Abstract
Purpose
Variation in soil microbial metabolism remains highly uncertain in predicting soil carbon (C) sequestration, and is particularly and poorly understood in agroecosystem with high soil phosphorus (P) variability.
Materials and methods
This study quantified metabolic limitation of microbes and their association with carbon use efficiency (CUE) via extracellular enzymatic stoichiometry and biogeochemical equilibrium models in field experiment employing five inorganic P gradients (0, 75, 150, 225, and 300 kg P ha−1) in farmland used to grow peas.
Results and discussion
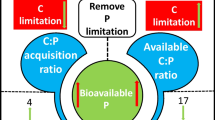
Results showed P fertilization significantly increased soil Olsen-P and NO3−-N contents, and enzyme activities (β-1,4-glucosidase and β-D-cellobiosidase) were significantly affected by P fertilization. It indicated that P fertilization significantly decreased microbial P limitation due to the increase of soil available P. Interestingly, P application also significantly decreased microbial nitrogen (N) limitation, a phenomenon primarily attributable to increasing NO3−-N content via increasing biological N fixation within the pea field. Furthermore, P fertilization increased microbial CUE because the reduction in microbial N and P limitation leads to higher C allocation to microbial growth. Partial least squares path modeling (PLS-PM) further revealed that the reduction of microbial metabolic limitation is conducive to soil C sequestration.
Conclusions
Our study revealed that P application in agroecosystem can alleviate not only microbial P limitation but also N limitation, which further reduces soil C loss via increasing microbial CUE. This study provides important insight into better understanding the mechanisms whereby fertilization mediates soil C cycling driven by microbial metabolism in agricultural ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agro-ecosystems are one of the most important components of terrestrial ecosystem and the most active part of global soil carbon (C) pool due to intense human activity and nutrient input (Lal 2011; Zhao et al. 2017). Phosphorus (P) is one of the main nutrient inputs applied to agroecosystems (Smil 2000). Although P application is a typical management practice used to alleviate soil nutrient deficiency in P-deficient farmland, the widespread use of P fertilization can affect soil C cycles, leading to great uncertainty of the soil C pool (Poeplau et al. 2016; Yue et al. 2017; Feng and Zhu 2019). Therefore, it is of great significance to investigate the response of soil C cycling to P addition to predict C storage and cycling mechanisms in agricultural ecosystems.
Soil microorganisms could drive nutrient cycling through soil organic matter (SOM) degradation and mineralization; their associated metabolic processes thus play a key role in soil C sequestration (Schimel et al. 2007; Zang et al. 2018; Cui et al. 2020a). Previous studies reported that microbial metabolism under P fertilization had dramatic but inconsistent influences on C sequestration. Wei et al. (2020) demonstrated that fertilizer increased soil organic carbon (SOC) content by influencing microbiota and enzyme activity. Zhang et al. (2015) also reported P applications have increased soil C pools by directly modifying the microbial stoichiometry of biomass to be C-limited. High P availability can potentially increase C sequestration by alleviating stoichiometric constraints (Manzoni et al. 2012). In contrast, some studies reported that P addition has accelerated the decomposition of recalcitrant C by stimulating microbial growth and increasing enzyme activities, resulting in negative impacts on soil C sequestration (Fisk et al. 2015; Luo et al. 2019). It has also been found that P addition leads to less efficient microbial utilization of C, thereby resulting in significant C loss through respiration (Thirukkumaran and Parkinson 2000; Poeplau et al. 2016). These findings suggest that potential mechanisms and factors related to effects of microbial metabolism alteration induced by P fertilization on soil C storage are contentious. Therefore, identifying the microbial metabolic responses to P addition in agroecosystems may be the key to revealing the effects of P fertilization on C sequestration in soil.
Nutrient stoichiometry is the key controlling driver of microbial metabolism (Sinsabaugh et al. 2009). Nutrient supplementation alters soil nutrient stoichiometry, which may cause shifts in microbial metabolic limitation (Zhang et al. 2015; Cui et al. 2020a). Changes in microbial metabolic limitation may be a critical juncture for the influence of microbial metabolism on C sequestration. Ecoenzymatic stoichiometry can reflect the relationships between microbial metabolic demands and soil nutrient supplies (Sinsabaugh et al. 2009; Cui et al. 2020b). Currently, more and more studies are employing ecoenzymatic stoichiometry to reflect microbial metabolic limitation (Sinsabaugh and Shah 2012; Zhang et al. 2019; Ma et al. 2021; Wu et al. 2021). Xiao et al. (2020) showed that the threshold elemental ratio (TER) revealed the microbial nutrient metabolisms were co-limited by nitrogen (N) and P during plant secondary succession. Additionally, Chen et al. (2018) also reported that N addition aggravated microbial C-limitation through ecoenzymatic stoichiometry. Therefore, this model can be used to elucidate changes in microbial metabolic limitation under P addition in agricultural ecosystems.
