Abstract
In this study, ornidazole xanthate (ONXT) ligand was successfully synthesized and its 99mTc-nitrido core, 99mTc-oxo core and [99mTc(CO)3]+ core complexes were prepared with high yields. The tumor cell experiments and the biodistribution in mice bearing S180 tumor showed that all of the complexes had a certain hypoxic selectivity and tumor uptake. Among them, 99mTcO-ONXT exhibited the highest tumor uptake and tumor/muscle ratio. Planar scintigraphic imaging studies showed the tumor detection was observable, suggesting it would be a potential radiotracer to target tumor hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the development of hypoxia imaging agent, nitroimidazole derivates are thought to undergo bioreduction reaction in the hypoxic cell and thus can be retained in hypoxic tissue [1, 2]. Therefore, different kinds of positron emission tomography (PET) and single photon emission computed tomography (SPECT) radiotracers containing nitroimidazole pharmacophore have been evaluated as hypoxia imaging agents [3–21]. At present, [18F]Fluoromisonidazole (18F-FMISO), a 2-nitroimidazole derivative, is one of the most widely used PET radiopharmaceuticals for clinical imaging of hypoxia. Compared to 18F, 99mTc is the most common radionuclide in routine nuclear medicine due to its ideal physical decay properties and the availability through 99Mo/99mTc generator. Thus, developing 99mTc-labeled nitroimidazole derivatives to target tumor hypoxia is still one of the major focuses of ongoing research.
Recently, we have reported the synthesis and biodistribution of a novel 99mTc-DMSAMe (DMSAMe: dimercaptosuccinic acid metronidazole ester) as a potential agent for imaging tumor hypoxia [16]. As reported, 99mTc-DMSAMe was synthesized through easy procedures and exhibited a certain tumor uptake and a relatively high tumor-to-muscle ratio. However, the tumor-to-blood ratio of 99mTc-DMSAMe was not satisfactory. Thus, it may be of great interest to probe other better 99mTc labeled nitroimidazole derivatives for imaging tumor hypoxia. Bearing in mind ornidazole is a commercial 5-nitroimidazole derivative and its molecular structure has a pendant –CH2OH group, thus making it possible to react with carbon disulfide in NaOH solutions to produce the ornidazole xanthate (ONXT). Based on our previous reported work [22–25], it can be assumed that ONXT can also be used to form stable 99mTcO, 99mTcN and [99mTc(CO)3] complexes on the basis of efficient binding of the group to four sulfur atoms. The above background encouraged us to prepare several 99mTc labeled ornidazole xanthate complexes by using different 99mTc cores to find good radiotracers for targeting tumor hypoxia. In this study, the synthesis and biological evaluation of novel 99mTc labeled ornidazole xanthate complexes for imaging tumor hypoxia are reported for the first time.
Experimental
Materials and methods
Ornidazole was purchased from J&K CHEMICA, China. Succinic dihydrazide (SDH) kit, which contains 0.05 mg of stannous chloride dihydrate, 5.0 mg of succinic dihydrazide (SDH) and 5.0 mg of propylenediamine tetraacetic acid (PDTA) and glucoheptonate (GH) kit containing 0.1 mg of stannous chloride dihydrate, 5.0 mg of GH were obtained from Beijing Shihong Pharmaceutical Center, Beijing Normal University, China. All other chemicals were of reagent grade and were used without further purification. 99Mo/99mTc generator was obtained from the China Institute of Atomic Energy (CIAE). IR spectrum was obtained with an AVATAR 360 FT-IR spectrometer using KBr pellets. NMR spectrum was recorded on a 500 MHz Bruker Avance spectrophotometer. Elemental analyses were performed on a Vario EL elemental analyzer model.
Synthesis of ornidazole xanthate (ONXT)
The synthesis of ONXT was carried out according to the literature [22]. In brief, 0.439 g of ornidazole, carbon disulfide (0.456 g) and NaOH (0.120 g) were dissolved in 25.0 mL water. The mixture was stirred at 3 °C for 2.0 h and continued to react overnight at room temperature. Most of the solvent was removed, and the precipitate was collected by filtration. The crude product was recrystallized from CH3OH/CH3CH2OCH2CH3 to produce ONXT as a yellow solid (0.279 g, 43.9 %).
