Abstract
Previous studies have found that root carbon inputs to the soil can stimulate the mineralization of existing soil carbon (C) pools. It is still uncertain, however, whether this “primed” C is derived from elevated rates of soil organic matter (SOM) decomposition, greater C release from microbial pools, or both. The goal of this research was to determine how the activities of the microbial exoenzymes that control SOM decomposition are affected by root C inputs. This was done by manipulating rhizodeposition with tree girdling in a coniferous subalpine forest in the Rocky Mountains of Colorado, USA, and following changes in the activities of nine exoenzymes involved in decomposition, as well as soil dissolved organic C, dissolved organic and inorganic nitrogen (N), and microbial biomass C and N. We found that rhizodeposition is high in the spring, when the soils are still snow-covered, and that there are large ephemeral populations of microorganisms dependent upon this C. Microbial N acquisition from peptide degradation increased with increases in microbial biomass when rhizodeposition was highest. However, our data indicate that the breakdown of cellulose, lignin, chitin, and organic phosphorus are not affected by springtime increases in soil microbial biomass associated with increases in rhizodeposition. We conclude that the priming of soil C mineralization by rhizodeposition is due to growth of the microbial biomass and an increase in the breakdown of N-rich proteins, but not due to increases in the degradation of plant litter constituents such as cellulose and lignin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Under natural conditions, the largest source of labile carbon (C) inputs to the soil is root exudates (Bertin et al. 2003; Hutsch et al. 2002; Kuzyakov 2002). Roots exude a variety of low-molecular weight organic compounds, including sugars, amino acids, organic acids, and phenolics (Bertin et al. 2003). These compounds are rapidly metabolized; for example, Hutsch et al. (2002) found that 64–86% of maize root exudates were respired by soil microorganisms.
Previous studies have demonstrated that labile carbon (C) inputs to the soil, such as root exudates, can stimulate the mineralization of existing C pools (Dalenberg and Jager 1989; Högberg and Ekblad 1996; Mary et al. 1993; Subke et al. 2004), resulting in a “priming” effect. In a review of the mechanisms of priming, Kuzyakov et al. (2000) defines rhizosphere priming as a short-term change in the intensity of soil organic matter (SOM) decomposition. These authors use a broad definition of soil organic matter (SOM) that includes microbial biomass, plant and animal derived organic compounds, and freshly deposited litter (Kuzyakov et al. 2000; Fontaine et al. 2004). An example of soil priming comes from Subke et al. (2004), who combined 13C-labeled litter addition and girdling of Norway spruce trees, and found that the difference in CO2 efflux between their litter-addition and litter-removal plots could not be accounted for entirely by root respiration, and suggested that rhizodeposition had a priming effect, increasing the decomposition component of soil respiration. This positive priming effect was responsible for 15–20% of the total soil CO2 flux in June and July (Subke et al. 2004). In another girdling study in a lodgepole pine forest in the Front Range of the Rocky Mountains in Colorado, USA, Scott-Denton et al. (2006) found that tree roots prime the soil with sugar-rich exudates beneath the spring snowpack. Unlike plant litter, which is largely comprised of polymers too large for microbial uptake, much of the C in exudates can be taken up without extracellular enzymatic decomposition.
While rhizodeposition has been found to stimulate additional decomposition of existing soil C pools, it is still unclear which pools and processes are affected. Kuzyakov (2002) hypothesized that elevated rates of decomposition are the ultimate cause of priming. According to this hypothesis, priming involves an enhancement of microbial C status and demand for other nutrients, which in turn stimulates decomposition. In support of this hypothesis, Subke et al. (2004) concluded that girdling slowed litter decomposition, indicating that rhizodeposition enhanced litter breakdown. Multiple studies, however, have concluded that primed C is derived from microbial C pools, not SOM breakdown, suggesting higher rates of microbial turnover and release of endocellular constituents as the principal sources (Dalenberg and Jager 1981, 1989; De Nobili et al. 2001). Thus, it is uncertain whether primed C is derived from enhanced decomposition, greater C release from microbial pools, or both.
Tree girdling has been shown to be an effective method of isolating the effects of rhizodeposition on soil processes without disturbing the soil environment (Bhupinderpal-Singh et al. 2003; Högberg et al. 2001; Scott-Denton et al. 2006; Subke et al. 2004). Girdling offers two significant advantages to soil trenching, the alternative and more commonly used method of inhibiting rhizodeposition. First, girdling does not result in the instantaneous death of roots, which causes a large root litter input to the soil; and second, girdling allows roots to maintain their ability to transport water, so that soil moisture is less affected by girdling than trenching (Subke et al. 2004). While girdling will ultimately result in root and tree death, roots can remain alive with virtually no growth for a considerable period of time (Subke et al. 2004). Based on our own observations in the subalpine forest ecosystem, it may take as long as 2–3 years for trees to die following girdling (Scott-Denton et al. 2006).