Variations in microbial metabolic restriction may affect soil C cycling processes, wherein the most critical characterization is microbial carbon use efficiency (CUE) (Spohn et al. 2016; Kallenbach et al. 2019; Widdig et al. 2020). CUE is a parameter to quantify the ratio between C allocated to growth and C taken up by microorganisms (Manzoni et al. 2012; Geyer et al. 2016; Mehnaz et al. 2018). Manzoni et al. (2012) reported that high P availability can potentially increase CUE by alleviating stoichiometric constraints. However, some studies have reported that intracellular C partitioning can change under high N availability, resulting in lower C allocation to respiration and higher C allocation to growth (Dijkstra et al. 2015; Spohn 2016). Our recent study also found that microbial metabolic limitation had a strong negative effect on microbial CUE (Cui et al. 2020b), which could be detrimental to soil C sequestration. Therefore, the way in which CUE responds to shifts in microbial metabolic limitation is crucial for understanding the effects of P addition on soil C dynamics in agricultural ecosystems.
For this study, a field experiment was conducted employing five inorganic P gradients (0, 75, 150, 225, and 300 kg P ha−1), while an ecoenzymatic stoichiometry model was used to determine microbial metabolic limitation and to explore how microbial metabolic limitation regulates microbial CUE. We hypothesized that (1) P fertilization could potentially alleviate microbial P limitation, but has no effect on microbial N limitation; (2) P addition could potentially lead to greater C requirements due to microbial stoichiometric homeostasis (i.e., where microbial metabolism maintains a balance among C, N, and P); and (3) the mitigation of microbial P limitation may increase microbial CUE because microorganisms may reduce energy (C) and resource investments during P acquisition. These results combined will improve our understanding of fertilizer mediate soil C cycling mechanisms.
2 Materials and methods
2.1 Site description
The study site is part of the Cao Xinzhuang Experimental Farm of Northwest A&F University, Xianyang City, Shaanxi Province, China (108˚ 04′ E, 34˚ 17′ N, 520 m a.s.l.), which is a flat triple-terraced cropland area situated within the Wei River plain. This area is influenced by a semi-humid and semiarid continental monsoon climate, with an average annual rainfall of 550–660 mm, mainly concentrated between seven and 9 months per annum. The annual average temperature is 12.9 ℃, for which the average temperature of the warmest month is 23.4 ℃, and the coolest month is 0.4 ℃.
2.2 Experimental design and treatments
Our field experiment adopted a randomized complete block design with a plot size of 35 m2 (5 m × 7 m). Prior to planting, all plots were laid out using 20 cm ridge furrows and 15 cm ridge heights. A total of 15 blocks (1 m × 1.5 m) were established. Plots were seeded with peas (Zhong Wan no. 8) in 2017 and then fertilized with superphosphate Ca(H2PO4)2 once per year in October. Five different P fertilization treatments were employed: 0, 75, 150, 225, and 300 kg P ha−1 (hereafter referred to as P0, P75, P150, P225, and P300, respectively). Five corresponding treatments were established whereby each treatment was replicated thrice.
Initially, P (Ca(H2PO4)2, containing 14–20% P was applied to each block. Fertilizer was then added once before the planting of the pea crop, which was artificially spread on the soil surface and then plowed into the ground before planting. For the treatments, all other agronomic practices employed were standard and uniform. Only natural rainfall was used for pea growth.
2.3 Soil sample collection
Soil samples were collected from the top 20 cm of the soil profile after litter was removed in July 2019. Ten soil cores were collected at intervals along an “S” shape pattern from each plot and mixed into one composite sample. After carefully removing roots, litter, debris, and stones, each soil sample was divided into two subsamples for future analysis. One subsample was immediately transported to the laboratory and then passed through a 2-mm sieve before being stored at 4 ℃ for analysis of microbial biomass and enzyme activities within a 2-week period. The other subsample was immediately air-dried to analyze physicochemical properties.
2.4 Analysis of soil properties
Soil moisture was determined using the gravimetric method in fresh soils at 105 ℃ for 24 h. Soil pH was measured in a 2.5:1 water-soil mixture using a glass electrode meter (InsMark™ IS126, Shanghai, China). Dissolved organic carbon (DOC) was extracted using 0.5 M K2SO4, and measured using a TOCII Liquid analyzer (Elementar, Germany) (Jones and Willett 2006). SOC content was determined through dichromate oxidation. Total nitrogen (TN) was measured by the Kjeldahl method (Bremner and Mulvaney 1982). Additionally, NO3−-N and NH4+-N content was measured using a Seal Auto Analyzer after being extracted using a 1:5 solution of 2 M KCl. Total phosphorus (TP) and available P (Olsen-P) were extracted using H2SO4-HClO4 and 0.5 M NaHCO3 and determined by the molybdenum blue method using an ultraviolet spectrophotometer (Hitachi UV2300) at 700 nm (Olsen and Sommers 1982).
2.5 Analysis of microbial biomass and soil enzyme activities
The chloroform-fumigation extraction method was used in this study to measure microbial C, N, and P biomass (i.e., MBC, MBN, and MBP, respectively). The experimental procedure used in this study was consistent with our previous study (Cui et al. 2018). Additionally, the conversion factors of MBC, MBN, and MBP were 0.45, 0.54, and 0.40, respectively (Joergensen 1996).