Radiolabeling and quality control techniques
The preparations of 99mTcO-ONXT, 99mTcN-ONXT and 99mTc(CO)3-ONXT were carried out according to our previous reported methods [25–27].
For 99mTcO-ONXT, 1 mL of saline containing [99mTcO4]− (37.0 MBq) was added to a GH kit containing 0.05 mg of stannous chloride dihydrate, 5.0 mg of glucoheptonate (GH). The mixture was kept at room temperature for 15 min. Then, 1.0 mg of ONXT dissolved in 1.0 mL water was added to the mixture and the resulting solution was heated at 100 °C for 30 min.
For 99mTcN-ONXT, 1 mL of saline containing [99mTcO4]− (37 MBq) was added to a SDH kit containing 0.05 mg of stannous chloride dihydrate, 5.0 mg of succinic dihydrazide (SDH), 5.0 mg of propylenediamine tetraacetic acid (PDTA). The mixture was kept at room temperature for 15 min. Successively, 1.0 mg of ONXT dissolved in 1.0 mL water was added and the reaction mixture was heated at 100 °C for 30 min.
For 99mTc(CO)3-ONXT, potassium sodium tartrate (15.0 mg), Na2CO3 (5.0 mg), and NaBH4 (10.0 mg) were added to a 10 mL glass vial. The vial was sealed and flushed with CO for 15 min, followed by the addition of 1.0 mL of saline containing [99mTcO4]− (370 MBq). The vial was heated at 80 °C for 30 min. After cooling to room temperature, 0.1 mol/L HCl was added to adjust the pH to approximately 8. Then 1 mL of a water solution containing 1.0 mg of the ONXT ligand was added and the reaction vial was incubated on a boiling water bath for 20 min.
The radiochemical purities of the complexes were assessed by HPLC. The HPLC analysis conditions are as follows. HPLC analysis was carried out with a reverse d-phase column (Kromasil 100-5C, 250 × 4.6 mm), Shimadzu SCL-10A VP series, working at a flow rate of 1.0 mL/min. For 99mTcO-ONXT and 99mTcN-ONXT, water (A) and acetonitrile (B) mixtures were used as the mobile phase. For 99mTc(CO)3-ONXT, water (containing 0.1 % TFA) (A) and acetonitrile (containing 0.1 % TFA) (B) mixtures were used as the mobile phase. The following gradient elution technique was adopted for the preparation: for 99mTcO-ONXT, 0 min 50 % B, 20 min 90 % B, 30 min 90 % B, 40 min 100 % B; for 99mTcN-ONXT, 0 min 70 % B, 10 min 70 % B, 15 min 90 % B, 40 min 100 % B; for 99mTc(CO)3-ONXT, 0 min 10 % B, 28 min 90 % B, 30 min 100 % B, 40 min 100 % B.
In vitro stability study
99mTcO-ONXT, 99mTcN-ONXT and 99mTc(CO)3-ONXT complexes were incubated in the labeling milieu at room temperature for 6 h. On the other hand, the stability in human serum albumin (HSA) was determined by incubating complexes in the solution of HSA at 37 °C for 6.0 h. The radiochemical purities of the complexes were assessed by HPLC.
In vitro cell uptake
In vitro uptake of the complexes both in hypoxic and aerobic conditions was evaluated by using the previous reported methods [17]. In brief, S180 cells at a concentration of 1.0 × 106 cells/mL were suspended in 20.0 mL DMEM containing 10 % (v/v) of fetal bovine serum and incubated at 37.0 °C. The hypoxic and aerobic conditions were conducted following the previous methods [17]. Then, 0.2 mL (0.74 MBq) of the complex was added to the suspension. 1000 μL aliquots were pipetted at 1.0, 2.0, 3.0 and 4.0 h post-incubation, and were centrifuged at 3000 rpm for 5.0 min. 900 μL supernatant medium was taken for counting (C out) and the left sample containing cells with 100 μL medium was also counted (C in). At each time point, three samples were determined. The cell accumulation, A, was calculated as the following equation:
The final results were expressed as mean ± SD.