Our goal was to determine the extent to which microbial exoenzymes associated with SOM breakdown are affected by rhizodeposition. We tested the hypothesis that enzyme activities will be reduced by girdling because the priming effect of rhizodeposition is channeled through the production of exoenzymes that depolymerize SOM substrates. To manipulate root C exudation, we girdled lodgepole pine trees in the Front Range of the Rocky Mountains, CO, USA, in the same forest as Scott-Denton et al. (2006). In order to determine the extent to which girdling affected soil C availability and microbial biomass, we measured soil dissolved organic C (DOC) and microbial biomass in both girdled and control plots. In order to determine the effect of rhizodeposition on soil nitrogen (N) status, we measured dissolved organic and inorganic N (DON and DIN). Finally, to determine the effects of rhizodeposition on SOM depolymerization, we measured the activities of nine different exoenzymes in the girdled and control plots, focusing on those involved in lignin and cellulose breakdown, and in the acquisition of N and P.
Materials and methods
Study site
The research was conducted at the Niwot Ridge AmeriFlux site, in the Front Range of the Rocky Mountains. The study site is approximately 25 km west of Boulder, Colorado (40°1′58″N; 105°32′47″W), at 3,050 m above sea level. The surrounding coniferous forest is dominated by Abies lasiocarpa (subalpine fir), Picea engelmannii (Engelmann spruce), and Pinus contorta (lodgepole pine). The forest is currently aggrading, having been logged ∼102 years ago. We conducted our experiment in a relatively pure stand of P. contorta to eliminate differential species effects. With an average gap fraction of 17%, the forest canopy (average height = 11.4 m) is relatively open (Monson et al. 2002). The site has little ground cover (<25%), mostly consisting of a low-growing shrub, Vaccinium sp. The soils are sandy inceptisols derived from granitic moraine with a thin organic horizon ranging from ∼2 to 6 cm. Past descriptions of the site can be found in Monson et al. (2006, 2002) and Scott-Denton et al. (2006, 2003).
Environmental measurements
Soil and air temperatures presented here are from a meteorological station ∼450 m from the study site, operated by the Niwot Ridge LTER program and the University of Colorado Mountain Research Station. The meteorological data presented here are daily means from a Campbell Instruments CR23X data logger recording at 5 s intervals (Campbell Scientific, Logan, UT, USA). The soil temperature data was collected at a depth of 5 cm. Soil moisture was determined gravimetrically after collecting 5 g of wet soil from the upper 5 cm and drying for a minimum of 48 h at 60 °C before reweighing the sample; it is calculated as (g wet–g dry)/g dry. Additional details of soil temperature and meteorological measurements can be found at: http://culter.colorado.edu/exec/.extracttoolA?c-1cr23x.ml.
Tree girdling
Our plots were set up in the manner of those in the girdling study conducted by Scott-Denton et al. (2006), and the girdling in July 2004 was timed to allow for the attenuation of transient treatment effects by the spring of 2005; our focal point was the springtime period of rhizodeposition. Plots were chosen in stands that contained 2–3 trees at least 10–15 cm in diameter at breast height, and in spots where the ground cover was sparse, so that the soil was dominated by root systems of the selected trees. The experimental plots were roughly circular in shape, being approximately 5–7 m in diameter, and were all within 200 m of each other on a shallow (5–6°) east-facing slope. Control plots were set up within 10 m of each of the girdled plots, and were similar in all other regards to the girdled plots. Over multiple days in the last week of July 2004, the trees were girdled using a saw to make two parallel incisions approximately 15 cm apart around the circumference of the tree at breast height, and the sharp edge of a hatchet to scrape away the bark and cambial layers between the incisions; there was no apparent regrowth of cambial tissue. We also trenched around the perimeter of the girdled plots to a depth of 15–20 cm to eliminate the intrusion of roots from trees surrounding the plots. There was no trenching around the control plots. In all, six girdled and six control plots were established.
Soil sampling
Samples from the organic horizon were collected from random locations within the plots using a 6-cm soil corer. When the organic horizon at the randomly selected location was ≥5 cm, then one 5 cm core was collected; if it was <5 cm, then two cores were collected to the bottom of the organic horizon (the organic horizon separates easily from the mineral soil) and composited. When the organic horizon was <2 cm, a different location was chosen. The entire depth of the organic horizon was kept from each core, and care was taken to avoid mineral soil. We focused on the organic layer because most fine roots are found within the upper 10–15 cm at this site (unpublished observations), thus we would expect rhizodeposition to be greatest in the surface soils (Scott-Denton et al. 2006). Within 24 h of collection the soils were homogenized by hand; we removed coarse plant litter and other debris, as well as larger (>1 mm) roots, but we were not able to remove all of the fine roots in the samples with relatively high root density (all samples had at least some roots in them). The soils were then immediately extracted for nutrients, fumigated for microbial biomass analysis, and frozen (for not longer than six months) for later enzyme analysis. Beginning with the week of girdling, we sampled every third month. During the spring, when rhizodeposition has been observed to occur at high rates (Scott-Denton et al. 2006), we increased our sampling to once every 1–3 weeks.
