Abstract
Globally, rain-fed wetlands provide critical habitat for a wide range of amphibian species, however, information on the use of rain-fed wetlands by Australian frog species is extremely limited. This study examined the distribution of frog breeding in rain-fed wetlands following the first significant rain event after a period of severe drought (2002–2009) in order to predict how frog communities may be affected in the future by changed climate. Tadpole communities along with vegetation and water quality variables were described in 35 rain-fed wetlands across the South West Slopes and Riverina bioregions of inland south-eastern Australia. In addition, weekly tadpole surveys were conducted in a subset of these wetlands to describe temporal patterns of occupancy. Despite the protracted dry period prior to this study 50% of the rain-fed wetlands surveyed contained tadpoles. However, frog communities were species poor with only five species recorded. The majority of wetlands were dominated be a single species, Limnodynastes tasmaniensis which is also common within permanent waterbodies such as farm dams and irrigation infrastructure in both bioregions. Tadpoles of two burrowing species L. interioris and Neobatrachus sudelli were restricted to a small number of wetlands mostly in the South West Slopes. The composition of tadpole communities changed over time, and Crinia parinsignifera was the only species that continued to breed over winter. The dominance of generalist species within rain-fed wetlands indicates that characteristics such as dispersal capability, flexibility in breeding times and the ability to utilise created habitats may be more important than burrowing ability and longevity when predicting vulnerability to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rain-fed wetlands are of major economic and ecological importance, providing many benefits such as surface water storage, floodwater protection, nutrient cycling and water quality maintenance. In addition, these wetland ecosystems provide vital habitat for unique biota in an otherwise dry environment where rainfall is highly variable (Jolly et al., 2008). Temporary rain-fed wetlands support a wide range of species including non-vascular and vascular plants (Casanova & Brock, 2000), microinvertebrates (Boix et al., 2001; Gleason et al., 2004), macroinvertebrates (Lee Foote & Rice Hornung, 2005) and amphibians (Homan et al., 2004; Trauth et al., 2006; Baldwin & deMaynadier, 2009).
Rain-fed wetlands are vulnerable to changes in rainfall and evaporation rates, including those driven by increases in atmospheric CO2 and enhanced green house effect, because their hydrological regime is driven largely by local weather patterns (Acreman et al., 2009; Brooks, 2009). The impacts of climate change on rain-fed wetlands and their dependent faunal communities are difficult to quantify due to the high level of uncertainty regarding future changes to local weather patterns, particularly changes in precipitation. However, a number of climate models for Australia predict a long-term shift to higher temperatures, increased evaporation rates and more extreme hydrological events (Smith & Chandler, 2010). For example, the drought experienced between 2002 and 2009 may have been more severe than previous drought events because reduced rainfall has also been accompanied by higher temperatures and evapotranspiration rates (Murphy & Timbal, 2008).
Rain-fed wetlands provide important habitat for a range of native frogs (Williams, 1985; Beja & Alcazar, 2003; Vignoli et al., 2007b; Babbitt et al., 2009; Gómez-Rodríguez et al., 2009). Unlike more persistent water bodies, rain-fed wetlands are typically free of large predators, particularly fish, making them important breeding habitat for species with predator-sensitive tadpoles (Gillespie & Hero, 1999; Adams, 2000; Baber & Babbitt, 2003; Goldingay, 2008). However, water level fluctuations result in variable physio-chemical conditions and frog species utilising rain-fed wetlands must trade-off the reduced risk of predation with the increased risk of breeding failure due to desiccation and declining water quality (Vignoli et al., 2007a; Gahl et al., 2009; Gómez-Rodríguez et al., 2009). Life-cycle adaptations such as plastic developmental times and bet-hedging in tadpoles (Dziminski & Alford, 2005), burrowing and aestivation (Penman et al., 2004; Tracy et al., 2007; Kayes et al., 2009), and emigration under adverse conditions (Wassens et al., 2008) are common characteristics of Australian frog species in temperate, semi-arid and arid environments. Dispersal capability may be a critical factor influencing the ability of both burrowing and non-burrowing species to recover following periods of drought because it enables them to rapidly recolonise newly created rain-fed wetlands. The spatial distribution of permanent waterbodies and soil types suitable for burrowing may also influence recolonisation of rain-fed wetlands (Mazerolle & Villard, 1999) although the latter issue has received little attention.
