Abstract
Context
Landscape-scale research quantifying ecological connectivity is required to maintain the viability of populations in dynamic environments increasingly impacted by anthropogenic modification and environmental change.
Objective
To evaluate how surface water network structure, landscape resistance to movement, and flooding affect the connectivity of amphibian habitats within the Murray–Darling Basin (MDB), a highly modified but ecologically significant region of south-eastern Australia.
Methods
We evaluated potential connectivity network graphs based on circuit theory, Euclidean and least-cost path distances for two amphibian species with different dispersal abilities, and used graph theory metrics to compare regional- and patch-scale connectivity across a range of flooding scenarios.
Results
Circuit theory graphs were more connected than Euclidean and least-cost equivalents in floodplain environments, and less connected in highly modified or semi-arid regions. Habitat networks were highly fragmented for both species, with flooding playing a crucial role in facilitating landscape-scale connectivity. Both formally and informally protected habitats were more likely to form important connectivity “hubs” or “stepping stones” compared to non-protected habitats, and increased in importance with flooding.
Conclusions
Surface water network structure and the quality of the intervening landscape matrix combine to affect the connectivity of MDB amphibian habitats in ways which vary spatially and in response to flooding. Our findings highlight the importance of utilising organism-relevant connectivity models which incorporate landscape resistance to movement, and accounting for dynamic landscape-scale processes such as flooding when quantifying connectivity to inform the conservation of dynamic and highly modified environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes to the ecological connectivity of habitat networks are a key threat to amphibian species, which have recently suffered dramatic global declines and extinctions (Stuart et al. 2004; Cushman 2006). The spatial structure and configuration of habitat networks can greatly affect amphibians by influencing the potential for dispersal between populations and breeding sites (Marsh and Trenham 2001; Smith and Green 2005). By restricting gene flow (McRae and Beier 2007; Semlitsch 2008; Baguette et al. 2013), altering metapopulation and source–sink dynamics (Hanski and Gilpin 1991; Smith and Green 2005), reducing the ability to adapt to changing environmental conditions (Nuñez et al. 2013; Saura et al. 2014), or by encouraging the spread of disease (Perkins et al. 2009), changes to connectivity can critically affect the persistence of amphibian populations (Marsh and Trenham 2001; Heard et al. 2012a).
There is an urgent need for landscape-scale amphibian research, particularly in highly modified landscapes where changes to dynamic landscape processes may have unknown impacts on habitat connectivity and dispersal (Hazell 2003). In addition to causing habitat loss, environmental change caused by development can increase the resistance of the landscape matrix to amphibian movement, potentially increasing the likelihood of local extinctions by restricting re-colonisation and rescue effects (Mazerolle and Desrochers 2005; Vos and Goedhart 2007; Murphy et al. 2010; Wassens 2010a; Van Buskirk 2012). For amphibian species inhabiting floodplain environments, connectivity may also be reduced by changes to dynamic landscape processes such as flooding which once provided transient opportunities for dispersal (Calabrese and Fagan 2004; Zeigler and Fagan 2014). Alternatively, connectivity may be maintained if the structure of remaining habitats and the intervening landscape matrix allow organisms to access well-connected refugia or “hub” habitats (Graham et al. 2010) or make successive “stepping stone” movements across the landscape (Kramer-Schadt et al. 2011; Leidner and Haddad 2011; Saura et al. 2014).
South-eastern Australia has been identified as a global hotspot of amphibian decline (Campbell 1999; Stuart et al. 2004). Although habitat fragmentation and isolation, changes to flooding regimes caused by river regulation, and the spread of disease through habitat networks (e.g., chytridiomycosis) have been suggested as contributing factors, many declines remain poorly understood (Hazell 2003; Stuart et al. 2004). Previous research has primarily assessed how changing connectivity and flooding affects amphibian ecology at the site or metapopulation scale rather than across regional-scale habitat networks (e.g., Wassens et al. 2008; Wassens and Maher 2011; McGinness et al. 2014; Ocock et al. 2014). Key doubts also remain about the effectiveness of Australia’s protected area systems for conserving the surface water habitats of species like amphibians, particularly given its poor coverage of freshwater ecosystems and the lack of consideration for landscape-scale processes such as flooding or connectivity (Kingsford 2011; Chessman 2013). Given this critical lack of knowledge, there is a need to guide and prioritise future conservation effects by quantifying regional- and habitat-scale patterns of connectivity across amphibian habitat networks.
This study used graph theory to model connectivity between amphibian habitats within the Murray–Darling Basin (MDB), Australia’s largest agricultural region and home to many of the country’s most significant amphibian habitats. Graph theory uses networks to model complex interacting systems, and has recently seen a rapid uptake in landscape ecology for analysing connectivity in terrestrial (e.g., Bunn et al. 2000; Urban and Keitt 2001; Minor and Urban 2007; Saura et al. 2014), marine (e.g., Treml et al. 2007; Kininmonth et al. 2009; Hock et al. 2014), and surface water systems (e.g., Fortuna et al. 2006; Wright 2010; McIntyre and Strauss 2013; McIntyre et al. 2014; Ruiz et al. 2014; Tulbure et al. 2014). When applied to habitat patches, amphibian habitats such as wetlands form network “nodes” which are linked by “edges” if ecologically connected (Urban and Keitt 2001; Urban et al. 2009; Galpern et al. 2011). This simple data structure can be used to combine measures of landscape structure (patch size, shape, and location) with ecological knowledge of organism movement and dispersal to efficiently study connectivity across large spatial extents (Urban and Keitt 2001; Minor and Urban 2007).
Although patch-based graph theory provides a powerful framework for studying connectivity, previous graph theory amphibian studies have overwhelmingly focused on how habitat patch structure and configuration influence connectivity, rather than assessing how the landscape matrix influences connectivity between patches themselves. Studies modelling Euclidean distance between habitat patches (e.g., Pyke 2005; Fortuna et al. 2006; Ribeiro et al. 2011; Peterman et al. 2013; Uden et al. 2014) may misrepresent connectivity by ignoring how landscape conditions influence an organism’s dispersal ability (Mazerolle and Desrochers 2005; Van Buskirk 2012; Pittman et al. 2014). Studies have used least-cost path modelling based on resistance to movement surfaces to incorporate how the landscape matrix can influence amphibian movement (e.g., Compton et al. 2007; Decout et al. 2012), but these approaches rely on two potentially simplistic assumptions: that movement potential can be related to a single optimal path between habitats, and that an organism can select that path based on a complete knowledge of the landscape they are traversing (Fahrig 2007; McRae et al. 2008; Pinto and Keitt 2009).
Algorithms from electrical circuit theory provide an improved technique for modelling the overland movement of organisms through heterogeneous landscapes (McRae 2006; McRae and Beier 2007; McRae et al. 2008). Increasing evidence suggests that amphibians do not rely on perceptual environment knowledge when dispersing from natal ponds but instead make largely random, incremental movement decisions in response to features encountered on their path (Semlitsch 2008; Matisziw et al. 2014; Pittman et al. 2014; Sinsch 2014). As the flow of current through electrical networks can be precisely related to random-walk theory (Doyle and Snell 1984; Chandra et al. 1996), circuit theory provides an ideal tool for modelling amphibian dispersal. By representing landscapes as conductive surfaces consisting of nodes connected by resistors, current flow (analogous to movement probabilities) can be modelled between source and target habitat patches. This process simulates multiple movement paths across the landscape and incorporates patch isolation, spatial structure, and landscape resistance to movement into a single effective “resistance distance” that provides a more realistic estimate of population-scale dispersal potential (McRae et al. 2008).
Circuit theory has been used extensively in amphibian genetic studies to model how landscape resistance to movement affects gene flow (e.g., Moore et al. 2011; Richardson 2012; Peterman et al. 2014; Nowakowski et al. 2015a). Comparisons of experimentally derived resistance surfaces suggest connectivity estimates based on circuit theory modelling can diverge significantly from Euclidean and least-cost equivalents, particularly in complex landscapes containing many potential paths for movement (Nowakowski et al. 2015b). Despite this, no study has yet combined circuit theory resistance distance modelling with patch-based graph theory analysis, nor compared how the use of resistance rather than Euclidean or least-cost distances affects estimates of connectivity across regional-scale habitat networks.
By using graph theory to model amphibian habitat networks within the MDB, this study aimed to evaluate how surface water network structure, landscape resistance to movement, and dynamic flooding events combine to affect ecological connectivity for two Australian frog species. In particular, we sought to answer the following research questions:
-
(1)
How do graph theory estimates of connectivity based on circuit theory modelling compare to other commonly used graph connection strategies (Euclidean and least-cost distances)?
-
(2)
How do network structure and dynamic flooding events combine to affect regional-scale ecological connectivity?
-
(3)
How is the distribution and importance of ecologically significant “hub” or “stepping stone” habitat patches affected by network structure and flooding?