Five soil enzyme activities, including two C-acquiring enzymes (β-1,4-glucosidase (BG) and β-D-cellobiosidase (CBH)), two N-acquiring enzymes (β-1,4-N-acetylglucosaminidase (NAG) and L-leucine aminopeptidase (LAP)), and one P-acquiring enzyme (alkaline phosphatase (AP)), were assayed using the method described by Saiya-Cork et al. (2002). The experimental procedure used in this study has been described in previous study (Cui et al. 2019a).
2.6 Threshold elemental ratios (TER) and CUE
We used the method described by Sinsabaugh et al. (2009) to calculate TER for C:N and C:P using the two following equations:
where TERC:N and TERC:P are the threshold ratios. (BG + CBH)/ (NAG + LAP) and (BG + CBH)/AP are the enzymatic activity ratios. BC:N and BC:P are the C/N and C/P ratios of microbial biomass N and P, respectively. n0 and p0 are normalization constants for N and P. n0 = eintercept in the standardized major axis (SMA) for ln(BG + CBH) vs. ln(NAG + LAP); p0 = eintercept in the SMA regressions for ln(BG + CBH) vs. ln(AP) (Tables S1 and S2).
Microbial CUE was calculated using the biogeochemical-equilibrium model (Sinsabaugh et al. 2013):
EEA was soil extracellular enzyme activity. EEAC:N and EEAC:P was calculated using (BG + CBH)/(NAG + LAP) and (BG + CBH)/AP, respectively. Molar C/X ratios of the labile substrate were calculated as estimates of LC:N and LC:P. KC:N and KC:P are half-saturation constants for CUE based on C, N, and P availability. For all models, we assumed that KC:N and KC:P were 0.5 and that CUEmax was 0.6, following the example of Sinsabaugh et al. (2013).
2.7 Statistical analysis
All statistical calculations (i.e., correlations and significant differences) were conducted using SPSS 20.0 (SPSS Inc., Chicago, USA). One-way analysis of variance (ANOVA) and least significant difference (LSD) multiple comparisons (P < 0.05) were used to examine P concentration effects on soil physicochemical properties, microbial biomass, extracellular enzyme activities, metabolic limitation, and CUE. Values were expressed as means ± standard errors (n = 3). Graphs were created using Origin 2020. Correlations among soil physicochemical properties, microbial biomass, microbial metabolic limitation, and CUE were calculated using a two-tailed Pearson rank-order correlation test. Heat map graphs were generated using the “corrplot” package in the R (Wei and Simko 2017). Relationships between enzyme activities were analyzed with type II SMA regression using the “smatr” package in the R (David et al. 2012). Data were loge-transformed prior to regression analysis to conform to stoichiometric analysis conventions and to normalize variance. Partial least squares path modeling (PLS-PM) was additionally used to identify potential pathways controlling microbial metabolic limitation and CUE. The model was constructed using the “innerplot” function from the “plspm” package in the R (v. 3.6.3) (Sanchez et al. 2017).
3 Results
3.1 Effects of P fertilization on soil physicochemical properties
P fertilization significantly affected soil moisture, pH, and available nutrients (P < 0.05; Table 1). Under P addition, neither SOC nor NH4+-N content showed significant effects (P > 0.05). However, DOC content decreased with an increase in P addition, wherein the P0 treatment yielded the highest value (103.4 ± 6.66 mg kg−1). Additionally, TP, Olsen-P, and NO3−-N content was higher in the P225 and P300 treatments compared to the P0, P75, and P150 treatments, wherein the highest concentrations in the P300 treatment were 1.00 ± 0.01 g kg−1, 9.60 ± 0.48 mg kg−1, and 15.22 ± 0.27 mg kg−1, respectively. Under the P supplement treatments, TN content also increased.
3.2 Influences of P fertilization on microbial biomass and extracellular enzyme activities
P fertilizer significantly affected microbial biomass (P < 0.05; Table 2). Moreover, MBC, MBN, and MBP content gradually increased under P addition, wherein the P300 treatment yielded the highest value (i.e., 123.70 ± 1.33 mg kg−1, 5.75 ± 0.51 mg kg−1, and 2.38 ± 0.16 mg kg−1, respectively). Additionally, P fertilization significantly affected C-acquisition enzyme activities (including BG and CBH) and P-acquisition enzyme activities (AP) (P < 0.001; Table 2). CBH content was higher in the P225 and P300 treatments compared to the P0, P75, and P150 treatments. However, P fertilization did not significantly affect N-acquisition associated with enzyme activities (including NAG and LAP).
