Abstract
The [99mTcN(PNP)]2+ core offers a unique route for the preparation of asymmetric 99mTc-complexes. Though bidentate chelators such as dithiocarbamates are most commonly used ligands in this approach, present study explores the possibility of using a monodentate ligand, a isocyanide derivative of metronidazole (MetroNC), for preparing a 99mTcN(PNP) complex for detecting tumor hypoxia. MetroNC could be prepared in good yield and subsequently radiolabeled with [99mTcN(PNP)]2+ precursor complex prepared from [99mTcN]2+ core and N-(2-methoxyethyl)-2-(diphenylphosphino)-N-(2-(diphenylphosphino)ethyl)ethanamine (PNP2) ligand. Preliminary biodistribution studies showed tumor uptake pattern similar to previous studies wherein, about 75 % of the tumor activity observed at 60 min post injection (p.i.) was still found to remain in tumor at 180 min p.i.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia is a condition where the tissue experience inadequate oxygen supply. Various physiological conditions which can result in tissue hypoxia are diabetes, cardiovascular diseases, anaemia, cancer etc. [1]. Presence of hypoxic region is one of the main reasons for the resistance of tumor to radiotherapy and chemotherapy [2, 3] and hence, detection of tumor hypoxia is currently a significant clinical problem. Detection of hypoxia and its extent in cancerous lesions can help physicians in patient selection for hypoxia directed treatment and to plan a treatment strategy for a better clinical outcome [3, 4]. In this direction, nitroimidazoles have received most attention as they are known to undergo oxygen-dependent reduction and accumulation in hypoxic tumor cells [5]. Several nitroimidazole-based radiopharmaceuticals using PET as well as SPECT isotopes are reported for the detection of tissue hypoxia [6–20]. Though, [18F]Fluoromisonidazole ([18F]FMISO) is currently the agent of choice for clinical detection of tumor hypoxia, its use is limited by number of PET centers and cyclotrons. Owing to a larger number of SPECT centers, a 99mTc-radiopharmaceutical for this purpose may find wider applicability. Though a number of 99mTc-labeled nitroimidazole-based agents have been prepared and evaluated for detecting hypoxia [14–20], no radiotracer has so far matched the pharmacokinetics of [18F]FMISO, the gold standard in a clinical set-up. This factor provided the necessary impetus for investigating new 99mTc-labeled nitroimidazole radiotracer for targeting tumor hypoxia.
99mTc labeling of biological vectors can be carried out following different routes using [99mTcO]3+, [99mTcO2]+, [99mTcN]2+, [99mTc(CO)3]+ etc. [21–23]. Among various approaches, use of [99mTcN]2+ core and [99mTc(CO)3]+ core are especially useful for radiolabeling molecules with 99mTc at low ligand concentration while yielding complexes with high specific activity and in vivo stability [24, 25].
There are two general approaches for radiolabeling molecules with [99mTcN]2+ core [26–31]. While the first approach involves two bidentate σ-donor ligands such as dithiocarbamates, xanthates, cysteine, etc., which form a symmetrical [2 + 2] complex [26–28], the second approach makes use of a combination of ‘pseudotridentate’ σ-donors π-acceptors diphosphinoamines (PNP) and bidentate σ-donor ligand (X–Y) forming an asymmetrical complex via the formation of [99mTcN(PNP)]2+ core [29–33]. The [99mTcN(PNP)]2+ core has two vacant coordination sites which can be occupied by the ligands having suitable donor atoms. Bidentate ligands containing (–NH2, –S), (–S, –S) and (–S, O) groups or atoms have been shown to form stable complexes with [99mTcN(PNP)]2+ core [30–33].
Though 99mTc-complexes with isocyanide ligands, such as [99mTc(MIBI)6]+, [99mTc(TBI)6]+, 99mTc-(4 + 1) mixed ligand complexes [19, 34–39], have been reported, use of isocyanide ligands to prepare 99mTcN(PNP) complex had not been explored. In the present study, we report the synthesis of isocyanide derivative of metronidazole and its use for the preparation of 99mTcN(PNP) complex. To the best of our knowledge, this constitutes the first report on the use of monodentate ligand such as isocyanide in combination with PNP for the preparation of a 99mTcN(PNP) complex. Preliminary biological evaluation of this complex is carried out in Swiss mice bearing fibrosarcoma tumor and the result obtained are also presented.
