Abstract
Aims
Alpine ecosystems are important terrestrial carbon (C) pools, and microbial decomposers play a key role in cycling soil C. Microbial metabolic limitations in these ecosystems, however, have rarely been studied. The objectives of this study are to reveal the characteristics of microbial nutrient limitation, and decipher the drivers in the alpine ecosystems.
Methods
Models of extracellular enzymatic stoichiometry were applied to examine and compare the metabolic limitations of the microbial communities in bulk and rhizosphere soils along an altitudinal gradient (2800–3500 m a.s.l.) under the same type of vegetation (Abies fabri) on Gongga Mountain, eastern Tibetan Plateau.
Results
The soil microbial communities suffered from relative C and phosphorus (P) limitations in the alpine ecosystem despite of high soil nutrient contents here. Partial least squares path modelling (PLS-PM) revealed that the limitations were directly regulated by soil nutrient stoichiometry, followed by nutrient availability. The C and P limitations were higher at the high altitudes (3000–3500 m) than that at the low altitude (2800 m), which mainly attribute to changes of soil temperature and moisture along the altitudinal gradient. This suggested that global warming may relieve microbial metabolic limitation in the alpine ecosystems, and then is conducive to the retention of organic C in soil. Furthermore, the C and P limitations varied significantly between the bulk and rhizosphere soils at the high altitudes (3200–3500 m), but not at the low altitudes. This indicated the influences of vegetation on the microbial metabolisms, while the influences could decrease under the scenario of global warming.
Conclusions
Our study suggests that the alpine ecosystems with high organic C storage harbour abundant microbial populations limited by relative C and P, which have sensitive metabolic characteristics. This could thus potentially lead to large fluctuations in the soil C turnover under climate change. The study provides important insights linking microbial metabolisms to the environmental gradients, and improves our understanding of C cycling in alpine ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alpine ecosystems provide various ecological services and are important pools of terrestrial carbon (C) (Chen et al. 2016; Li et al. 2018). Microbial decomposers play a key role in global C and nutrient cycles by the degradation and mineralization of soil organic matter (Manzoni 2017). Climate (e.g., temperature), vegetation, and soil properties in alpine ecosystem vary drastically over short distances and along altitudinal gradients (Mccain 2007). A growing number of studies have concluded that the changes of soil properties predominantly altered microbial activities and distributional patterns in soils (Shen et al. 2013; Yang et al. 2014; Chen et al. 2017; Li et al. 2018). Microbial decomposers consequently could strongly regulate the cycling of soil C in alpine ecosystems (Wang et al. 2016). Therefore, a better understanding of nutrient acquisition and metabolic characteristics of microbes along an environmental gradient would help to elucidate the mechanisms of C cycling in alpine ecosystems, which would improve our ability to predict the dynamics of C pools under a changing climate.
The rhizosphere, as a hotspot of microbial activity, plays a vital role in microbial metabolisms and functions because of frequent microbial-plant interactions and active nutrient flows (Kuzyakov et al. 2007; Ge et al. 2017; Cui et al. 2018; Zhang et al. 2019). Bulk soils generally have relatively oligotrophic conditions, with low rates of nutrient transformation and microbial activities compared to the active rhizospheric environment (Ai et al. 2012). The structures of microbial communities in rhizosphere also differ considerably from those in non-rhizosphere due to the strong selectivity of the root systems (Kuzyakov et al. 2007; Kielak et al. 2010; Ai et al. 2012), which may markedly affect microbial metabolic patterns. Most studies in alpine ecosystems, however, have focused on microbial communities in bulk soils (e.g., Shen et al. 2013; Wang et al. 2016; Chen et al. 2017; Li et al. 2018). Further, microbial communities in alpine ecosystems have special habitats, such as large diurnal temperature differences and undisturbed native vegetation, compared to communities in other ecosystems (Ai et al. 20,012; Tkacz et al. 2015). These differences suggest distinct variations in the metabolisms of microbial communities between bulk and rhizosphere soils, and the responses of microbial communities to the rhizospheric conditions in alpine ecosystems may differ greatly from the responses in other ecosystems. Our previous study suggested that the low environmental temperature and high nutrient contents lead to the similar microbial communities between the rhizosphere and bulk soils in the alpine ecosystems (Cui et al. 2019b). Identifying the metabolic characteristics of microbial communities in the rhizospheric microenvironment is consequently vital for our understanding the roles of root systems in the cycles of soil C in alpine ecosystems.
Soil microorganisms acquire C and nutrients mainly by decomposing organic matter. Extracellular enzymes are key participants in microbial metabolisms and soil organic matter decomposition (Sinsabaugh et al. 2009; Duan et al. 2018; Cui et al. 2019a). Ecoenzymatic stoichiometry can determine the relationships between microbial nutrient demands and soil nutrient supplies (Sinsabaugh et al. 2009; Sinsabaugh and Shah 2012) and can thus be an indicator of the ability of microorganisms to acquire nutrients. Ecoenzymatic stoichiometry is also generally used to assess the characteristics of soil nutrient limitation of microbial metabolism represented by C, nitrogen (N) or P (Sinsabaugh et al. 2009; Tapia-Torres et al. 2015; Cui et al. 2018, 2019a). Carbon from decomposed compounds is partly used for the growth of new cells and partly respired for energy production (Manzoni 2017). The partitioning of C between these anabolic and catabolic processes affects the rate of C accumulation in soils, because C allocated to microbial growth may remain in the system, whereas respired C is lost (Sinsabaugh et al. 2013). Consequently, the microbial metabolic limitation could also greatly mediate microbial C use efficiency, and then affect soil C sequestration.