Determination of the partition coefficient
The partition coefficient (log P) between 1-octanol and phosphate buffer (0.025 mol/L, pH 7.4) of the complex was measured in order to evaluate their lipophilicity. In a centrifuge tube, containing 2 mL of each phase, 0.1 mL of the labeled complex solution was added, and the mixture was shacked on a Vortex mixer for 1 min and then centrifuged at 5000 g for 5 min. Three samples (0.1 mL each) from each layer were counted in a well gamma γ-counter. The partition coefficient, P, was calculated as the mean value of counts per minute in octanol divided by that of the buffer. Usually the final partition coefficient value was expressed as log P. The log P value was reported as an average of three measurements plus the standard deviation.
Biodistribution study
The Kunming male mice (18–20 g, five animals per group) bearing S180 tumor were injected via a tail vein with 99mTcO-ONXT (0.1 mL, 7.4 × 105 Bq). At 2 h and 4 h post-injection, the mice were sacrificed by neck dislocation. The tumor, other organs of interest and blood were collected, weighed and assayed for radioactivity. The results were expressed as the percent uptake of injected dose per gram of tissue (% ID/g). The final results are expressed as mean ± SD. Animal studies were performed in compliance with the national laws related to the ethics during experimentation. The biodistribution studies of 99mTcN-ONXT and 99mTc(CO)3-ONXT were conducted in the same way.
SPECT imaging studies
0.2 mL of 99mTcO-ONXT (111 MBq) was injected intravenously through trail vein in mice (18–22 g) bearing S180 tumor. A dual-head SPECT (Skylight; Philips, Milpitas, CA, USA), using a low-energy parallel-hole collimator (diameter 3.5 mm), was used for SPECT imaging studies. Static images were acquired at 4 h after injection.
Results and discussion
Synthesis and radiolabeling
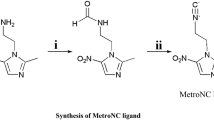
ONXT was prepared by reacting ornidazole with carbon disulfide in NaOH solutions. The reaction equation is shown in Scheme 1. It was characterized by IR, 1H NMR, 13C NMR and Elemental analysis. IR(KBr)/cm−1: νC–O:3421.2, νNO2:1636.0,1354.6, νC=S:1083.8. 1H-NMR (D2O): δ7.76(s, 1H, CH), δ4.53–4.50(d, 2H, CH2), δ3.74–3.74 (d, 2H, CH2), δ3.08–3.02 (tt, H, CH), δ2.06 (s, 3H, CH3); 13C-NMR (D2O): δ208.47 (CS2); δ160.36 (C); δ150.80 (C); δ127.85 (CH); δ61.14 (CH2); δ47.78 (CH); δ42.48 (CH2); δ18.29 (CH3). Elemental analysis calculated (%) for C8H9N3NaO3S2Cl: C, 30.24; N, 13.22; H, 2.85. Found: C, 30.07; N, 13.49; H, 3.02.
The preparations of 99mTcO-ONXT, 99mTcN-ONXT and 99mTc(CO)3-ONXT can be carried out by using the following procedures in Scheme 2.
For labeling, 99mTcO-ONXT was prepared by ligand-exchange reaction with 99mTc-glucoheptonate (GH) [24]. 99mTc-GH is an ideal substrate for the substitution reaction with ONXT to produce the final complex 99mTcO-ONXT.
99mTcN-ONXT was prepared by adding ONXT to the [99mTcN]2+ intermediate, which was produced by the reaction of [99mTcO4]− with succinic dihydrazide (SDH) in the presence of stannous chloride as reducing agent. The [99mTcN]2+ core is a proper substrate for the substitution reaction with ONXT to prepare 99mTcN-ONXT with high yield [23]. As for preparing 99mTc(CO)3-ONXT, the H2O molecule in the fac-[99mTc(CO)3(H2O)3]+ precursor is readily substituted by sulfur atoms in the ONXT ligand [25]. The ONXT ligand displaces the two H2O molecules of the fac-[99mTc(CO)3(H2O)3]+ precursor.