-
(4)
Do current protected area systems successfully protect surface water habitats critical for facilitating ecological connectivity?
Method
Study area
The study area encompassed the entire MDB in south-eastern Australia (Fig. 1). The MDB is Australia’s largest river basin (occupying over 1 million km2) and consists of a fragmented mosaic of natural and highly modified intensive agricultural landscapes (Rogers and Ralph 2010a). The MDB contains Australia’s three longest river systems which together drain over 14 % of the nation’s landmass: the Darling (approximately 2740 km long), Murray (2530 km), and Murrumbidgee (1690 km). Although dry and semi-arid across much of its western margins, the MDB simultaneously provides 39 % of Australia’s entire agricultural output while supporting some of the nation’s most biologically diverse and ecologically significant wetland ecosystems (Finlayson et al. 2011). These include permanent and ephemeral freshwater lakes, lagoons, swamps, marshes, billabongs, and waterholes, which together provide crucially important habitat for a wide array of organisms including 29 amphibian species (Rogers and Ralph 2010a; Wassens 2010b).
Surface water environments of the MDB study area (GA 2006) and the distribution (inset) of the focal species: Limnodynastes fletcheri and Crinia parinsignifera (Atlas of Living Australia; http://www.ala.org). Grey lines indicate MDB surface water resource plan areas (Murray–Darling Basin Authority, MDBA 2012). Numbered labels identify discussed surface water habitats including (1) the Lower Lakes of the Murray including Lakes Alexandrina and Albert, (2) Spectacle Lakes, (3) Gurra Lakes and Pike-Mundic wetlands, (4) Riverland and Chowilla wetlands, (5) Lake Victoria, (6) Murray–Murrumbidgee Rivers confluence, (7) Barmah–Millewa Forest, (8) Macquarie Marshes, and (9) Paroo River and Cuttaburra Creek
The majority of the MDB’s surface water habitats are entirely reliant on variable river flows, creating highly dynamic environments which vary greatly in response to water availability and flooding (Ballinger and Mac Nally 2006). Despite their ecological significance, decreased flows and changes to flooding regimes resulting from river regulation, earthwork construction, and the diversion of water for consumptive use have caused many surface water ecosystems to suffer severe ecological declines (Kingsford 2000; Rogers and Ralph 2010b; Finlayson et al. 2011). Although some habitats have been formally protected through listing on the Collaborative Australian Protected Areas Database (CAPAD) or the International Ramsar Convention, the majority (94.6 %) remain unprotected or provided with only informal protection under the Directory of Important Wetlands of Australia (DIWA; Nairn and Kingsford 2012).
Focal species
We selected two amphibian species highly dependent on flooding for breeding and dispersal and with ranges following the entire extent of the MDB (Fig. 1, inset). Both are ground-dwelling, non-burrowing, and generalists in their ability to occupy most wetland types. The Barking Marsh Frog (Limnodynastes fletcheri) is a medium-sized frog (up to 50 mm in length) found throughout the MDB (Anstis 2013). Despite occurring in dry–semi-arid regions, L. fletcheri lacks water-conserving features and is typically associated with semi-permanent lake, wetland, pond, and creek habitats (Amey and Grigg 1995). The species is regarded as highly mobile and dispersive (Gonzalez et al. 2011), with radio-tagging studies recording movements of up to 383 m in a single 24-h period (Ocock et al. 2014).
The Eastern Sign-bearing Froglet (Crinia parinsignifera) is a small species (<20 mm) found most frequently within the MDB’s temperate southern and eastern regions. Although little is known about the species’ ability to resist dry conditions, C. parinsignifera is believed to be relatively resilient to changes in wetland hydrology and breeds opportunistically in ephemeral still-water habitats containing diverse aquatic or submerged vegetation (Wassens 2010b). C. parinsignifera is thought to have a relatively limited dispersal ability based on its small size and high degree of philopatry (Mac Nally 1984). Although neither L. fletcheri or C. parinsignifera are listed as threatened in the MDB, decreases and shifts in the timing of water availability are believed to be responsible for an up to tenfold decrease in the average density of C. parinsignifera observed in southern MDB sites since the late 1970s (Mac Nally et al. 2009).
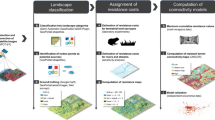
Graph and resistance surface creation
We created network graphs for L. fletcheri and C. parinsignifera by considering Geoscience Australia (GA) lake, wetland, and reservoir topographic features (Table 1) as potential surface water habitat patches or ‘nodes’, and potential ecological connections between these habitats as ‘edges’ (Urban and Keitt 2001). To assess how landscape resistance to movement and the strategy used to connect graphs influence connectivity estimates, graph edges were created using three unique distance measures: Euclidean, least-cost and circuit theory resistance distances (Fig. 2). As measures of effective distance, least-cost and circuit theory graph connection strategies use spatial resistance surfaces to provide a quantitative estimate of the costs faced by an organism when moving across features in the landscape. This potentially allows distant patches to be considered connected if separated by optimal movement terrain, or disconnected despite being in close proximity if the intervening landscape is hostile to movement (Spear et al. 2010; Zeller et al. 2012).
A comparison of the three connection strategies (Euclidean, least-cost and resistance distance) used to construct graphs (networks) from a surface water habitat patches. Graphs constructed using (b, c) the Euclidean distance strategy considered habitat patches connected if separated by less than an organism’s maximum dispersal distance, ignoring the influence of landscape resistance to movement. Resistance to movement surfaces (d) were used to calculate (e, f) least-cost and (g, h) resistance distance graphs. These may be less connected than Euclidean graphs where terrain between habitats offers high resistance to amphibian movement (i.e., highly modified agricultural land to north of example landscape) or more connected within optimal movement terrain (i.e., floodplain to the south). Graph nodes (c, f, h) are symbolised by their degree centrality (the number of connections between the node and its neighbours; see Table 2), and annotated to illustrate several common graph theory concepts
We used surface water, land cover (including vegetation), and transportation features to develop resistance surfaces as these have been found to strongly affect the distribution and movement of Australian floodplain amphibian species (Jansen and Healey 2003; Hazell et al. 2004; Wassens et al. 2008; Heard et al. 2012b; Ocock et al. 2014). Resistance surfaces were developed at the resolution of the Australian 250 m Dynamic Land Cover Dataset (DLCD), the highest resolution land cover data available for the entire MDB (Table 1). Although circuit theory approaches have been shown to be highly resilient to the choice of study resolution (McRae et al. 2008), we combined DLCD mapping with linear hydrological and transport features sourced from GA topographic mapping and the MDB Waterbodies Project (Table 1) to ensure these narrow but potentially significant movement barriers or corridors were accounted for in our analysis.
Landscape features were consolidated into 27 unique units (see Supplementary material) by combining similar features (e.g., “shrub” and “chenopod shrub” to “arid or semi-arid shrubland”). To account for climatic variation in vegetation communities, vegetation units were divided further into temperate and arid–semi-arid subunits using a current condition WorldClim 500 mm annual rainfall threshold (Hijmans et al. 2005). Saline waterbodies unsuitable for either amphibian habitat or movement terrain (Smith et al. 2007) were differentiated from freshwater by identifying surface water patches intersecting DLCD “salt lake” cells.
Given the lack of empirical movement data available for Australian amphibian species, we parameterised resistance surfaces using literature and the expert opinion of six researchers with a combined 130 years of research or field experience with the focal species and study area. Landscape units were rated on a consistent scale indicating their resistance to movement relative to hypothetically optimal movement terrain, with ratings based on the species’ ability or willingness to cross a unit and any physiological costs or risks of mortality associated with the crossing (Zeller et al. 2012). Literature ratings were weighted to assign the highest certainty to research studying dispersal or movement of the focal species or closely related taxa within the study area, whereas expert opinion weightings took into account expert-specific estimates of certainty and years of experience with the focal species (see Supplementary material for additional details).
To study how flooding affects amphibian connectivity, we combined parameterised resistance surfaces with CSIRO MDB-FIM2 present-condition flood inundation modelling (Table 1) for eight inundation scenarios: no flooding and 1, 2, 5, 10, 20, 50, and 100 year average recurrence interval (ARI) floods. We kept habitat patches consistent across flooding scenarios and instead modified resistance surfaces within inundated areas to focus on how flooding alters landscape resistance to movement between habitats. Locations with a >50 % modelled likelihood of inundation were assigned minimum resistance to movement (resistance of 1), resulting in 16 resistance to movement surfaces unique to each focal species and flooding scenario.
Graph connection strategy
A Euclidean distance graph connection strategy ignored landscape resistance to movement and flooding, and assumed that any two habitats separated by less than a maximum distance threshold were connected. Pairwise Euclidean distances between the edges of habitat patches were measured using the gDistance package (Van Etten 2014) within the statistical software R v3.0.3 (R Development Core Team 2014), and converted to graph edges by preserving connections lower than the maximum dispersal distance of each species as estimated from literature and expert opinion (3250 m for L. fletcheri and 960 m for C. parinsignifera).