Despite their many adaptations to the use of variable resources, amphibians that preferentially breed in rain-fed and temporary pools are considered to be extremely sensitive to changes in temperature and precipitation occurring as a result of climate change (Blaustein et al., 2010). Whilst long-term shifts in precipitation are difficult to predict, a number of recent studies have demonstrated the negative impact of severe drought on amphibian community composition and breeding success (Rohr & Madison, 2003; Piha et al., 2007; McMenamin et al., 2008; Mac Nally et al., 2009; Rohr & Raffel, 2010). Increased climatic variability and increased frequency of extreme weather events can have a number of direct and indirect impacts on amphibian populations, including increased mortality during extreme dry or extreme cold events (Blaustein et al., 2010), reduced breeding success and recruitment (Babbitt & Tanner, 2000; Piha et al., 2007) and increased susceptibility to disease (Rohr & Raffel, 2010). It can, however, be difficult to separate the normal inter-annual variability in amphibian population dynamics and distributions from potential long-term shifts caused by climate change. For example, some population declines attributed to extreme drought and climate change may have been a result of normal variability in occupancy patterns caused by individuals retreating back to areas of core habitat during extended drought, only to recolonise formerly occupied wetlands with a return to favourable conditions (Patla et al., 2009).
Much of our understanding of the impacts of climate change on amphibians in rain-fed waterbodies is derived from studies in the northern hemisphere, where vernal pools are important habitat for a wide range of species (e.g. Blaustein et al., 2010; Blaustein et al., 2011). Knowledge of the importance of rain-fed wetlands for frogs in Australian temperate and semi-arid regions is extremely limited making predictions on the impacts of future climate change difficult. Frog communities in the dry temperate and semi-arid regions of south-eastern Australia are typically species poor compared with the wetter temperate, tropical and monsoonal regions of Australia (Caughley & Gall, 1985). The Riverina and South West Slopes Bioregions support 15 frog species, the majority of which occur across a range of aquatic habitats including seasonally flooded wetlands associated with the Murrumbidgee River system (Wassens, 2006). Only two species Notaden bennettii and Pseudophryne bibronii are considered to be rain-fed wetland specialists and are active only after heavy rain (Cogger, 2000).
The use of rain-fed wetlands by resident frog species is primarily driven by the timing of filling and drying (seasonality of rainfall) and the hydrological regime (duration of inundation and the length time between filling events). A large number of frog species have restricted activity periods which are often linked to temperature (Carroll et al., 2009) and as a result tadpole communities within the same rain-fed wetland can vary considerably between years depending on the timing of inundation (Paton & Crouch, 2002; Jakob et al., 2003). Those species which follow specific breeding times may suffer more from altered seasonality of rainfall under climate change, than those with long breeding periods or opportunistic breeding.
Tadpole development times are species specific and this is reflected in the hydroperiod of the wetlands that these species inhabit. Species with rapid tadpole development times typically have a higher breeding success rate in temporary waterbodies, whilst species with long development times may be excluded (Lane & Mahony, 2002; Popescu & Gibbs, 2009). Tadpole mortalities due to desiccation are a common result of hydroperiods which fail to extend over the given species complete developmental time frame (Williamson & Bull, 1999; Babbitt & Tanner, 2000; Brodman et al., 2003; Jakob et al., 2003). Predicted increases in potential evapotranspiration, especially during summer may contribute to reduced hydroperiods within rain-fed wetlands and increased frequency of recruitment failure due to tadpole desiccation.
The aims of this study were to:
-
(1)
identify which frog species utilise rain-fed wetlands after a period of severe and extended droughts and
-
(2)
describe temporal patterns of breeding activity by frogs within rain-fed wetlands in order to better understand the impacts of potential shifts in the timing of significant rain events.
Methods
Study area
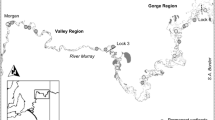
Regional scale surveys were conducted at a total of 35 rain-fed wetlands, located in the South West Slopes bioregion (n = 18) and in the Riverina bioregion centred on the Hay Plain (n = 17) in south west New South Wales, Australia (Fig. 1). Vegetation of the South West Slopes is diverse, with the original vegetation dominated by open woodlands containing white box (Eucalyptus albens, Benth), yellow box (E. melliodora, Cunn. ex Schauer) and blakelys red gum (E. blakelyi, Maiden) the vast majority of which has been cleared. Black box (E. largiflorens, Muell) communities occur along intermittent creeks and wetlands and river red gum forests (E. camaldulensis, Dehnh) occur close to permanent rivers and seasonally flooded wetlands in both bioregions. The Riverina bioregion is a flat, largely treeless plain dominated by Atriplex communities and native grasslands. The dominant land use is pastoral grazing and along with extensive areas of irrigated crop production.
Climate and rainfall patterns
The annual mean rainfall in the South West Slopes is 572 mm, summer temperatures are hot, averaging between 29 and 32°C and winter temperatures range from overnight minimums of three to daily maximums of 12 to 14°C (Bureau of Meteorology, 2010). The climate in the Riverina is semi-arid (mean annual rainfall 365 mm), with very hot summers, mean maximum temperatures of 33°C, and cool winters (average min. 3.5 to average max. of 15°C) (Bureau of Meteorology, 2010). Rainfall is slightly winter dominant, although high intensity summer thunderstorms of short duration are common during wetter years.