Experimental
Succinic dihydrazide, anhydrous pyridine, p-toluene sulfonyl chloride (p-TsCl) and anhydrous dichloromethane were procured from Sigma Aldrich. Ethylformate was purchased from M/s. S.D. Fine Chemicals, Mumbai. Flexible silica gel plates used for thin layer chromatography (TLC) were obtained from Bakerflex Chemical Company, Germany. HPLC analyses were carried out on a JASCO PU 2080 Plus dual pump HPLC system, Japan, with a JASCO 2075 Plus tunable absorption detector and a Gina Star radiometric detector system, using a C18 reversed phase HiQ Sil column (5 μm, 4 × 250 mm). All the solvents used were of HPLC grade and filtered prior to use. IR spectra of the synthesized compounds were recorded on JASCO-FT/IR-420 spectrometer, Japan. 1H NMR spectra were recorded using 300 MHz Bruker Avance II, spectrometer, Germany.
Synthesis
Synthesis of N-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)formamide) (1)
The compound 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanamine hydrochloride (500 mg, 1.91 mmol) and triethylamine (482 mg, 4.77 mmol) was dissolved in ethylformate (2 mL) and the reaction mixture was refluxed. Upon completion of the reaction (Cf. TLC), excess ethylformate was removed under vacuum and the crude product was purified by silica gel column chromatography, eluting with ethyl acetate, to obtain compound 1 [40]. R f (ethyl acetate) = 0.15. IR (neat, cm−1) 3243(s); 3122(m); 3044(m); 2912(w); 2852(w); 1674(s); 1518(s); 1464(s); 1428(s); 1386(s); 1357(s). 1H NMR (δ ppm, CDCl3) 2.55 (s, 3H, –CH3), 3.68 (q, 2H, –CH2CH 2 NHCHO), 4.51 (t, 2H, –CH 2 CH2NHCHO), 7.98 (s, 1H, metronidazole-C4-H), 8.23 (s, 1H, –CH2CH2NHCHO).
Synthesis of 1-(2-isocyanoethyl)-2-methyl-5-nitro-1H-imidazole [MetroNC] (2)
The synthesis of compound 2 from compound 1 was carried out following a reported procedure [41]. To compound 1 (50 mg, 0.25 mmol) and dry pyridine (39 mg, 0.5 mmol) dissolved in anhydrous dichloromethane (2 mL), p-TsCl (57 mg, 0.3 mmol) was added and the mixture was stirred at room temperature under nitrogen atmosphere. Upon completion of the reaction (Cf. TLC), saturated NaHCO3 solution (10 mL) was added to the reaction mixture and stirring was continued for another 2 h. The crude compound was extracted with chloroform (3 × 10 mL) and further purified by silica gel column chromatography eluting with ethyl acetate. R f (ethyl acetate) = 0.6. IR (neat, cm−1) 3124(m); 3045(m); 2916(w); 2850(w); 2150(s); 1530(s); 1462(s); 1427(s); 1366(s); 1356(s). 1H NMR (δ ppm, CDCl3) 2.64 (s, 3H, –CH3), 3.92 (t, 2H, –CH 2 CH2NC), 4.59 (t, 2H, –CH2CH 2 NC), 8.02 (s, 1H, metronidazole-C4-H).
Radiolabeling
[99mTcN]2+ intermediate was prepared by adding freshly eluted Na99mTcO4 (750 µL, 37 MBq) to a mixture of succinic dihydrazide (5 mg) and stannous chloride (0.1 mg) in ethanol (250 µL), and incubating the reaction mixture at room temperature for 30 min [30]. To this intermediate, PNP2 ligand (2 mg) [29] was added and incubated at room temperature for another 5 min. The precursor complex [99mTcN(PNP)]2+ thus prepared (900 µL) was added to compound 2 (1 mg in 100 µL ethanol) and incubated at 100 °C for 30 min. The complex thus obtained was analyzed by HPLC.
Quality control studies
HPLC
HPLC analysis was carried out using a C18 reversed phase HiQ Sil column (5 μm, 4 × 250 mm) using the following gradient elution method. Trifluoroacetic acid 0.1 % in water (A) and trifluoroacetic acid 0.1 % in acetonitrile (B) 0 min—90 % A; 28 min—10 % A, 30 min—90 % A. About 15 µL of the test solution was injected into the column for analysis. Flow rate was maintained at 1 mL/min. The radiochemical purity (RCP) of the complex was determined by peak area measurements from the chromatogram.
Octanol–water partition co-efficient
The radiolabeled compound (0.1 mL, ~3.7 MBq) was mixed with double distilled water (0.9 mL) and n-octanol (1 mL) on a vortex mixer for about 1 min and then centrifuged (3500 g) for 5 min to effect clear separation of the two layers. The n-octanol layer (0.8 mL) was withdrawn and equal volume of fresh double distilled water was added. The mixture was vortexed again and then centrifuged as described above. Equal aliquots of the two layers were withdrawn and measured for associated radioactivity. The readings thus obtained were used to calculate the LogP o/w of the complex.