Effective distances between habitat patches based on resistance surfaces for each species and flooding scenario were measured using least-cost path (Adriaensen et al. 2003) and circuit theory models of landscape connectivity (McRae 2006; McRae et al. 2008). Least-cost distances were calculated using the gDistance R package (Van Etten 2014), and reflected the minimum cost accumulated along the shortest path between two habitats (measured in cost units). Resistance distances were calculated using the Circuitscape v4.04 software package (Shah and McRae 2008), and incorporated both minimum movement costs and the availability of alternative pathways between habitats (McRae et al. 2008). Both least-cost and resistance distances were calculated between patch edges with raster cells connected to their eight immediate neighbours. To reduce computing time across the 1 million km2 study area, we restricted neighbouring patches and resistance surfaces to a 15 km buffered analysis window around each focal patch (St-Louis et al. 2014). This also minimised edge effects and accounted for connectivity between surface water habitats located near but outside the MDB boundary.
To construct least-cost and resistance distance graphs which could be compared to Euclidean distance equivalents, we identified least-cost and resistance cut-off values at which 95 % of corresponding Euclidean distances between each pair of nodes were below the estimated maximum dispersal distance of the focal species. This 95 % threshold was selected to mimic the output of 95th percentile dispersal kernels frequently used in metapopulation ecology (Hanski 1998). Unique graphs for each flooding scenario were then generated by connecting all habitat patch pairs separated by effective distances lower than the least-cost or resistance distance cut-off values.
Global and local connectivity analyses
We used the igraph R package (Csardi and Nepusz 2006) to compute graph theory metrics for each connection strategy (Euclidean, least-cost, and resistance distance), species (L. fletcheri and C. parinsignifera) and flooding scenario (no flood to a 100 year ARI). Global metrics evaluated connectivity at a global or regional scale, allowing us to characterise and compare habitat network resilience, fragmentation, and potential implications for long-distance dispersal, gene flow, and metapopulation dynamics across the study area (Bunn et al. 2000; Urban and Keitt 2001). Global metrics (Table 2) quantified total potential connections between habitats across the entire network (total number of graph edges), the proportion of habitats forming into groups of interconnected nodes (or “components” in graph theory terminology), the size in patches of the largest group of connected habitats (maximum component size), and the degree to which habitats formed tightly connected clusters (global clustering coefficient). For more detailed descriptions of global graph metrics, readers are referred to Newman (2003) and references in Table 2.
Local metrics operated at the patch-scale, and identified individual patches critical for maintaining connectivity across habitat networks (Minor and Urban 2007). We calculated degree centrality (DC) for each individual habitat patch, a local metric quantifying the number of edges (connections) between a node and its neighbours (Table 2). Nodes with degrees in the highest 10 % were taken to represent “hubs” of connectivity which are likely to be important for facilitating organism movement within the patch’s local neighbourhood (Estrada and Bodin 2008; Urban et al. 2009). The ability of a habitat patch to facilitate connectivity beyond its immediate neighbourhood can be quantified by measuring its betweenness centrality (BC), or the number of times a node lies on the shortest path through the network between any other two nodes (Table 2). Betweenness values in the top 10 % were identified to highlight crucial “stepping stones” which enable long-distance dispersal across the landscape or connect otherwise disconnected groups of habitat patches (Newman and Girvan 2004). To visualise the spatial distribution of local metric values across the study area, we converted habitat polygons to continuous 15 × 15 km cell raster surfaces based on mean DC or BC values.
To assess how successfully protected area schemes maintain connectivity within the MDB, we compared mean local metric values between unprotected habitats and those within three protected area classifications: habitats formally protected within Australia’s national reserve system (Collaborative Australian Protected Areas Database or CAPAD), informally protected at a national level (Australian Directory of Important Wetlands or DIWA), and formally protected internationally (Ramsar Convention).
Results
Global or region-scale connectivity
At a regional-scale, connectivity varied greatly depending on species, flooding scenario, and graph connection strategy (Euclidean, least-cost, and resistance distance). As a consequence of L. fletcheri’s greater dispersal ability, L. fletcheri networks exhibited higher values (inferring increased connectivity) for all global graph metrics and flooding scenarios compared to C. parinsignifera (Fig. 3). During smaller flooding scenarios (no flooding–20 year ARI), overall connectivity measured by graph edge counts was higher for Euclidean than least-cost and resistance distance networks for both species (Fig. 3a). Both least-cost and resistance network connectivity increased as optimal movement conditions associated with flooding allowed for additional movement between habitat patches, although resistance graphs gained new connections faster than least-cost equivalents. Edge counts for resistance networks overtook their Euclidean equivalents during large flooding scenarios, with 50 % higher values observed for L. fletcheri during a 100 year flood (Fig. 3a).
Global graph metrics for two MDB amphibian species: Crinia parinsignifera (first column of panels) and Limnodynastes fletcheri (second column) for eight flooding scenarios (no flooding–100 year ARI flood). Connectivity metrics include a number of edges, b proportion of nodes forming components, c global clustering coefficient, and d size of the largest graph component. Results compare resistance distance (orange) with Euclidean (blue), and least-cost (green) connection strategies (for interpretation of references to colours in this figure caption, readers are referred to the web version of this article). (Color figure online)
Networks for both species were highly fragmented, particularly under the resistance distance no flooding scenario. During this scenario only 33 and 58 % of C. parinsignifera and L. fletcheri habitats formed components, respectively (Fig. 3b), inferring that a large proportion of potential habitats remained completely isolated for these species in the absence of flooding. Although this proportion steadily increased for both species during small floods (1–10 year ARIs), the largest increase occurred between 50 and 100 year events. During the largest flooding scenario, 68 % of L. fletcheri habitat patches formed components compared to only 45 % for C. parinsignifera (Fig. 3b). A lower proportion of habitat patches formed into connected components under all resistance and least-cost graphs compared to Euclidean graphs.
At the expense of forming new components, both species’ resistance graphs displayed the highest values of the global clustering coefficient for all floods with recurrence intervals over 5 years (Fig. 3c). Clustering rose rapidly with minor flooding as new connections appeared between neighbouring floodplain habitats. Although clustering was consistently higher for L. fletcheri, values for C. parinsignifera continued to rise with increased flooding while L. fletcheri clustering largely levelled off by a 20 year event. Least-cost graphs exhibited a different pattern, starting with higher initial clustering than Euclidean equivalents during small flooding scenarios and increasing only slightly with additional flooding.
Maximum component sizes were smaller for resistance graphs compared to both Euclidean and least-cost equivalents during small flooding scenarios and increased by less than four habitat patches between a no flooding and 10 year ARI scenario for both species (Fig. 3d). Larger floods had little effect on the size of C. parinsignifera’s largest component, which added only six additional habitat patches between a 20 and 100 year flood to reach a maximum of 146 habitat patches, or less than 1.5 % of the 9959 habitat patches in the study area. In contrast, the maximum component size for L. fletcheri increased rapidly from 226 to 806 connected habitat patches during the same floods, causing over 8 % of all habitat patches to join into a single connected component. This large increase in maximum component size was not replicated for least-cost graphs, which instead saw the greatest increase between 20 and 50 year floods (256–409) and did not increase greatly during a 100 year event (409–431).
Local or patch-scale connectivity
We calculated local graph metrics for each surface water habitat patch to identify potential “hubs” (using the DC metric) and “stepping stones” (using the BC metric). Local metric values varied spatially and in response to flooding. Both DC and BC values were consistently lower for C. parinsignifera (Fig. 4a, b), as fewer overall connections between habitat patches (Fig. 3a) ensured that each patch had a lower number of neighbours and were less likely to be situated on as many shortest paths between other patches.
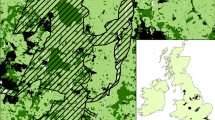
Spatial distribution of resistance distance graph a degree and b betweenness centrality local metrics for two MDB amphibian species: Crinia parinsignifera (first and third column of panels) and Limnodynastes fletcheri (second and fourth columns). Results are shown for three flooding scenarios (no flooding, 10, and 100 year ARI flood; panel rows) and visualised by quantile-classified average values within 15 × 15 km raster cells. Grey lines indicate MDB surface water resource plan areas (MDBA 2012). Numbered labels identify (1) the Lower Lakes, (2) Lake Victoria and the Spectacle Lakes, Gurra Lakes, Pike-Mundic, Riverland and Chowilla wetlands, (3) Murray–Murrumbidgee confluence, (4) Barmah–Millewa Forest, (5) Macquarie Marshes, and (6) Paroo River and Cuttaburra Creek
During small or no flooding scenarios, resistance graph DC metric values revealed highly connected habitat patches that represented important “hubs” of connectivity in the absence of flooding. These patches were typically well-connected to many smaller neighbours given their large size, location, and spatial configuration, and included Lakes Alexandrina and Albert on the lower Murray and large wetlands associated with the Paroo River and Cuttaburra Creek in the north-west of the MDB (Fig. 4a). During these dry scenarios, patches connected using resistance distances consistently had fewer neighbours than Euclidean graphs (Fig. 5a). This discrepancy was particularly apparent in highly modified or semi-arid regions, with exceptions occurring only in locations characterised by optimal landscape conditions for amphibian movement such as the Macquarie Marshes in the central MDB for L. fletcheri (Fig. 5a). Although smaller differences in DC were apparent between resistance and least-cost graphs within non-floodplain regions, least-cost networks were more connected than resistance equivalents along much of the Murray River in the absence of flooding (Fig. 5b).