Moorhead et al. (2016) proposed calculating the ‘length’ and ‘angle’ of vectors in a plot of proportional activities of enzymatic C:N versus C:P acquisition to quantify the relative investments in C vs nutrient acquisition (vector length) or P vs N acquisition (vector angle) for illustrating the characteristics of microbial metabolism. Converting these ratios into vector lengths and angles identified the simultaneous, relative resource demands of the communities, independent of variations in total enzymatic activity, and provided clear metrics of relative C limitation (length) and relative P vs N limitation (angle) (Moorhead et al. 2016). Therefore, the application of this method is conducive to understand microbial metabolic characteristics in alpine ecosystems and reveal the relationships of microbial metabolic limitation with environmental variables under in the context of global climate change.
Gongga Mountain is the highest mountain on the eastern Tibetan Plateau and features a large altitudinal range, undisturbed native vegetation, and complex climatic conditions (He and Tang 2008; Wu et al. 2013; Bing et al. 2016a). Soil properties on Gongga Mountain vary dramatically along the altitudinal gradient due to different soil development time and local climate conditions, although the parent materials are generally uniform (Wu et al. 2013). As a result, this area provides a unique natural laboratory for both identifying microbial metabolic characteristics in alpine ecosystems and assessing potential responses of microbial metabolism to climate change. In this study, we investigated the nutrient limitation of microbial communities in the bulk and rhizosphere soils from the same type of vegetation (Abies fabri) along an altitudinal gradient from 2800 to 3500 m a.s.l. on the eastern slope of Gongga Mountain. We expected to observe distinct characteristics of microbial metabolisms along the varied environmental gradient in this alpine ecosystem. We hypothesized that the microbial C metabolism could be more limited in high altitudes than that in low altitudes due to low temperature in high altitude regions. Similarly, the N and P metabolisms would be more limited at rhizosphere than that at bulk soils because of the rhizodeposition as well as the N and P competition between plants and microbes. The main objectives of this study are therefore to (1) determine the characteristics of microbial metabolic limitation along the altitudinal gradient, (2) reveal the variation of microbial metabolic limitation between the bulk and rhizosphere soils, and (3) decipher the driving factors of microbial metabolic limitation in the alpine ecosystem.
Materials and methods
Study area and samples collection
The study area is in the Hailuogou catchment of Gongga Mountain (29°30′-30°20′N, 101°30′-102°15′E: 2800–3500 m a.s.l.) (Figure S1). Gongga Mountain is located in the transition zone between the Tibetan Plateau Frigid Zone and the Warm-humid Subtropic Monsoon Zone. The climate in the area is mainly controlled by the Asian monsoon. Mean annual temperature (MAT) and precipitation are 4.2 °C and 1947 mm, respectively (Wu et al. 2013). The soils are mainly developed from glacial debris and colluvial deposits derived from weathered Cenozoic feldspar granite and Permian quartz schist. The specific types of soil and vegetation with altitude have been described in detail elsewhere (Bing et al. 2016b).
Soil samples were collected in the subalpine coniferous forests at four altitudes (2800, 3000, 3200, and 3500 m a.s.l.) where A. fabri is the dominant plant species. Three 10 × 10 m plots were established at each altitude in October 2017. The distance between the plots was >15 m, 10–12 bulk and rhizosphere soils were respectively collected from each plot, and then mixed into a composite sample as one replicate. Specifically, 10–12 fibrous roots of A. fabri were excavated at each plot. The bulk soils not directly attached to the roots was collected by removing several roots and gently shaking them to release the soils. The rhizosphere soils tightly adhered to the root surface was then physically brushed with a sterile soft-bristled paintbrush (Garcia et al. 2005). Each composite sample was divided into two subsamples. One subsample was immediately placed in an ice box, transported to the laboratory, and then stored at 4 °C for the analysis of enzymatic activity within two weeks. The other subsample was passed through a 2-mm sieve and air-dried for physicochemical analysis.
