Abstract
Conventional 99mTc-radiopharmaceuticals for the detection of tumor hypoxia generally possess a single nitroimidazole moiety. Herein, we report the synthesis and evaluation of a 99mTc-complex with three-nitroimidazole moieties in an attempt to improve hypoxic cell detection. Isocyanide derivative of metronidazole (MetroNC) was synthesized and subsequently radiolabeled with [99mTc(CO)3(H2O)3]+ precursor complex, wherein the three labile water molecules were replaced with MetroNC ligand to form a pseudo-octahedral [99mTc(CO)3(MetroNC)3]+ complex. Analysis of corresponding Re(CO)3-analog prepared in macroscopic scale confirmed the formation of expected complex. Cyclic voltammetric studies of [Re(CO)3(MetroNC)3]+ complex showed no significant change in single-electron reduction potential (SERP) of MetroNC ligand (− 0.96 V) upon forming the [Re(CO)3(MetroNC)3]+ complex (− 0.90 V). In vitro studies in Chinese hamster ovary (CHO) cells showed three-fold preferential accumulation of [99mTc(CO)3(MetroNC)3]+ complex in hypoxic cells over normoxic cells. Biodistribution studies of [99mTc(CO)3(MetroNC)3]+ complex in Swiss mice bearing fibrosarcoma tumor showed tumor uptake and steady retention till 60 min post injection. Present study constitutes a novel design approach towards development of a 99mTc-radiopharmaceutical for hypoxia imaging application, which could be extended to other potential nitroimidazole ligands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The negative influence of hypoxia in the clinical management of cancer is well documented [1] and the possible reasons for the formation of hypoxic cancerous lesions are thoroughly understood [2, 3]. Poor prognosis has been associated with the presence of hypoxia in several types cancers such as advanced cancer of the uterine cervix, advanced squamous cell carcinoma of cervix [3,4,5], head and neck cancers [6,7,8], adenocarcinoma of pancreas [9] etc. Determination of hypoxic status in cancerous lesions can help in modifying therapeutic strategy for a better clinical outcome. Information on hypoxic status of cancer lesions can also help in selecting patients for hypoxia-directed radiotherapy [10, 11]. Invasive procedures to determine hypoxic status of cancerous lesions, which are also predictive of response to therapy, are available [12]. However, routine use of invasive procedures in a clinical set-up is severely limited due to their technical complexity, inconvenience and the inability to obtain repetitive measurements in target tissue. In addition, due to heterogeneous nature of hypoxia, results obtained through invasive methods are often inconsistent [12]. These factors favor non-invasive techniques for detecting tissue hypoxia.
Nitroimidazoles have been extensively used for non-invasive detection of tissue hypoxia [13, 14]. Due to their selective, oxygen-dependent, reduction and trapping in hypoxic cells, these molecules are suitable biological vectors for developing hypoxia detecting radiopharmaceuticals. At present, [18F]Fluoromisonidazole, a PET radiopharmaceutical, is used for clinical imaging of tissue hypoxia [15]. However, SPECT facilities being more common in developing countries, a SPECT-radiopharmaceutical for this application may find wider applicability. Among different SPECT-radioisotopes, owing to favourable nuclear characteristics and easy availability, 99mTc is often the preferred choice for developing SPECT-radiopharmaceuticals. Therefore, significant efforts have been directed towards the development of a 99mTc-radiopharmaceutical for hypoxia imaging.
Most of the 99mTc-radiopharmaceuticals evaluated for the detection of hypoxia contain a single nitroimidazole molecule [16,17,18,19,20,21,22]. Our group had extensively evaluated several nitroimidazole-99mTc(CO)3 complexes with different overall charge, single-electron reduction potential and lipophilicity [20]. Although, misonidazole-99mTc(CO)3 complex, an analogue of clinically used hypoxia imaging agent [18F]fluromisonidazole, showed encouraging results in animal experiments, overall pharmacokinetics was not at par.