Difference in degree centrality between resistance distance and a Euclidean and b least-cost graphs for two MDB amphibian species: Crinia parinsignifera (first and third column of panels) and Limnodynastes fletcheri (second and fourth columns). Results are shown for three flooding scenarios (no flooding, 10, and 100 year ARI flood; panel rows) and visualised by quantile-classified average values within 15 × 15 km raster cells. Grey lines indicate MDB surface water resource plan areas (MDBA 2012). Numbered labels identify (1) the Lower Lakes, (2) Lake Victoria and the Spectacle Lakes, Gurra Lakes, Pike-Mundic, Riverland and Chowilla wetlands, (3) Murray–Murrumbidgee confluence, (4) Barmah–Millewa Forest, (5) Macquarie Marshes, and (6) Paroo River and Cuttaburra Creek
As a Euclidean distance connection strategy ignored landscape resistance to movement, the spatial distribution of local metric values did not change with flooding scenario for these networks. In contrast, the importance and distribution of “hub” habitats varied greatly for least-cost and resistance networks as additional optimal flooded movement terrain increased the likelihood of connections between floodplain habitats. The largest differences between resistance graphs and other connection strategies occurred within floodplain regions during larger flooding events, where extensive regions of inundated terrain allowed habitat patches to accumulate many more connections than indicated by either Euclidean or least-cost strategies. The largest increases in DC occurred in areas where extensive flooding coincided with groups of highly clustered habitat patches, including the Gurra Lakes, Pike-Mundic, Riverland and Chowilla wetland complexes on the Murray, the Murray–Murrumbidgee confluence, and the Barmah–Millewa Forest (Fig. 4a).
Compared to DC which increased positively with increasing flooding extent, resistance graph BC values exhibited a more complex response based on a patch’s location in its wider network and the spatial configuration of flooding during each scenario. As habitat patches with very high DC values have many connections between their neighbours, several potential “hubs” (e.g., Paroo River and Cuttaburra Creek wetlands) also exhibited high BC values (Fig. 4b). In addition to habitats located in less flood-prone regions (e.g., Lakes Alexandrina and Albert), these habitats consistently served as important “stepping stones” regardless of flooding extent. Other habitat patches were important during small floods but lost their significance as larger flooding created redundant pathways through neighbouring habitat patches. These habitats were typically located at the centre of floodplains and included patches within the Spectacle Lakes, Gurra Lakes, Pike-Mundic, and Riverland wetland complexes (Fig. 4b).
The highest BC values were observed along a large stretch of the Murray River extending westward from the Murray–Murrumbidgee confluence (Fig. 4b). For L. fletcheri, large flooding in this region (100 year ARI) caused the entire floodplain to connect into a single 400 km long graph component containing over 800 habitat patches (Figs. 3d, 4b). Within this component, 12 small lakes and the larger Lake Victoria located along 130 km of the Murray exhibited extremely high BC values (>150,000). Despite being more fragmented overall, C. parinsignifera networks also displayed the highest BC values along a similar stretch of the Murray where extensive flooding provided abundant movement paths for the species despite its lower dispersal ability.
Importance by protected status
We evaluated the success of national and international schemes for protecting the MDB’s “hub” and “stepping-stone” habitats by comparing the mean local metric values of protected and non-protected habitats. For both species, habitat patches formally protected within Australia’s national reserve system (CAPAD) exhibited significantly higher DC (Fig. 6a) and BC scores (Fig. 6b) than non-protected habitat patches. This remained true during all flooding scenarios but was particularly apparent during moderate–large floods (20–100 year ARI). Informally protected nationally important wetlands (DIWA) provided additional connectivity, displaying higher local metric values compared to CAPAD habitats for all flooding scenarios. Formally protected habitats listed on the International Ramsar Convention were typically associated with the highest overall metric values, but also displayed the most variability of any classification (Fig. 6a, b).
Comparison of habitat conservation listing by resistance distance graph a degree and b betweenness centrality local metrics for two MDB amphibian species: Crinia parinsignifera (first column of panels) and Limnodynastes fletcheri (second column). Unprotected habitats (black), those listed on the Collaborative Australian Protected Area Database (CAPAD; blue), Directory of Important Wetlands (DIWA; green), and Ramsar Convention (orange) are compared by mean metric value and shaded 95 % confidence intervals. Betweenness centrality values are plotted on a log scale. (Color figure online)
Discussion
Amphibians are of critical conservation concern, with over one third of species threatened with extinction globally (Stuart et al. 2004; Vié et al. 2008). Although both our focal species are currently believed to be widespread across the MDB, many formerly common and widespread Australian amphibian species have recently suffered severe and unpredicted declines (Mahony 1996; Hines et al. 1999; Mac Nally et al. 2009). Decreases in extensively distributed amphibian species may go undetected until they reach a considerable magnitude and can be obscured through often considerable time lags associated with habitat isolation and other reductions in ecological connectivity (Tyler 1991; Hazell 2003). We used a graph theory approach to generate regional-scale assessments of ecological connectivity for two MDB amphibian species and spatially explicit estimates of the importance of individual habitats as critical connectivity providers. By doing so, this study aimed to increase current understanding of how habitat network structure, landscape resistance to movement, and dynamic flooding events may combine to influence the ongoing viability and persistence of amphibian populations.
Effect of graph connection strategy
We combined patch-based graph theory with circuit theory, an improved model for amphibian movement previously used for modelling amphibian connectivity in genetic studies. Although previous graph theory studies have used resistance surfaces to study amphibian ecological connectivity (e.g., Compton et al. 2007; Decout et al. 2012), our work is the first to evaluate graphs based on circuit theory resistance distances. We compared graphs created using resistance distances with Euclidean and least-cost approaches, finding that the influence of graph connection strategy varied strongly both spatially and in response to dynamic processes such as flooding.
Previous graph theory studies have assessed structural connectivity by measuring Euclidean distances between habitats (e.g., Pyke 2005; Fortuna et al. 2006; Ribeiro et al. 2011; Peterman et al. 2013; Ruiz et al. 2014; Tulbure et al. 2014; Uden et al. 2014). Euclidean distance graph connection strategies may correlate well with amphibian species richness in relatively undisturbed areas with optimal movement terrain (Ribeiro et al. 2011), but regularly over-predict connectivity in complex heterogeneous landscapes (Fletcher et al. 2011). We found Euclidean graphs consistently provided higher connectivity estimates compared to resistance and least-cost equivalents during small flooding and in highly modified or semi-arid regions where terrain offered high resistance to amphibian movement. In these situations, connection strategies based only on distance will greatly overestimate the likelihood of dispersal between habitat patches. In addition, the inability of Euclidean graphs to reflect changes in landscape resistance to movement associated with flooding may result in large underestimates of connectivity in floodplain regions, particularly during larger flooding events.
Least-cost path modelling provides an alternative approach which incorporates landscape resistance to movement, but which may also misrepresent connectivity by imposing a potentially inappropriate directed movement model on organisms such as amphibians which may favour more random, undirected dispersal throughout much of their life cycle (Pittman et al. 2014; Sinsch 2014). Although least-cost graphs produced similar results to resistance equivalents in regions with high resistance to amphibian movement, key differences occurred in areas such as along the Murray River during small flooding scenarios. Here, narrow but low resistance linear river features allowed least-cost networks to become connected without the presence of large areas of innundated floodplain required by resistance equivalents, causing least-cost graphs to predict a higher initial availability of dispersal paths and greater habitat clustering. As least-cost distances are measured along single optimal paths through the landscape, the addition of optimal movement terrain associated with increased flooding in areas surrounding habitat patches did little to increase the connectivity of least-cost graphs, causing global metric values to increase at lower rates than for equivalent resistance networks.
These results highlight the utility of incorporating landscape resistance to movement and organism-relevant modelling approaches in network analyses. For amphibian species reliant on random-walk dispersal strategies, a circuit theory model is likely to better reflect the landscape-scale influence of flooding by producing lower resistance values in the presence of abundant undirected movement pathways through the landscape (McRae et al. 2008; Pittman et al. 2014; Sinsch 2014). Where uncertainty exists about an organism’s dispersal mode (i.e., random or directed), our results indicate that comparing graphs constructed using multiple strategies may be more informative than relying on a single method. We suggest that a multiple graph construction approach may be used to complement already common multi-species approaches (e.g., Moilanen et al. 2005), minimising the likelihood that connectivity estimates are impacted by the selection of effective distance measure. This is likely to be particularly important in highly dynamic landscapes like the MDB or for focal species whose dispersal is strongly affected by the quality of the matrix between habitat patches (Prevedello and Vieira 2010).