Nutrients and microbial biomass
In order to extract the soils for DOC, DON, and DIN, 25 ml of 0.5 M potassium sulfate (K2SO4) was added to 5 g of each homogenized sample and agitated on an orbital shaker table at 120 rpm for 1 h. The samples were then vacuum-filtered through Pall A/E glass fiber filters and frozen until analysis. At the same time, samples were fumigated with chloroform to measure soil microbial biomass using the method described by Scott-Denton et al. (2006). Briefly, 2 ml of ethanol free chloroform was added directly to 5 g of each sample in a 250-ml Erlenmeyer flask, which was then stoppered and incubated for 24 h, unstoppered and vented for 30 min, and then extracted in 0.5 M K2SO4 as described above. Concentrations of DOC and DON in these fumigated samples were used to quantify soil microbial biomass C and N (MB-C and MB-N) by subtracting the concentrations of DOC and DON in the unfumigated samples, and in soil-free controls that underwent an identical fumigation and extraction process, from the fumigants (Brookes et al. 1985; Sparling and West 1988; Vance et al. 1987). We did not apply an extraction efficiency correction factor (e.g., K ec) to the calculated values.
Dissolved inorganic N (DIN) in the K2SO4 extracts was analyzed with a Lachat QuikChem Nutrient Analyzer using the standard phenolate chemistry for \( {\text{NH}}^{ + }_{4} \) and the cadmium reduction/sulfanilamide chemistry for \( {\text{NO}}^{ - }_{3} \) (Lachat Instruments, Milwaukee, WI, USA). DOC and DON in the K2SO4 extracts and the fumigated soil K2SO4 extracts were analyzed using a Shimadzu total organic carbon analyzer (TOC 5000) equipped with a total dissolved nitrogen (TDN) module (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA). All samples were analyzed using the non-purgable-organic-C protocol on the TOC analyzer. DON was calculated as TDN–DIN. All concentrations were corrected using measurements from blanks.
Enzyme assays
The enzyme assays were conducted using the procedures outlined by Saiya-Cork et al. (2002). We analyzed our samples for the activities of nine different enzymes involved in the breakdown of plant litter, and nitrogen and phosphorus cycling (Table 1). Sample slurries were prepared by homogenizing 1 g of soil in 125 ml of 50 mM sodium acetate buffer, pH 5.0 (a typical pH for these soils) using a Virtex 45 tissue homogenizer (Virtex Inc., Yonkers, NY, USA). The slurries were continuously stirred using a magnetic stir plate while 200-μl aliquots were pipetted into 96-well microplates. For each assay and sample, 16 replicate wells were created.
All of the enzyme assays except phenol oxidase and peroxidase were fluorimetric (see Table 1 for a list of substrates). All of the fluorimetric assays were set up in black 96-well microplates. We added 50 μl of 200 μM substrate solution (Table 1) to each sample well. Blank wells were created with 50 μl of buffer and 200 μl of sample slurry. Negative control wells were created with 50 μl substrate solution and 200 μl of buffer. Quench standards were created with 50 μl of standard (10 mM 4-methylumbelliferone, or 7-amino-4-methylcoumarin in the case of leucine aminopeptidase) and 200 μl of the sample slurry. Reference standards were created with 50 μl of standard and 200 μl of buffer. Eight replicate wells were set up for each blank, negative control, and quench standard. The assay plates were incubated in the dark at 13 °C for up to 24 h. At the end of the incubation, 10 μl aliquots of 1.0M NaOH were added to each well to raise the pH, as 4-methylumbelliferone and 7-amino-4-methylcoumarin fluoresce more strongly at higher pH. Fluorescence was measured using a Fluoroskan II microplate fluorometer (Thermo Labsystems, Franklin, MA, USA) with 365 nm excitation and 460 nm emission filters. After correcting for quenching and for the negative controls, enzyme activities were expressed as nmol reaction product h−1 g dry soil−1 (nmol h−1 g−1).
