Abstract
Aims
Cropland revegetation is an effective measure to curb soil erosion on eroding hillslopes and increase farmers’ income at depositional check dams. However, how soil microbial metabolic limitation responds to cropland revegetation in erosion and deposition landscapes remains poorly understood, which has substantial implications for carbon (C) retention and nutrient cycling in the eroding environment.
Methods
We sampled 0–2 m soils in cropland and revegetated forest and grassland at upslopes and check dams in the hilly-gully region of the Loess Plateau, China. The activities of soil C-, nitrogen (N)-, and phosphorus (P)-acquiring enzymes were analyzed. The improved enzyme vector model (V-T model) based on balance points was used to quantify microbial metabolic limitations based on ecoenzymatic stoichiometry.
Results
Microorganisms suffered from no energy (C) limitation, but the relative microbial C limitation was greater in revegetated forest than in cropland. At upslopes, the revegetated forest was primarily limited by P, while the revegetated grassland and cropland were limited by N, which was indicated by the VTN/P limitation values of 4.35, -2.74, and − 1.87, respectively. Microbial P limitation was greater in deep soils of revegetated forest due to the assimilation of P by the root system. At check dams, land-use change had no significant influence on microbial N/P limitations owing to abundant soil C and nutrients and a wet environment.
Conclusion
Concludingly, cropland revegetation had a weaker influence on microbial metabolic limitation at the lower-lying topography, which compensates for the current understanding of resource restrictions on microorganisms at slopes or flat areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cropland revegetation in eroding landscapes is a widely used strategy that substantially improves soil quality and ecosystem carbon (C) sequestration (Borrelli et al. 2017; Lutter et al. 2022). The conversion of cropland to forest or grassland would lead to the accumulation of soil organic matter and may result in a stoichiometric imbalance that does not meet microbial elemental demands (Zinn et al. 2018). Microbial metabolism serves as the base of soil organic matter decomposition and controls C and nutrient turnover of plant-soil ecosystems (Houghton 2007; Sinsabaugh and Shah 2012). Therefore, understanding soil resource limitations for microbial metabolism is crucial to identifying C retention and nutrient cycling in agriculture and revegetation ecosystems.
Microbial metabolic limitation is due to the fact that soil C and nutrient supply cannot meet the demand for microbes (Sinsabaugh et al. 2008, 2009). At global scales, microbial metabolism in high-latitude grasslands and low-latitude forests was predominantly limited by soil nitrogen (N) and phosphorus (P), while these limitations were alleviated in agricultural ecosystems due to fertilization (Camenzind et al. 2018; Cui et al. 2021b; Turner et al. 2018). Previous site-level studies have investigated microbial metabolic limitations focusing on land-use type (Mganga et al. 2016), plant species (Wobeng et al. 2020), rhizosphere (Cui et al. 2021a), natural succession stage (Xue et al. 2022), vegetation restoration period after anthropogenic forest clearcut (Kang et al. 2022) or farmland abandonment (Deng et al. 2019). For example, Deng et al. (2019) reported a downward unimodal trend of microbial C limitation and a reversed pattern of microbial P limitation during the 30 years of vegetation restoration in an abandoned agroecosystem. However, these site studies were generally conducted in flat areas or on hillslopes suffering from erosion; how soil microbial metabolism responds to land-use change in the lower-lying topographies remains less understood.
Topography is the major driving factor for soil resource distribution. Soil nutrients are transported away with runoff from the eroding slopes, and the majority of which accumulates in the deposition topographies (Berhe et al. 2018; Stallard 1998; Yao et al. 2022). The accelerated nutrient loss due to erosion would augment soil microbial P limitation (Alewell et al. 2020; Deng et al. 2019) since P is primarily derived from the weathering of parent materials (Yao et al. 2018). Apart from C and nutrient redistribution and the correspondingly changed stoichiometric ratios, the different soil moisture, ventilation conditions, and pH contributed to the variations of microbial metabolic limitations between erosion and deposition landscapes (Berhe et al. 2018; Doetterl et al. 2016; Yao et al. 2022). Based on the 2,200 samples collected across the US, Sinsabaugh and Shah (2012) reported that the microbial metabolisms of stream sediments were less restrained by N and P but more limited by C than terrestrial soils. Therefore, we may infer that converting cropland to grassland or forest may have a different influence on microbial metabolic limitations between erosion and deposition topographies; however, solid evidence from field studies has rarely been reported.
Extracellular enzymes catalyze the breakdown of complex macromolecules into simpler polymers, which can be readily assimilated by microbes (Sinsabaugh et al. 2008). The integration of enzyme stoichiometric ratios and metabolic theory provides a promising avenue for identifying resource limitations in soil microbial metabolism (Sinsabaugh et al. 2009). There are two commonly used models to quantify microbial metabolic limitations. The threshold element ratio (TER) model can predict simultaneous co-limitations by N and P based on the elemental ratio of C/N or C/P, but it cannot identify the most limiting nutrient and fails to determine microbial C limitation (Mori 2020). The enzyme vector model can quantify the relative C limitation with vector length and N/P limitation with vector angle; however, this model relies on the empirical global-scale ecoenzymatic stoichiometry, which varies among ecosystems due to local factors (Cui et al. 2022; Moorhead et al. 2016; Sinsabaugh et al. 2008, 2009). A new enzyme vector (V-T) model has been recently developed based on balance points of P and N acquisition that are not constrained by soil resources (Cui et al. 2021b). The improved V-T model has been successfully used to estimate microbial metabolic limitations in varied ecosystems globally (Asada et al. 2022; Kang et al. 2022; Xu et al. 2022).