Regional-scale connectivity and flooding
By altering the resistance of the landscape matrix to movement, dynamic processes such as flooding may create “transient connectivity windows” where conditions temporarily increase the probability of individuals or populations moving successfully between habitat patches (Zeigler and Fagan 2014). Our findings suggest that dynamic opportunities for dispersal created by flooding are vital for maintaining regional-scale ecological connectivity across the highly fragmented habitat networks of the MDB, and may play a key role in determining the persistence and dynamics of water-dependent amphibian metapopulations (Keymer and Marquet 2000; Johst et al. 2002; Wassens 2010a).
During small or absent flooding, a large proportion of potential L. fletcheri and C. parinsignifera habitats existed as single patches completely isolated from any neighbours. Without access to dispersal from neighbouring populations, organisms in these habitats may be at particularly high risk of demographic or genetic isolation, inbreeding depression, or stochastic local extinction events (Hanski 1999; Keller and Waller 2002). As flooding increased, the number of potential ecological connections between habitats rose greatly for L. fletcheri, and to a smaller extent the less mobile C. parinsignifera. In the MDB, this effect of infrequent but large flooding may play a crucial role in facilitating rare dispersal events, potentially allowing usually isolated populations to avoid local extinction through rescue effects or recolonization (Hanski 1999; Johst et al. 2002).
Climate change, river regulation and agricultural development are expected to affect both the availability of surface water and the frequency of future flooding within the MDB (Pittock et al. 2006; Finlayson et al. 2011). L. fletcheri is believed to be particularly vulnerable to changes in water availability, with flooding playing a key role in maximising recruitment by encouraging breeding and dispersal (Gonzalez et al. 2011; McGinness et al. 2014; Ocock et al. 2014). Our results indicate that during large flooding events, L. fletcheri habitats connect into large groups of tightly clustered habitats which may extent hundreds of kilometres across the MDB, potentially providing long distance dispersal pathways that may be vital for enabling shift ranges in response to environmental change (Wright 2010). This network structure suggests the vagile and dispersive L. fletcheri may be relatively resilient to changes in habitat distribution and the quality of the intervening landscape matrix, but only if large flooding events continue to provide access to permanent refugia and enable regular dispersal between floodplain habitats (Ocock et al. 2014).
While large floods combined with L. fletcheri’s greater dispersal ability to form large groups of connected patches by driving new connections between geographically isolated habitat clusters, these connections were not available to the less vagile C. parinsignifera. Our findings indicate that even large flooding is unlikely to facilitate similar long-distance dispersal opportunities for C. parinsignifera, supporting recent speculation that dispersal limitations may make this species particularly vulnerable to climatic and environmental change (Gonzalez et al. 2011). Optimistically, habitat clustering compared to other metrics increased at a similar rate with flooding for C. parinsignifera as for L. fletcheri, and continued to rise during larger flooding events. Although C. parinsignifera is believed to be relatively resistant to changes in flooding regimes (Wassens 2010a), flooding is still likely to play a significant role in maintaining metapopulation dynamics and increasing local genetic diversity for this species by providing regular access to resilient, highly clustered groups of connected habitats.
Patch-scale connectivity
Our study identified individual surface water habitats critical for maintaining connectivity within the MDB during different flooding events, providing a powerful approach for prioritising conservation or allocating limited and contested environmental flows (i.e., environmental water released into rivers and wetlands to maintain their ecological health). We identified habitats that served as highly connected “hubs” during even dry scenarios, including the relatively undeveloped Paroo River and Cuttaburra Creek catchments in the MDB’s north-west and the internationally significant Lower Lakes of the Murray. In a dynamic surface water system like the MDB, these large, well-connected, and relatively permanent habitats may serve as essential refugia for organisms dispersing from ephemeral habitats during drought periods (Davis et al. 2013). During increased flooding, many of the most important “hubs” were located along the floodplains of the Murray River. These habitats included the Ramsar-listed Barmah–Millewa Forest, recently the site of the largest environmental water release in Australia’s history (McGinness et al. 2014). Our results suggest that the continued provision of environmental flows to such habitats is likely not only to support the ongoing viability of local amphibian populations, but also assist in maintaining regional-scale ecological connectivity.
We identified “stepping stone” habitats that contributed disproportionately to overall network connectivity. “Stepping stones” increased in importance as flooding caused regions of the MDB to join into long chains of connected habitats. “Stepping stone” habitats often included large, well-connected “hubs” (e.g., Lake Alexandrina on the lower Murray), but others were small in size and located strategically along the central Murray River. By providing transient links between amphibians populations located along Australia’s longest inland river system, these habitats may be vital for enhancing regional-scale gene flow and for enabling water-dependent organisms to move effectively through the highly fragmented MDB landscape.
Although “stepping stone” habitats may increase connectivity for amphibian species themselves, they may also facilitate the spread of disease through amphibian habitat networks. Recent research suggests that the amphibian fungal disease chytridiomycosis may be widespread in semi-arid–arid surface water habitats of Australia (Fisher et al. 2009; Ocock et al. 2013). L. fletcheri had the highest infection prevalence of seven amphibian species found to be infected with the disease in a central MDB site (Ocock et al. 2013). Resource managers attempting to monitor or prevent the spread of the disease through amphibian habitat networks are likely to benefit greatly from the identification of “stepping stone” habitats that represent important choke points in the landscape through which disease vectors may pass (Brooks et al. 2008; Perkins et al. 2009).
Protected areas and ecological connectivity
This study is one of the first to use local graph metrics to assess how existing protected areas maintain landscape-scale ecological connectivity (Minor and Lookingbill 2010; Uden et al. 2014). Our findings indicate that both formal and informal protected area listings provide valuable frameworks for maintaining ecological connectivity within the MDB. Surface water habitats protected formally under Australia’s system of reserves (CAPAD) were considerably more likely to serve as important connectivity providers compared to non-protected habitats. This result is particularly significant given how poorly Australia’s reserve system represents surface water environments (Kingsford et al. 2004, 2011; Fitzsimons and Robertson 2005; Nairn and Kingsford 2012).
Internationally significant Ramsar-listed wetlands exhibited the highest overall local connectivity values of any protected area listing. As many of the MDB’s Ramsar sites support diverse and abundant amphibian populations (McGinness et al. 2014; Ocock et al. 2014), these habitats are likely to play significant roles both as population sources and as facilitators of connectivity through their wider habitat network. Although Ramsar wetlands represented particularly significant “hubs” and “stepping stones”, nationally important but unprotected wetlands (DIWA) also exhibited connectivity values significantly higher than both unprotected and protected CAPAD habitats. This suggests that DIWA wetlands previously identified as significant based on non-connectivity ecological criteria may represent ideal candidates for future formal protection, providing a balance between preserving ecologically significant habitat at the site scale while enhancing ecological connectivity across the wider MDB.
Our findings also support recent calls for more comprehensive approaches to the conservation of fresh water habitats that combine site-scale habitat protection with the maintenance of dynamic catchment-scale processes such as flooding (Fitzsimons and Robertson 2005; Kingsford 2011; Chessman 2013). The importance of CAPAD, DIWA and Ramsar habitats was even more pronounced as flooding extent increased. It is likely that these sites will only reach their full potential as connectivity providers when floods are sufficient to provide extensive areas of optimal movement terrain between neighbouring habitats. Formal protection alone is unlikely to be enough to allow protected areas to facilitate ecological connectivity across the MDB’s habitat networks. Conserving or improving flooding regimes through provision of environmental flows will be critical for maintaining connectivity and the long-term resilience and persistence of the MDB’s flood-dependent amphibian species.
Limitations and future work
Although the graph theory approach used here represents a powerful, flexible and spatially consistent framework for the analysis of ecological connectivity, some important limitations should be noted. The resistance to movement component of this study relied on expert opinion, which may produce connectivity estimates which differ from those based on empirically derived resistance values (Zeller et al. 2012). Our findings should therefore be viewed as spatially explicit hypotheses of “potential” rather than “functional” connectivity, and tested by field-based tracking, relocation, or genetic studies before being used to guide conservation policy. To ensure connectivity analyses are based on best-available ecological knowledge, our model uses reproducible scripts that can easily accept improved input data. This will allow our results to be re-run and re-assessed as new empirical movement data becomes available (Zeller et al. 2012).