The phenol oxidase and peroxidase assays were colorimetric, using L-3,4-dihydroxyphenylalanine (L-DOPA) as the substrate. All of the colorimetric assays were set up in clear 96-well microplates. For phenol oxidase, 50 μl of 25 mM L-DOPA was added to each sample well, containing 200 μl of sample slurry. The peroxidase assays received 50 μl of 25 mM L-DOPA, plus 10 μl of 0.3% H2O2. For the phenol oxidase assays, the negative control wells received 200 μl of buffer and 50 μl of the L-DOPA solution. The blank wells received 200 μl of sample slurry and 50 μl of acetate buffer. For the peroxidase assays, the negative control and blank wells also received 10 μl of 0.3% H2O2. Sixteen replicate sample wells were created for each assay and eight replicate wells were set up for the blanks and controls. The microplates were incubated in the dark at 13 °C for up to 24 h. Enzyme activity was quantified by measuring absorbance at 460 nm using a Molecular Devices Spectramax 340pc microplate spectrophotometer (Molecular Devices, Inc., Sunnyvale, CA, USA) and expressed as nmol h−1 g−1. Phenol oxidase activity was subtracted from peroxidase activity to calculate the net peroxidase activities reported here.
Statistics
Data were analyzed using two-way multivariate analysis of variance (MANOVA) with treatment and date as factors (N = 6 for each treatment, N = 9 dates). Data were also analyzed using one-way MANOVAs for each individual date with treatment as the factor. For MB-C and MB-N there were not enough data for the two-way MANOVAs (there are no MB-C or MB-N data for 30 July 2004). In those cases, separate one-way MANOVAs were performed with each date and treatment as factors. In order to account for the possibility of finding significant results simply by chance, which is a concern with such a large number of statistical comparisons, the Bonferroni correction was used with all of the MANOVA tests, and all of the P values reported here are Bonferroni-corrected. For all analyses, differences were considered significant at P ≤ 0.05. Systat version 11 (Systat Software Inc., Richmond, CA, USA) was employed for all of the statistical analyses, which were conducted on untransformed data.
Results
Climate
Average daily soil temperatures ranged from −1 to 20 °C over the course of the study, while average daily air temperatures ranged from −21 to 19 °C (Fig. 1). Soil temperatures were at or below 0 °C from 25 November 2004 through 15 May 2005, and were typically high enough for there to be unfrozen soil water (Patterson and Smith 1981), and therefore biological activity, throughout the winter (Fig. 1). There was a measurable snowpack beginning in late September, which had largely disappeared by the beginning of June. While variable, the snowpack was generally deepest in the spring of 2005. Precipitation was highly variable, but was generally greatest in the late fall of 2004 and in late April to early May 2005, and lowest in July 2005.
Soil temperatures from a depth of 5 cm, air temperatures, snow depth, and precipitation from the C1 metereological station at the Niwot Ridge LTER (available at http://culter.colorado.edu/NWT/), approximately 500 m from our girdled plots, and soil moisture (g H2O g dry soil−1) from the means of our girdled and control plots. Moisture contents in the two treatments were statistically indistinguishable, so the overall means of all samples are plotted here. Error bars for soil moisture are the standard error of the mean
Soil moisture was relatively low at 1.3 g H2O g−1 dry soil in our 2004 samples, and it then increased slowly through the spring, reaching a maximum of 3.0 g H2O g−1 dry soil on 26 April 2005. Soil moisture peaked in late April after above-freezing temperatures throughout the third week in April caused snowmelt water to percolate down into the soil. Soil moisture then decreased steadily for the remainder of the experiment, reaching a low of 0.3 g H2O g−1 dry soil on 20 July 2005.
Nutrients and microbial biomass
We observed higher dissolved organic carbon (DOC) in the control plots, overall (P = 0.05; Table 2). The seasonal time-course (Fig. 2) suggests that this was caused by differences between girdled and nongirdled plots during the spring, when DOC was elevated in the control plots but not the girdled plots. In May, DOC concentrations decreased, especially in the control plots, and by June concentrations in the girdled and nongirdled plots had converged. In June and July, DOC increased again in both treatments.
The effect of girdling on soil microbial biomass (MB-C) was similar to DOC (Fig. 2), and the decrease in MB-C was statistically significant (P ≤ 0.01). This treatment effect was driven by decreased MB-C in the girdled plots in the spring of 2005, with significantly lower MB-C in the girdled plots on 21 April 2005 (P ≤ 0.01; Fig. 2, Table 3).
The pattern for dissolved organic nitrogen (DON) was similar to that of DOC, although the overall difference between the girdled and nongirdled plots not statistically significant (P = 0.11; Table 2). However, there was a strong trend toward higher DON concentrations in the control soils in the spring of 2005 (Fig. 2). As a result of high variability over time in both treatments, date had a significant effect on DON concentrations (P = 0.04; Table 2).
The pattern for microbial biomass N (MB-N) was fairly similar to that of MB-C, as MB-N was higher in the control plots at the same time as MB-C. Across all dates, MB-N was significantly higher in the control plots (P = 0.03; Table 2). The seasonal time-courses suggest that the differences in MB-N we observed between treatments are similar to those for MB-C, but that MB-N varies more than MB-C (Fig. 2).