China’s Loess Plateau is one of the most ecologically vulnerable areas in the world, suffering from severe soil erosion and land degradation. “Grain for Green” (revegetation of steep croplands) and check dam construction (trapping and storing eroded sediments) programs have been implemented in this region to curb serious soil erosion (Wang et al. 2016; Yao et al. 2022). Here, we used the improved enzymatic stoichiometry model (V-T model) to investigate patterns of soil microbial metabolic limitations after converting cropland to forest and grassland in contrasting topographies (i.e., eroding slope lands and depositional check dams) and at different soil depths in the hilly-gully region on the Loess Plateau of China. The objectives of this study are to (1) explore how soil microbial metabolic limitations changed after cropland revegetation; (2) determine whether the pattern was different in the erosional upslopes and depositional check dams; (3) identify the potential mechanisms that affect microbial metabolic limitations after vegetation restoration in different topographies.
Materials and methods
Study sites and soil sampling
Study sites are located in the Jiuyuangou watershed (110°16′-110°26′E, 37°33′-37°38′N, 820–1180 m a.s.l.), Suide county, Yulin city, Shaanxi province, China. The watershed is a typical representative of the first sub-region of the loess hilly-gully area, with undulating hills, crisscross gullies, and broken terrain. The gully density of the watershed is 5.34 km/km2. The climate is classified as a semi-arid temperate continental monsoon climate, with a mean air temperature of 8℃, a mean annual precipitation of 475 mm, with approximately 60% fall as rainstorms from July to September. The soil is dominated by Huangmian soil (a Calcaric Cambisol in FAO classification), characterized by sandy loam texture, low soil fertility, and high erodibility.
Soil and water conservation projects have been carried out in the Jiuyuangou watershed since 1952. Trees and grasses have been planted on steep cropland where slope gradients are over 25°; and check dams have been built on the gully channels to store the sediments eroded from upslopes. Currently, the vegetation coverage is 48%, and there are 241 check dams, which have contributed to 66% flood reduction and 88% sediment reduction. To increase local farmers' income, the check dams have developed productive agriculture, economic forests, and grasslands.
To examine the effects of cropland revegetation on soil microbial nutrient limitations in topographies suffering from erosion or deposition processes, the upslope and check dam were selected to represent erosion and deposition topographies, respectively. Cropland and revegetated forest and revegetated grassland on former cropland were chosen for both upslopes and check dams. The crops at upslopes and downslope check dams were Solanum tuberosum L. and Zea mays L., respectively. The chemical N and P fertilizers used in cropland were diammonium hydrogen phosphate and dipotassium hydrogen phosphate, with a mean rate of about 300 kg N ha− 1 yr− 1 and 100 kg P ha− 1 yr− 1, respectively. To control the severe soil erosion in this area, the slope cropland was converted to forest and grassland. Revegetated plant species on upslope were Pinus tabuliformis Carr. and Armeniaca vulgaris Lam. for forest, and Bothriochloa ischcemum (L.) Keng, Poa sphondylodes Trin., and Cynanchum chinense R. Br. for grassland. The check dams were originally used for grain production, and some of the check dams were planted with grass or trees for economic reasons. The revegetated species in check dams were Sophora japonica Linn. for forest and Phragmites australis (Cav.) Trin. ex Steud. for grassland. No fertilizer was applied to the forest and grassland at upslopes or check dams.
Three replicate sampling plots (20 m × 20 m) were established in cropland, forest, and grassland both at upslopes and at check dams in July 2021. In each plot, three representative sampling points were randomly selected. Soils were collected at the surface soil layer (0–10 and 10–20 cm) and deep soil layer (60–80 and 180–200 cm) with a 9-cm soil auger, and then combined into a composite sample from these three points at each depth. Soil samples were divided into two parts. One part was passed through a 2-mm sieve in the field, placed in an ice chest, and transported to the laboratory. These fresh soil samples were used for the measurement of extracellular enzyme activity, microbial biomass C, N, and P (MBC, MBN, and MBP, respectively), dissolved organic C (DOC), available N, and soil moisture. Another part was transported to the laboratory and air-dried for the measurement of soil physicochemical properties. In the center of each plot, a 0.8 × 0.8 × 2 m pit was dug, and undisturbed soil cores (5 cm in diameter and 5 cm in height) were collected at each depth for the measurement of soil bulk density.
Soil physical and chemical analysis
Soil particle size distribution was determined by a laser particle sizer (Malvern Instruments Ltd. UK). Soil organic C (OC), total P (TN), and total P (TP) were determined using the Walkley-Black, the Kjeldahl, and the sulfuric acid and perchloric acid digestion methods, respectively (Page et al. 1982). DOC was extracted with 0.5 M K2SO4 and shaken for 60 min at 200 rpm, and the extractions were measured using a TOC analyzer (TOC-VCPH, Shimadzu, Japan). Soil ammonium (NH4+) and nitrate (NO3−) were measured by a continuous flow analyzer (AutoAnalyzer-AA3, Seal Analytical, Norderstedt, Germany) after extraction with 2 mol L− 1 KCL. Soil mineral N (Min-N) was the sum of NH4+ and NO3−. Soil available phosphorus (EP) was determined by the Olsen method. Soil pH was measured in a 1:2.5 soil water mixture using a glass-electrode meter (PHS-3 C, Leici, China). The undisturbed soil cores were used to determine the bulk density using the drying method. Information about soil physical and chemical properties was presented in Tables S1-S3.
Soil microbial biomass C, N, P and extracellular enzyme activity
Soil MBC, MBN, and MBP were determined using the fumigation extraction method (Brookes et al. 1985). Data about MBC, MBN, and MBP are presented in Table S4. Extracellular enzyme activities were measured using the microplate-scale fluorometric method (Giacometti et al. 2014). The C-acquiring enzymes (EEAC) were β-D-cellobiosidase (CBH) and β-1,4glucosidase (BG), the N-acquiring enzymes (EEAN) were β-1,4-N-acetylglucosaminidase (NAG) and L-leucine aminopeptidase (LAP), and the P-acquiring enzyme (EEAP) was alkaline phosphatase (AP). The enzyme commission classification of CBH, BG, NAG, LAP, and AP was 3.2.1.91, 3.2.1.21, 3.2.1.14, 3.4.11.1, and 3.1.3.1, respectively (Sinsabaugh et al. 2009), and their meanings were presented in Table S5.