Soil physiochemical analysis
Soil-moisture content was determined by oven-drying 10 g of fresh soil at 105 °C for 48 h. Soil pH was measured in a 1:2.5 soil:water (w/v) mixture using a glass-electrode meter (InsMark™ IS126, Shanghai, China). Soil organic C (SOC) content was determined using dichromate oxidation; approximately 0.10 g of air-dried soils was digested with 5 ml of 0.8 M K2Cr2O7 and 5 ml of H2SO4 at 170–180 °C for 5 min, and the digestate was then titrated with 0.2 M FeSO4. The experimentally derived conversion factors were 1.724. Dissolved organic C (DOC) was extracted with 0.5 M K2SO4 and shaken for 60 min at 200 rpm on a reciprocal shaker. The extracts were filtered through a Millipore 0.45-μm filter, and then measured using a Liqui TOCII analyzer (Elementar, Germany) (Jones and Willett 2006). Total N (TN) content was measured by the Kjeldahl method using the Kjeltec 8400 (FOSS, Denmark) (Bremner and Mulvaney 1982). Briefly, approximately 0.70 g of air-dried soil was digested with 1.85 g mixed catalyst (K2SO4:CuSO4:Se = 100:10:1) and 5 ml of H2SO4 at 385 °C for 45 min, and the digestate was then titrated with 0.02 M HCl. The contents of NO3−-N and NH4+-N were measured using a Seal Auto Analyzer after extraction with 2 M KCl with a 1:5 ratio. Total P (TP) and available P (Olsen-P) were extracted with H2SO4-HClO4 and 0.5 M NaHCO3 (Olsen and Sommers 1982), respectively. The extracts of Olsen-P were filtered through a Millipore 0.45-μm filter. The contents of TP and Olsen-P were then determined by the molybdenum blue method using an ultraviolet spectrophotometer (Hitachi UV2300).
Assays of extracellular enzyme activities
The potential activities of two extracellular C-acquiring enzymes (β-1,4-glucosidase (BG) and β-D-cellobiosidase (CBH)), two extracellular N-acquiring enzymes (β-1,4-N-acetylglucosaminidase (NAG) and L-leucine aminopeptidase (LAP)), and one extracellular organic P-acquiring enzyme (acid phosphatase (AP)) were determined using the method of Saiya-Cork et al. (2002). The extracellular enzyme activities were measured fluorometrically using a 200-μM solution of substrates labelled with 4-methylumbelliferone (MUB) or 7-amino-4-methylcoumarin (AMC). Fifty microlitres of 50 mM buffer were pipetted into wells of black 96-well microplates to serve as blanks (buffer + slurry) and 200 μl of 50 mM buffer were pipetted into wells as the reference standard (buffer + standard) and negative control ((buffer + substrate) (eight analytical replicates per soil per assay). One gram of fresh soil was homogenized in 125 ml of 50 mM buffer on a constant-temperature shaker for 2 h. The soil suspension (slurry) was continuously stirred as 200-μl aliquots were dispensed into the microplate wells that served as the sample assay (16 analytical replicate suspensions for each sample per assay) and as the blank and quench standard (slurry + standard) (eight analytical replicates each). Twenty-five microlitres of a fluorescence standard solution (10 μM 4-methylumbelliferone-MUB or 7-amino-4-methylcoumarin-AMC for the LAP assay) were dispensed into microplate wells that served as a reference standard (buffer + standard) and as a quench standard. Finally, the sample assays (slurry + substrate) and negative controls (buffer + substrate) also received 25 μl of a 200-μM substrate solution in a final reaction volume of 125 μl. Prepared plates were incubated at 25 °C in the dark for up to 4 h following substrate addition. Fluorescence was measured without addition of NaOH using a microplate reader with 365-nm excitation and 450-nm emission filters (German et al. 2011). The measurements of well fluorescence for the negative controls, blanks, and quench standards were corrected, and the enzymatic activity was expressed as nanomoles of substrate released per hour per gram of soil organic matter (SOM) (nmol g SOM−1 h−1, SOM = 1.724 × SOC).
Quantification of microbial metabolic limitation
The microbial metabolic limitation was quantified by calculating the vector lengths and angles of enzymatic activity for all data based on untransformed proportional activities (e.g. [BG + CBH]/[BG + CBH + NAG + LAP]). Vector length, representing C limitation, was calculated as the square root of the sum of x2 and y2 (Eq. 1), where x represents the relative activity of C- versus P-acquiring enzymes, and y represents the relative activity of C- versus N-acquiring enzymes (Moorhead et al. 2013, 2016). Vector angle, representing N or P limitation, was calculated as the arctangent of the line extending from the plot origin to point (x, y) (Eq. 2). Microbial C limitation increases with the vector length. Vector angles >45° represent microbial P limitation, and vector angles <45° represent N limitation. Microbial P limitation increases with the vector angle, and microbial N limitation decreases with the vector angle.
Statistical analysis
A two-way ANOVA was used to analyze the effects of altitude, location (bulk and rhizosphere soils), and their interactions on microbial metabolic limitation, and mean comparisons were then determined with Tukey’s multiple comparison test (P < 0.05). Generalized linear models were adopted to determine the relationships of the microbial metabolic limitation with environmental variables. Significant environmental variables were selected to divide into four categories (physical property, total nutrients, nutrient ratios, and available nutrients) for conducting a downstream analysis. Partial least squares path modelling (PLS-PM) was used to further identify the possible pathways by which attributes controlled microbial metabolic limitation in this alpine ecosystem. The model was constructed using the “innerplot” function in the “plspm” package. All statistical analyses were performed using the R software package v.3.3.2.
