261–269
Verbruggen E, Struyf E, Vicca S (2021) Can arbuscular mycorrhizal fungi speed up carbon sequestration by enhanced weathering? Plants People Planet 3:445–453
Villegas J, Fortin JA (2001) Phosphorus solubilization and pH changes as a result of the interactions between soil bacteria and arbuscular mycorrhizal fungi on a medium containing NH4+ as nitrogen source. Can J Bot 79:865–870
Villegas J, Fortin JA (2002) Phosphorus solubilization and pH changes as a result of the interactions between soil bacteria and arbuscular mycorrhizal fungi on a medium containing NO3- as nitrogen source. Can J Bot 80:571–576
Villegas J, Williams RD, Nantais L, Archambault J, Fortin JA (1996) Effects of N source on pH and nutrient exchange of extramatrical mycelium in a mycorrhizal Ri T-DNA transformed root system. Mycorrhiza 6:247–251
Vodnik D, Grčman H, Maček I, van Elteren JT, Kovačevič M (2008) The contribution of glomalin-related soil protein to Pb and Zn sequestration in polluted soil. Sci Total Environ 392:130–136
Wang F, Jiang R, Kertesz MA, Zhang F, Feng G (2013) Arbuscular mycorrhizal fungal hyphae mediating acidification can promote phytate mineralization in the hyphosphere of maize (Zea mays L.). Soil Biol Biochem 65:69–74
Wang F, Kertesz MA, Feng G (2019) Phosphorus forms affect the hyphosphere bacterial community involved in soil organic phosphorus turnover. Mycorrhiza 29:351–362
Wang F, Shi N, Jiang R, Zhang F, Feng G (2016) In situ stable isotope probing of phosphate-solubilizing bacteria in the hyphosphere. J Exp Bot 67:1689–1701
Watts-Williams SJ, Smith FA, McLaughlin MJ, Patti AF, Cavagnaro TR (2015) How important is the mycorrhizal pathway for plant Zn uptake? Plant Soil 390:157–166
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107
Xavier LJC, Germida JJ (2003) Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol Biochem 35:471–478
Yao Q, Li X, Feng G, Christie P (2001) Mobilization of sparingly soluble inorganic phosphates by the external mycelium of an abuscular mycorrhizal fungus. Plant Soil 230:279–285
York LM, Carminati A, Mooney SJ, Ritz K, Bennett MJ (2016) The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. J Exp Bot 67:3629–3643
Zhai S, Wu Y, Xu C, Chen W, Feng J, Zheng Q, Meng Y, Yang H (2021) Symbiotic soil fungi suppress N2O emissions but facilitate nitrogen remobilization to grains in sandy but not clay soils under organic amendments. Appl Soil Ecol 167:104012
Zhang F, Shen J, Zhang J, Zuo Y, Li L, Chen X (2010) Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: implications for China. Adv Agron 107:1–32
Zhang L, Chu Q, Zhou J, Rengel Z, Feng G (2021) Soil phosphorus availability determines the preference for direct or mycorrhizal phosphorus uptake pathway in maize. Geoderma 403:115261
Zhang L, Fan J, Ding X, He X, Zhang F, Feng G (2014) Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol Biochem 74:177–183
Zhang L, Feng G, Declerck S (2018a) Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J 12:2339–2351
Zhang L, Peng Y, Zhou J, George TS, Feng G (2020) Addition of fructose to the maize hyphosphere increases phosphatase activity by changing bacterial community structure. Soil Biol Biochem 142:107724
Zhang L, Shi N, Fan J, Wang F, George TS, Feng G (2018b) Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ Microbiol 20:2639–2651
Zhang L, Xu M, Liu Y, Zhang F, Hodge A, Feng G (2016) Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol 210:1022–1032
Zhang S, Ding X (2018) The competitive vs. complementary bacteria-fungi interactions promote microbial release of Fe(III)-fixed phosphorus: the roles of exogenous C application. J Plant Nutr Soil Sci 181:566–574
Zhou J, Chai X, Zhang L, George TS, Wang F, Feng G (2020) Different arbuscular mycorrhizal fungi cocolonizing on a single plant root system recruit distinct microbiomes. mSystems 5:e00929–00920
Acknowledgements
This study was funded by the National Key Research and Development Program of China (2021YFD190090104), the National Natural Science Foundation of China (U1703232, 31872184) and the Natural Science Foundation of Henan Province (222300420044). TS George contribution through The James Hutton Institute was supported by funds from the Rural and Environment Science and Analytical Services Division of the Scottish Government.
Funding
This study was funded by the National Key Research and Development Program of China (2021YFD190090104), the National Natural Science Foundation of China (U1703232, 31872184) and the Natural Science Foundation of Henan Province (222300420044). TS George contribution through The James Hutton Institute was supported by funds from the Rural and Environment Science and Analytical Services Division of the Scottish Government.
Author information
Authors and Affiliations
Contributions
GF, FW and LZ planned and designed the framework of this review. All authors performed literature search, analysis and writing. The first draft of the manuscript was written by FW and GF, and all authors wrote and commented on successive versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Ismail Cakmak.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Zhang, L., Zhou, J. et al. Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant Soil 481, 1–22 (2022). https://doi.org/10.1007/s11104-022-05621-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05621-z