Calculation
Elemental homeostasis for microorganisms
The elemental homeostasis for microorganisms was identified according to Eq. (1).
where 1/H is the slope of the elemental stoichiometry of microbial biomass and resource (total or available). According to the 1/H value, it was defined as homeostatic (0-0.25), weakly homeostatic (0.25–0.5), weakly plastic (0.5–0.75) or plastic (> 0.75) (Sterner and Elser 2002). And when the regression slope was not significant (P > 0.05), it was classified as strictly homeostatic.
Traditional vector model to quantify microbial metabolic limitation
Based on the vector model proposed by Moorhead et al. (2016), the vector length and angle were created to quantify relative microbial C limitation and microbial N/P limitation, respectively. Based on the stoichiometric and metabolic theories (Sterner and Elser 2002), the higher vector length the greater relative C limitation; values of vector angle > 45° are P limited, while values < 45° are N limited (Moorhead et al. 2016). In the plots of x(EEAC/(EEAC+EEAP) and y(EEAC/(EEAC+EEAN), the vector length is the length of the point to the origin, and the vector angle is the angle from the x-axis to the connecting line with point and origin. They were calculated based on the following equations:
where x represents EEAC/(EEAC+EEAP), and y denotes EEAC/(EEAC+EEAN).
Improved V-T model to quantify microbial metabolic limitation
The traditional vector model is based on the empirical evidence that EEAC : EEAN : EEAP nearly equals to 1:1:1 (Sinsabaugh et al. 2008, 2009), and thus the slopes of their regressions are close to 45°. However, the empirical patterns of ecoenzymatic stoichiometry strongly interacted with local factors (Cui et al. 2021b). In this circumstance, Cui et al. (2021b) developed an improved model (V-T) of balance points (X0, Y0) based on the traditional vector model and ΔTER1C:P or ΔTER1C:N. The V-T model assumed that microbial metabolism was not limited by either N or P when ΔTER1C:P and ΔTER1C:N were both equal to zero. The calculations of ΔTER1C:P and ΔTER1C:N were in supplementary materials and methods.
Values of VTC limitation > 0 indicate that microbial C limitation occurs no matter how much N and P are available. Values of VTN/P limitation greater than zero mean that microbial metabolism is primarily limited by P, while values less than zero denote the primary limitation by N. In this study, we calculated the VTC limitation and VTN/P limitation based on balance points derived from our database (n = 72) and from the empirical global values in varied ecosystems (n = 2,667) in Cui et al. (2021b).
Statistical analysis
The regressions for EEAC/(EEAC+EEAP) vs. ΔTER1C:P and EEAC/(EEAC+EEAN) vs. ΔTER1C:P were conducted using the Type II standardized major axis regressions (Type II SMA), and X0 and Y0 were the intercepts of these regressions, respectively. The Type II SMA was conducted using the “lmodel2” function from the “lomdel2” package in R. A two-way ANOVA was conducted to analyze the effects of land use, soil depth, and their interactions on the tested variables at upslopes and check dams. Multivariable linear regression analysis (MLRA) was conducted to quantify the contributions of soil variables to microbial C and N/P limitations. The contribution of each variable was a proportion of error sum of squares (Sum Sq) to the sum of the Sum Sq of all variables and residuals. In the MLRA, sand content was not defined because of singularities and was not included in further statistical analysis. To explore the cascading relationships among soil variables and microbial C and N/P limitations, the partial least squares path model (PLS-PM) was used to identify possible pathways. The PLS-PM was conducted using the “innerplot” function in the “plspm” package (Sanchez et al. 2016). All statistical analyses were done using the R environment (v4.0.5, http://www.rproject.org/).
Results
Stoichiometric homeostasis
The C/N ratios were generally lower than the C/P ratios for soil total resources (9.70 and 13.60, respectively, P < 0.001) and available resources (15.01 and 34.13, respectively, P < 0.01), while there were no significant differences for microbial biomass (11.53 and 9.53, respectively, P > 0.05) (Fig. 1). The regression slope (1/H) of resource stoichiometry between resource (total or available) and microbial biomass was less than 0.25, and these correlations were statistically not significant (P > 0.05). Our database confirmed the strict homeostasis for soil microorganisms, which justified the requirement for stoichiometric model applications.
Soil enzymatic activities and stoichiometry
Soil enzymatic activities per OC varied significantly among land uses (Table 1). At upslopes, soil C-, N-, and P-acquiring enzymatic activities were higher in revegetated forest and grassland than in cropland; these variations were significant at surface soils for EEAC and EEAN and at both surface and deep soils for EEAP (P < 0.05). At check dams, cropland revegetation also led to higher soil C-, N-, and P-acquiring enzyme activities, but the influences of land use on EEAC and EEAP were lower than those at upslopes, which can be proved by the lower F values. And the variations among land uses were significant at 0–10 cm, 0–20 cm, and 0–20 cm depth for EEAC, EEAN, and EEAP, respectively (P < 0.05). Therefore, revegetation in cropland increased soil enzymatic activities, which was more pronounced at upslopes.
Regardless of soil depth, enzymatic stoichiometry EEAC:N followed the pattern of forest (0.40) > grassland (0.32) > cropland (0.28) at upslopes (P < 0.05), but differed not significantly at check dams (P > 0.05). There were no significant differences in EEAC:P among land uses either at upslopes or at check dams (P > 0.05). The EEAN:P was significantly lower in forest (0.29) than in grassland (0.44) and cropland (0.45) at upslopes, but the differences were only significant at 180–200 cm depth at check dams (P < 0.05). Thus, the revegetated forest had a higher EEAC:N, but a lower EEAN:P especially at upslopes.