Results
Soil physiochemical properties and nutrient stoichiometry
The contents of DOC, NO4+-N and Olsen-P and the C:P ratio were significantly higher at the sites of 2800 and 3000 m a.s.l. (low altitude regions) than that at the sites of 3200 and 3500 m a.s.l. (high altitude regions) (P < 0.05; Table 1). For example, the contents of DOC (806 ± 103 mg kg−1) and NO4+-N (216 ± 10.1 mg kg−1) and the C:P ratio (467 ± 13.2) were highest in the rhizosphere soils at the site of 2800 m a.s.l. Altitude and location had significant main and interactive effects on the NO3−-N and NO4+-N contents and the C:N and C:P ratios (P < 0.05). The other physiochemical properties varied significantly between the bulk and rhizosphere soils along the altitudinal gradient (P < 0.05; Table S1). Two-way ANOVAs showed that SOC, TN, and soil-moisture contents and soil temperature were significantly higher at the sites of 2800 and 3000 m a.s.l. than that at the sites of 3200 and 3500 m a.s.l. (P < 0.05). The contents of TN, TP and soil-moisture differed significantly between the bulk and rhizosphere soils. Altitude and location had significant main and interactive effects on SOC, TP and pH (P < 0.05). Also, there were significantly correlations among the soil physicochemical properties (P < 0.05; Table S2). Particularly, the soil temperature was significantly correlated positively with SOC, DOC, TN, NH4+-N, Olsen-P, soil moisture, and nutrient ratios (P < 0.01).
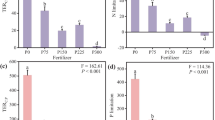
Extracellular enzyme activities (EEAs)
Altitude and location had significant main and interactive effects on the EEAs (P < 0.001; Table 2). Different from the variation patterns of soil physicochemical properties, the activities of the C-, N- and P- acquiring enzymes were significantly higher at the site of 3200 and 3500 m a.s.l. than those at the sites of 2800 and 3000 m a.s.l. (P < 0.05). For example, the activities of N-acquiring enzymes (622 ± 65.6 nmol g SOM−1 h−1) and P-acquiring enzyme (2.90 × 103 ± 255 nmol g SOM−1 h−1) were highest in the bulk soils at the site of 3200 m a.s.l. Furthermore, the soil properties (including SOC, DOC, TN, NO3−-N, NH4+-N, Olsen-P, C:N ratio, C:P ratio, soil moisture and pH) were significantly correlated with the EEAs (P < 0.05; Table S3).
Vector characteristics of extracellular enzyme stoichiometry
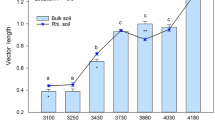
The characteristics of ecoenzymatic stoichiometry differed among altitudinal gradients and sampling location (bulk and rhizosphere) soils (Fig. 1a). All data points were above the line (1:1 line), indicating a strong P limitation in the microbial community in our study area. Altitude and location had significant main and interactive effects on the microbial metabolic characteristics (P < 0.01; Fig. 1b and c). Vector lengths (microbial C limitation) were largest and smallest for the rhizosphere soils at the site of 3200 m a.s.l. (1.12 ± 0.015) and the bulk soils at the site of 2800 m a.s.l. (0.593 ± 0.025), respectively (Fig. 1b). Vector angles were > 45°, indicating that the microbial metabolisms are limited by soil P (Fig. 1c). Altitude had a significant effect, and altitude and location had significant interactive effects, on the vector angles (microbial P limitation). Microbial P limitation was largest (69.6 ± 2.3°) for the rhizosphere soils at the site of 3500 m a.s.l. and smallest (51.6 ± 3.1°) for the bulk soils at the site of 2800 m a.s.l. Generally, the C and P limitations were higher at the high altitudes (3000–3500 m a.s.l.) than that at the low altitude (2800 m a.s.l.). Moreover, the limitations significantly varied between the bulk and rhizosphere soils at the high altitudes (3200–3500 m a.s.l.) but not at the low altitudes. In addition, the microbial C limitation was significantly correlated negatively with microbial P limitation (vector angle) in altitude 3000–3500 m a.s.l. (P < 0.01; Fig. 1d).
Extracellular enzyme stoichiometry of the relative proportions of C to N acquisition versus C to P acquisition (a), the variation of vector length and angle (b and c) and their relationships (d). a: BG, β-1,4-glucosidase; CBH, β-D-cellobiosidase; NAG, β-1,4-N-acetylglucosaminidase; LAP, L-leucine aminopeptidase; AP, alkaline phosphatase; vector length represents soil C limitation for microbes, vector angle represents soil N/P limitation for microbes. b and c: Values are the means ± standard error (n = 3). Different letters indicate significant differences (P < 0.05) amongst the altitudes within the bulk or rhizosphere soils based on two-way ANOVA followed by Tukey’s test. Asterisk indicates significant differences (P < 0.05) between the bulk soils (Bulk) and rhizosphere soils (Rhi.) at an altitude based on T test. d: Linear-regression analysis to identify the relationships of microbial C limitation with microbial N/P limitation
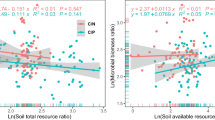
The results of linear regression analysis further showed that microbial C limitation was significantly correlated negatively with SOC, TN, DOC, NO3−-N and NH4+-N, and soil-moisture contents and the C:P and N:P ratios and positively with TP content (P < 0.05; Fig. 2). The microbial P limitation was significantly correlated negatively with TN, DOC, NO3−-N, and NH4+-N contents, the C:P and N:P ratios, and soil temperature and positively with TP content (P < 0.05; Fig. 3). Correlation analysis also showed that the microbial P limitation was significantly correlated with soil temperature (P < 0.05; Table S3).