Girdling resulted in a significant overall difference in \( {\text{NH}}^{ + }_{4} \), compared to the control plots (P = 0.02; Table 2). In May 2005, \( {\text{NH}}^{ + }_{4} \) was significantly higher in the control plots (P = 0.01; Table 3) after increasing in late April (Fig. 3). \( {\text{NH}}^{ + }_{4} \) began to accumulate in the girdled plots in June 2005, however, and was significantly higher in this treatment from 22 June onward (P = 0.05; Table 3). As a result of this variability, the effect of date on \( {\text{NH}}^{ + }_{4} \) concentrations was significant (P ≤ 0.01).
\( {\text{NO}}^{ - }_{3} \) was typically low in both girdled and control plots, and girdling did not significantly affect \( {\text{NO}}^{ - }_{3} \) concentrations (P = 0.12; Table 2). The effect of date on \( {\text{NO}}^{ - }_{3} \) concentrations was not significant (P = 0.07; Table 2).
Exoenzyme activity
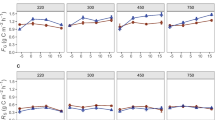
The girdling treatment did not have a significant overall effect on the activities of any of the enzymes involved in the breakdown of relatively easy-to-degrade C polymers (Fig. 4), except β-xylosidase (P = 0.04; Table 2). Their patterns of activity varied over time, but were similar for β-glucosidase (BG), α-glucosidase (AG), β-D-cellobiosidase (BC), and β-xylosidase (BX), and were much higher in the summer of 2004 than the summer of 2005 (Fig. 4). BG was the most active of these enzymes, with catalytic activities peaking at over 1,300 nmol h−1 g−1 in October 2004. Its activity had decreased to less than 150 nmol h−1 g−1 by July 2005, however. The activities of AG, BC, and BX were lower than those for BG, but they had similar seasonal dynamics; date was a significant factor affecting the activities of β-glucosidase, β-xylosidase, and β-D-cellobiosidase (Table 2).
Timecourses of β-glucosidase, α-glucosidase, β-xylosidase, and β-D-cellobiosidase in the girdled and control plots. Values are expressed as nmol reaction product catalyzed h−1 g dry soil−1. Error bars represent the standard error of the mean; asterisks represent a significant difference; P ≤ 0.05 unless marked otherwise
Girdling did not have a significant effect on N-acetyl-β-glucosaminidase (NAG) overall (P = 0.99; Table 2), although NAG activity was significantly higher in the girdled plots on 27 October 2004 (P = 0.04; Table 3). NAG was one of the most active enzymes we measured, with activities averaging nearly 500 nmol h−1 g−1 (Fig. 5). Similar to the other enzymes, NAG activity was highest in the summer and fall of 2004, and lowest in July 2005.
Timecourses of N-acetyl-β-glucosaminidase, leucine amino peptidase, and acid phosphatase in the girdled and control plots. Values are expressed as nmol reaction product catalyzed h−1 g dry soil−1. Error bars represent the standard error of the mean; asterisks represent a significant difference; P ≤ 0.05 unless marked otherwise
Leucine amino peptidase (LAP) was not significantly affected by girdling overall (P = 0.37; Table 2). However, LAP activity was significantly lower in the girdled plots on April 26 (P ≤ 0.01; Table 3). LAP had the lowest activity of any of the enzymes in our study, with a mean activity of less than 20 nmol h−1 g−1 (Fig. 5). In contrast with the other enzymes, LAP activity did not decline substantially after October, and unlike the other enzymes, its activity decreased sharply in both control and girdled plots in late April. Like the other enzymes, though, its activity was much lower in the summer of 2005 than in the summer of 2004. LAP was the only enzyme for which activity exhibited a significant interaction between date and treatment (P = 0.05; Table 2).
There was no significant effect of girdling on acid phosphatase (AP) activity (P = 0.75; Table 2). This was one of the most active enzymes in our study, with activities averaging nearly 800 nmol h−1 g−1 (Fig. 5). The dynamics of AP activity over time were similar to those for of BG and BX, except that AP activity increased on 16 May 2005, while activity for the others decreased. As with most of the other enzymes, AP was highest in the summer and fall of 2004, and lowest in the summer of 2005. As a result, date had a significant effect on AP activity (P ≤ 0.01).
Phenol oxidase (PO) activity was not significantly affected by girdling overall (P = 0.38; Table 2). However, its activity was significantly lower in the girdled plots on 30 July 2004, despite being relatively low in both girdled and nongirdled plots at this time (P = 0.04; Table 3). PO activity was also significantly reduced by girdling on 1 June 2005 (P = 0.02; Table 3). PO had a different pattern of activity than any of the other enzymes, and was lowest in the summer and fall of 2004, and highest in the spring of 2005 (Fig. 6). As a result of this variability, PO activity was significantly affected by date (P ≤ 0.01; Table 2).