The radiochemical purities of the complexes were assessed by HPLC. The retention time of [99mTcO]3+, [99mTcN]2+ and [99mTc(CO)3(H2O)3]+ was 4.13, 4.73, and 15.60 min, respectively, while that of 99mTcO-ONXT, 99mTcN-ONXT and 99mTc(CO)3-ONXT were found to be 13.38, 17.11 and 8.66 min (Fig. 1). The mean radiochemical purities of the products were all over 95 % immediately after the preparation.
In vitro stability study
The complexes were stable over 6 h in the reaction mixture at room temperature. On the other hand, no decomposition of the above complexes occurred over 6 h at 37 °C in HSA, suggesting they had good in vitro stability.
In vitro cell uptake
The effect of hypoxic and aerobic conditions on the accumulation of the three 99mTc complexes in S180 cells as a function of time is illustrated in Fig. 2. From Fig. 2, it is shown that the uptake of the three 99mTc complexes in hypoxic cells is constantly more than that in aerobic cells, suggesting all of them exhibit preferential uptake in hypoxic conditions.
Partition coefficient (log P)
The logP values of 99mTcO-ONXT, 99mTcN-ONXT and 99mTc(CO)3-ONXT were found to be −1.42 ± 0.01, −1.29 ± 0.01 and −0.63 ± 0.01, respectively, indicating all of them were hydrophilic. Moreover, 99mTcO-ONXT and 99mTcN-ONXT were more hydrophilic than 99mTc(CO)3-ONXT.
Biodistribution study
The results of biodistribution of the complexes in mice bearing S180 tumor are shown in Table 1. Results of biodistribution in mice bearing S180 tumor of recently reported 99mTc complexes as hypoxia imaging agents are shown in Table 2 for comparison.
As noted from Table 1, 99mTcO-ONXT, 99mTcN-ONXT and 99mTc(CO)3-ONXT have a relatively high tumor uptake and good tumor retention. The blood and muscle uptakes are low so the T/B and T/N ratios are better. The high concentration in the liver and kidney shows that the major route of excretion is renal and hepatobiliary. By comparison, 99mTcO-ONXT has a lower muscle uptake and higher tumor uptake, so the T/N ratio of 99mTcO-ONXT is much higher than that of 99mTcN-ONXT and 99mTc(CO)3-ONXT. However, owing to the higher blood uptake, the T/B ratio of 99mTcO-ONXT is lower than that of 99mTcN-ONXT and 99mTc(CO)3-ONXT at 4 h post-injection. The above facts prove that different 99mTc core for preparing the complexes may exhibit significant impact on the tumor uptake, T/B and T/N ratios.
As seen from Table 2, 99mTcO-ONXT exhibits a much higher tumor uptake when compared to the other nine complexes. With regard to T/N ratio, 99mTcO-ONXT is superior to the other complexes except 99mTc-N2IPA. Moreover, 99mTcO-ONXT also has the higher T/B ratio than other complexes except for 99mTc-HYNIC-MN and 99mTc(CO)3-ONXT. As a good tumor hypoxia imaging agent, its detectability of tumor depends on both the absolute tumor uptake and tumor to background ratio. From the above point of views, 99mTcO-ONXT exhibits more potential usefulness as a radiotracer for imaging tumor hypoxia.
SPECT imaging studies
The SPECT imaging results showed the tumor uptake was observable (Fig. 3), however, the high uptake of 99mTcO-ONXT in the liver and kidneys is the drawback of the complex. The imaging findings were similar to the biodistribution results in mice. Low uptake in the thyroid is suggestive of in vivo stability of 99mTcO-MNXT. The imaging results of 99mTcO-ONXT exhibits its very promising property for further studies in more extensive preclinical animal models.