Vector lengths in relation to total nutrient contents (a, b, c), nutrient ratios (d, e, f), available nutrient contents (g, h, i), and soil physical properties (j, k, l). Solid lines indicate the model fits between the vector lengths and the properties, and grey areas are the 95% confidence intervals of the models
Vector angles in relation to total nutrient contents (a, b, c), nutrient ratios (d, e, f), available nutrient contents (g, h, i), and soil physical properties (j, k, l). Solid lines indicate the model fits between the vector angle and the properties, and grey areas are the 95% confidence intervals of these models
The PLS-PM identified direct and indirect effects of soil physical properties, total and available nutrient contents, and nutrient ratios on the microbial C and P limitations (Fig. 4a and c). The physical properties (−0.501), total nutrient contents (−0.264) and nutrient ratios (−2.488) had negative total-effects on the microbial C limitation, while the available nutrient contents (1.024) showed positive total-effect on it (Fig. 4b). Moreover, the physical properties (−0.414) and available nutrient contents (−0.628) had negative total-effects on the microbial P limitation, while the total nutrient contents (0.638) and nutrient ratios (0.659) induced positive total-effects on it (Fig. 4d). Nutrient ratios were the major factor affecting the microbial C limitation (−2.488) and microbial P limitation (0.659).
Cascading relationships of microbial nutrient limitation with soil physicochemical properties. Partial least squares path modelling disentangling major pathways of the influences of soil physicochemical properties on microbial C limitation (represented by vector length, a and b), microbial P limitation (represented by vector angle, c and d). Blue and red arrows indicate positive and negative flows of causality (P < 0.05), respectively. Numbers on the arrow indicate significant standardized path coefficients. R2 indicates the variance of dependent variable explained by the model. Soil physicochemical properties are categorized into physical property, total and available nutrients, nutrient ratios
Discussion
Relative metabolic limitations of microorganisms in the alpine ecosystems
This study identified the relative C and P limitations on soil microbial metabolisms in the alpine ecosystem of Gongga Mountain (Fig. 1). The C and P limitations were higher at the high altitudes (3000–3500 m a.s.l.) than those at the low altitude (2800 m a.s.l.), which supported our hypothesis. Local climate such as temperature plays an important role in soil microbial activities and consequently the soil nutrient availability (Melillo et al. 2002; Mccain 2007; Grandy et al. 2007). The high altitude area features many restraining factors such as low temperature, low thermal energy and high viscosity (Jansson and Taş 2014), which may provide a relatively stable habitat and lead to low nutrient circulation. Specially, our results observed the effects of soil moisture and temperature on microbial C and P limitations (Figs. 2, 3 and 4). Soil temperature, an important driver of microbial-community structure and activity (Zhou et al. 2013; Yang et al. 2014; Zhou et al. 2016), was much lower at the high altitudes than that at the low altitudes (Table S1). The low environmental temperature thus suppresses the metabolic activity and nutrient acquisition of microorganisms at the high altitudes. In addition, the high soil moisture in our study areas inhibits the microbial activities due to the lack of oxygen (Table S1; Grandy et al. 2007), which thus affects the microbial metabolic limitation (Figs. 2 and 3).
A strong effect of root systems on microbial metabolic limitation cannot be ignored in the alpine ecosystem. Different from our previous study that found the similar microbial community structures between bulk and rhizosphere soils in the study area (Cui et al. 2019b), the present study showed that the microbial metabolic limitations differed significantly between the bulk and rhizosphere soils especially at the high altitudes (3200–3500 m a.s.l.) (Fig. 1). Previous studies have identified that microbial and vegetation communities strongly compete for soil nutrients (Kielak et al. 2010; Ai et al. 2012; Cui et al. 2018). Nutrient competition by root systems can reduce the availability of soil nutrients and impede nutrient acquisition by microorganisms. Furthermore, at the high altitudes, the relative low nutrient contents such as DOC and Olsen-P may strengthen the nutrient competition between plants and microbes (Tables 1 and 2). In addition, rhizodeposition can decrease microbial relative C limitation due to increasing C source, while increasing microbial relative P limitation (Weintraub et al. 2007). This effect could decline with altitude as rhizodeposition decreases (productivity decreases).
Soil nutrient availability and nutrient stoichiometry mediate microbial C and P limitations
Although temperature variation and rhizosphere effects can strongly affect microbial nutrient acquisition, and thus induce relative C and P limitation of microorganisms, the most direct drivers of microbial nutrient limitation remain to be identified. The levels of both soil available nutrients and total nutrients especially for C and N in the study area, however, were markedly high (Tables 1 and S1), much higher than those in arid and semi-arid regions where microbial metabolism is universally limited by C, N or P (Tapia-Torres et al. 2015; Cui et al. 2018). Why is the microbial metabolism in this alpine ecosystem limited by soil C and P even though nutrient sources are highly available? Two possible explanations may be responsible.