There are few reports on synthesis and evaluation of bis-nitroimidazole-radiometal complex for targeting tissue hypoxia [23,24,25,26]. Our group had synthesized and evaluated a 99mTcN(PNP)-complex containing two metronidazole moieties for targeting hypoxic cells [26]. The purpose of introducing more than one nitroimidazole groups in a radiotracer is to improve its chances of oxygen dependent reduction in hypoxic cells, in vivo. We realized that the [99mTc(CO)3(H2O)3]+ precursor complex introduced by Alberto et al. offered an excellent route for the preparation of such a complex [27], wherein, the substitution labile water molecules could be easily replaced by suitable nitroimidazole ligands to obtain a stable pseudo-octahedral complex. Recognizing that isocyanides are excellent mono-dentate ligands suitable for replacing labile water molecules in [99mTc(CO)3(H2O)3]+ precursor complex [27], we synthesized isocyanide derivative of metronidazole, a 5-nitroimidazole, which is known to target hypoxic cells. Present work describes the preparation of a 99mTc(CO)3 complex containing three metronidazole moieties and their preliminary biological evaluation in vitro as well as in vivo.
Materials and methods
General
The compound 1-(2-isocyanoethyl)-2-methyl-5-nitro-1H-imidazole (MetroNC) was synthesized following a procedure reported earlier [26]. Bis-tetraethylammonium Rheniumtricarbonyl tribromide ([NEt4]2[Re(CO)3(Br3)]) was also synthesized following a reported procedure [28]. All reagents were of analytical grade and used without additional purification. Sodium pertechnetate was obtained from 99Mo/99mTc alumina column generator, constructed in-house. The [99mTc(CO)3(H2O)3]+ precursor was prepared using Isolink® carbonyl kit vial obtained as a gift from Mallinckrodt Medical B. V. HPLC analyses were performed on a JASCO PU 2080 Plus dual pump HPLC system, Japan, with a JASCO 2075 Plus tunable absorption detector and a Gina Star radiometric detector system, using a C18 reversed phase HiQ Sil (5 μm, 4 × 250 mm) column. Aqueous 0.05 M triethylammonium phosphate (TEAP) buffer, pH = 2.5 (Solvent A) and methanol (Solvent B) were used as the mobile phase for HPLC. The HPLC solvents were filtered through 0.22 μ filter before use. The elution started with 100% A from 0 to 6 min. At 6 min the eluent switched to 75% A and 25% B and at 9 min to 66% A and 34% B followed by a linear gradient 66% A/34% B to 100% B from 9 to 20 min. Up to 30 min, the eluent remained at 100% B before switching back to the initial conditions. Flow rate was maintained at 1 mL/min. Infrared spectra were recorded on a JASCO-FT/IR-420 spectrophotometer, Japan. 1H-NMR spectrum was recorded on a 300 MHz Bruker AvanceII NMR spectrometer. High-resolution mass spectra of the Re(CO)3(MetroNC)3 complex was recorded on a Bruker MicroTOFQ-2, Germany, by ESI. Cyclic voltammograms of MetroNC ligand and Re(CO)3(MetroNC)3 complex were recorded on a CH instrument (CHI760D), USA. The CHO cells used for in vitro studies were obtained from National Centre for Cell Science (NCCS), Pune.
Synthesis
Synthesis of Re(CO)3 complex of 1-(2-isocyanoethyl)-2-methyl-5-nitro-1H-imidazole
MetroNC ligand (16 mg, 0.09 mmol) was refluxed with 0.3 eq of [NEt4]2[Re(CO)3(Br3)] (19 mg, 0.03 mmol) in acetonitrile for 4 h. Subsequently, solvent from the reaction mixture was removed, water was added and extracted with ethyl acetate (3 × 7 mL). Organic extracts were pooled together, washed with saline and dried. Upon removal of the organic solvent, complex 2 could be obtained in practically pure form (21 mg, 91%). Rf (ethyl acetate) = 0.4. IR (neat, cm−1) 3127 (w); 2958 (w); 2923 (w); 2853 (w); 2221 (m); 2197 (m); 2037 (s); 1977 (s); 1923 (s); 1531 (m); 1470 (m); 1427 (m); 1364 (m); 1260 (m); 1189 (m); 1148 (w); 1011 (m); 824 (w); 743 (w); 679(w). 1H-NMR (δ ppm, CDCl3) 2.63 (s, 3H, –CH3), 4.39 (m, 2H, –CH2CH2NC), 4.72 (m, 2H, –CH2CH2NC), 8.01 (s, 1H, metronidazole-C4-H). HRMS (ESI+) m/z: 811.1802 (M+H)+.