Inter-annual variability in precipitation is extremely high in both bioregions, between 1941 and 2010 the annual precipitation ranged from 164.9 to 836.8 mm in the Riverina (variance 21094.9) measured at Hay and between 165.7 and 1019.2 mm in the South West Slopes (variance = 28345.1) measured at the nearby town of Wagga Wagga (Bureau of Meteorology, 2010). In both bioregions, 2002, 2006 and 2008 were in the top 12 driest years between 1941 and 2010, whilst 2010 was the wettest year for that period in Wagga Wagga and the fourth wettest year in Hay (Fig. 2) (Bureau of Meteorology, 2010).
Annual precipitation over a 50-year period (1940–2010) in the Riverina at Hay (75031) and South West Slopes at Wagga Wagga (72150). Reference line shows median precipitation for the same period. Source: Commonwealth Bureau of Meteorology (2010)
As the distribution of rain-fed wetlands is poorly documented, air photos and consultation with property owners and Murrumbidgee Catchment Management Authority staff were initially employed to identify potential wetlands for investigation. In February 2010, a low pressure system brought significant rainfall to the Riverina and the South West Slopes; this was followed by an inland trough in early March which brought a second significant rain event (record highest rainfall in Wagga Wagga) to both regions (Bureau of Meteorology, 2010) (Fig. 3). Further significant rain events occurred in May, July and August. All wetlands included in this study first filled in February 2010 with subsequent top-up falls in March and May after which time the smaller shallower wetlands dried out, before refilling in June. Larger wetlands retained water throughout the study period for 6–9 months. All wetlands were dry during the severe drought conditions experienced through 2009.
Total monthly rainfall (mm) and mean daily air temperature recorded at 3 pm at Wagga Wagga (72150) and Hay (75031) between February 1st 2010 and August 31st 2010. Source: Commonwealth Bureau of Meteorology (2010)
Habitat parameters
Regional scale surveys of tadpole communities, vegetation characteristics and water quality were conducted between 15th and 31st of March 2010. In addition to regional scale surveys, repeat weekly sampling was conducted at a subset of wetlands between 31st of March and 10th of August, 2010. Wetland type and size were classified according to (Semeniuk & Semeniuk, 1995) as Playa (n = 20) (intermittently flooded basin), Sumpland (n = 7) (seasonally inundated basin) or Wadi (n = 8) (intermittently flooded channel). Wetlands fell into one of two size classes, Leptoscale (n = 24) less than 100 × 100 m, and mesoscale (n = 3) between 500 × 500 m to 1,000 × 1,000 m. Wadi channels were all classified as microscale (n = 8) less than 10 m across and 100 m long (Semeniuk & Semeniuk, 1995).
Three replicate measures of water quality (turbidity, conductivity, pH, dissolved oxygen and temperature) were taken on each survey occasion using a hand held YSI 6820 V2 multi-parameter sonde at approximately 1 m from the water’s edge. Vegetation was assessed by visual estimation of the proportion of microhabitats (fringing vegetation, tall emergent vegetation, short emergent vegetation, submerged vegetation, floating vegetation and timber within the water body) comprising 5 m wide bank to bank sections of the wetland. Fringing vegetation was considered to include all plant classes present within 2 m of the shoreline, or where there was an abrupt change in vegetation communities from semi-aquatic and riparian vegetation to terrestrial grassland and Atriplex communities. Tall emergent vegetation was classified as vegetation greater than 20 cm above the water level (e.g. Typha sp.), whilst short emergent vegetation included sedges and short rushes less than 20 cm above the water level. Submerged vegetation included all plant species present below the water surface, including vegetation recently inundated due to rising water levels. Floating vegetation was any vegetation that floated on the water’s surface and included both attached and free floating species.
Tadpole surveys
Tadpoles were surveyed using sweep nets, targeting the range of water depths and habitats within the wetland. This is a common and effective technique for sampling tadpoles within rain-fed water bodies (Anstis, 2002). Sweeping was conducted at each wetland for 20 min. Visual searches for tadpoles and metamorphs were also conducted by walking the entire wetland perimeter, or in the case of larger systems, walking a 50 m transect. Tadpoles were collected in a soft mesh sweep net (mesh size 2 mm, 40 cm wide), and immediately transferred to a container where they were identified to species, measured and their development stage described based on the extent of limb development (Grosner, 1960) before being released at the point of capture.
Temporal occupancy patterns
Change in tadpole communities over time was assessed at a subset of five wetlands on 14 occasions (weekly), from the 31st of March to the 10th of August, 2010. Wetlands were selected on the basis of their tadpole communities with the aim of including all species likely to occur in both the South West Slopes and Riverina bioregion sites. In order to conduct intensive surveys of wetlands on a weekly basis and to minimise potential variability in tadpole responses caused by regional variation in temperature and rainfall, we aimed to select wetlands within a 15 km radius of one another which contained all five species. Three key species, Crina parinsignifera Main, Litoria peronii Tschudi and Neobatrachus sudelli Lamb, were restricted to a small number of wetlands in the South West Slopes, as a result wetlands containing these three species formed the basis for our search area with the remaining wetlands selected from within the 15 km radius that contained the more common and widespread species.