3.3 Influences of P fertilization on threshold elemental ratios, microbial metabolic limitation, and CUE
Based on stoichiometric microbial C:N:P values procured from enzyme data, estimated TERC:N and TERC:P significantly differed among the different P treatments (P < 0.001: Fig. 1). For example, TERC:N significantly decreased under P treatments, wherein the P300 treatment yielded the lowest value (1.43 ± 0.20) (Fig. 1a). TERC:P also significantly decreased under the P addition, wherein the P300 treatment yielded the lowest value (0.01 ± 0.00) while the P150, P225, and P300 treatments exhibited no significant differences (Fig. 1c). In this study, TER values subtracted by corresponding available soil nutrient ratios were used to calculate the nutrient limitation of microbes (Fig. 1b, d), wherein negative values indicated no microbial nutrient limitation. Results indicated that soil microbial nutrient limitation under the P treatments significantly decreased (P < 0.001: Fig. 1b, d). Furthermore, N limitation decreased under the P addition, after which N was no longer limited under the P300 treatment (−4.17 ± 0.23) (Fig. 1b). Results also showed that P limitation significantly decreased, wherein the P150, P225, and P300 treatments were lower than the P0 and P75 treatments, at which point the P150 treatment was no longer limited by P (Fig. 1d).
The threshold elemental ratio TERC:N and TERC:P (a and c, respectively) and N limitation and P limitation (c and d, respectively) of different P fertilizer treatments. P0, P75, P150, P225, and P300 represent P fertilizer addition of 0, 75, 150, 225, and 300 kg P ha−1, respectively. Different lowercase letters indicate significant differences (P < 0.05) among the fertilizer addition treatments based on one-way ANOVA followed by LSD test
Microbial CUE varied significantly among the different P treatments (P < 0.05: Fig. 2). For example, CUE values increased along with an increase in P addition, wherein the P300 treatment yielded the highest microbial CUE value (0.55 ± 0.01), the P0 treatment yielded the lowest (0.45 ± 0.16), and the P75, P150, P225, and P300 treatments exhibited no significant differences.
Carbon use efficiency (CUE) under different P fertilizer treatments. P0, P75, P150, P225, and P300 represent P fertilizer treatments of 0, 75, 150, 225, and 300 kg P ha−1, respectively. Different lowercase letters indicate significant differences (P < 0.05) among the fertilizer addition treatments based on one-way ANOVA followed by LSD test
3.4 Relationships between microbial metabolic limitation and CUE with soil physicochemical properties
This study found that both P limitation and N limitation were negatively correlated with pH, MBC, MBN, MBP, Olsen-P, and NO3−-N, and P limitation and N limitation were positively correlated (P < 0.001: Fig. 3). Moreover, CUE was positively correlated with MBC, MBN, MBP, Olsen-P, and NO3−-N and negatively correlated with both P limitation and N limitation (P < 0.001). Additionally, PLS-PM analysis identified direct and indirect effects of P fertilization on soil available nutrients, microbial biomass, microbial metabolic limitation, and CUE (Fig. 4 a). Microbial biomass included MBC, MBN, and MBP, and it is used as a latent variable in our PLS-PM model. The quality and performance of the model are mainly judged by goodness of fit (GOF) and R2. When GOF is more than 0.7, it is fine of the model that we constructed. In our model, the GOF was 0.83, indicating that the model is reasonable. R2 is acceptable between 0.3 and 0.6, and more than 0.6 the results are better. In our model, R2 was between 0.3 and 0.95. Therefore, the PLS-PM model that we constructed was reasonable. The PLS-PM analysis identified that P fertilization positively affected Olsen-P content (0.95 of the direct effects) and microbial biomass (0.94 of the direct effects). Microbial biomass directly affected NO3−-N content (0.83 of the direct effects). Olsen-P content and NO3−-N content further negatively affected microbial P limitation and microbial N limitation (−0.89 and −0.80 of the direct effects, respectively). Finally, microbial P limitation and microbial N limitation directly affected CUE (−0.30 and −0.61 of the direct effects, respectively).
Correlation heat map results for the soil physicochemical properties, microbial biomass (MBC, MBN, and MBP), microbial metabolic limitation, and CUE. MBC microbial biomass carbon, MBN microbial biomass nitrogen, MBP microbial biomass phosphorus, Olsen-P soil available phosphorus, P limitation microbial phosphorus limitation, N limitation microbial nitrogen limitation, CUE carbon use efficiency. *Correlation is significant at P < 0.05 (two-tailed); **Correlation is significant at P < 0.01 (two-tailed); ***Correlation is significant at P < 0.001 (two-tailed)
Cascading relationships of microbial metabolic limitation and CUE along with soil physicochemical properties. Partial least squares path modeling (PLS-PM) elucidating major pathways of the influence of soil physicochemical properties on microbial N and P limitations. Solid and dashed lines indicate positive and negative causality flow (P < 0.05), respectively. Numbers on arrows indicate significant standardized path coefficients. R2 indicates the variance of dependent variables explained by the model. Microbial biomass: microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), and microbial biomass phosphorus (MBP); Olsen-P soil available phosphorus; P limitation microbial phosphorus limitation; N limitation microbial nitrogen limitation; CUE carbon use efficiency
Overall, P fertilization negatively affected microbial P limitation and N limitation (−0.85 and −0.62 of the total effects, respectively) and positively affected CUE (0.63 of the total effects: Fig. 4b). Olsen-P had the greatest and most negative effect on microbial P limitation (−0.89 of the total effects), while NO3−-N content and microbial biomass significantly affected microbial N limitation (−0.80 and −0.66 of the total effects, respectively). Both microbial P limitation and microbial N limitation also significantly affected CUE (−0.30 and −0.62 of the total effects, respectively).