Microbial metabolic limitations predicted by the traditional vector model
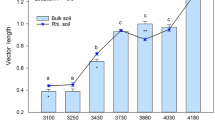
Based on the traditional vector model, the vector angles were all greater than 45° for all the data, implying a general P limitation in the study area (Fig. 2). The vector angles were higher in the revegetated forest than in revegetated grassland and cropland regardless of the depth at upslopes (P < 0.05), while the vector angle differed not significantly among land uses at check dams (P > 0.05) (Fig. S1). The vector length was significantly greater in surface soils (0–10 and 10–20 cm) than in deep soils (60–80 and 180–200 cm), indicating a higher relative C limitation at surface soils (Fig. S1). The vector length followed the decreasing pattern of the forest, grassland, and cropland both at upslopes and at check dams. These results indicated that revegetation increased relative microbial C limitation and microbial P limitation at 0-200 cm, with greater effects at upslopes.
Enzymatic stoichiometry of the relative proportions of carbon to nitrogen acquisition versus carbon to phosphorus acquisition for cropland and revegetated forest and grassland at 0–10, 10–20, 60–80 and 180–200 cm depth.0 EEAC: CBH + BG; EEAN: NAG + LAP; EEAP: AP. The balance points (X0, Y0) were from data in this study and from data in cropland, forest, and grassland in Cui et al. (2021b) at global scale. Detailed information was in Table S6
Microbial metabolic limitations predicted by the improved V-T model
Based on the balance points derived from our study (Fig. 2), the improved V-T model showed that VTC limitation was generally less than zero, and VTN/P limitation was generally less than zero in surface soils and greater than zero in deep soils (Fig. 3). These results indicated that soil microorganisms were not limited by C at 0-200 cm depth and were more limited by N at 0–20 cm but were more limited by P in soils below 20 cm. With the empirical balance points derived from the global database (Fig. 2) (Cui et al. 2021b), the VTC limitation was generally less than zero, and the VTN/P limitation was generally less than zero in cropland (Fig. S2). According to the balance points from your study and from the global database, these results indicated that the traditional vector model may overestimate relative microbial C and microbial P limitations.
The microbial C limitation (VTC limitation) and microbial N/P limitation (VTN/P limitation) quantified by the V-T model based on the balance point for cropland and revegetated forest and grassland along 0-200 cm depth at upslopes and check dams. The balance point was derived from database in our study (n = 72) (Fig. 2; Table S6). Values of VTC limitation > 0 indicate that microbial C limitation occurs no matter how much N and P are available. Values of VTN/P limitation > 0 are P limited, while values < 0 are N limited. *, ***, and ns represent effects at P < 0.05, P < 0.001, and P > 0.05, respectively
The improved V-T model with the database from our study showed that revegetated forest had a higher VTC limitation than grassland and cropland, with higher differences at upslopes (F = 5.039, P < 0.05) than at check dams (F = 4.267, P < 0.05) (Fig. 3). At upslopes, the VTN/P limitation was significantly higher in forest (4.35 ± 2.21) than in grassland (-2.74 ± 1.76) or cropland (-1.87 ± 2.71), indicating that forest was primarily limited by P, especially in deep soils, while cropland and grassland were mainly restricted by N, especially in surface soils. At check dams, however, there were no significant differences for VTN/P limitation among cropland (0.92 ± 1.78), forest (-0.68 ± 1.98), and grassland (-0.44 ± 1.84), regardless of depth (P > 0.05).
Key drivers and possible pathways of microbial metabolic limitations
The contributions of soil properties explained 76% of the relative microbial C limitation and 58% of the microbial N/P limitation (Fig. 4). The C, N, P and their stoichiometry of total soil organic matter explained the maximum variations of VTC limitation (34%) and VTN/P limitation (26%). According to the MLRA analysis, OC, clay, TP, MBP, SM, C/P, and silt had significant contributions to VTC limitation with a decreasing relative effect (P < 0.05). In terms of VTN/P limitation, OC, C/N, pH, BD, C/NL, and N/PL had significantly contributions with their importance in a decreasing pattern (P < 0.05).
Contributions of soil physical properties (clay, silt, pH, BD, SM), total C, N, P and their stoichiometry (OC, TN, TP, C/N, C/P, N/P), labile C, N, P and their stoichiometry (DOC, Min-N, EP, C/NL, C/PL, N/PL), microbial biomass C, N, P and their stoichiometry (MBC, MBN, MBP, C/NB, C/PB, N/PB) on the microbial C limitation (VTC limitation) (a) and microbial N/P limitation (VTN/P limitation) (b) based multivariable linear regression analysis. BD: bulk density; SM: soil moisture; OC: organic carbon; TN: total nitrogen; TP: total phosphorus; DOC: dissolved organic matter; Min-N: mineral nitrogen; EP: Olsen phosphorus; MBC: microbial biomass carbon; MBN: microbial biomass nitrogen; MBP: microbial biomass phosphorus; C/N, C/NL, C/NB: carbon to nitrogen ratio for total soil resources, available soil resources and microbial biomass, respectively; C/P, C/PL, C/PB: carbon to phosphorus ratio for total soil resources, available soil resources and microbial biomass, respectively; N/P, N/PL, N/PB: nitrogen to phosphorus ratio for total soil resources, available soil resources and microbial biomass, respectively
The combination of the five types of soil variables explained 56% of the variance in VTC limitation and 30% of the variance in VTN/P limitation as revealed by PLS-PM analysis (Fig. 5); and VTC limitation was negatively related with VTN/P limitation (r = -0.40, P < 0.001). Total C, N, P and their stoichiometry had a direct influence on VTC limitation (0.53) and an indirect influence on VTC limitation (0.26) through the labile and microbial biomass variables, with a total effect of 0.79. Soil physical properties mainly affected VTC limitation with an indirect influence, while labile variables and microbial biomass variables mainly had a direct influence, with the total effects of -0.48, 0.20, and 0.15, respectively. As to VTN/P limitation, total C, N, P and their stoichiometry had direct and indirect influences with standardized path coefficients of -0.47 and − 0.12, respectively. Soil physical properties, labile C, N, P and their stoichiometry, and microbial biomass C, N, P and their stoichiometry had total effects of 0.29, -0.19, and − 0.01, respectively.