Radiolabeling
Preparation of [99mTc(CO)3(H2O)3]+ precursor complex
The [99mTc(CO)3(H2O)3]+ precursor complex was prepared using Isolink® kit vial by adding ~ 1 mL (~ 37 MBq) of freshly eluted Na99mTcO4 from 99Mo/99mTc alumina column generator and heating the vial at 95 °C for 20 min. Thereafter, the vial was cooled, re-equilibrated to atmospheric pressure and the pH was adjusted to 7 using 1:3 mixture of 0.5 M phosphate buffer (pH 7.4):1 M HCl.
Radiolabeling of MetroNC with [99mTc(CO)3(H2O)3]+ precursor complex
Under optimized conditions, 900 μL of 10−3M 5% aqueous ethanolic solution of MetroNC was mixed with 100 μL (~ 3 MBq) of freshly prepared [99mTc(CO)3(H2O)3]+ precursor complex and the reaction mixture was incubated at 75 °C for 45 min. Thereafter, the preparation was cooled to room temperature and analyzed by HPLC.
Physicochemical studies
Electrochemical studies
Cyclic voltammogram of relevant nitroimidazole ligands and [Re(CO)3(MetroNC)3]+ complex were obtained using a glassy carbon working electrode, platinum counter electrode and Ag/Ag+ as reference electrode, following a procedure reported earlier [24]. Before recording the voltammograms, the test solutions were thoroughly purged with high purity argon to remove tracers of dissolved oxygen. The voltammograms was recorded within the potential range of 0 to − 2.5 volts. Scan rate was maintained at 50 mV/s.
HPLC
The radiochemical purity (RCP) of the [99mTc(CO)3(H2O)3]+ precursor complex as well as the [99mTc(CO)3(MetroNC)3]+ complex were analyzed by HPLC. About 15 μL of the radioactive preparation was injected into the HPLC column and the UV/radioactivity profile was monitored as a function of time.
Determination of octanol–water partition coefficient (LogPo/w)
Octanol–water partition coefficients of the [99mTc(CO)3(MetroNC)3]+ complex was determined following a reported procedure [29]. The study was carried out in triplicate and average LogPo/w was reported along with standard deviation.
Serum stability studies
Stability of [99mTc(CO)3(MetroNC)3]+ complex in human serum was evaluated by incubating the radiolabeled complex (~ 0.37 MBq) in human serum (500 μL) at 37 °C. A 100 μL aliquot was withdrawn after 3 h and serum proteins are precipitated by adding equal volume of ethanol. The mixture was centrifuged and the supernatant was analyzed by HPLC to assess stability of the complex in serum.
In vitro cell accumulation study under normoxic and hypoxic conditions
Accumulation of [99mTc(CO)3(MetroNC)3]+ complex in CHO cells under normoxic and hypoxic conditions were studied following a reported procedure [30, 31]. About 15 mL aliquot of CHO cells (1 × 106 cells/mL) in suspension culture at 37 °C in RPMI medium, supplemented with 2 mM l-glutamine and 10% heat-inactivated fetal bovine serum, was kept stirring in a glass vial. The glass vial was flushed with a gentle, continuous flow of warm, humidified gas mixture of 95% air/5% carbon dioxide (aerobic exposure) or 95% nitrogen/5% carbon dioxide (hypoxic exposure). After equilibration for 45 min, about 0.1 mL of freshly prepared [99mTc(CO)3(MetroNC)3]+ complex was added to the vial such that final activity of 0.3 MBq/mL was obtained. Under these conditions, concentration of nitroimidazole in the vial was approximately 1 μg/mL. At 0.5, 1 and 3 h post incubation, 1 mL aliquots in triplicate was withdrawn from the vial. Subsequently, the ratio of activity in cells to that of supernatant was determined following a reported procedure [32].