Conclusion
In present study, a novel ligand ONXT was synthesized and its 99mTc-oxo core, 99mTc-nitrido core and 99mTc tricarbonyl core complexes were successfully prepared with high yields through ligand-exchange reactions. The preliminary studies showed all of them had a certain hypoxic selectivity and a relatively high tumor uptake and good target to non-target ratios. Especially for 99mTcO-ONXT, it is prepared from a kit without the need for purification and shows high tumor uptake, tumor/blood and tumor/muscle ratios, suggesting it would be a potential radiotracer to target tumor hypoxia.
References
Nunn A, Linder K, Strauss HW (1995) Nitroimidazoles and imaging hypoxia. Eur J Nucl Med 22:265–280
Ballinger JR (2001) Imaging hypoxia in tumors. Semin Nucl Med 31:321–329
Ballinger JR, Kee JWM, Rauth AM (1996) In vitro and in vivo evaluation of a 99mTc-labelled 2-nitromidazole (BMS181321) as a maker of tumor hypoxia. J Nucl Med 37:1023–1031
Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ (1999) Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med 40:177–183
Hoigebazar L, Jeong JM, Lee JY, Shetty D, Yang BY, Lee YS, Lee DS, Chung JK, Lee MC (2012) Syntheses of 2-nitroimidazole derivatives conjugated with 1,4,7-triazacyclononane-N, N′-diacetic acid labeled with 18F using an aluminum complex method for hypoxia imaging. J Med Chem 55:3155–3162
Yang DJ, Wallace S, Cherif A, Li C, Gretzer MB, Kim EE, Podoloff DA (1995) Development of 18F-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology 194:795–800
Mei L, Wang Y, Chu TW (2012) 99mTc/Re complexes bearing bisnitroimidazole or mononitroimidazole as potential bioreductive markers for tumor: synthesis, physicochemical characterization and biological evaluation. Eur J Med Chem 58:50–63
Mallia MB, Kumar C, Mathur A, Sarma HD, Banerjee S (2012) On the structural modification of 2-nitroimidazole-99mTc(CO)3 complex, a hypoxia marker, for improving in vivo pharmacokinetics. Nucl Med Biol 39:1236–1242
Joyard Y, Joncour VL, Castel H, Diouf CB, Bischoff L, Papamicaël C, Levacher V, Vera P, Bohn P (2013) Synthesis and biological evaluation of a novel 99mTc labeled 2-nitroimidazole derivative as a potential agent for imaging tumor hypoxia. Bioorg Med Chem Lett 23:3704–3708
Li N, Zhu H, Chu TW, Yang Z (2013) Preparation and biological evaluation of 99mTc-N4IPA for single photon emission computerized tomography imaging of hypoxia in mouse tumor. Eur J Med Chem 69:223–231
Mallia MB, Subramanian S, Mathur A, Sarma HD, Banerjee S (2014) A study on nitroimidazole-99mTc(CO)3 complexes as hypoxia marker: some observations towards possible improvement in in vivo efficacy. Nucl Med Biol 41:600–610
Giglio J, Dematteis S, Fernández S, Cerecetto H, Rey A (2011) Synthesis and biological characterisation of novel dithiocarbamate containing 5-nitroimidazole 99mTc-complexes as potential agents for targeting hypoxia. Bioorg Med Chem Lett 21:394–397
Giglio J, Fernández S, Pietzsch HD, Dematteis S, Moreno M, Pacheco JP, Cerecetto H, Rey A (2012) Synthesis, in vitro and in vivo characterization of novel 99mTc-‘4 + 1’-labeled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl Med Biol 39:679–686
Giglio J, Dematteis S, Fernández S, Cerecetto H, Rey A (2014) Synthesis and evaluation of a new 99mTc(I)-tricarbonyl complex bearing the 5-nitroimidazol-1-yl moiety as potential hypoxia imaging agent. J Label Compd Radiopharm 57:403–409