In order to limit potential confounding effects caused by land use disturbance, all wetlands were selected from within nature reserves. Vegetation and water quality data were collected on each survey occasion, as described for the broad-scale occupancy surveys, along with collection of additional tadpole data for body size. Tadpoles were identified to species and stage of development via visual assessment (Anstis, 2002).
Data analysis
General linear models (GLMs) and Bonferroni post hoc tests were employed to analyse univariate data using SPSS (SPSS for Windows Release 17.0 SPSS Inc 2005). The mean values of temperature, salinity, turbidity, dissolved oxygen, conductivity and pH were compared between regions and among wetland types. Differences in the composition of tadpole communities between bioregions and between seasons, autumn (n = 7) and winter (n = 7) (temporal occupancy patterns) were tested using analysis of similarities (ANOSIM) with a Bray Curtis Similarity matrix. Data were square-root transformed prior to analysis (Clarke & Warwick, 2001).
Two-tailed t tests were used to compare the means for conductivity, pH, turbidity, per cent cover of open water, floating, submerged, fringing and short emergent vegetation, and total per cent cover of aquatic vegetation, total per cent cover of aquatic vegetation for vacant and occupied wetlands from the broad-scale data.
Results
Habitat parameters
Water quality was variable between regions and among wetlands, reflecting the wide geographic spread of wetlands included in this study. Conductivity was high in playa wetlands (mean 1.088 mScm−1 SE 0.086) within the Riverina compared to range of 0.054–0.426 mScm−1 for other wetland types resulting in significant differences among wetland types (GLM; F = 3.779, P = 0.039), however, there was no significant difference in conductivity between bioregions. In contrast pH differed significantly between bioregions, with mean pH significantly higher in the Riverina (mean 10.20 SE 0.04) than in the South West Slopes (mean 8.38 SE 0.11) (GLM; F = 15.711, P = 0.001). Aquatic vegetation communities were variable across wetlands in both regions and there were no significant differences in the structural diversity of aquatic vegetation, aquatic or fringing vegetation cover between bioregions or among wetland types.
Broad-scale patterns of tadpole occupancy
A total of 310 tadpoles of five species were collected from 17 of the 35 wetlands (49%) surveyed. The proportion of wetlands containing tadpoles was similar across the two bioregions, seven out of 17 wetlands in the Riverina and nine out of 18 wetlands in the South West Slopes contained tadpoles. Three species, N. sudelli (n = 3), C. parinsignifera (n = 2) and L. peronii (n = 1) occurred only in the South West Slopes (Fig. 4). Of the remaining two species Limnodynastes interioris Fry occurred in both bioregions but was rare (n = 4) whilst L. tasmaniensis Günther, was widespread and abundant in both the bioregions. Consequently, species richness was on average higher in the South Western Slopes (0.94) than the Riverina (0.47) but this difference was not significant (GLM; F = 1.680, P = 0.204). It was not possible to identify statistically significant differences in community composition between the two bioregions due to lack of statistical power as the vast majority of wetlands in both bioregions contained just one species (L. tasmaniensis) (ANOSIM; Global R = 0.011, P = 0.435).
As the majority of species were very rare, we were only able to analyse the relationship between presence/absence and habitat and water quality variables for L. tasmaniensis. There were no significant relationships for mean water quality and vegetation attributes measured in wetlands, where L. tasmaniensis tadpoles were present and where they were absent.
Temporal patterns in tadpole occupancy
Significant rain events occurred from 13th to 16th of February (60 mm) and 6th to 8th of March (164 mm), the smallest of the five wetlands dried out in late May and was refilled in June (see Fig. 3). Water temperature decreased over time at the five weekly monitoring sites from a mean of 25°C in March to 10°C in late July and August. Whilst conductivity also decreased from a mean high of 0.099 mScm−1 in March to a low of 0.062 mScm−1 in August. The per cent cover of aquatic vegetation also decreased over time mainly due to fluctuating water levels, ranging from a mean of 37% in March to 6% in August.
Tadpoles of five frog species (L. peronii, C. parinsignifera, L. interioris, N. sudelli and L. tasmaniensis) were monitored over the duration of the study within the five wetlands. The tadpole communities differed significantly between autumn and winter (ANOSIM; Global R = 0.278, P = 0.01) and according to location (discrete wetlands) (ANOSIM; Global R = 0.683, P = 0.01). Tadpole communities in autumn were typically more diverse, with four species, C. parinsignifera, N. sudelli, L. tasmaniensis and L. interioris present, whilst the winter tadpole communities were generally dominated by C. parinsignifera (Fig. 5).