The unbalance of elemental stoichiometry in our study area could be a main reason for the microbial metabolic limitation due to the homeostasis of the microbial biomass (Cleveland and Liptzin 2007; Sinsabaugh et al. 2009; Cui et al. 2018). The C:P and N:P ratios were significantly correlated with microbial C and P limitations (Figs. 2 and 3), indicating the strong influence of nutrient stoichiometry on microbial nutrient acquisition. Furthermore, the results of PLS-PM showed that the soil nutrient stoichiometry explained the maximum variations of microbial nutrient limitation in our study (Fig. 4b and d). Our previous study of nutrient stoichiometry also suggested that the soil microbial communities in the coniferous forest of this mountain may be limited by P (Bing et al. 2016b). The microbial C limitation was correlated negatively with SOC and TN but positively with TP (Fig. 2), suggesting different effects of soil C, N and P on microbial nutrient limitation. The results of PLS-PM further supported that the soil total nutrient contents directly determined the soil nutrient stoichiometry, and indirectly caused the microbial metabolic limitation (Fig. 4a and c). In terms of the total-effects, the soil total nutrient contents and nutrient ratios negatively and positively affected microbial C and P limitations, respectively (Fig. 4b and d). The microbial C and P limitations are thus mostly due to the nutrient stoichiometric variation that led to the unbalanced supply of soil nutrients.
The availability of soil nutrients also regulates the microbial C and P limitations in the alpine ecosystem. Microbial nutrient demand is determined by elemental stoichiometry of microbial biomass relative to environmental nutrient availability (Sinsabaugh et al. 2009). The contents of DOC, NO3−-N, and NH4+-N were negatively related to the microbial C and P limitations (Figs. 2 and 3), suggesting the important roles of soil available nutrients in microbial nutrient acquisition. In the alpine ecosystems, the high leaching of TP and available P immobilized by iron and aluminum is commonly present in acid soils under the high precipitation and humid conditions (Haynes and Mokolobate 2001; Manzoni et al. 2010; He et al. 2018). This can lead to the microbial metabolism limited by P, which is supported by the proportion of the available nutrients to soil total nutrient on the Gongga Mountain. The proportions of available C and P to their total contents were much smaller than that of available N to TN (Tables 1 and S1). The results of PLS-PM further confirmed that the microbial C and P limitations were strongly affected by the availability of soil nutrients (Fig. 4).
Implications of microbial C and P limitations for soil C pools of the alpine ecosystems
The relative C and P limitations of microbial community play important roles in soil nutrient cycling, especially in organic C decomposition (Sinsabaugh et al. 2009; Cui et al. 2019a). The present study showed the significant correlation between microbial C and P limitations at the high altitude (P < 0.01; Fig. 1). The metabolic limitations thus could strengthen the decomposition of SOM to provide available C and P (Sinsabaugh et al. 2009), which, would stimulate the release of soil C. Our previous study suggested that microbial metabolic limitations were directly associated with the variation of SOM (Cui et al. 2019a). The earth system models also indicated that implementing N and P limitation reduced the land C sink, transforming some regional sinks into net sources over historical period (Exbrayat et al. 2013). As microbial C and P limitation increase, the metabolisms of microbial communities will shift from growth to maintenance respiration and increased investment in the production of enzymes as decomposition progresses (Manzoni et al. 2012; Luo et al. 2017). Consequently, the microbial metabolic limitations could be detrimental to the assimilation of SOC by microorganisms, and then affect soil C sequestration.
Furthermore, in the alpine ecosystem, the soil available nutrients were significantly higher at the low altitude than that at the high altitude (Table 1), whereas the microbial metabolic limitations did not show the similar patterns (Fig. 1). Also, our results showed that the microbial metabolic limitations significantly varied between the bulk and rhizosphere soils (Fig. 1), but not for microbial community structures (Cui et al. 2019b). These suggest that the microbial metabolisms be more flexible and sensitive than soil properties and community structures in the alpine ecosystems. Our previous study also revealed that the alpine ecosystem harbored many microbial taxa with diverse nutrient preferences and metabolic characteristics (Cui et al. 2019b). The variations of microbial metabolic limitation thus can not only depend on the soil nutrient availability and the rhizosphere effects, but also the responses of microbial metabolisms to climate change (Allison et al. 2010). Consequently, the process of microbial metabolic limitation with great sensitivity would potentially offset some of the C fixed by plants in the alpine ecosystems, and would increase the difficulty in precisely estimating the soil C sequestration in the context of global climate change.
Conclusions
Our study revealed that soil microbial communities suffered from relative C and P limitations in the typical alpine ecosystem of eastern Tibetan Plateau despite of high soil nutrient contents here. The limitations were directly mediated by soil nutrient stoichiometry and nutrient availability. The C and P limitations were higher at the high altitudes (3000–3500 m) than those at the low altitude (2800 m), which mainly attribute to changes of soil temperature and moisture along the altitudinal gradient. This suggested that global warming may relieve microbial metabolic limitation in the alpine ecosystems, and then is beneficial to soil C sequestration. The relative C and P limitations in microbial communities also varied significantly between bulk and rhizosphere soils at the high altitudes. This indicated the influences of vegetation on the microbial metabolisms, while the influences may be weakened under a scenario of climate warming. The study provides important insights linking microbial metabolic characteristics to the environmental gradients, and improves our understanding of soil C turnover in alpine ecosystems.