Partial least squares path modeling (PLS-PM) disentangling major pathways of the influence of soil variables on the microbial C limitation (VTC limitation) (a) and microbial N/P limitation (VTN/P limitation) (b). ↑ and ↓ indicate the positive and negative loadings within each group of soil variables, respectively. Solid and dashed lines indicate positive and negative flows of causality, respectively. Numbers with arrows indicate the standardized path coefficients. The thicker the single arrow line the greater the effect on soil microbial C and N/P limitation. BD: bulk density; SM: soil moisture; OC: organic carbon; TN: total nitrogen; TP: total phosphorus; DOC: dissolved organic matter; Min-N: mineral nitrogen; EP: Olsen phosphorus; MBC: microbial biomass carbon; MBN: microbial biomass nitrogen; MBP: microbial biomass phosphorus; C/N, C/NL, C/NB: carbon to nitrogen ratio for total soil resources, available soil resources and microbial biomass, respectively; C/P, C/PL, C/PB: carbon to phosphorus ratio for total soil resources, available soil resources and microbial biomass, respectively; N/P, N/PL, N/PB: nitrogen to phosphorus ratio for total soil resources, available soil resources and microbial biomass, respectively. *, **, and *** represent effects at P < 0.05, P < 0.01, and P < 0.001, respectively
Discussion
Effects of cropland revegetation on soil enzymatic activities
Soil enzymes are sensitive indicators for monitoring changes in microbial community structure and activity as well as soil organic matter dynamics. In this study, soil C-, N-, and P-acquiring enzyme activities (expressed as per unit of OC) increased after converting cropland to forest or grassland (Table 1). Generally, soil C and nutrients increase with vegetation restoration due to higher organic matter inputs from plant litter and root residues. However, in our study, soil available C, N, and P decreased after cropland revegetation (Table S3), which may be due to the cession of fertilization in the revegetated land. To acquire available nutrients, soil microorganisms produce extracellular enzymes to catalyze the decomposition of organic matter and convert nutrients from organic into inorganic forms (Burns et al. 2013). A previous study reported that N fertilization in cropland reduced the activity of the N-acquiring enzymes NAG and LAP per unit of soil organic N (Cenini et al. 2016). There are studies reporting both increased (Zhang et al. 2021; Guan et al. 2022), decreased (Xiao et al. 2020), and unchanged (de Oliveira Silva et al. 2019) enzymatic activities per OC after cropland revegetation. These discrepancies may be due to the revegetation species, year, and climate of the study area. For example, Xiao et al. (2020) documented that soil enzymatic activities per OC increased in the first 17 years of plant secondary succession and then decreased in the longer term of succession. Therefore, the responses of soil enzymatic activities to cropland revegetation were closely related to soil nutrient status. In addition, we found that the influence of land-use change on soil enzymatic activities was greater at upslopes than at check dams, implying the importance of topography in regulating soil nutrients and thus enzymatic activities.
Comparisons between the traditional vector model and the improved V-T model to predict microbial metabolic limitations
The improved V-T model showed that microbial metabolism was restrained by N in surface soils and P in deep soils (Fig. 3), while the traditional vector model predicted a general microbial P limitation with the vector angle value greater than 45° (Fig. S1). These results indicated that the traditional vector model may overestimate microbial P limitation. The traditional model was based on the assumption that the ratios of C-, N-, and P-acquiring enzymes were 1:1:1, and thus the boundary between N and P limitation was 45° of vector angle. However, the global generic 1:1:1 from all ecosystems was not true in individual ecosystems both on the global database (Cui et al. 2021b) and for regional or site-level studies (Cao et al. 2022; Sinsabaugh et al. 2012; Xu et al. 2022). In our study, the ratios of enzyme activities for C versus P and C versus N acquisition were different from 1 (Table 1). The improved V-T model considers the differences in enzymatic stoichiometry among specific ecosystems by integrating the balance points of microbial resource demands underlying the metabolic theory and ecological stoichiometry theory (Cui et al. 2021b). The balance point in our study (0.21, 0.48) was close to that in agricultural land with soil OC content less than 10 g kg− 1 in the global database (0.19, 0.46) (Cui et al. 2021b) (Fig. 2, Table S6). And the VTC limitation and VTN/P limitation predicted by the improved V-T model with balance points from our study and from the global database were significantly positively related (P < 0.001). Therefore, the prediction of microbial metabolic limitations using the improved V-T model with the balance point was more reliable.
Microbial metabolism was not limited by C both at upslopes and at check dams
The improved V-T model showed that microbial metabolism was not restrained by C with negative VTC limitation values both at upslopes and at check dams (Fig. 3), meaning that soil organic matter can satisfy the energy demand of microorganisms (Cui et al. 2021b). From the perspective of enzymatic stoichiometry, the EEAC:N and EEAC:P at 0–2 m depth were far less than 1 (Table 1). This result meant that microorganisms excreted more enzymes associated with N and P metabolism compared with C (Burns et al. 2013), indicating that microbial metabolism was more likely to be limited by nutrients (N or P) than energy (C). We also found that the concentration of available C (DOC, 45.87 mg kg− 1) was much greater than that of available N (Min-N, 7.52 mg kg− 1) and phosphorus (EP, 6.18 mg kg− 1) (Table S3). For sediments in the depositional check dam, the VTC limitation values (-0.17 ± 0.02) were greater than those for soils in the erosional upslopes (-0.28 ± 0.03), and the C-acquiring enzyme activities were also significantly higher at check dams (6.96 ± 1.12) than at upslopes (5.28 ± 0.81). These results suggested that depositional sediments could contain more recalcitrant organic C that is less readily available to microorganisms than upslope soils (Andersen et al. 2013), although they both did not suffer from microbial C limitation.