4 Discussion
4.1 P fertilization alleviates microbial metabolic limitation in agricultural ecosystems
Extracellular enzymatic stoichiometry revealed that microorganisms were strongly limited by N and P in P0 and P75 (Fig. 1b, d). P fertilization (0–300 kg P ha−1) negatively affected microbial P limitation (−0.85 of the total effects: Fig. 4b), and soil Olsen-P was negatively correlated with microbial P limitation (P < 0.001: Fig. 3), namely, that P fertilization alleviates microbial P limitation. Fisk et al. (2015) reported that P application can be used to directly alleviate microbial P limitation. Therefore, it can be concluded that P fertilization alleviates microbial P limitation by increasing Olsen-P content in soil (Fig. 5).
It is interesting to note that P fertilization also alleviates microbial N limitation (Fig. 1b), which contradicts our first hypothesis. The decrease of microbial N limitation was attributed to an increase in NO3−-N content (−0.80 of the total effects: Fig. 4). In this study, the increase in NO3−-N content may be attributed to two factors. On the one hand, mitigation of microbial metabolic limitation can increase microbial biomass (Fig. 4; Table 2) (Cheng et al. 2018), which would further stimulate the growth of nitrifying bacteria to increase nitrification rates, thereby increasing the inorganic N concentration in soil (Mori et al. 2010; Chen et al. 2017; Cheng et al. 2018). On the other hand, P availability can potentially significantly alter soil N availability by controlling N fixation (Vera-Nunez et al. 2007; Aleixo et al. 2020; Miguez-Montero et al. 2020). The crops we grew were peas in this study, which are known to fix N. The most important and efficient processes of N fixation are the formation of root nodules on legumes (Santi et al. 2013). Symbiotic association of soybean root system with soil bacteria can realize N fixation (Jensen et al. 2020). Alfalfa can biologically fixate N through symbiotic association of its root nodules with soil bacteria (Mahmud et al. 2020). Chickpea also affects soil microbial population through symbiotic N fixation with root nodules (Stagnari et al. 2017). In addition, P fertilizers may stimulate the growth of legumes and increase nodule production (Reed et al. 2007; Zheng et al. 2016). Additionally, studies have reported that P fertilization significantly stimulates activity and community abundance of N-fixing bacteria in soil, thus increasing biological N fixation (Vesterdal and Raulund-Rasmussen 2002; Houlton et al. 2008; Pang et al. 2011). Therefore, P addition increases NO3−-N content by increasing nitrification rates and biological N fixation, and then alleviating microbial N limitation (Fig. 5).
4.2 P fertilization increases microbial CUE in agricultural ecosystems
CUE is pivotal for the understanding of soil C turnover driven by microbial metabolism (Manzoni et al. 2012; Spohn and Chodak 2015; Wang et al. 2015). In this study, P fertilization increased C-acquiring enzyme activities (including BG and CBH) (Table 2), showing that microorganisms secreted more enzymes to stimulate SOM decomposition to obtain nutrients after P addition due to the microbial stoichiometric homeostasis mechanism (Zhu et al. 2021), which supports our second hypothesis, namely, that P supplementation leads to greater C requirements for microorganisms. Widdig et al. (2020) also showed that P addition increased C requirements for microorganisms, which was consistent with our results. Additionally, P fertilization also significantly increased microbial CUE (Fig. 2), which was potentially due to the transition of intracellular C allocation in microorganisms (Manzoni et al. 2012; Sinsabaugh et al. 2016).
PLS-PM analysis further deciphered the key pathways of microbial metabolic limitation that drives soil C sequestration (Fig. 4). The decrease in microbial P and N limitation contributed to higher microbial CUE by affecting microbial growth and metabolism processes (Figs. 4 and 5). When both microbial N and P limitations were alleviated, microorganisms would reduce the energy (C) and resource investment in N and P acquisition and would devote more energy (C) and resources to growth, which supports our third hypothesis that the mitigation of microbial metabolic limitation increases microbial CUE (Sinsabaugh and Shah 2012; Cui et al. 2019b). Similarly, Spohn et al. (2016) also observed that intracellular C partitioning changes under high nutrient availability, leading to the lower allocation of C to respiration and the higher allocation of C to growth, resulting in an increase in CUE that promotes soil C sequestration. Our results showed that P fertilization can promote soil C sequestration by increasing CUE in farmland, which is in accordance with the results from Zhao et al. (2017). In addition, Sinsabaugh et al. (2013) also showed that microbial CUE may increase as a result of changing microbial community composition, when both N and P availability are increasing. Therefore, P fertilization alleviates microbial metabolic limitation, which further promotes soil C sequestration via allocating more C to growth-increasing microbial CUE in agricultural ecosystems (Fig. 5).