Fernández S, Giglio J, Rey A, Cerecetto H (2012) Influence of ligand denticity on the properties of novel 99mTc(I)-carbonyl complexes. Application to the development of radiopharmaceuticals for imaging hypoxic tissue. Bioorg Med Chem 20:4040–4048
Zhang JB, Yu Q, Huo JF, Pang Y, Yang S, He YN, Tang TT, Yang CC, Wang XB (2010) Synthesis and biodistribution of a novel 99mTc-DMSA-metronidazole ester as a potential tumor hypoxia imaging agent. J Radioanal Nucl Chem 283:481–485
Zhang Y, Chu TW, Gao XG, Liu XQ, Yang Z, Guo ZQ, Wang XY (2006) Synthesis and preliminary biological evaluation of the 99mTc labeled nitrobenzoimidazole and nitrotriazole as tumor hypoxia markers. Bioorg Med Chem Lett 16:1831–1833
Chu TW, Li RJ, Hu SW, Liu XQ, Wang XY (2004) Preparation and biodistribution of 99mTc-labeled 1-(2-nitroimidazole-1-yl)-propanhydroxyiminoamide (N2IPA) as a tumor hypoxia marker. Nucl Med Biol 31:199–203
Liu LQ, Zhang M, Zhong GR, Wang XB (2011) Synthesis and biological evaluation of novel 99mTc-labeled HYNIC-d-glucose as a potential tumor imaging agent. J Radioanal Nucl Chem 287:847–852
Wang JJ, Tian Y, Duan XJ, Mao HN, Tan CM, Wu WS (2012) Synthesis, radiolabeling and biodistribution studies of [99mTc(CO)3(MN-TZ-BPA)]+in tumor-bearing mice. J Radioanal Nucl Chem 292:177–181
Wang JJ, Zheng XB, Wu WS, Yang WJ, Liu Y (2014) Synthesis and preliminary biological evaluation of 99mTc(CO)3-labeled pegylated 2-nitroimidazoles. J Radioanal Nucl Chem 300:1013–1020
Zhang JB, Lin Y, Sheng X, Wang XB (2009) Synthesis of a novel 99mTc nitrido radiopharmaceutical with isopropyl xanthate, showing brain uptake. Appl Radiat Isot 67:79–82
Zhang JB, Ren JL, Lin X, Wang XB (2009) Synthesis and biological evaluation of a novel 99mTc nitrido radiopharmaceutical with deoxyglucose dithiocarbamate, showing tumor uptake. Bioorg Med Chem Lett 19:2752–2754
Lin X, Jin ZH, Ren JL, Pang Y, Zhang WF, Huo JF, Wang XB, Zhang JB, Zhang YY (2012) Synthesis and biodistribution of a new 99mTc-oxo complex with deoxyglucose dithiocarbamate for tumor imaging. Chem Biol Drug Des 79:239–245
Lin X, Chao XY, Zhang JB, Jin ZH, Zhang YY (2014) Preparation and biodistribution of a 99mTc tricarbonyl complex with deoxyglucose dithiocarbamate as a tumor imaging agent for SPECT. Bioorg Med Chem Lett 24:3964–3967
Liu M, Lin X, Song XQ, Cui Y, Li PW, Wang XB, Zhang JB (2013) Synthesis and biodistribution of a novel 99mTc nitrido radiopharmaceutical with proline dithiocarbamate as a potential tumor imaging agent. J Radioanal Nucl Chem 298:1659–1663
Zhu JJ, Wang Y, Li ZX, Fang SA, Zhang JB (2014) Synthesis and biological evaluation of novel 99mTc-oxo and 99mTc-nitrido complexes with phenylalanine dithiocarbamate for tumor imaging. J Radioanal Nucl Chem 302:211–216
Acknowledgments
The work was financially supported, in part, by National Natural Science Foundation of China (21171024, 81101069), Beijing Natural Science Foundation (7112035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Song, X. & Zhang, J. Synthesis and biological evaluation of novel 99mTc labeled ornidazole xanthate complexes as potential hypoxia imaging agents. J Radioanal Nucl Chem 306, 535–542 (2015). https://doi.org/10.1007/s10967-015-4125-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4125-2