Girdling did not have a significant effect on peroxidase (PX) activity, overall (P = 0.36; Table 2). PX activity peaked in the girdled plots on 11 January 2005 at over 2000 nmol h−1 g−1. Its pattern of activity was most similar to that of PO in that both had low rates of activity in the summer and fall of 2004, when the activities of the other enzymes were high, and higher rates in the spring of 2005, when the activities of the other enzymes were low. The peak in PX activity in the girdled plots on 11 January 2005 was unique, however. As a result of its variability over time, date had a significant overall effect on PX activity (P = 0.05).
Discussion
We observed elevated soil DOC in the control plots in late April 2005 (Fig. 2). There was no similar elevation in the girdled plots, suggesting that this increase in DOC was caused by the presence of an active root system (Högberg and Hogberg 2002). Spring increases in DOC in the control plots attenuated rapidly, and by early summer there was no longer any difference between the control and girdled plots. Another result that suggests girdling was effective in suppressing root activity is the accumulation of \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} \) we observed in the girdled plots in June 2005, which may reflect reduced tree N uptake.
The patterns of DOC we observed are in accord with those from a previous girdling study in the same forest. In their control plots, Scott-Denton et al. (2006) observed a late winter pulse in soil sucrose, which they attributed to root leakage associated with mechanical damage to roots as a result of winter soil heaving, and late April increases in soil DOC that they attributed to active root exudation. This conclusion is supported by eddy covariance data from the same forest, which indicates that photosynthesis began in this forest in mid-April in both 2002 and 2003, prior to their sampling (Scott-Denton et al. 2006). They also found that girdling caused little difference in soil sugars at other times. While we do not have sufficient data to determine if a similar release of root sugars also occurred in the late winter of 2004, the timing of our late-April samples in 2005 was similar to that of Scott-Denton et al. (2006) in 2002 and 2003, and also began after the start of tree photosynthesis, which was on April 19 in 2005 (data not shown). This suggests that the difference in DOC between our girdled and control plots in late April was also attributable to root exudation.
Because some fine roots were present in the soil samples, it is possible that leakage from severed roots was at least partly responsible for the difference in DOC between the girdled and control plots in the spring. However, if leakage from severed roots was a significant contributor to the DOC pool, it likely would have caused a more consistent elevation in control DOC concentrations than we observed. The fact that DOC concentrations in both treatments were similar in the winter, early spring, and again in July, at the height of the growing season, when Scott-Denton et al. (2006) found root respiration to be highest, suggests that the contribution from severed roots to the DOC concentrations we observed in the control plots was minimal. Because late April is when root exudation has previously been observed to be highest in this forest (Scott-Denton et al. 2006), the temporal pattern in DOC that we observed suggests that girdling caused differences in root C inputs to the soil.
As with DOC, we observed springtime increases in microbial biomass in only the control plots. This indicates that there are dynamic populations of soil microorganisms whose growth beneath the spring snow-pack is associated with active roots. In a girdling study of Scots pine, Högberg and Hogberg (2002) also found that girdling caused a significant reduction in microbial biomass, and concluded that the group of microorganisms most responsible for this reduction was ectomycorrhizal fungi. Since the lodgepole pines in our experimental plots are also ectomycorrhizal (unpublished observations), it is likely that a significant proportion of the microbial biomass lost in response to girdling was also from ectomycorrhizal fungi.
We observed substantial declines in microbial biomass in the control plots in late April 2005 coincident with sharp decreases in DOC, just after the peak in rhizodeposition. That this decrease did not occur in the girdled plots suggests microbial turnover was higher in the control plots in late April, which would result in higher rates of C mineralization in the control plots at this time. This suggests that a significant proportion of the priming effect in this soil is associated with rhizodeposition that initially stimulates microbial growth, and then is released as microbial biomass turns over during snowmelt.
Despite the fact that girdling resulted in a significant reduction in DOC and microbial biomass, there was little effect on exoenzyme activity. The only enzyme to show a significant overall response was BX, which was stimulated by girdling after the initial three months, but was unaffected by the following spring, when girdling had the largest impact on DOC and microbial biomass. We hypothesized that tree girdling would cause a decrease in soil enzyme activities because rhizodeposition has a positive priming effect on soil organic matter decomposition. This hypothesis was not supported by our observations. If anything, girdling caused an increase in soil enzyme activities, especially during the initial three months after initiation of the treatment.