5 Conclusions
This study revealed that P fertilization can alleviate not only microbial P limitation but also microbial N limitation due to an increase in both Olsen-P and NO3−-N content after the application of P. Additionally, P fertilization increased microbial CUE because the reduction in both microbial N and P limitation led to more C allocation to growth. Our study suggests that agro-ecosystem with fertilizer is conducive to soil C storage by alleviating microbial metabolic limitation. This study offers important insight into better understanding the effects of fertilization on soil C pools in agricultural ecosystems. Further studies should therefore design relevant incubation experiments to study the response of microbial community structure to fertilizer addition, which can further explain potential microbial mechanisms related to fertilizer enrichment on soil C sequestration.
References
Aleixo S, Gama-Rodrigues AC, Gama-Rodrigues EF, Campello EFC, Silva EC, Schripsema J (2020) Can soil phosphorus availability in tropical forest systems be increased by nitrogen-fixing leguminous trees? Sci Total Environ 712. https://doi.org/10.1016/j.scitotenv.2019.136405
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, Chemical and Microbial Properties. Agronomy Society of America, Madison, pp 595–624
Chen H, Zhang W, Gurmesa GA, Zhu X, Li D, Mo J (2017) Phosphorus addition affects soil nitrogen dynamics in a nitrogen-saturated and two nitrogen-limited forests. Eur J Soil Sci 68:472–479. https://doi.org/10.1111/ejss.12428
Chen H, Li DJ, Zhao J, Zhang W, Xiao KC, Wang KL (2018) Nitrogen addition aggravates microbial carbon limitation: Evidence from ecoenzymatic stoichiometry. Geoderma 329:61–64. https://doi.org/10.1016/j.geoderma.2018.05.019
Cheng Y, Wang J, Sun N, Xu MG, Zhang JB, Cai ZC, Wang SQ (2018) Phosphorus addition enhances gross microbial N cycling in phosphorus-poor soils: a 15N study from two long-term fertilization experiments. Biol Fertil Soils 54:783–789. https://doi.org/10.1007/s00374-018-1294-5
Cui YX, Fang LC, Guo XB, Wang X, Zhang YJ, Li PF, Zhang XC (2018) Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol Biochem 116:11–21. https://doi.org/10.1016/j.soilbio.2017.09.025
Cui YX, Bing HJ, Fang LC, Wu YH, Yu JL, Shen GT, Jiang M, Wang X, Zhang XC (2019) Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 338:118–127. https://doi.org/10.1016/j.geoderma.2018.11.047
Cui YX, Fang LC, Deng L, Guo XB, Han F, Ju WL, Wang X, Chen HS, Tan WF, Zhang XC (2019) Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci Total Environ 658:1440–1451. https://doi.org/10.1016/j.scitotenv.2018.12.289
Cui YX, Wang X, Zhang XC, Ju WL, Duan CJ, Guo XB, Wang YQ, Fang LC (2020a) Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol Biochem 147. https://doi.org/10.1016/j.soilbio.2020.107814
Cui YX, Zhang YL, Duan CJ, Wang X, Zhang XC, Ju WL, Chen HS, Yue SC, Wang YQ, Li SQ, Fang LC (2020b) Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res 197. https://doi.org/10.1016/j.still.2019.104463
David IW, Remko AD, Daniel SF, Sara T (2012) smatr 3 - an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259
Dijkstra P, Salpas E, Fairbanks D, Miller EB, Hagerty SB, van Groenigen KJ, Hungate BA, Marks JC, Koch GW, Schwartz E (2015) High carbon use efficiency in soil microbial communities is related to balanced growth, not storage compound synthesis. Soil Biol Biochem 89:35–43. https://doi.org/10.1016/j.soilbio.2015.06.021
Feng JG, Zhu B (2019) A global meta-analysis of soil respiration and its components in response to phosphorus addition. Soil Biol Biochem 135:38–47. https://doi.org/10.1016/j.soilbio.2019.04.008
Fisk M, Santangelo S, Minick K (2015) Carbon mineralization is promoted by phosphorus and reduced by nitrogen addition in the organic horizon of northern hardwood forests. Soil Biol Biochem 81:212–218. https://doi.org/10.1016/j.soilbio.2014.11.022
Geyer KM, Kyker-Snowman E, Grandy AS, Frey SD (2016) Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 127:173–188. https://doi.org/10.1007/s10533-016-0191-y
Houlton BZ, Wang YP, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330. https://doi.org/10.1038/nature07028
Jensen ES, Carlsson G, Hauggaard-Nielsen H (2020) Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: a global-scale analysis. Agron Sustain Dev 40. https://doi.org/10.1007/s13593-020-0607-x
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k (EC) value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999. https://doi.org/10.1016/j.soilbio.2005.08.012
Kallenbach CM, Wallenstein MD, Schipanksi ME, Grandy AS (2019) Managing agroecosystems for soil microbial carbon use efficiency: ecological unknowns, potential outcomes, and a path forward. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.01146