Biodistribution studies
The procedures performed herein were in strict compliance with the national laws governing the conduct of animal experiments. Solid tumor models were developed in Swiss mice by implantation of HSDM1C1 murine fibrosarcoma cells obtained from National Centre for Cell Science (NCCS), Pune, India. About 106 cells were injected subcutaneously on the dorsum of the Swiss mice. The tumors were grown to a size of 10 mm in diameter before the animals were used for the experiment. Individual sets of animals (n = 3) were administered with the HPLC purified radioactive preparation (~ 1.1 MBq per animal in 100 μL volume) through the lateral tail vein and incubated for different time points (30, 60, and 180 min). At the end of the respective time intervals, the animals were sacrificed and the relevant organs excised for measurement of retained activity. The organs were weighed and the activity associated with them was measured in a flat-bed type NaI(Tl) counter with energy window adjusted for 99mTc. The activity associated with each organ/tissue was expressed as percentage injected dose per gram (%ID/g).
Results and discussion
Mediated by nitroreductase enzymes, nitroimidazoles undergo a series of one-electron reduction in hypoxic cells leading to permanent or transient trapping inside hypoxic cells. The presence of oxygen, however, prevents such reduction, which explains non-accumulation of nitroimidazole in normoxic cells. One of our previous studies had shown that the time spend by the radiotracer inside hypoxic cell is an important parameter that decides its efficacy to target hypoxic cells. To be an effective agent for hypoxic cell detection, the radiotracer should undergo reduction within the limited time that it spends in hypoxic cells. To increase the chances of hypoxia specific reduction we envisaged a radiometal complex with multiple nitroimidazole moieties. We recognized that the [99mTc(CO)3(H2O)3]+ precursor complex offered a simple and attractive route to achieve this objective [27]. This precursor complex allowed substitution of the three labile water molecules with suitable monodentate ligands, such as isocyanide, resulting in a pseudo-octahedral 99mTc(CO)3 complex of the type [99mTc(CO)3(nitroimidazoleNC)3]+. A similar complex was reported by Kothari et al., wherein the three labile water molecules in [99mTc(CO)3(H2O)3]+ precursor complex were replaced by three tert-butyl isocyanide ligands [33]. We envisaged preparation of a 99mTc(CO)3 complex with three metronidazole isocyanide molecules from [99mTc(CO)3(H2O)3]+ precursor complex and MetroNC ligand.
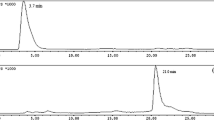
The synthesis of MetroNC ligand was carried out following a procedure reported by our group earlier (Fig. 1) [26]. Radiolabeling of the MetroNC ligand was carried out using freshly prepared [99mTc(CO)3(H2O)3]+ precursor complex. The [99mTc(CO)3(H2O)3]+ precursor complex as well as the [99mTc(CO)3(MetroNC)3]+ complex were analyzed by HPLC. Typical radioactivity elution profile of the [99mTc(CO)3(H2O)3]+ precursor complex with retention time 3.7 ± 0.2 min was reported earlier [22]. Under similar conditions, the [99mTc(CO)3(MetroNC)3]+ complex appeared at 15.4 ± 0.3 min (n = 3) in the HPLC chromatogram (Fig. 2a). From the peak area measurements radiochemical purity of the complex was found to be > 95%.
We prepared [Re(CO)3(MetroNC)3]+ complex in macroscopic level for structural characterization. The precursor complex, [NEt4]2[Re(CO)3(Br3)], prepared following a reported procedure, was used as the source for Re(CO)3 core [27]. Figure 2b shows the UV-elution profile of [Re(CO)3(MetroNC)3]+ complex, which matched with the radioactivity profile of [99mTc(CO)3(MetroNC)3]+ complex [Fig. 2a]. This expected result indicated the formation of similar type of complexes both at the no carrier added level as well as macroscopic level.