An initial increase in enzyme activities is what we would expect to happen if tree girdling enhanced turnover of roots and their associated ectomycorrhizal fungi. The elevated NAG activity that we observed in the girdled plots is consistent with the hypothesis that tree girdling caused an initial increase in mycorrhizal fungal turnover (Högberg and Hogberg 2002). The observed increases in BX three months after girdling, and in PX activity another three months later, are also consistent with the hypothesis that root turnover in the girdled plots initially stimulated decomposition and exoenzyme activity. Existing models of litter decomposition indicate that we would expect a delay in the decomposition of lignin from dead roots relative to cellulose and other labile litter constituents (Sinsabaugh et al. 2002). Our data suggest that shortly following girdling, the breakdown of accessible polysaccharides such as chitin and xylan (a plant structural polymer less tightly associated with cell walls than cellulose, acted upon by BX) was stimulated. During mid-winter, approximately five months after girdling, the enhancement of enzyme activities in the girdled plots was minimal, suggesting that the the decomposition pulse related to turnover labile substrates had attenuated; however, there was a significant increase in PX activity, which is associated with degradation of the less labile substrate, lignin.
We hypothesized that enzyme activity would be reduced in the girdled plots, particularly during the spring, when rhizodeposition is greatest. However, we observed no spring reductions in exoenzyme activities except LAP. Girdling significantly reduced LAP activity in late April and the trend continued to early June 2005 (Table 3). The reduction in the girdled plots persisted despite a drop in activity affecting both types of plots in late April (Fig. 5). That the difference in LAP between the girdled and control plots grew when microbial biomass in the controls decreased suggests that LAP may contribute to the mineralization of microbial N.
Our finding that peptide decomposition and microbial biomass were reduced by girdling in the spring, while cellulose and lignin breakdown were not, suggests that rhizodeposition enhances microbial N acquisition, but has no significant relation to the breakdown of cellulose and lignin. This supports the hypotheses stated in past studies that enhanced mineralization of soil C pools in the presence of rhizodeposition is due not to enhanced breakdown of litter or complex SOM, but rather to the mineralization of microbial endocellular reserves (De Nobili et al. 2001), the turnover of microbial biomass (Dalenberg and Jager 1981; Dalenberg and Jager 1989; Wu et al. 1993), and greater microbial demand for N and other elements that are required to balance the elevated C availability from rhizodeposition (Kuzyakov 2002). Thus, rhizosphere microorganisms are able to respond positively to the presence of rhizodeposited C (Cheng et al. 1996), but this response requires them to allocate resources toward peptide degradation in order to enhance N availability. This conclusion is consistent with the hypothesis stated in Kuzyakov (2002) that greater microbial N acquisition is one of the principal causes of positive rhizosphere priming effects.
The reduction in DON in the girdled plots that we observed in the spring suggests that the activity of microorganisms growing on rhizodeposited C contributes to increases in N availability. Previous studies have also found that root exudation is associated with increased N availability in the rhizosphere (e.g., Hamilton and Frank 2001; reviewed in Bardgett 2005; Paterson 2003). This is caused by a stimulation of microbial N immobilization in response to elevated C availability, followed by microbial N mineralization due to C starvation as a result of reductions in root C inputs, and/or protozoans grazing rhizosphere microorganisms and excreting microbial N as \( {\text{NH}}^{ + }_{4} \) (Bonkowski 2004; Clarholm 1985). However, we did not analyze the composition of rhizodeposits and thus we cannot rule out the possibility that the difference in DON between treatments was the result of direct root N inputs to the soil. We note, however, that rhizodeposits are known to have high C/N ratios relative to microbial biomass and have previously been shown to cause N limitation as a result (Kuzyakov 2002). This causes us to doubt that the difference in DON between treatments was the result of direct root N inputs to the soil.
While the significant April increases in microbial biomass associated with rhizodeposition generally did not significantly alter enzyme activity, we found that the seasonal changes in climate exerted strong effects. Most enzymes had relatively high activities in the summer and fall of 2004, the summer of girdling, in large part due to a relatively wet season. The activities of most enzymes decreased with the onset of winter, but were still detectable in the soil despite the low temperatures; enzyme activities increased again in the spring, and then decreased steadily in the summer after girdling (Figs. 4, 5, 6) as the soils dried (Fig. 1) in response to drought. As a result, sampling date was a significant factor affecting the activities of all of the enzymes we measured except AG.
Conclusions
The results of this study indicate that the soil microbial biomass in a subalpine coniferous forest benefits from root C inputs in the spring, when the soils are still covered with snow. Our results suggest that the microorganisms growing on rhizodeposited C contribute to increases in N availability by increasing protein breakdown. While enhanced protein degradation is likely to have a small priming effect on soil C mineralization, our data suggest that rhizodeposition does not stimulate the decomposition of cellulose and lignin. Because the microbial community growing underneath the late snowpack includes populations that depend on rhizodeposition rather than the breakdown of organic polymers, the microbial biomass can grow beyond the constraints allowed by exoenzyme-mediated C acquisition. Thus, in the fall and winter microbial dynamics are controlled by rates of C acquisition from decomposition, but they are controlled by both decomposition and rhizodeposition in the spring. Our results support the hypothesis that the priming effect of rhizodeposition is due to influences on microbial biomass, turnover, and demand for other nutrients required to balance the higher availability of C, rather than the direct stimulation of exoenzymes required for depolymerization of soil organic matter.