Lal R (2011) Sequestering carbon in soils of agro-ecosystems. Food Policy 36:S33–S39. https://doi.org/10.1016/j.foodpol.2010.12.001
Luo RY, Fan JL, Wang WJ, Luo JF, Kuzyakov Y, He JS, Chu HY, Ding WX (2019) Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau. Sci Total Environ 650:303–312. https://doi.org/10.1016/j.scitotenv.2018.09.038
Ma ZZ, Zhang XC, Zheng BY, Yue SC, Zhang XC, Zhai BN, Wang ZH, Zheng W, Li ZY, Zamanian K, Razavi BS (2021) Effects of plastic and straw mulching on soil microbial P limitations in maize fields: dependency on soil organic carbon demonstrated by ecoenzymatic stoichiometry. Geoderma 388:12. https://doi.org/10.1016/j.geoderma.2021.114928
Mahmud K, Makaju S, Ibrahim R, Missaoui A (2020) Current progress in nitrogen fixing plants and microbiome research. Plants-Basel 9. https://doi.org/10.3390/plants9010097
Manzoni S, Taylor P, Richter A, Porporato A, Agren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Mehnaz KR, Keitel C, Dijkstra FA (2018) Effects of carbon and phosphorus addition on microbial respiration, N2O emission, and gross nitrogen mineralization in a phosphorus-limited grassland soil. Biol Fert Soils 54:481–493. https://doi.org/10.1007/s00374-018-1274-9
Miguez-Montero MA, Valentine A, Perez-Fernandez MA (2020) Regulatory effect of phosphorus and nitrogen on nodulation and plant performance of leguminous shrubs. AoB Plants 12. https://doi.org/10.1093/aobpla/plz047
Mori T, Ohta S, Ishizuka S, Konda R, Wicaksono A, Heriyanto J, Hardjono A (2010) Effects of phosphorus addition on N2O and NO emissions from soils of an Acacia mangium plantation. Soil Sci Plant Nutr 56:782–788. https://doi.org/10.1111/j.1747-0765.2010.00501.x
Olsen SR, Sommers LE (1982) Phosphorous. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, Chemical and Microbial Properties. Agronomy Society of America, Madison, pp 403–430
Pang J, Tibbett M, Denton MD, Lambers H, Siddique KHM, Ryan MH (2011) Soil phosphorus supply affects nodulation and N: P ratio in 11 perennial legume seedlings. Crop Pasture Sci 62:992–1001. https://doi.org/10.1071/CP11229
Poeplau C, Herrmann AM, Katterer T (2016) Opposing effects of nitrogen and phosphorus on soil microbial metabolism and the implications for soil carbon storage. Soil Biol Biochem 100:83–91. https://doi.org/10.1016/j.soilbio.2016.05.021
Reed SC, Seastedt TR, Mann CM, Suding KN, Townsend AR, Cherwin KL (2007) Phosphorus fertilization stimulates nitrogen fixation and increases inorganic nitrogen concentrations in a restored prairie. Appl Soil Ecol 36:238–242. https://doi.org/10.1016/j.apsoil.2007.02.002
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Sanchez G, Trinchera L, Russolillo G (2017) Plspm: tools for partial least squares path modeling (PLS-PM). https://CRAN.R-project.org/package=plspm
Santi C, Bogusz D, Franche C (2013) Biological nitrogen fixation in non-legume plants. Ann Bot 111:743–767. https://doi.org/10.1093/aob/mct048
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394. https://doi.org/10.1890/06-0219
Sinsabaugh RL, Hill BH, Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–799. https://doi.org/10.1038/nature08632
Sinsabaugh RL, Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343. https://doi.org/10.1146/annurev-ecolsys-071112-124414
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16:930–939. https://doi.org/10.1111/ele.12113
Sinsabaugh RL, Turner BL, Talbot JM, Waring BG, Powers JS, Kuske CR, Moorhead DL, Shah JJ (2016) Stoichiometry of microbial carbon use efficiency in soils. Ecol Monogr 86:172–189. https://doi.org/10.1890/15-2110.1
Smil V (2000) Phosphorus in the environment: natural flows and human interferences. Annu Rev Energ Environ 25:53–88. https://doi.org/10.1146/annurev.energy.25.1.53
Spohn M, Chodak M (2015) Microbial respiration per unit biomass increases with carbon-to-nutrient ratios in forest soils. Soil Biol Biochem 81:128–133. https://doi.org/10.1016/j.soilbio.2014.11.008
Spohn M, Potsch EM, Eichorst SA, Woebken D, Wanek W, Richter A (2016) Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol Biochem 97:168–175. https://doi.org/10.1016/j.soilbio.2016.03.008
Stagnari F, Maggio A, Galieni A, Pisante M (2017) Multiple benefits of legumes for agriculture sustainability: an overview. Chem Biol Technol Ag 4. https://doi.org/10.1186/s40538-016-0085-1
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol Biochem 32:59–66. https://doi.org/10.1016/S0038-0717(99)00129-7
Vesterdal L, Raulund-Rasmussen K (2002) Availability of nitrogen and phosphorus in Norway spruce forest floors fertilized with nitrogen and other essential nutrients. Soil Biol Biochem 34:1243–1251. https://doi.org/10.1016/S0038-0717(02)00064-0