The VTC limitation value was significantly lower in deep soils below 20 cm (-0.35) than the 0–20 cm surface oils (-0.09), and the EEAC:N and EEAC:P were significantly lower in deep soils (0.21 and 0.07, respectively) than surface soils (0.65 and 0.26, respectively). Thus, the non-energy limitation phenomenon for microorganisms strengthened in deep soils. Although not C limited, the VTC limitation value was significantly higher in revegetated forest than cropland but similar in revegetated grassland and cropland, indicating that energy was relatively more needed in forests than in grassland and cropland (Fig. 3). In our study, soil OC was the most important factor accounting for VTC limitation (Fig. 4). Soil microorganisms would secrete excess enzymes to compensate for the decreased organic matter (Wallenius et al. 2011), which can be proved by the increased C-acquiring enzyme activity per OC for revegetated forest compared with cropland at upslopes (Table 1). Compared with the perennial trees, herbaceous litter is more easily decomposed since it contains less cellulose, hemicellulose, and lignin (Seastedt et al. 2011), and soil OC concentration in grassland was similar to or higher than in cropland (Table S2). Converting cropland to forest did not result in more OC but led to higher microbial biomass as indicated by MBC (Table S4), therefore, the soil C resources may not feed the soil microorganisms and subsequently increase microbial C restrictions in revegetated forest. Similarly, intensified relative C limitation has been reported in the early stages of natural vegetation restoration on the Loess Plateau (Deng et al. 2019; Xiao et al. 2020).
Converting cropland to forest increased microbial P limitation at upslopes but not at check dams
At upslopes, the microbial metabolisms of cropland and revegetated grassland were primarily limited by N, as evidenced by the negative VTN/P limitation values (-1.87 and − 2.74, respectively), while the microorganisms of revegetated forest were primarily limited by P, as indicated by the positive VTN/P limitation value (4.35), especially in deep soils (Fig. 3). These results indicated that microbial P limitation occurred after converting cropland to forest. Similarly, the VTN/P limitation value was close to or less than zero in agriculture and grassland ecosystems but greater than zero in forest ecosystems under the same OC level (< 10 g kg− 1) at the global scale (Cui et al. 2021b). The occurrence of microbial P limitation after converting cropland to forest was primarily caused by the intensified P competition between plants and microbes. The P consumption by root systems in revegetated forest would aggravate the competition for P and impede P acquisition by microorganisms (Pinsonneault et al. 2016), which can be proved by the significantly lower EP concentration and higher P-acquiring enzyme activity in revegetated forest than cropland (Table 1, S3). In addition, the EEAN:P was significantly lower in the revegetated forest than cropland and grassland (Table 1), meaning that microorganisms produced more P-acquiring enzymes compared with C- or N-acquiring enzymes. In our study, we found that microbial P limitation was more dramatic in deep soils (Fig. 3), which may be explained by the greater uptake of P by root systems with depth. With the investigation of microbial P limitation in forest ecosystems in China, Cui et al. (2022) found that the positive influence of the vegetation index on microbial P limitation increased with depth, implying an accelerated competition of P between microorganisms and plants with increasing root density. In addition, the loess soils have a high soil pH and a low soil P availability (Wei et al. 2011), and the cessation of fertilization in revegetated ecosystems excluded anthropogenic inputs of P (Cui et al. 2021b; DeForest et al. 2021). Previous studies have reported a subsequent P limitation of plants after the microbial P limitation (Cui et al. 2022; Xue et al. 2022); therefore, the P deficiency issue of the revegetated forest on hillslopes should be considered by the local government. It is especially important since the increasing vegetation biomass and water deficiency (Jia et al. 2019) would aggravate microbial P limitation with the development of the revegetated forest.
At check dams, the VTN/P limitation values differed not significantly among land uses and they were all close to zero (Fig. 3). Converting cropland to forest did not increase microbial P limitations at the depositional topographies, which was different to the erosional upslopes. At upslopes, soil erosion processes would move soil nutrients away (Alewell et al. 2020) and thus augment microbial nutrient limitation, especially P, which was mostly derived from parent materials (Yao et al. 2018). The eroded soils and associated soil nutrients accumulated at the lower-lying check dams, which resulted in a better environment for microorganisms with abundant nutrients (Berhe et al. 2018; Yao et al. 2022). For example, soil EP concentration in revegetated forest was significantly higher at check dams (5.85 mg kg− 1) than at upslope (3.81 mg kg− 1) (Table S3). The relatively abundant nutrients in the depositional landscape would alleviate nutrient competition between plants and microbes (Pinsonneault et al. 2016); it would also result in a weaker response of microbial metabolic limitations to land-use change. In addition, soil moisture was significantly higher in the depositional area (Table S1), and the higher soil moisture was conducive to the assimilation of available P by microorganisms and thus alleviated microbial P limitation (Bell et al. 2008). Thus, the relatively abundant soil nutrient conditions and wet environment can better meet the nutrient demand of revegetated plants and microbes and thus weaken the influence of cropland revegetation on microbial nutrient limitations. In this study, soil total C, N, P and their stoichiometry were the most important factors regulating microbial N/P limitations (Fig. 5). Soil erosion is one of the most important contributors to the movement of soil and water in terrestrial ecosystems, which in turn triggers the redistribution of soil C and nutrients (Berhe et al. 2018; Doetterl et al. 2016; Yao et al. 2022). Therefore, we need to consider topography in the evaluation of soil microbial metabolic limitation in response to land-use change.