The IR spectra of [Re(CO)3(MetroNC)3]+ complex showed absence of peak at 2150 cm−1 representing –NC stretching in the free MetroNC ligand. Instead, two peaks (2220 and 2196 cm−1) appeared at higher frequency, consistent with the coordination of isocyanide group to Re(CO)3 core. Similar observations were made with alkyl isocyanide-Cr(CO)3 complexes reported earlier [34]. Three strong IR peaks in the range 2034–1916 cm−1 marked the presence of fac-Re(CO)3 core. The 1H-NMR of the [Re(CO)3(MetroNC)3]+ complexes also had signatures indicative of coordination of –NC group with the Re(CO)3 core. The methylene protons linking the nitroimidazole group and Re(CO)3 core in the complex showed a downfield shift compared to the corresponding methylene protons in the free MetroNC ligand. This could be attributed to the decreased electron density on N-atom of the –NC group, consequent to its coordination to Re-metal centre. Observable changes in the 1H-NMR peak splitting is evidence indicating ligand–metal coordination in the complex. The two methylene protons, which appeared as triplets in free MetroNC ligand, were seen as multiplets in the [Re(CO)3(MetroNC)3]+ complex. This could be attributed to the hindrance in free rotation over the C–C bond of the linker, which rendered the methylene protons diastereotopic. A four peak isotopic cluster observed in the high resolution mass spectrum of [185/187Re(CO)3(MetroNC)3]+ complex with ion peaks at m/z 809.1761, 810.1791, 811.1802 and 812.1815 provided the confirmatory evidence for the formation of expected, pseudo-octahedral complex containing three MetroNC molecules (Fig. 3).
The single electron reduction potential (SERP) of nitroimidazole is an important molecular parameter, which decides its efficiency of reduction in hypoxic cells [35,36,37,38]. Any modification to the nitroimidazole ring or its electronic environment can potentially alter its SERP and consequently, its efficiency of reduction in hypoxic cells. In the present work, metronidazole was synthetically modified to an isocyanide derivative for radiolabeling with 99mTc(CO)3 core. Though Adams et al. [36, 38] had shown that any synthetic modifications two or more carbon–carbon bonds away from the nitroimidazole ring had insignificant effect on its SERP, we carried out cyclic voltammetric studies of MetroNC as well as [Re(CO)3(MetroNC)3]+ complex for confirmation. A single-electron reduction peak at − 0.99 V was observed in the cyclic voltammogram (CV) of unmodified metronidazole, while MetroNC ligand showed single electron reduction peak at − 0.96 V. These observations are completely in agreement with the observations of Adams et al. [36, 38].
Formation of a complex is another process which can potentially alter the electronic environment of the coordinating nitroimidazole ligand and consequently, its SERP. The Fig. 4 shows the cyclic voltammogram of MetroNC ligand and corresponding [Re(CO)3(MetroNC)3]+ complex. It could be noted that the [Re(CO)3(MetroNC)3]+ complex showed a single-electron reduction peak at − 0.90 V, compared to − 0.96 V shown by free MetroNC ligand. The small shift in SERP value of Re(CO)3(MetroNC)3 complex indicates possible influence of the metal centre on the nitroimidazole moiety.
The LogPo/w value of the [99mTc(CO)3(MetroNC)3]+ complex determined following a reported procedure [29] was 0.46 (0.04). Stability of the [99mTc(CO)3(MetroNC)3]+ complex in human serum was analyzed following a reported protocol and it was found to the stable in human serum during the period of study (3 h).
Accumulation of [99mTc(CO)3(MetroNC)3]+ complex in CHO cells under normoxic and hypoxic conditions were studied to evaluated hypoxic cell selectivity of the radiotracer. Figure 5 shows the accumulation of activity in CHO cells as a function of time under normoxic and hypoxic conditions. It could be noted that accumulation of [99mTc(CO)3(MetroNC)3]+ complex consistently increases under hypoxic conditions. The Cin/Cout ratio, the ratio of activity associated with the cell pellet to that of supernatant, under hypoxic conditions was more than twice the Cin/Cout ratio under normoxic condition, which is indicative of the hypoxia selectivity of the radiotracer.
Biodistribution of the [99mTc(CO)3(MetroNC)3]+ complex was carried out in Swiss mice bearing fibrosarcoma tumor. Table 1 shows the distribution of the complex in tumor bearing Swiss mice at different time points. The [99mTc(CO)3(MetroNC)3]+ complex showed a steady retention of activity in tumor (~ 0.67%ID/g) till 1 h p.i., before decreasing to 0.31%ID/g at 3 h p.i. Major clearance of the activity from the body was through hepatobiliary route. This is evident from the presence of significant levels of activity in liver and gastrointestinal tract. The maximum tumor/blood ratio obtained for [99mTc(CO)3(MetroNC)3]+ complex was 1, at 60 min p.i. Similarly, peak tumor to muscle ratio of the complex, 4.38 (0.51), was also observed at 60 min p.i.