Vera-Nunez JA, Infante-Santiago JP, Velasco-Velasco V, Salgado-Garcia S, Palma-Lopez DJ, Grageda-Cabrera OA, Cardenas R, Pena-Cabriales JJ (2007) Influence of P fertilization on biological nitrogen fixation in herbaceous legumes grown in acid savannah soils from the Tabasco State, Mexico. J Sustainable Agric 31:25–42. https://doi.org/10.1300/J064v31n03_04
Wang YD, Hu N, Xu MG, Li ZF, Lou YL, Chen Y, Wu CY, Wang ZL (2015) 23-year manure and fertilizer application increases soil organic carbon sequestration of a rice-barley cropping system. Biol Fertil Soils 51:583–591. https://doi.org/10.1007/s00374-015-1007-2
Wei TY, Simko V (2017) R package “corrplot”: visualization of a correlation matrix (Version 0.84). https://github.com/taiyun/corrplot
Wei XM, Zhu ZK, Liu Y, Luo Y, Deng YW, Xu XL, Liu SL, Richter A, Shibistova O, Guggenberger G, Wu JS, Ge TD (2020) C:N: P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol Fert Soils 56:1093–1107. https://doi.org/10.1007/s00374-020-01468-7
Widdig M, Schleuss PM, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Seabloom EW, Wragg PD, Spohn M (2020) Microbial carbon use efficiency in grassland soils subjected to nitrogen and phosphorus additions. Soil Biol Biochem 146. https://doi.org/10.1016/j.soilbio.2020.107815
Wu Y, Chen WJ, Li Q, Guo ZQ, Li YZ, Zhao ZW, Zhai JY, Liu GB, Xue S (2021) Ecoenzymatic stoichiometry and nutrient limitation under a natural secondary succession of vegetation on the Loess Plateau, China. Land Degrad Dev 32:399–409. https://doi.org/10.1002/ldr.3723
Xiao L, Liu GB, Li P, Li Q, Xue S (2020) Ecoenzymatic stoichiometry and microbial nutrient limitation during secondary succession of natural grassland on the Loess Plateau. China Soil Tillage Res 200:9. https://doi.org/10.1016/j.still.2020.104605
Yue K, Fornara DA, Yang WQ, Peng Y, Peng CH, Liu ZL, Wu FZ (2017) Influence of multiple global change drivers on terrestrial carbon storage: additive effects are common. Ecol Lett 20:663–672. https://doi.org/10.1111/ele.12767
Zang HD, Blagodatskaya E, Wen Y, Xu XL, Dyckmans J, Kuzyakov Y (2018) Carbon sequestration and turnover in soil under the energy crop Miscanthus: repeated 13C natural abundance approach and literature synthesis. GCB Bioenergy 10:262–271. https://doi.org/10.1111/gcbb.12485
Zhao H, Sun BF, Lu F, Wang XK, Zhuang T, Zhang G, Ouyang ZY (2017) Roles of nitrogen, phosphorus, and potassium fertilizers in carbon sequestration in a Chinese agricultural ecosystem. Clim Change 142:587–596. https://doi.org/10.1007/s10584-017-1976-2
Zhang W, Xu YD, Gao DX, Wang X, Liu WC, Deng J, Han XH, Yang GH, Feng YZ, Ren GX (2019) Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil Biol Biochem 134:1–14. https://doi.org/10.1016/j.soilblo.2019.03.017
Zhang ZJ, Li HY, Hu J, Li X, He Q, Tian GM, Wang H, Wang SY, Wang B (2015) Do microorganism stoichiometric alterations affect carbon sequestration in paddy soil subjected to phosphorus input? Ecol Appl 25:866–879. https://doi.org/10.1890/14-0189.1
Zheng MH, Li DJ, Lu X, Zhu XM, Zhang W, Huang J, Fu SL, Lu XK, Mo JM (2016) Effects of phosphorus addition with and without nitrogen addition on biological nitrogen fixation in tropical legume and non-legume tree plantations. Biogeochemistry 131:65–76. https://doi.org/10.1007/s10533-016-0265-x
Zhu ZK, Zhou J, Shahbaz M, Tang HM, Liu SL, Zhang WJ, Yuan HZ, Zhou V, Alharbi H, Wu JS, Kuzyakov Y, Ge TD (2021) Microorganisms maintain C: N stoichiometric balance by regulating the priming effect in long-term fertilized soils. Appl Soil Ecol 167. https://doi.org/10.1016/j.apsoil.2021.104033
Funding
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDB40000000), the National Natural Science Foundation of China (41977031), and the Program of State Key Laboratory of Loess and Quaternary Geology of CAS (SKLLQGZR1803).
Author information
Authors and Affiliations
Contributions
The manuscript is approved by all the authors for publication. All the authors have agreed to be listed and have seen the manuscript, and approved the submission to Journal of Soils and Sediments.
Corresponding author
Ethics declarations
Ethics approval
This study does not involve human participants and/or animals.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Jianming Xue
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Cui, Y., Wang, Y. et al. Ecoenzymatic stoichiometry reveals phosphorus addition alleviates microbial nutrient limitation and promotes soil carbon sequestration in agricultural ecosystems. J Soils Sediments 22, 536–546 (2022). https://doi.org/10.1007/s11368-021-03094-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03094-8