We relied on present-condition modelled flooding scenarios in the absence of historic inundation mapping covering our study area (Chen et al. 2012). These datasets restricted our analysis to the dynamics of the MDB’s floodplains, potentially ignoring the influence of non-floodplain phenomenon such as the flooding of rain-fed wetlands (Wassens et al. 2013). The modelled scenarios provide composite estimates of flooding across the entire MDB, whereas actual flooding events may be temporally disjointed or restricted to individual catchments. Although the effects of this are likely to be minimal given the fragmented nature of both our focal species’ habitat networks, our flooding scenarios should nevertheless be interpreted as “best case” flooding events which potentially overestimate connectivity. Our model additionally considered only how flooding affects movement between existing surface water habitats rather than incorporating changes to the structure and availability of habitats themselves. Although this allowed us to focus on an important but so far little-studied aspect of connectivity (Zeigler and Fagan 2014), future work investigating the effect of spatial habitat change will be required to produce a more comprehensive model of connectivity for the MDB’s dynamic floodplain environments (e.g., Wassens 2010a).
Recently, remotely sensed time series of surface water extent and distribution have been used to study connectivity in spatiotemporally dynamic landscapes (Ruiz et al. 2014; Tulbure et al. 2014). Such an approach would greatly benefit future work studying ecological connectivity at regional scales by providing improved spatial data on potential organism habitats and flooding inundation, and by incorporating the temporality of landscape dynamics. Combing the modelling techniques used in this study (graph and circuit theory) with spatially explicit and temporally dynamic remotely sensed surface water datasets would allow regional- and site-scale connectivity trends to be quantified in high resolution across both space and time. This may provide an invaluable means to explore and predict the influence of climate change, land use intensification and evolving environmental policies on the ecological connectivity of Australia’s increasingly vulnerable surface water ecosystems.
References
Adriaensen F, Chardon JP, De Blust G, Swinnen E, Villalba S, Gulinck H, Matthysen E (2003) The application of “least-cost” modelling as a functional landscape model. Landsc Urban Plan 64:233–247. doi:10.1016/S0169-2046(02)00242-6
Amey A, Grigg G (1995) Lipid-reduced evaporative water loss in two arboreal hylid frogs. Comp Biochem Physiol A 111:283–291
Anstis M (2013) Tadpoles and frogs of Australia. New Holland Publishers, Sydney
Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C (2013) Individual dispersal, landscape connectivity and ecological networks. Biol Rev Camb Philos Soc 88:310–326. doi:10.1111/brv.12000
Ballinger A, Mac Nally RC (2006) The landscape context of flooding in the Murray–Darling Basin. Adv Ecol Res 39:85–105. doi:10.1016/S0065-2504(06)39005-8
Brooks CP, Antonovics J, Keitt TH (2008) Spatial and temporal heterogeneity explain disease dynamics in a spatially explicit network model. Am Nat 172:149–159. doi:10.1086/589451
Bunn A, Urban DL, Keitt TH (2000) Landscape connectivity: a conservation application of graph theory. J Environ Manag 59:265–278. doi:10.1006/jema.2000.0373
Calabrese JM, Fagan WF (2004) A comparison-shopper’s guide to connectivity metrics. Front Ecol Environ 2:529–536. doi:10.1890/1540-9295(2004)002[0529:ACGTCM]2.0.CO;2
Campbell A (1999) Declines and disappearances of Australian frogs. Environment Australia, Canberra. http://www.environment.gov.au/biodiversity/threatened/publications/declines-and-disappearances-australian-frogs. Accessed Nov 2014
Chandra AK, Raghavan P, Ruzzo WL, Smolensky R, Tiwari P (1996) The electrical resistance of a graph captures its commute and cover times. Comput Complex 6:312–340. doi:10.1007/BF01270385
Chen Y, Cuddy SM, Merrin LE, Huang C, Pollock D, Sims N, Wang B, Bai Q (2012) Murray–Darling Basin floodplain inundation model version 2.0 (MDB-FIM2). Technical Report. CSIRO Water for a Healthy Country Flagship, Australia. https://publications.csiro.au/rpr/download?pid=csiro:EP124995&dsid=DS8. Accessed Nov 2014
Chessman BC (2013) Do protected areas benefit freshwater species? A broad-scale assessment for fish in Australia’s Murray–Darling Basin. J Appl Ecol 50:969–976. doi:10.1111/1365-2664.12104
Compton BW, McGarigal K, Cushman SA, Gamble LR (2007) A resistant-kernel model of connectivity for amphibians that breed in vernal pools. Conserv Biol 21:788–799. doi:10.1111/j.1523-1739.2007.00674.x
Csardi G, Nepusz T (2006) The igraph software package for complex network research. Inter J Complex Syst 1695. http://cran.r-project.org/web/packages/igraph/. Accessed Nov 2014
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240. doi:10.1016/j.biocon.2005.09.031
Davis J, Pavlova A, Thompson R, Sunnucks P (2013) Evolutionary refugia and ecological refuges: key concepts for conserving Australian arid zone freshwater biodiversity under climate change. Glob Change Biol 19:1970–1984. doi:10.1111/gcb.12203
Decout S, Manel S, Miaud C, Luque S (2012) Integrative approach for landscape-based graph connectivity analysis: a case study with the common frog (Rana temporaria) in human-dominated landscapes. Landscape Ecol 27:267–279. doi:10.1007/s10980-011-9694-z
Doyle PG, Snell JL (1984) Random walks and electric networks. Mathematical Association of America, Washington, DC
Estrada E, Bodin Ö (2008) Using network centrality measures to manage landscape connectivity. Ecol Appl 18:1810–1825
Fahrig L (2007) Non-optimal animal movement in human-altered landscapes. Funct Ecol 21:1003–1015. doi:10.1111/j.1365-2435.2007.01326.x
Finlayson CM, Davis JA, Gell PA, Kingsford RT, Parton KA (2011) The status of wetlands and the predicted effects of global climate change: the situation in Australia. Aquat Sci 75:73–93. doi:10.1007/s00027-011-0232-5
Fisher M, Garner T, Walker S (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310
Fitzsimons JA, Robertson HA (2005) Freshwater reserves in Australia: directions and challenges for the development of a comprehensive, adequate and representative system of protected areas. Hydrobiologia 552:87–97. doi:10.1007/s10750-005-1507-4
Fletcher RJ, Acevedo MA, Reichert BE, Pias KE, Kitchens WM (2011) Social network models predict movement and connectivity in ecological landscapes. Proc Natl Acad Sci USA 108:19282–19287. doi:10.1073/pnas.1107549108
Fortuna MA, Gómez-Rodríguez C, Bascompte J (2006) Spatial network structure and amphibian persistence in stochastic environments. Proc R Soc Lond B 273:1429–1434. doi:10.1098/rspb.2005.3448
Galpern P, Manseau M, Fall A (2011) Patch-based graphs of landscape connectivity: a guide to construction, analysis and application for conservation. Biol Conserv 144:44–55. doi:10.1016/j.biocon.2010.09.002
Geoscience Australia (GA, 2006) GEODATA TOPO 250K Series 3 (Packaged-Shape file format). http://www.ga.gov.au/metadata-gateway/metadata/record/gcat_63999. Accessed Nov 2014
Geoscience Australia (GA, 2010) Murray Darling Basin waterbodies project. Spatial data and metadata prepared for Murray Darling Basin Authority, February 2010. http://www.mdba.gov.au/kid/kid-view.php?key=QdAtzXIWWzLYlbit178av5KqbBWdj8e3BR2qkwutSlo. Accessed Nov 2014
Geoscience Australia (GA), Australian Bureau of Agricultural and Resource Economics and Sciences (ABARES, 2011) The national dynamic land cover dataset. http://www.ga.gov.au/metadata-gateway/metadata/record/gcat_71071. Accessed Nov 2014
Gonzalez D, Scott A, Miles M (2011) Assessing the vulnerability of native vertebrate fauna under climate change to inform wetland and floodplain management of the River Murray in South Australia. Report prepared for the South Australian Murray–Darling Basin Natural Resources Management Board. https://data.environment.sa.gov.au/Content/Publications/amph_rep_mam_vulnerabl_2011.pdf. Accessed May 2015
Graham CH, Vanderwal J, Phillips SJ, Moritz C, Williams SE (2010) Dynamic refugia and species persistence: tracking spatial shifts in habitat through time. Ecography 33:1062–1069. doi:10.1111/j.1600-0587.2010.06430.x