Tadpole abundance for the majority of species declined over time with the exception of C. parinsignifera which declined and then increased following winter rainfall. N. sudelli was present for the shortest period of time (no longer recorded after the 15th of May) and its abundance was initially high and steadily decreased over time with late stage tadpoles (grosner stages 37 and above) recorded in early May. C. parinsignifera was the only species to undergo two breeding events, early stage tadpoles were recorded in March and April, with later stage tadpoles (grosner stages 37 and above) were collected in mid-May. The site containing C. parinsignifera dried out briefly and then refilled following significant rain events on 26 and 29 of May (26 and 20 mm, respectively) (see Fig. 4). Egg masses and very early stage tadpoles (stage 26) located from 2nd of June through July, mid-stage tadpoles (grosner stages 32) (Grosner, 1960) were present when surveys ceased on 10th August.
Discussion
Tadpoles were relatively widespread in the rain-fed wetlands of both bioregions, but communities were species poor. Only five of the 15 species known to occur in both bioregions were recorded within rain-fed wetlands in this study (Wassens, 2006). The vast majority of wetlands contained only one species, the habitat generalist Limnodynastes tasmaniensis. Despite the lower survey effort in this study, tadpoles occurred at a far higher proportion of wetlands than reported by Mac Nally et al. (2009) in their study in south-eastern Victoria. This probably reflects the different focus of that study on more persistent waterbodies which were present during the drought period in 2006 and 2007.
Wetlands are subject to a wide range of disturbances and threatening processes including draining, damming, grazing and cultivation (Finlayson & Rea, 1999). The spatial context of rain-fed wetlands has also been altered within agricultural landscapes with dams and canals increasing the availability of permanent habitats, whilst the availability of temporary waterbodies has decreased (Brock et al., 1999). These shifts in habitat availability have undoubtedly altered frog communities (e.g. Mac Nally et al., 2009), with a potential shift towards generalist species capable of utilising more persistent waterbodies as well as the opportunistic use of rain-fed waterbodies when they are available.
The results of this study suggest that dispersive generalist frog species, rather than burrowing frog species tend to dominate rain-fed wetlands in modified agricultural landscapes after periods of extended drought. Limnodynastes tasmaniensis has a number of adaptations that make it highly suited to the opportunistic use of rain-fed wetlands, it has comparably good dispersal ability (Schauble, 2004), and utilises a wide range of aquatic habitats including farm dams (Hazell et al., 2001) and irrigation infrastructure (Wassens, 2006) which provide a more constant source of water during drought periods. Studies of other vertebrate taxa consider dispersal ability to be a critical factor in determining the risk from climate change, however, this typically relates to large scale movements which reflect range shifts (Pearson & Dawson, 2003; Thomas et al., 2004). At local scales dispersal ability may be a key factor influencing the ability of aquatic and semi-aquatic fauna to recover from severe climate perturbations such as drought because it influences their ability to seek out permanent refuge sites and recolonise waterbodies following local extinctions (Harrison, 1994; Smith & Green, 2005).
Burrowing ability is a specific adaptation of frogs to variable rainfall and allows individuals to persist for extended dry periods (Tracy et al., 2007; Kayes et al., 2009). On this basis burrowing ability might be seen as lowering the susceptibility of species to future climate changes, with large-bodied, long-lived species such as L. interioris being comparatively resilient to drought compared to the shorter lived, non-burrowing species. However, there have been few studies on the actual capacity of these species to aestivate for extended periods. Many burrowing species are active throughout the year, but this activity is closely linked to meteorological conditions, particularly rain and humidity (Penman et al., 2004). Extended drought may limit the ability of these species to forage and this, along with their requirement for wetlands with extended hydroperiods to support breeding, may explain their rarity in rain-fed wetlands in the drier Riverina bioregion. Non-cocoon forming species such as L. interioris may also burrow deeper during extended dry periods in order to maintain moisture balance (Thomas et al., 2004) and retreating water tables or increased groundwater salinity could potentially limit the survival of these species during periods of extended drought.
Weekly monitoring of tadpole communities within selected wetlands was used in this study to assess change in tadpole communities over time. This study was conducted outside the reported core calling periods of L. tasmaniensis (Lemckert & Mahony, 2008) but despite this, tadpoles of this species were abundant, which demonstrates the ability of this species to respond to unusual weather conditions. C. parinsignifera was the only species able to respond to winter rain events and underwent two distinct breeding events between March and August. This high level of flexibility and rapid breeding times may increase the resilience of this species to changes in the seasonality of rain events. Studies conducted in the Northern Hemisphere have reported on changes in breeding phenology, including earlier calling times in response to increased spring temperatures (Neveu, 2009; Blaustein et al., 2010; Todd et al., 2010). These studies focused on areas where the availability of wetland habitat was unchanged but where changes in temperature altered breeding cues. Assessing the impacts of altered rainfall patterns on frog breeding is more complex because both the temporal patterns of habitat availability, and the temperature driven breeding cues are altered. For frogs to respond to a rain event it must occur within their activity window and for tadpoles to successfully develop, wetlands must retain water for a sufficient period of time. Shifts towards summer dominated rainfall may allow breeding for many species but higher temperature and evaporation rates may reduce wetland hydroperiod excluding those species with longer and less flexible development times, such as L. interioris.