Conclusion
In this study, the influence of cropland revegetation on microbial metabolic limitations at erosion and deposition topographies was determined with the improved V-T model in a hilly-gully area on the Loess Plateau. Microbial metabolism was not limited by energy (C) but by nutrients (N and/or P) both at upslopes and at check dams. At upslopes, the cropland and revegetated grassland were primarily limited by N, while the revegetated forest was mainly restricted by P, especially at deep soils below 20 cm. The strengthened microbial P limitation for the revegetated forest would be due to the competition for P between microorganisms and plants at upslopes, where the erosion processes accelerated nutrient loss. At check dams, converting cropland to forest did not result in greater microbial P limitation, since the higher available P concentration and higher moisture content alleviated nutrient competition between microorganisms and plants. Soil total C, N, P and their stoichiometry were the most important factors in explaining soil metabolic resource limitations. Results of this study showed the weakened influence of cropland revegetation on soil microbial metabolic limitations at lower-lying landscape positions than at upslopes, which highlighted the importance of topography in the evaluation of microbial metabolism in response to land-use changes.
References
Alewell C, Ringeval B, Ballabio C et al (2020) Global phosphorus shortage will be aggravated by soil erosion. Nat Commun 11:4546. https://doi.org/10.1038/s41467-020-18326-7
Andersen R, Chapman SJ, Artz RRE (2013) Microbial communities in natural and disturbed peatlands: a review. Soil Biol Biochem 57:979–994. https://doi.org/10.1016/j.soilbio.2012.10.003
Asada K, Kanda T, Yamashita N et al (2022) Interpreting stoichiometric homeostasis and flexibility of soil microbial biomass carbon, nitrogen, and phosphorus. Ecol Model 470:110018. https://doi.org/10.1016/j.ecolmodel.2022.110018
Bell C, McIntyre N, Cox S et al (2008) Soil microbial responses to temporal variations of moisture and temperature in a Chihuahuan Desert Grassland. Microb Ecol 56:153–167. https://doi.org/10.1007/s00248-007-9333-z
Berhe AA, Barnes RT, Six J, Marín-Spiotta E (2018) Role of soil erosion in biogeochemical cycling of essential elements: carbon, nitrogen, and phosphorus. Annu Rev Earth Planet Sci 46:521–548. https://doi.org/10.1146/annurev-earth-082517-010018
Borrelli P, Robinson DA, Fleischer LR et al (2017) An assessment of the global impact of 21st century land use change on soil erosion. Nat Commun 8:2013. https://doi.org/10.1038/s41467-017-02142-7
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Burns RG, DeForest JL, Marxsen J et al (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234. https://doi.org/10.1016/j.soilbio.2012.11.009
Camenzind T, Hättenschwiler S, Treseder KK et al (2018) Nutrient limitation of soil microbial processes in tropical forests. Ecol Monogr 88:4–21. https://doi.org/10.1002/ecm.1279
Cao X, Shi Z, Chen J et al (2022) Extracellular enzyme characteristics and microbial metabolic limitation in soil of subalpine forest ecosystems on the eastern Qinghai-Tibetan Plateau. Plant Soil. https://doi.org/10.1007/s11104-022-05521-2
Cenini VL, Fornara D, McMullan G et al (2016) Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol Biochem 96:198–206. https://doi.org/10.1016/j.soilbio.2016.02.015
Cui Y, Bing H, Fang L et al (2021a) Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 458:7–20. https://doi.org/10.1007/s11104-019-04159-x
Cui Y, Moorhead DL, Guo X et al (2021b) Stoichiometric models of microbial metabolic limitation in soil systems. Global Ecol Biogeogr 30:2297–2311. https://doi.org/10.1111/geb.13378
Cui Y, Bing H, Moorhead DL et al (2022) Ecoenzymatic stoichiometry reveals widespread soil phosphorus limitation to microbial metabolism across Chinese forests. Commun Earth Environ 3:184. https://doi.org/10.1038/s43247-022-00523-5
de Oliveira Silva É, de Medeiros EV, Duda GP et al (2019) Seasonal effect of land use type on soil absolute and specific enzyme activities in a brazilian semi-arid region. Catena 172:397–407. https://doi.org/10.1016/j.catena.2018.09.007
DeForest JL, Dorkoski R, Freedman ZB, Smemo KA (2021) Multi-year soil microbial and extracellular phosphorus enzyme response to lime and phosphate addition in temperate hardwood forests. Plant Soil 464:391–404. https://doi.org/10.1007/s11104-021-04947-4
Deng L, Peng C, Huang C et al (2019) Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 353:188–200. https://doi.org/10.1016/j.geoderma.2019.06.037
Doetterl S, Berhe AA, Nadeu E et al (2016) Erosion, deposition and soil carbon: a review of process-level controls, experimental tools and models to address C cycling in dynamic landscapes. Earth-Sci Rev 154:102–122. https://doi.org/10.1016/j.earscirev.2015.12.005
Giacometti C, Cavani L, Baldoni G et al (2014) Microplate-scale fluorometric soil enzyme assays as tools to assess soil quality in a long-term agricultural field experiment. Appl Soil Ecol 75:80–85. https://doi.org/10.1016/j.apsoil.2013.10.009
Guan HL, Fan JW, Lu X (2022) Soil specific enzyme stoichiometry reflects nitrogen limitation of microorganisms under different types of vegetation restoration in the karst areas. Appl Soil Ecol 169:104253. https://doi.org/10.1016/j.apsoil.2021.104253
Houghton RA (2007) Balancing the global carbon budget. Annu Rev Earth Planet Sci 35:313–347. https://doi.org/10.1146/annurev.earth.35.031306.140057
Jia X, Shao M, Yu D et al (2019) Spatial variations in soil-water carrying capacity of three typical revegetation species on the Loess Plateau, China. Agric Ecosyst Environ 273:25–35. https://doi.org/10.1016/j.agee.2018.12.008