Room temperature stability and cysteine challenge
The in vitro stability of the complex was checked by incubating the complex at room temperature for 6 h and evaluating the radiochemical purity using HPLC. The MetroNC-[99mTcN(PNP)] complex was also challenged with excess of cysteine to check the stability of the complex in vivo. To the radiolabeled compound (18.5 MBq, 500 μL), 100 fold excess of cysteine was added and incubated at 37 °C for 1 h. The complex was then analyzed by HPLC to assess its RCP.
Serum stability
The radiolabled complex (1.85 MBq, 50 μL) was added to 250 μL of serum and incubated at 37 °C. About 100 μL of the mixture was taken after 1 h and to that 100 μL of acetonitrile was added to precipitate the serum proteins and centrifuged at 3500 g for 10 min. The supernatant was analyzed by HPLC to check the stability of the complex in serum. After 6 h another 100 μL of the mixture was withdrawn and analyzed following the procedure mentioned above.
Biodistribution studies
Biodistribution study of the radiolabeled compound was carried out in Swiss mice bearing fibrosarcoma tumor. The fibrosarcoma tumor was induced by injecting about 100 μL of HSDM1C1 murine fibrosarcoma cells (~1 × 106 cells) on the dorsum of the Swiss mice. About 14 days after transplantation of the tumor cells, animals with tumor size in the range 0.8–1.0 cm diameter were obtained. These animals were used for biodistribution studies. Radioactive preparation (~3.7 MBq per animal in 100 µL volume) was injected into the mice via lateral tail vein. After injections, the animals (n = 3) were incubated for different time intervals (30 min, 60 min and 180 min). At the end of respective time interval, the animals were sacrificed. The relevant organs/tissue were excised, weighed and activity associated with them were measured in a flat-bed type NaI(Tl) counter with energy window set for 99mTc. Results were expressed as percentage injected activity per gram of organ (%IA/g).
Results and discussion
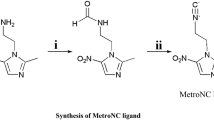
The target compound, MetroNC (2) was synthesized in a two step synthetic procedure shown in Fig. 1. MetroNC could be prepared in good yield (~75 %) and it was characterized by appropriate spectroscopic techniques. Infra red spectrum of MetroNC showed a strong absorption peak at 2150 cm−1 confirming the presence of isocyanide group in the target compound. The 1H NMR of the compound was consistent with the expected structure. Subsequently, compound 2 was radiolabeled with [99mTc(N)PNP]2+ precursor complex and analyzed by HPLC. The HPLC profile of [99mTc(N)PNP]2+ precursor complex showed two peaks, a minor peak (25 %) at 20.05 min and a major peak (75 %) at 27.52 min. Upon incubating the intermediate [99mTc(N)PNP]2+ complex with MetroNC ligand, however, the two peaks (at 20.05 and 27.52 min) disappeared completely and a single sharp peak at 26.40 min, presumed to be that of MetroNC-[99mTcN(PNP)] complex, was obtained (Fig. 2a, b). Complete conversion of the two peaks representing intermediate [99mTc(N)PNP]2+ complex into a single peak possibly indicates the presence of 99mTc(N)PNP-group common in them with the other two coordination sites occupied by different labile ligands. Upon incubation with MetroNC ligand, the labile ligands may be replaced with MetroNC, thus forming the complex which appeared as a single sharp peak. No attempt was made, however, to establish the identity of the individual peak representing [99mTc(N)PNP]2+ complex.
The LogP o/w value of the complex, a measure of its lipophilicity, was determined following a reported procedure and it was found to be 1.1 [15]. The complex was found to be stable at room temperature for 6 h. The complex did not showed appreciable degradation upon incubation with human serum for 1 h at 37 °C. However, about 22 % degradation was observed after 6 h under similar conditions. Challenging studies with cysteine showed ~25 % decomposition with 100 fold excess cysteine. The transchelation in the presence of excess of cysteine shows that the complex formed using monodentate isocyanide group is not very stable in the presence of excess of bidentate cysteine molecule.