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hanski I (1999) Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 87:209–219
Hanski I, Gilpin M (1991) Metapopulation dynamics: brief history and conceptual domain. Biol J Linn Soc 42:3–16. doi:10.1111/j.1095-8312.1991.tb00548.x
Hazell D (2003) Frog ecology in modified Australian landscapes: a review. Wildl Res 30:193–205. doi:10.1071/WR02075
Hazell D, Hero JM, Lindenmayer D, Cunningham R (2004) A comparison of constructed and natural habitat for frog conservation in an Australian agricultural landscape. Biol Conserv 119:61–71. doi:10.1016/j.biocon.2003.10.022
Heard GW, Scroggie MP, Malone BS (2012a) Classical metapopulation theory as a useful paradigm for the conservation of an endangered amphibian. Biol Conserv 148:156–166. doi:10.1016/j.biocon.2012.01.018
Heard GW, Scroggie MP, Malone BS (2012b) The life history and decline of the threatened Australian frog, Litoria raniformis. Austral Ecol 37:276–284. doi:10.1111/j.1442-9993.2011.02275.x
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hines H, Mahony M, McDonald K (1999) An assessment of frog declines in wet subtropical Australia. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra, pp 44–63. http://www.environment.gov.au/biodiversity/threatened/publications/declines-and-disappearances-australian-frogs. Accessed Aug 2014
Hock K, Wolff NH, Condie SA, Anthony KRN, Mumby PJ (2014) Connectivity networks reveal the risks of crown-of-thorns starfish outbreaks on the Great Barrier Reef. J Appl Ecol 51:1188–1196. doi:10.1111/1365-2664.12320
Jansen A, Healey M (2003) Frog communities and wetland condition: relationships with grazing by domestic livestock along an Australian floodplain river. Biol Conserv 109:207–219. doi:10.1016/S0006-3207(02)00148-9
Johst K, Brandl R, Eber S (2002) Metapopulation persistence in dynamic landscapes: the role of dispersal distance. Oikos 2:263–270
Keller L, Waller D (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Keymer J, Marquet P (2000) Extinction thresholds and metapopulation persistence in dynamic landscapes. Am Nat 156:478–494
Kingsford RT (2000) Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol 25:109–127
Kingsford RT (2011) Conservation management of rivers and wetlands under climate change—a synthesis. Mar Freshw Res 62:217–222. doi:10.1071/MF11029
Kingsford RT, Brandis K, Thomas RF, Crighton P, Knowles E, Gale E (2004) Classifying landform at broad spatial scales: the distribution and conservation of wetlands in New South Wales, Australia. Mar Freshw Res 55:17–31. doi:10.1071/MF03075
Kingsford RT, Biggs HC, Pollard SR (2011) Strategic Adaptive Management in freshwater protected areas and their rivers. Biol Conserv 144:1194–1203. doi:10.1016/j.biocon.2010.09.022
Kininmonth SJ, De’ath G, Possingham HP (2009) Graph theoretic topology of the Great but small Barrier Reef world. Theor Ecol 3:75–88. doi:10.1007/s12080-009-0055-3
Kramer-Schadt S, Kaiser T, Frank K, Wiegand T (2011) Analyzing the effect of stepping stones on target patch colonisation in structured landscapes for Eurasian lynx. Landscape Ecol 26:501–513. doi:10.1007/s10980-011-9576-4
Leidner AK, Haddad NM (2011) Combining measures of dispersal to identify conservation strategies in fragmented landscapes. Conserv Biol 25:1022–1031. doi:10.1111/j.1523-1739.2011.01720.x
Mac Nally RC (1984) Chorus dynamics of two sympatric species of Ranidella (anura): within-year and between-year variability in organisation and their determination. Zeitshriftfür Tierpsychol 65:134–151
Mac Nally R, Horrocks G, Lada H, Lake PS, Thomson JR, Taylor AC (2009) Distribution of anuran amphibians in massively altered landscapes in south-eastern Australia: effects of climate change in an aridifying region. Glob Ecol Biogeogr 18:575–585. doi:10.1111/j.1466-8238.2009.00469.x
Mahony M (1996) The decline of the green and golden bell frog Litoria aurea viewed in the context of declines and disappearances of other Australian frogs. Aust Zool 30:237–247
Marsh DM, Trenham PC (2001) Metapopulation dynamics and amphibian conservation. Conserv Biol 15:40–49. doi:10.1046/j.1523-1739.2001.00129.x
Matisziw TC, Alam M, Trauth KM, Inniss EC, Semlitsch RD, McIntosh S, Horton J (2014) A vector approach for modeling landscape corridors and habitat connectivity. Environ Model Assess 20:1–15. doi:10.1007/s10666-014-9412-8
Mazerolle MJ, Desrochers A (2005) Landscape resistance to frog movements. Can J Zool 464:455–464. doi:10.1139/Z05-032
McGinness H, Arthur A, Ward K, Ward P (2014) Floodplain amphibian abundance: responses to flooding and habitat type in Barmah Forest, Murray River, Australia. Wildl Res 41:149–162. doi:10.1071/WR13224
McIntyre NE, Strauss RE (2013) A new, multi-scaled graph visualization approach: an example within the playa wetland network of the Great Plains. Landscape Ecol 28:769–782. doi:10.1007/s10980-013-9862-4
McIntyre NE, Wright CK, Swain S, Hayhoe K, Liu G, Schwartz FW, Henebry GM (2014) Climate forcing of wetland landscape connectivity in the Great Plains. Front Ecol Environ 12:59–64. doi:10.1890/120369
McRae BH (2006) Isolation by resistance. Evolution 60:1551–1561. doi:10.1554/05-321.1
McRae BH, Beier P (2007) Circuit theory predicts gene flow in plant and animal populations. Proc Natl Acad Sci USA 104:19885–19890. doi:10.1073/pnas.0706568104
McRae BH, Dickson BG, Keitt TH, Shah VB (2008) Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 89:2712–2724
Minor ES, Lookingbill TR (2010) A multiscale network analysis of protected-area connectivity for mammals in the United States. Conserv Biol 24:1549–1558. doi:10.1111/j.1523-1739.2010.01558.x
Minor ES, Urban DL (2007) Graph theory as a proxy for spatially explicit population models in conservation planning. Ecol Appl 17:1771–1782. doi:10.1890/06-1073.1
Moilanen A, Franco AMA, Early RI, Fox R, Wintle BA, Thomas CD (2005) Prioritizing multiple-use landscapes for conservation: methods for large multi-species planning problems. Proc R Soc B 272:1885–1891. doi:10.1098/rspb.2005.3164
Montoya JM, Sol RV (2002) Small world patterns in food webs. J Theor Biol 214:405–412. doi:10.1006/jtbi.2001.2460
Moore JA, Tallmon DA, Nielsen J, Pyare S (2011) Effects of the landscape on boreal toad gene flow: does the pattern–process relationship hold true across distinct landscapes at the northern range margin? Mol Ecol 20:4858–4869. doi:10.1111/j.1365-294X.2011.05313.x
Murphy MA, Dezzani R, Pilliod DS, Storfer A (2010) Landscape genetics of high mountain frog metapopulations. Mol Ecol 19:3634–3649. doi:10.1111/j.1365-294X.2010.04723.x
Murray–Darling Basin Authority, MDBA (2012) Water resource plan areas and water accounting periods. Basin Plan. Murray–Darling Basin Authority, Canberra, pp 15–19
Nairn LC, Kingsford RT (2012) Wetland distribution and land use in the Murray–Darling Basin. A report to the Australian Floodplain Association. Australian Wetlands, Rivers and Landscapes Centre, UNSW, Sydney. https://www.ecosystem.unsw.edu.au/content/wetland-distribution-and-land-use-in-the-murray-darling-basin. Accessed Nov 2014
Newman MEJ (2003) The structure and function of complex networks. SIAM Rev 45:167–256
Newman MEJ, Girvan M (2004) Finding and evaluating community structure in networks. Phys Rev E. doi:10.1103/PhysRevE.69.026113
Nowakowski AJ, DeWoody JA, Fagan ME, Willoughby JR, Donnelly MA (2015a) Mechanistic insights into landscape genetic structure of two tropical amphibians using field-derived resistance surfaces. Mol Ecol 24:580–595. doi:10.1111/mec.13052
Nowakowski J, Veiman-Echeverria M, Kurz D, Donnelly MA (2015b) Evaluating connectivity for tropical amphibians using empirically derived resistance surfaces. Ecol Appl. doi:10.1890/14-0833.1
Nuñez TA, Lawler JJ, McRae BH, Pierce DJ, Krosby MB, Kavanagh DM, Singleton PH, Tewksbury JJ (2013) Connectivity planning to address climate change. Conserv Biol 27:407–416. doi:10.1111/cobi.12014
Ocock J, Rowley J, Penman T (2013) Amphibian chytrid prevalence in an amphibian community in arid Australia. EcoHealth 10:77–81. doi:10.1007/s10393-013-0824-8
Ocock JF, Kingsford RT, Penman TD, Rowley JJL (2014) Frogs during the flood: differential behaviours of two amphibian species in a dryland floodplain wetland. Austral Ecol 39:929–940. doi:10.1111/aec.12158
Perkins SE, Cagnacci F, Stradiotto A, Arnoldi D, Hudson PJ (2009) Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. J Anim Ecol 78:1015–1022. doi:10.1111/j.1365-2656.2009.01557.x