Conclusions
Present study reports the synthesis and evaluation of a 99mTc-complex with three-nitroimidazole groups to detect tumor hypoxia. The 99mTc(CO)3 chemistry was effectively utilized to achieve this objective. In vitro studies showed consistent increase in accumulation of the radiotracer in CHO cells under hypoxic conditions, indicating its ability to target hypoxic cells. Present study also constitutes a novel design approach towards development of a 99mTc-radiopharmaceutical for hypoxia imaging application.
References
Gray LH, Conger AD, Ebert M (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 26:638–648
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic and molecular aspects. J Natl Cancer Inst 93:266–276
Mees G, Dierckx R, Vangestel C, Van de Wiele C (2009) Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging 36:1674–1686
Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P (1996) Association between tumor hypoxia and malignant progression. Cancer Res 56:4509–4515
Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A, Chapman W, Levin W, Manchul L, Keane TJ, Hill RP (1998) Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol 48:149–156
Nordsmark M, Overgaard M, Overgaard J (1996) Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 41(1):31–39
Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW (1997) Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38(2):285–289
Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, Terris DJ, Overgaard J (2005) Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 77(1):18–24
Duffy JP, Eibl G, Reber HA, Hines OJ (2003) Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer 2:12
Hay MP, Hicks KO, Wang J (2014) Hypoxia-directed drug strategies to target the tumor microenvironment. Adv Exp Med Biol 772:111–145
Lin A, Hahn SM (2012) Hypoxia imaging markers and applications for radiation treatment planning. Semin Nucl Med 42(5):343–352
Ballinger JR (2001) Imaging hypoxia in tumors. Semin Nucl Med 31(4):321–329
Adams GE, Stratford IJ (1986) Hypoxia-mediated nitro-heterocyclic drugs in the radio- and chemotherapy of cancer. An overview. Biochem Pharmacol 35(1):71–76
Stratford IJ (1992) Bioreductive drugs in cancer therapy. BJR Suppl 24:128–136
Lee ST, Scott AM (2007) Hypoxia positron emission tomography imaging with 18F-fluoro misonidazole. Semin Nucl Med 37(6):451–461
Li Z, Chu T (2012) Recent advances on radionuclide labeled hypoxia-imaging agents. Curr Pharm Des 18(8):1084–1097
Hoigebazar L, Jeong JM (2013) Hypoxia imaging agents labeled with positron emitters. Recent Results Cancer Res 194:285–299
Mallia MB, Kumar C, Mathur A, Sarma HD, Banerjee S (2012) On the structural modification of 2-nitroimidazole-99 mTc(CO)3 complex, a hypoxia marker, for improving in vivo pharmacokinetics. Nucl Med Biol 39(8):1236–1242
Mallia MB, Subramanian S, Banerjee S, Sarma HD, Venkatesh M (2006) Evaluation of 99mTc(CO)3 complex of 2-methyl-5-nitroimidazole as an agent for targeting tumor hypoxia. Bioorg Med Chem 14(23):7666–7670
Mallia MB, Subramanian S, Mathur A, Sarma HD, Banerjee S (2014) A study on nitroimidazole-99mTc(CO)3 complexes as hypoxia marker: some observations towards possible improvement in in vivo efficacy. Nucl Med Biol 41(7):600–610
Giglio J, Fernández S, Pietzsch HJ, Dematteis S, Moreno M, Pacheco JP, Cerecetto H, Rey A (2012) Synthesis, in vitro and in vivo characterization of novel 99mTc-‘4+1’-labeled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl Med Biol 39(5):679–686
Mallia MB, Mathur A, Sarma HD, Banerjee S (2015) A (99m)Tc-labeled misonidazole analogue: step toward a (99m)Tc-alternative to [18F]fluromisonidazole for detecting tumor hypoxia. Cancer Biother Radiopharm 30(2):79–86