References
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173–174(2):330–338. https://doi.org/10.1016/j.geoderma.2011.07.020
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3(5):336–340. https://doi.org/10.1038/ngeo846
Bing HJ, Wu YH, Zhou J, Li R, Luo J, Yu D (2016a) Vegetation and cold trapping modulating elevation-dependent distribution of trace metals in soils of a high mountain in eastern Tibetan Plateau. Sci Rep 6:24081. https://doi.org/10.1038/srep24081
Bing HJ, Wu YH, Zhou J, Sun HY, Luo J, Wang JP, Yu D (2016b) Stoichiometric variation of carbon, nitrogen and phosphorus in soils and its implication for nutrient limitation in alpine ecosystem of Eastern Tibetan Plateau. J Soils Sediments 16:405–416. https://doi.org/10.1007/s11368-015-1200-9
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page, A.L., Miller, R.H., Keeney, D.R. (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbial Properties, pp. 595–624. Agronomy Society of America, Agronomy Monograph 9, Madison, Wisconsin
Chen LY, Liang JY, Qin SQ, Li L, Kai F, Xu YP, Ding JZ, Luo YQ, Yang YH (2016) Determinants of carbon release from the active layer and permafrost deposits on the Tibetan Plateau. Nat Commun 7:13046. https://doi.org/10.1038/ncomms13046
Chen YL, Deng Y, Ding JZ, Hu H, Xu T, Li F, Yang GB, Yang YH (2017) Distinct microbial communities in the active and permafrost layers on the Tibetan Plateau. Mol Ecol 26(23):6608–6620. https://doi.org/10.1111/mec.14396
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a "Redfield ratio" for the microbial biomass? Biogeochemistry 85(3):235–252. https://doi.org/10.1007/s10533-007-9132-0
Cui YX, Fang LC, Guo XB, Wang X, Zhang YJ, Li PF, Zhang XC (2018) Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern loess Plateau, China. Soil Biol Biochem 116:11–21. https://doi.org/10.1016/j.soilbio.2017.09.025
Cui YX, Fang LC, Deng L, Guo XB, Han F, Ju WL, Wang X, Chen HS, Tan WF, Zhang XC (2019a) Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci Total Environ 658:1440–1451. https://doi.org/10.1016/j.scitotenv.2018.12.289
Cui YX, Bing HJ, Fang LC, Wu YH, Yu JL, Shen GT, Jiang M, Wang X, Zhang XC (2019b) Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 338:118–127. https://doi.org/10.1016/j.geoderma.2018.11.047
Duan CJ, Fang LC, Yang CL, Chen WB, Cui YX, Li SQ (2018) Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol Environ Saf 156:106–115. https://doi.org/10.1016/j.ecoenv.2018.03.015
Exbrayat JF, Pitman AJ, Zhang Q, Abramowitz G, Wang YP (2013) Examining soil carbon uncertainty in a global model: response of microbial decomposition to temperature, moisture and nutrient limitation. Biogeosciences 10(11):7095–7108. https://doi.org/10.5194/bg-10-7095-2013
Garcia C, Roldan A, Hernandez T (2005) Ability of different plant species to promote microbiological processes in semiarid soil. Geoderma 124(1–2):193–202. https://doi.org/10.1016/j.geoderma.2004.04.013
Ge T, Wei X, Razavi BS, Zhu Z, Hu Y, Kuzyakov Y, Jones DL, Wu J (2017) Stability and dynamics of enzyme activity patterns in the rice rhizosphere: effects of plant growth and temperature. Soil Biol Biochem 113:108–115. https://doi.org/10.1016/j.soilbio.2017.06.005
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43(7):1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Grandy AS, Neff JC, Weintraub MN (2007) Carbon structure and enzyme activities in alpine and forest ecosystems. Soil Biol Biochem 39(11):2701–2711. https://doi.org/10.1016/j.soilbio.2007.05.009
Haynes RJ, Mokolobate MS (2001) Amelioration of al toxicity and p deficiency in acid soils by additions of organic residues: a critical review of the phenomenon and the mechanisms involved. Nutr Cycl Agroecosyst 59(1):47–63. https://doi.org/10.1023/A:1009823600950
He L, Tang Y (2008) Soil development along primary succession sequences on moraines of Hailuogou Glacier, Gongga Mountain, Sichuan, China. Catena 72(2):259–269. https://doi.org/10.1016/j.catena.2007.05.010
He XL, Zhou J, Wu YH, Bing HJ, Sun HY, Wang JP (2018) Leaching disturbed the altitudinal distribution of soil organic phosphorus in subalpine coniferous forests on Mt. Gongga, SW China. Geoderma 326:144–155. https://doi.org/10.1016/j.geoderma.2018.04.015
Jansson JK, Taş N (2014) The microbial ecology of permafrost. Nat Rev Microbiol 12(6):414–425. https://doi.org/10.1038/nrmicro3262
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38(5):991–999. https://doi.org/10.1016/j.soilbio.2005.08.012
Kielak A, Pijl AS, Veen JAV, Kowalchuk GA (2010) Differences in vegetation composition and plant species identity lead to only minor changes in soilborne microbial communities in a former arable field. FEMS Microbiol Ecol 63:372–382. https://doi.org/10.1111/j.1574-6941.2007.00428.x
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290(1–2):293–305. https://doi.org/10.1007/s11104-006-9162-8
Li JB, Shen ZH, Li CN, Kou YP, Wang YS, Tu B, Zhang SH, Li XZ (2018) Stair-step pattern of soil bacterial diversity mainly driven by pH and vegetation types along the elevational gradients of Gongga Mountain, China. Front Microbiol 9:569. https://doi.org/10.3389/fmicb.2018.00569