Peterman WE, Rittenhouse TAG, Earl JE, Semlitsch RD (2013) Demographic network and multi-season occupancy modeling of Rana sylvatica reveal spatial and temporal patterns of population connectivity and persistence. Landscape Ecol 28:1601–1613. doi:10.1007/s10980-013-9906-9
Peterman WE, Connette GM, Semlitsch RD, Eggert LS (2014) Ecological resistance surfaces predict fine-scale genetic differentiation in a terrestrial woodland salamander. Mol Ecol 23:2402–2413. doi:10.1111/mec.12747
Pinto N, Keitt TH (2009) Beyond the least-cost path: evaluating corridor redundancy using a graph-theoretic approach. Landscape Ecol 24:253–266. doi:10.1007/s10980-008-9303-y
Pittman SE, Osbourn MS, Semlitsch RD (2014) Movement ecology of amphibians: a missing component for understanding population declines. Biol Conserv 169:44–53. doi:10.1016/j.biocon.2013.10.020
Pittock B, Abbs D, Suppiah R, Jones R (2006) Climatic background to past and future floods in Australia. Adv Ecol Res 39:13–39. doi:10.1016/S0065-2504(06)39002-2
Prevedello JA, Vieira MV (2010) Does the type of matrix matter? A quantitative review of the evidence. Biodivers Conserv 19:1205–1223. doi:10.1007/s10531-009-9750-z
Pyke C (2005) Assessing suitability for conservation action: prioritizing interpond linkages for the California tiger salamander. Conserv Biol 19:492–503
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org/. Accessed April 2014
Ribeiro R, Carretero MA, Sillero N, Alarcos G, Ortiz-Santaliestra M, Lizana M, Llorente GA (2011) The pond network: can structural connectivity reflect on (amphibian) biodiversity patterns? Landscape Ecol 26:673–682. doi:10.1007/s10980-011-9592-4
Richardson JL (2012) Divergent landscape effects on population connectivity in two co-occurring amphibian species. Mol Ecol 21:4437–4451. doi:10.1111/j.1365-294X.2012.05708.x
Rogers K, Ralph TJ (2010a) Floodplain wetlands of the Murray–Darling Basin and their freshwater biota. In: Rogers K, Ralph TJ (eds) Floodplain wetland biota in the Murray–Darling Basin water habitat requirements. CSIRO Publishing, Collingwood, pp 1–16
Rogers K, Ralph TJ (2010b) Impacts of hydrological changes on floodplain wetland biota. In: Rogers K, Ralph TJ (eds) Floodplain wetland biota in the Murray–Darling Basin water habitat requirements. CSIRO Publishing, Collingwood, pp 311–328
Ruiz L, Parikh N, Heintzman LJ, Collins SD, Starr SM, Wright CK, Henebry GM, van Gestel N, McIntyre NE (2014) Dynamic connectivity of temporary wetlands in the southern Great Plains. Landscape Ecol 29:507–516. doi:10.1007/s10980-013-9980-z
Saura S, Bodin Ö, Fortin MJ (2014) Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J Appl Ecol 51:171–182. doi:10.1111/1365-2664.12179
Semlitsch RD (2008) Differentiating migration and dispersal processes for pond-breeding amphibians. J Wildl Manag 72:260–267. doi:10.2193/2007-082
Shah VB, McRae BH (2008) Circuitscape: a tool for landscape ecology. In: Varoquaux G, Vaught T, Millman J (eds) Proceedings of the 7th Python in science conference (SciPy 2008), Pasadena, CA, pp 62–66. http://gauss.cs.ucsb.edu/publication/Circuitscape_Python_Scipy08.pdf. Accessed April 2014
Sinsch U (2014) Movement ecology of amphibians: from individual migratory behaviour to spatially structured populations in heterogeneous landscapes. Can J Zool 502:491–502
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:110–128. doi:10.1111/j.0906-7590.2005.04042.x
Smith MJ, Schreiber ESG, Scroggie MP, Kohout M, Ough K, Potts J, Lennie R, Turnbull D, Jin C, Clancy T (2007) Associations between anuran tadpoles and salinity in a landscape mosaic of wetlands impacted by secondary salinisation. Freshw Biol 52:75–84. doi:10.1111/j.1365-2427.2006.01672.x
Spear SF, Balkenhol N, Fortin MJ, McRae BH, Scribner K (2010) Use of resistance surfaces for landscape genetic studies: considerations for parameterization and analysis. Mol Ecol 19:3576–3591. doi:10.1111/j.1365-294X.2010.04657.x
St-Louis V, Forester JD, Pelletier D, Bélisle M, Desrochers A, Rayfield B, Wulder MA, Cardille JA (2014) Circuit theory emphasizes the importance of edge-crossing decisions in dispersal-scale movements of a forest passerine. Landscape Ecol 29:831–841. doi:10.1007/s10980-014-0019-x
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786. doi:10.1126/science.1103538
Treml EA, Halpin PN, Urban DL, Pratson LF (2007) Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landscape Ecol 23:19–36. doi:10.1007/s10980-007-9138-y
Tulbure MG, Kininmonth SJ, Broich M (2014) Spatiotemporal dynamics of surface water networks across a global biodiversity hotspot—implications for conservation. Environ Res Lett 9:114012. doi:10.1088/1748-9326/9/11/114012
Tyler M (1991) Declining amphibian populations—a global phenomenon? An Australian perspective. Alytes 9:43–50
Uden D, Hellman M, Angeler DG, Allen CR (2014) The role of reserves and anthropogenic habitats for functional connectivity and resilience of ephemeral wetlands. Ecol Appl 24:1569–1582
Urban DL, Keitt TH (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82:1205–1218
Urban DL, Minor ES, Treml EA, Schick RS (2009) Graph models of habitat mosaics. Ecol Lett 12:260–273. doi:10.1111/j.1461-0248.2008.01271.x
Van Buskirk J (2012) Permeability of the landscape matrix between amphibian breeding sites. Ecol Evol 2:3160–3167. doi:10.1002/ece3.424
Van Etten J (2014) gdistance: distances and routes on geographical grids. R package version 1.1-5. http://cran.r-project.org/web/packages/gdistance/index.html. Accessed Nov 2014
Vié J-C, Hilton-Taylor C, Stuart SN (2008) Wildlife in a changing world: an analysis of the 2008 IUCN Red List of Threatened Species. IUCN-The World Conservation Union, Gland
Vos CC, Goedhart P (2007) Matrix permeability of agricultural landscapes: an analysis of movements of the common frog (Rana temporaria). Herpetol J 17:174–182
Wassens S (2010a) Flooding regimes for frogs in lowland rivers of the Murray–Darling Basin. In: Saintilan N, Overton I (eds) Ecosystem response modelling in the Murray–Darling Basin. CSIRO Publishing, Collingwood, pp 213–227
Wassens S (2010b) Frogs. In: Rogers K, Ralph T (eds) Floodplain wetland biota of the Murray–Darling Basin: water and habitat requirements. CSIRO Publishing, Collingwood, pp 253–274
Wassens S, Maher M (2011) River regulation influences the composition and distribution of inland frog communities. River Res Appl 27:238–246. doi:10.1002/rra.1347
Wassens S, Watts RJ, Jansen A, Roshier D (2008) Movement patterns of southern bell frogs (Litoria raniformis) in response to flooding. Wildl Res 35:50–58. doi:10.1071/WR07095
Wassens S, Walcott A, Wilson A, Freire R (2013) Frog breeding in rain-fed wetlands after a period of severe drought: implications for predicting the impacts of climate change. Hydrobiologia 708:69–80. doi:10.1007/s10750-011-0955-2
Wright CK (2010) Spatiotemporal dynamics of prairie wetland networks: power-law scaling and implications for conservation planning. Ecology 91:1924–1930
Zeigler SL, Fagan WF (2014) Transient windows for connectivity in a changing world. Mov Ecol 2:1. doi:10.1186/2051-3933-2-1
Zeller KA, McGarigal K, Whiteley AR (2012) Estimating landscape resistance to movement: a review. Landscape Ecol 27:777–797. doi:10.1007/s10980-012-9737-0
Acknowledgments
This work was funded through an Australian Research Council Discovery Early Career Researcher Award (DE140101608) to Tulbure. Flood inundation modelling was based on the Murray–Darling Basin Floodplain Inundation Model (MDB-FIM) provided by the Commonwealth Scientific and Industrial Research Organisation, as described in its metadata statement (http://www.clw.csiro.au/products/mdb-fim/). South Australian Ramsar boundaries were provided by the Department of Environment, Water and Natural Resources. For estimating landscape resistance to movement for our focal species, we thank M. Anstis, A. Hamer, G. Heard, F. Lemckert, J. Ocock, and M. Scroggie.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bishop-Taylor, R., Tulbure, M.G. & Broich, M. Surface water network structure, landscape resistance to movement and flooding vital for maintaining ecological connectivity across Australia’s largest river basin. Landscape Ecol 30, 2045–2065 (2015). https://doi.org/10.1007/s10980-015-0230-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0230-4