Marked inter-annual variation in rainfall, wetland hydrology and subsequent variability in frog breeding and community composition has also been widely reported for rain-fed wetlands systems (Jakob et al., 2003; Piha et al., 2007; Gómez-Rodríguez et al., 2009). This high level of variability presents a significant challenge for predicting impacts of future climate change on frogs within rain-fed wetland systems. In this respect predictive models that consider species distributions with respect to climate variability, for example, variability in the duration of wet-dry periods, may be more informative when predicting the impacts of climate change on species in variable landscapes than models based on temperature shifts alone. The long-term study of frog communities in rain-fed wetlands and better assessment of their hydrological requirements and distributions will also greatly assist our understanding of population processes in systems that already have a high level of inter-annual variability.
References
Acreman, M. C., J. R. Blake, D. J. Booker, R. J. Harding, N. Reynard, J. O. Mountford & C. J. Stratford, 2009. A simple framework for evaluating regional wetland ecohydrological response to climate change with case studies from Great Britain. Ecohydrology 2: 1–17.
Adams, M. J., 2000. Pond permanence and the effects of exotic vertebrates on anurans. Ecological Applications 10: 559–568.
Anstis, M., 2002. Tadpoles of South-eastern Australia: A Guide with Keys. Reed New Holland, Sydney.
Babbitt, K. J. & G. W. Tanner, 2000. Use of temporary wetlands by anurans in a hydrologically modified landscape. Wetlands 20: 313–322.
Babbitt, K. J., M. J. Baber, D. L. Childers & D. Hocking, 2009. Influence of agricultural upland habitat type on larval anuran assemblages in seasonally inundated wetlands. Wetlands 29: 294–301.
Baber, M. J. & K. J. Babbitt, 2003. The relative impacts of native and introduced predatory fish on a temporary wetland tadpole assemblage. Oecologia 136: 289–295.
Baldwin, R. F. & P. G. deMaynadier, 2009. Assessing threats to pool-breeding amphibian habitat in an urbanizing landscape. Biological Conservation 142: 1628–1638.
Beja, P. & R. Alcazar, 2003. Conservation of Mediterranean temporary ponds under agricultural intensification: an evaluation using amphibians. Biological Conservation 114: 317–326.
Blaustein, A. R., S. C. Walls, B. A. Bancroft, J. J. Lawler, C. L. Searle & S. S. Gervasi, 2010. Direct and indirect effects of climate change on amphibian populations. Diversity 2: 281–313.
Blaustein, A. R., B. A. Han, R. A. Relyea, P. T. J. Johnson, J. C. Buck, S. S. Gervasi & L. B. Kats, 2011. The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Annals of the New York Academy of Sciences 1223: 108–119.
Boix, D., J. Sala & R. Moreno-Amich, 2001. The faunal composition of Espolla pond (ne Iberian Peninsula): the neglected biodiversity of temporary waters. Wetlands 21: 577–592.
Brock, M. A., R. G. Smith & P. J. Jarman, 1999. Drain it, dam it: alteration of water regime in shallow wetlands on the New England Tableland of New South Wales, Australia. Wetlands Ecology and Management 7: 37–46.
Brodman, R., J. Ogger, T. Bogard, A. J. Long, R. A. Pulver, K. Mancuso & D. Falk, 2003. Multivariate analyses of the influences of water chemistry and habitat parameters on the abundances of pond-breeding amphibians. Journal of Freshwater Ecology 18: 425–436.
Brooks, R., 2009. Potential impacts of global climate change on the hydrology and ecology of ephemeral freshwater systems of the forests of the northeastern United States. Climatic Change 95: 469–483.
Bureau of Meteorology, 2010. Climate Data Online. Commonwealth of Australia, Canberra. http://www.bom.gov.au/climate/data/.
Carroll, E. A., T. H. Sparks, N. Collinson & T. J. C. Beebee, 2009. Influence of temperature on the spatial distribution of first spawning dates of the common frog (Rana temporaria) in the UK. Global Change Biology 15: 467–473.
Casanova, M. T. & M. A. Brock, 2000. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecology 147: 237–250.
Caughley, J. & B. Gall, 1985. Relevance of zoogeographical transition to conservation of fauna: amphibians and reptiles in the south-western slopes of NSW. Australian Zoology 21: 513–527.