Kang H, Xue Y, Yan C et al (2022) Contrasting patterns of microbial nutrient limitations between rhizosphere and bulk soil during stump sprout restoration in a clear-cut oak forest. For Ecol Manag 515:120241. https://doi.org/10.1016/j.foreco.2022.120241
Lutter R, Tullus A, Vares A et al (2022) Twenty years of afforestation of former agricultural lands with silver birch plantations affects vertical distribution of SOC and macronutrients in the topsoil layer. Plant Soil. https://doi.org/10.1007/s11104-022-05695-9
Mganga KZ, Razavi BS, Kuzyakov Y (2016) Land use affects soil biochemical properties in Mt. Kilimanjaro region Catena 141:22–29. https://doi.org/10.1016/j.catena.2016.02.013
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7. https://doi.org/10.1016/j.soilbio.2015.10.019
Mori T (2020) Does ecoenzymatic stoichiometry really determine microbial nutrient limitations? Soil Biol Biochem 146:107816. https://doi.org/10.1016/j.soilbio.2020.107816
Page AL, Miller RH, Kenney DR (1982) Methods of Soil Analysis Part 2 (agronomy monographs 9). American Society of Agronomy, Madison
Pinsonneault AJ, Moore TR, Roulet NT (2016) Effects of long-term fertilization on peat stoichiometry and associated microbial enzyme activity in an ombrotrophic bog. Biogeochemistry 129:149–164. https://doi.org/10.1007/s10533-016-0224-6
Sanchez G, Trinchera L, Russolillo G (2016) plspm: Tools for Partial Least Squares Path Modeling (PLS-PM). R package version 0.4.7 edn
Seastedt T, Parton W, Ojima D (2011) Mass loss and nitrogen dynamics of decaying litter of grasslands: the apparent low nitrogen immobilization potential of root detritus. Can J Bot 70:384–391. https://doi.org/10.1139/b92-052
Sinsabaugh RL, Lauber CL, Weintraub MN et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. https://doi.org/10.1038/nature08632
Sinsabaugh RL, Shah JJF (2012) Ecoenzymatic stoichiometry and ecological theory. In: Futuyma DJ (ed) Annual Review of Ecology, Evolution, and Systematics, vol 43. Annual Reviews, Palo Alto, pp 313–343
Sinsabaugh RL, Follstad Shah JJ, Hill BH, Elonen CM (2012) Ecoenzymatic stoichiometry of stream sediments with comparison to terrestrial soils. Biogeochemistry 111:455–467. https://doi.org/10.1007/s10533-011-9676-x
Stallard RF (1998) Terrestrial sedimentation and the carbon cycle: coupling weathering and erosion to carbon burial. Glob Biogeochem Cycles 12:231–257. https://doi.org/10.1029/98GB00741
Sterner RW, Elser JJ (2002) Stoichiometry and homeostasis. In: Sterner RW, Elser JJ (eds) Ecological stoichiometry: the biology of elements from molecules to the bio-sphere. Princeton University Press, Princeton, pp 1–43
Turner BL, Brenes-Arguedas T, Condit R (2018) Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555:367–370. https://doi.org/10.1038/nature25789
Wallenius K, Rita H, Mikkonen A et al (2011) Effects of land use on the level, variation and spatial structure of soil enzyme activities and bacterial communities. Soil Biol Biochem 43:1464–1473. https://doi.org/10.1016/j.soilbio.2011.03.018
Wang S, Fu B, Piao S et al (2016) Reduced sediment transport in the Yellow River due to anthropogenic changes. Nat Geosci 9:38–41. https://doi.org/10.1038/ngeo2602
Wei X, Shao M, Shao H et al (2011) Fractions and bioavailability of soil inorganic phosphorus in the Loess Plateau of China under different vegetations. Acta Geologica Sinica - English Edition 85:263–270
Wobeng NBM, Banfield CC, Megueni C et al (2020) Impact of legumes on soil microbial activity and C cycle functions in two contrasting Cameroonian agro-ecological zones. Pedobiologia 81–82:150662. https://doi.org/10.1016/j.pedobi.2020.150662
Xiao L, Liu G, Li P et al (2020) Ecoenzymatic stoichiometry and microbial nutrient limitation during secondary succession of natural grassland on the Loess Plateau, China. Soil Tillage Res 200:104605. https://doi.org/10.1016/j.still.2020.104605
Xu M, Li W, Wang J et al (2022) Soil ecoenzymatic stoichiometry reveals microbial phosphorus limitation after vegetation restoration on the Loess Plateau, China. Sci Total Environ 815:152918. https://doi.org/10.1016/j.scitotenv.2022.152918
Xue Y, Kang H, Cui Y et al (2022) Consistent plant and microbe nutrient limitation patterns during natural vegetation restoration. Front Plant Sci 13:885984. https://doi.org/10.3389/fpls.2022.885984
Yao Q, Li Z, Song Y et al (2018) Community proteogenomics reveals the systemic impact of phosphorus availability on microbial functions in tropical soil. Nat Ecol Evol 2:499–509. https://doi.org/10.1038/s41559-017-0463-5
Yao Y, Song J, Wei X (2022) The fate of carbon in check dam sediments. Earth-Sci Rev 224:103889. https://doi.org/10.1016/j.earscirev.2021.103889
Zhang Q, Zhang D, Wu J et al (2021) Soil nitrogen-hydrolyzing enzyme activity and stoichiometry following a subtropical land use change. Land Degrad Dev 32:4277–4287. https://doi.org/10.1002/ldr.4034
Zinn YL, Marrenjo GJ, Silva CA (2018) Soil C:N ratios are unresponsive to land use change in Brazil: a comparative analysis. Agric Ecosyst Environ 255:62–72. https://doi.org/10.1016/j.agee.2017.12.019
Acknowledgements
This study was supported by the National Natural Science Foundation of China (42107344, 42041004), the China Postdoctoral Science Foundation (2021T140558, 2020M683699XB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No.
Additional information
Responsible Editor: Tim S. George.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Y., Tang, F., Wang, C. et al. Divergent responses of soil microbial metabolic limitations to cropland revegetation at erosion and deposition topographies in the hilly-gully region of the northern Loess Plateau, China. Plant Soil 487, 213–227 (2023). https://doi.org/10.1007/s11104-023-05915-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05915-w