Huang H, Zhou H, Li Z, Wang X, Chu T (2012) Effect of a second nitroimidazole redox centre on the accumulation of a hypoxia marker: synthesis and in vitro evaluation of 99mTc-labeled bisnitroimidazole propylene amine oxime complexes. Bioorg Med Chem Lett 22(1):172–177
Mei L, Wang Y, Chu T (2012) 99mTc/Re complexes bearing bisnitroimidazole or mononitroimidazole as potential bioreductive markers for tumor: synthesis, physicochemical characterization and biological evaluation. Eur J Med Chem 58:50–63
Seelam SR, Lee JY, Lee YS, Hong MK, Kim YJ, Banka VK, Lee DS, Chung JK, Jeong JM (2015) Development of (68)Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Bioorg Med Chem 23(24):7743–7750
Vats K, Mallia MB, Mathur A, Sarma HD, Banerjee S (2016) Synthesis and evaluation of a novel 99mTcN(PNP)-complex with metronidazole isocyanide ligand as a marker for tumor hypoxia. J Radioanal Nucl Chem 308(1):363–369
Alberto R, Schibli R, Egli A, Schubiger AP (1998) A novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in aqueous solution and its reaction with a bifunctional ligand. J Am Chem Soc 120(31):7987–7988
Alberto R, Schibli R, Schubiger PA, Abram U, Kaden TA (1996) Reactions with the technetium and rhenium carbonyl complexes (NEt4)2[MX3(CO)3]. Synthesis and structure of [Tc(CN-But)3(CO)3](NO3) and (NEt4)[Tc2(μ-SCH2CH2OH)3(CO)6]. Polyhedron 15:1079–1089
Troutner DE, Volkert WA, Hoffman TJ, Holmes RA (1984) A neutral lipophilic complex of 99mTc with a multidentate amine oxime. Int J Appl Radiat Isot 35(6):467–470
Zhang Y, Chu TW, Gao XG, Liu XQ, Yang Z, Guo ZQ, Wang XY (2006) Bioorg Med Chem Lett 16:1831–1833
Zhang JB, Yu Q, Huo JF, Pang Y, Yang S, He YI, Tang TT, Yang CC, Wang XB (2010) J Radioanal Nucl Chem 283:481–485
Liu L, Zhang M, Zhong G, Wang X (2011) Synthesis and biodistribution of a novel 99mTc complex of HYNIC-conjugated metronidazole as a potential tumor hypoxia imaging agent. J Radioanal Nucl Chem 287:847–852
Kothari KK, Satpati D, Joshi S, Venkatesh M, Ramamoorthy N, Pillai MR (2005) 99mTc carbonyl t-butyl isonitrile: a potential new agent for myocardial perfusion imaging. Nucl Med Commun 26(2):155–161
Ekkehardt Hahn F (1993) The coordination chemistry of multidentate isocyanide ligands. Angew Chem Int Ed Engl 32:650–665
Adams GE, Dewey DL (1963) Hydrated electrons and radiobiological sensitisation. Biochem Biophys Res Commun 12:473–477
Adams GE, Cooke MS (1969) Electron-affinic sensitization. I. A structural basis for chemical radiosensitizers in bacteria. Int J Radiat Biol Relat Stud Phys Chem Med 15(5):457–471
Simic M, Powers EL (1974) Correlation of the efficiencies of some radiation sensitizers and their redox potentials. Int J Radiat Biol Relat Stud Phys Chem Med 26(1):87–90
Adams GE, Flockhart IR, Smithen CE, Stratford IJ, Wardman P, Watts ME (1976) Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res 67(1):9–20
Acknowledgements
Authors thank Radiochemicals Section of Radiopharmaceuticals Division (RPhD) for providing 99Mo activity. The authors also acknowledge the help received from the staff of the animal house facility, Bhabha Atomic Research Centre (BARC), Dr. Manoj Kumar Sharma and Dr. Pranaw Kumar of Fuel Chemistry Division (FCD), BARC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mallia, M.B., Mathur, A., Sharma, R. et al. Preparation and preliminary evaluation of a tris-metronidazole-99mTc(CO)3 complex for targeting tumor hypoxia. J Radioanal Nucl Chem 317, 1203–1210 (2018). https://doi.org/10.1007/s10967-018-6012-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6012-0