Clarke, K. R. & R. M. Warwick, 2001. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation. PRIMER-E, Plymouth, UK.
Cogger, H. A., 2000. Reptiles and Amphibians of Australia. Reed New Holland, Sydney.
Dziminski, M. & R. Alford, 2005. Patterns and fitness consequences of intraclutch variation in egg provisioning in tropical Australian frogs. Oecologia 146: 98–109.
Finlayson, C. M. & N. Rea, 1999. Reasons for the loss and degradation of Australian wetlands. Wetlands Ecology and Management 7: 1–11.
Gahl, M. K., A. J. K. Calhoun & R. Graves, 2009. Facultative use of seasonal pools by American bullfrogs (Rana catesbeiana). Wetlands 29: 697–703.
Gillespie, G. & J. Hero, 1999. Potential impacts of introduced fish and fish translocations on Australian amphibians. In Campbell, A. (ed.), Declines and Disappearances of Australian Frogs. Environment Australia, Canberra: 131–144.
Gleason, R., N. Euliss, D. Hubbard & W. Duffy, 2004. Invertebrate egg banks of restored, natural, and drained wetlands in the prairie pothole region of the United States. Wetlands 24: 562–572.
Goldingay, R. L., 2008. Conservation of the endangered Green and Golden Bell Frog: what contribution has ecological research made since 1996? Australian Zoologist 34: 334–349.
Gómez-Rodríguez, C., C. Díaz-Paniagua, L. Serrano, M. Florencio & A. Portheault, 2009. Mediterranean temporary ponds as amphibian breeding habitats: the importance of preserving pond networks. Aquatic Ecology 43: 1179–1191.
Grosner, K. L., 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–189.
Harrison, S., 1994. Metapopulations and conservation. In Edwards, P. J., R. M. May & N. R. Webb (eds), Large-Scale Ecology and Conservation Biology. Blackwell Science, Oxford: 111–128.
Hazell, D., R. Cunnningham, D. Lindenmayer, B. Mackey & W. Osborne, 2001. Use of farm dams as frog habitat in an Australian agricultural landscape: factors affecting species richness and distribution. Biological Conservation 102: 155–169.
Homan, R. N., B. S. Windmiller & J. M. Reed, 2004. Critical thresholds associated with habitat loss for two vernal pool-breeding amphibians. Ecological Applications 14: 1547–1553.
Jakob, C., G. Poizat, M. Veith, A. Seitz & A. J. Crivelli, 2003. Breeding phenology and larval distribution of amphibians in a Mediterranean pond network with unpredictable hydrology. Hydrobiologia 499: 51–61.
Jolly, I. D., K. L. McEwan & K. L. Holland, 2008. A review of groundwater-surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 1: 43–58.
Kayes, S. M., R. L. Cramp, N. J. Hudson & C. E. Franklin, 2009. Surviving the drought: burrowing frogs save energy by increasing mitochondrial coupling. Journal of Experimental Biology 212: 2248–2253.
Lane, S. J. & M. J. Mahony, 2002. Larval Anurans with synchronous and asynchronous development periods: contrasting responses to water reduction and predator presence. Journal of Animal Ecology 71: 780–792.
Lee Foote, A. & C. L. Rice Hornung, 2005. Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecological Entomology 30: 273–283.
Lemckert, F. & M. Mahony, 2008. Core calling periods of the frogs of temperate New South Wales, Australia. Herpetological Conservation and Biology 3: 71–76.
Mac Nally, R., G. Horrocks, H. Lada, P. S. Lake, J. R. Thomson & A. C. Taylor, 2009. Distribution of anuran amphibians in massively altered landscapes in south-eastern Australia: effects of climate change in an aridifying region. Global Ecology and Biogeography 18: 575–585.
Mazerolle, M. J. & M. A. Villard, 1999. Patch characteristics and landscape context as predictors of species presence and abundance: a review. Ecoscience 6: 117–124.
McMenamin, S. K., E. A. Hadly & C. K. Wright, 2008. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. P. Natl. Acad. Sci. USA 105: 16988–16993.
Murphy, B. F. & B. Timbal, 2008. A review of recent climate variability and climate change in southeastern Australia. International Journal of Climatology 28: 859–879.
Neveu, A., 2009. Incidence of climate on common frog breeding: long-term and short-term changes. Acta Oecologica 35: 671–678.
Patla, D. A., C. R. Peterson & P. S. Corn, 2009. Amphibian decline in Yellowstone National Park. Proceedings of the National Academy of Sciences 106: E22.
Paton, P. W. C. & W. B. Crouch, 2002. Using the phenology of pond-breeding amphibians to develop conservation strategies. Conservation Biology 16: 194–204.
Pearson, R. G. & T. P. Dawson, 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography 12: 361–371.