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35. https://doi.org/10.1016/j.soilbio.2016.12.006
Manzoni S (2017) Flexible carbon-use efficiency across litter types and during decomposition partly compensates nutrient imbalances-results from analytical stoichiometric models. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.00661
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80(1):89–106. https://doi.org/10.1890/09-0179.1
Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196(1):79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Mccain CM (2007) Could temperature and water availability drive elevational species richness patterns? A global case study for bats. Glob Ecol Biogeogr 16(1):1–13. https://doi.org/10.1111/j.1466-8238.2006.00263.x
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Morrisseau S (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298(5601):2173–2176. https://doi.org/10.1126/science.1074153
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models. Front Microbiol 4(4):223. https://doi.org/10.3389/fmicb.2013.00223
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7. https://doi.org/10.1016/j.soilbio.2015.10.019
Olsen SR, Sommers LE (1982) Phosphorous. In: Page, A.L., Miller, R.H., Keeney, D.R.(Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbial Properties, pp. 403e430. Agronomy Society of America, Agronomy Monograph 9, Madison, Wisconsin
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an acer saccharum, forest soil. Soil Biol Biochem 34(9):1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Shen CC, Xiong JB, Zhang HY, Feng YZ, Lin XG, Li XY, Liang WJ, Chu HY (2013) Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem 57(00):204–211. https://doi.org/10.1016/j.soilbio.2012.07.013
Sinsabaugh RL, Shah JJF (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43(1):313–343. https://doi.org/10.1146/annurev-ecolsys-071112-124414
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798. https://doi.org/10.1038/nature08632
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16(7):930–939. https://doi.org/10.1111/ele.12113
Tapia-Torres Y, Elser JJ, Souza V, García-Oliva F (2015) Ecoenzymatic stoichiometry at the extremes: how microbes cope in an ultra-oligotrophic desert soil. Soil Biol Biochem 87:34–42. https://doi.org/10.1016/j.soilbio.2015.04.007
Tkacz A, Cheema J, Chandra G, Grant A, Poole PS (2015) Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J 9(11):2349–2359. https://doi.org/10.1038/ismej.2015.41
Wang JP, Wu YH, Zhou J, Bing HJ, Sun HY (2016) Carbon demand drives microbial mineralization of organic phosphorus during the early stage of soil development. Biol Fertil Soils 52(6):825–839. https://doi.org/10.1007/s00374-016-1123-7
Weintraub MN, Scott-Denton LE, Monson SRK (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154(2):327–338. https://doi.org/10.1007/s00442-007-0804-1
Wu YH, Li W, Zhou J, Cao Y (2013) Temperature and precipitation variations at two meteorological stations on eastern slope of Gongga Mountain, SW China in the past two decades. J Mt Sci 10(3):370–377. https://doi.org/10.1007/s11629-013-2328-y
Yang YF, Gao Y, Wang SP, Xu DP, Yu H, Wu LW, Lin QY, Hu YG, Li XZ, He ZL, Deng Y, Zhou JZ (2014) The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J 8(2):430–440. https://doi.org/10.1038/ismej.2013.146
Zhang C, Wang J, Liu GB, Song ZL, Fang LC (2019) Impact of soil leachate on microbial biomass and diversity affected by plant diversity. Plant Soil. https://doi.org/10.1007/s11104-019-04032-x
Zhou XQ, Chen CR, Wang YF, Xu ZH, Han HY, Li LH, Wan SQ (2013) Warming and increased precipitation have differential effects on soil extracellular enzyme activities in a temperate grassland. Sci Total Environ 444(2):552–558. https://doi.org/10.1016/j.scitotenv.2012.12.023
Zhou JZ, Deng Y, Shen LN, Wen CQ, Yan QY, Ning DL, Brown JH (2016) Temperature mediates continental-scale diversity of microbes in forest soils. Nat Commun 7:12083. https://doi.org/10.1038/ncomms12083
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (41630751 and 41571314), State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, CAS (SKLLQGZR1803), CAS “Light of West China” Program (XAB2016A03), and State Key Research & Development Plan Project (2017YFC0504504).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yunfeng Yang.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
The supplementary information provides additional tables and one figure demonstrating the effects of altitude, location (bulk and rhizosphere), and their interactions on soil parameters; the correlation analyses of EEAs and microbial nutrient limitations with soil properties; a map of the Tibetan Plateau and the location of the study area; and a schematic of the sampling sites along the altitudinal gradient on Gongga Mountain. (DOCX 2352 kb)
Rights and permissions
About this article
Cite this article
Cui, Y., Bing, H., Fang, L. et al. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 458, 7–20 (2021). https://doi.org/10.1007/s11104-019-04159-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04159-x