References
Bardgett RD (2005) The biology of soil: a community and ecosystem approach. Oxford University Press, New York, pp 120–121
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Bhupinderpal-Singh, Nordgren A, Lofvenius MO, Högberg MN, Mellander PE, Högberg P (2003) Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environ 26:1287–1296
Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162:617–631
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Cheng W, Zhang Q, Coleman DC, Carroll CR, Hoffman CA (1996) Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol Biochem 28:1283–1288
Clarholm M (1985) Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol Biochem 17:181–188
Dalenberg JW, Jager G (1981) Priming effect of small glucose additions to C-14-labeled soil. Soil Biol Biochem 13:219–223
Dalenberg JW, Jager G (1989) Priming effect of some organic additions to C-14-labeled soil. Soil Biol Biochem 21:443–448
De Nobili M, Contin M, Mondini C, Brookes PC (2001) Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33:1163
Fontaine S, Bardoux G, Benest D, Verdier B, Mariotti A, Abbadie L (2004) Mechanisms of the priming effect in a savannah soil amended with cellulose. Soil Sci Soc Am J 68:125–131
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Högberg P, Ekblad A (1996) Substrate-induced respiration measured in situ in a C3-plant ecosystem using additions of C4-sucrose. Soil Biol Biochem 28:1131
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154:791–795
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Hutsch BW, Augustin J, Merbach W (2002) Plant rhizodeposition: an important source for carbon turnover in soils. J Plant Nutr Soil Sci 165:397–407
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sc 165:382–396
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Mary B, Fresneau C, Morel JL, Mariotti A (1993) C and N cycling during decomposition of root mucilage, roots and glucose in soil. Soil Biol Biochem 25:1005
Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK (2006) Winter forest soil respiration controlled by climate and microbial community composition. Nature 439:711
Monson RK, Turnipseed AA, Sparks JP, Harley PC, Scott-Denton LE, Sparks K, Huxman TE (2002) Carbon sequestration in a high-elevation, subalpine forest. Global Change Biol 8:459–478
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750
Patterson DE, Smith MW (1981) The measurement of unfrozen water-content by time domain reflectometry: results from laboratory tests. Can Geotech J 18:131–144
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Scott-Denton LE, Rosenstiel TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Global Change Biol 12:205–216
Scott-Denton LE, Sparks KL, Monson RK (2003) Spatial and temporal controls of soil respiration rate in a high-elevation, subalpine forest. Soil Biol Biochem 35:525–534
Sinsabaugh RL, Carreiro MM, Alvarez S (2002) Enzyme and microbial dynamics of litter decomposition. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology, and applications. Marcel Dekker, New York, pp 249–265
Sparling GP, West AW (1988) A direct extraction method to estimate soil microbial carbon: calibration in situ using microbial respiration and carbon-14 labelled cells. Soil Biol Biochem 20:337–344
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass carbon. Soil Biol Biochem 19:703–708
Wu J, Brookes PC, Jenkinson DS (1993) Formation and destruction of microbial biomass during the decomposition of glucose and ryegrass in soil. Soil Biol Biochem 25:1435–1441
Acknowledgments
Thanks to the Niwot Ridge LTER program for providing measurement support for the inorganic N analyses. We thank Dr. Bill Bowman (University of Colorado Mountain Research Station) for providing valued logistical support in establishing the girdled and nongirdled plots. This work was financially supported by a grant from the South-Central Section of the National Institute for Global Environmental Change (NIGEC) through the US Department of Energy (BER Program) (Cooperative Agreement No. DE-FC03-90ER61010), and grants from the US National Science Foundation’s Ecological and Evolutionary Physiology (IBN-0212267) and Microbial Observatories (MCB-0455606) programs. We thank Laura Brotherton, Sean Burns, Peter Casey, Eric Reinsvold, and Lisa Tiemann for assistance in the field and lab. We would also like to thank Robert Sinsabaugh, Christian Lauber, and Marcy Gallo for assistance with the enzyme assays, and Jason Neff and Jon Carrasco for assistance with the DOC and DON analyses. We are also grateful for Bruce Caldwell's help in reviewing this manuscript and providing valuable input on table 1. Climate data were provided by the Niwot Ridge Long-Term Ecological Research project (NSF DEB 0423662) and the Mountain Research Station (BIR 9115097).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Zoe Cardon.
Rights and permissions
About this article
Cite this article
Weintraub, M.N., Scott-Denton, L.E., Schmidt, S.K. et al. The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154, 327–338 (2007). https://doi.org/10.1007/s00442-007-0804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0804-1