Penman, T. D., F. L. Lemckert & M. J. Mahony, 2004. Meteorological effects on the activity of the giant burrowing frog (Heleioporus australiacus) in south-eastern Australia. Wildlife Research 33: 35–40.
Piha, H., M. Luoto, M. Piha & J. Merila, 2007. Anuran abundance and persistence in agricultural landscapes during a climatic extreme. Global Change Biology 13: 300–311.
Popescu, V. D. & J. P. Gibbs, 2009. Interactions between climate, beaver activity, and pond occupancy by the cold-adapted mink frog in New York State, USA. Biological Conservation 142: 2059–2068.
Rohr, J. R. & D. M. Madison, 2003. Dryness increases predation risk in Efts: support for an amphibian decline hypothesis. Oecologia 135: 657–664.
Rohr, J. R. & T. R. Raffel, 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proceedings of the National Academy of Sciences 107: 8269–8274.
Schauble, C. S., 2004. Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biological Journal of the Linnean Society 82: 39–56.
Semeniuk, C. A. & V. Semeniuk, 1995. A geomorphic approach to global classification for inland wetlands. Plant Ecology 118: 103–124.
Smith, I. & E. Chandler, 2010. Refining rainfall projections for the Murray Darling Basin of south-east Australia—the effect of sampling model results based on performance. Climatic Change 102: 377–393.
Smith, M. A. & D. M. Green, 2005. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28: 110–128.
Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B. F. N. Erasmus, M. F. de Siqueira, A. Grainger, L. Hannah, L. Hughes, B. Huntley, A. S. van Jaarsveld, G. F. Midgley, L. Miles, M. A. Ortega-Huerta, A. Townsend Peterson, O. L. Phillips & S. E. Williams, 2004. Extinction risk from climate change. Nature 427: 145–148.
Todd, B. D., D. E. Scott, J. H. K. Pechmann & J. W. Gibbons, 2010. Climate change correlates with rapid delays and advancements in reproductive timing in an amphibian community. Proceedings of the Royal Society B: Biological Sciences: Published online before print December 15, 2010.
Tracy, C. R., S. J. Reynolds, M. C. McArthur, R. Tracy & K. A. Christian, 2007. Ecology of Aestivation in a cocoon-forming frog, Cyclorana australis (Hylidae). Copeia 4: 901–912.
Trauth, J. B., S. E. Trauth & R. L. Johnson, 2006. Best management practices and drought combine to silence the Illinois Chorus Frog in Arkansas. Wildlife Society Bulletin 34: 514–518.
Vignoli, L., M. A. Bologna & L. Luiselli, 2007a. Seasonal patterns of activity and community structure in an amphibian assemblage at a pond network with variable hydrology. Acta Oecologica-International Journal of Ecology 31: 185–192.
Vignoli, L., M. A. Bologna & L. Luiselli, 2007b. Seasonal patterns of activity and community structure in an amphibian assemblage at a pond network with variable hydrology. Acta Oecologica 31: 185–192.
Wassens, S., 2006. Frog communities of the Murrumbidgee Irrigation Area, NSW. In Taylor, I. R., P. A. Murray & S. G. Taylor (eds), Wetlands of the Murrumbidgee River Catchment: Practical Management in an Altered Environment. Fivebough and Tuckerbil Wetlands Trust, Leeton, NSW: 86–95.
Wassens, S., R. J. Watts, A. Jansen & D. Roshier, 2008. Movement patterns of Southern Bell Frogs (Litoria raniformis) in response to flooding. Wildlife Research 35: 50–58.
Williams, W. D., 1985. Biotic adaptations in temporary lentic waters, with special reference to those in semi-arid and arid regions. Hydrobiologia 125: 85–110.
Williamson, I. & C. M. Bull, 1999. Population ecology of the Australian frog Crinia signifera: larvae. Wildlife Research 26: 81–99.
Acknowledgements
David Read (Wagga Wagga City Council) and Dr Patricia Murray (Murrumbidgee Catchment Management Authority) greatly assisted in the identification and selection of survey wetlands. Two anonymous reviewers provided valuable advice which greatly improved the quality of this article. Vanessa Griese and Sarah Cordell assisted with data collection. We thank land holders for giving us access to study sites on their property. This research was conducted under a NSW National Parks and Wildlife Service Licence (S12393). Ethics approval was granted by Charles Sturt University Animal Care and Ethics Committee (10/042).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editor: C. Max Finlayson / Wetlands and climate change: ecological outcomes and adaptation as shown by Australian case studies
Rights and permissions
About this article
Cite this article
Wassens, S., Walcott, A., Wilson, A. et al. Frog breeding in rain-fed wetlands after a period of severe drought: implications for predicting the impacts of climate change. Hydrobiologia 708, 69–80 (2013). https://doi.org/10.1007/s10750-011-0955-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0955-2