Biological evaluation of MetroNC-[99mTcN(PNP)] complex was carried out in Swiss mice bearing fibrosarcoma tumor. The uptake of complex in tumor and different organs/tissue is summarized in Table 1. The complex showed an initial tumor uptake of 0.85 (0.07) %IA/g at 30 min p.i. which reduced to 0.66 (0.05) %IA/g at 60 min p.i. Clearance of activity from tumor was slow after 60 min p.i., with only 25 % of the tumor activity observed at 60 min p.i. cleared at 180 min p.i. [Figure 3]. Comparison of results obtained for MetroNC-[99mTcN(PNP)] complex with the previously reported 99mTcN-metronidazole complexes is summarized in Table 2. Among these, the 99mTc-MNZ-xanthate derivative [42] and 99mTcN-metronidazoleDTC1 [43] showed tumor uptake of 1.36 (0.29) %IA/g at 180 min p.i. and 1.0 (0.2) %IA/g at 240 min p.i. respectively. However another derivative of metronidazole with a piperazine linker, radiolabeled with [99mTcN]2+ core (99mTcN-metronidazoleDTC2) [43] showed only 0.4 (0.1) %IA/g tumor uptake at 240 min p.i. with fast clearance from blood via hepatobiliary pathway due to its more lipophilic nature (LogP o/w ~ 0.7). In the present study, in line with the aforementioned findings, the MetroNC-[99mTcN(PNP)] complex showed a fast clearance from blood through the hepatobiliary system, which could be due to its more lipophilic nature (LogP o/w ~ 1.1). Though the tumor uptake of MetroNC-[99mTcN(PNP)] complex was lower than 99mTc-MNZ-xanthate and 99mTcN-metronidazoleDTC1, fast clearance of activity from blood resulted in better tumor to blood ratio compared to previously reported 99mTcN-metronidazole complexes (Table 2). For hypoxia imaging agents, adequate lipophilicity is essential for entry of the radiotracer into the cell by passive diffusion and subsequently be retained in blood for sufficient period of time. A radiotracer that clears slowly from blood gets sufficient time to distribute better in tumor by passive diffusion along the concentration gradient between blood and intracellular environment of tumor [18]. Observed fast clearance from blood may be contributing to lower uptake in tumor for the present complex.
The tumor to blood ratio and tumor to muscle ratio obtained with the present complex are shown in Fig. 4. It could be observed that the tumor to blood ratio increased from 0.5 (0.04) at 30 min p.i. to 1.36 (0.14) at 180 min p.i. and the tumor to muscle ratio remained almost constant at ~1.4 throughout the period of study.
The distribution of activity in other organs is shown in Table 1. A small fraction of the injected activity was excreted through renal route. Activity associated with other vital organs like heart, lungs and spleen showing 1.08 (0.05) % IA/g, 1.97 (0.49) %IA/g and 2.72 (0.46) %IA/g respectively at 30 min p.i. which cleared with time.
Conclusion
Metronidazole isocyanide derivative was synthesized and subsequently radiolabled with [99mTc(N)PNP]2+ precursor complex. Present study, probably, may be the first of its kind where a monodentate isocyanide ligand was used in combination with PNP ligand for the preparation of a 99mTcN(PNP) complex. Preliminary studies indicated the formation of a MetroNC-[99mTcN(PNP)] complex with high radiochemical purity. Observations derived from in vivo distribution studies of the radiotracer in tumor bearing mice shows that although the tumor to blood ratio was better at 180 min p.i., the uptake of activity in liver and intestine was high. The in vivo pharmacokinetics of the complex can be probably improved by envisaging a similar radiotracer using bis[(diethoxypropylphosphanyl)ethyl]ethoxyethylamine (PNP6) instead of PNP2 [33] wherein the ether linkages present in PNP6 is expected to be metabolized faster in liver leading to reduced uptake in liver.
References
Wiebe LI, Machulla HJ (1999) In: Machulla HJ (ed) Imaging hypoxia. Kluwer Academic Publishers, Netherlands
Ballinger JR (2001) Imaging hypoxia in tumors. Semin Nucl Med 31:321–329
Mees G, Dierckx R, Vangestel C, Van de Wiele C (2009) Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging 36:1674–1686
Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56:941–943
Krohn KA, Link JM, Mason RP (2008) Molecular imaging of hypoxia. J Nucl Med 49(Suppl 2):129S–148S
Grunbaum Z, Freauff SJ, Krohn KA, Wilbur DS, Magee S, Rasey JS (1987) Synthesis and characterization of congeners of misonidazole for imaging hypoxia. J Nucl Med 28:68–75
Sorger D, Patt M, Kumar P, Wiebe LI, Barthel H, Seese A, Dannenberg C, Tannapfel A, Kluge R, Sabri O (2003) [18F]Fluoroazomycinarabinofuranoside (18FAZA) and [18F]fluoromisonidazole (18FMISO): a comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl Med Biol 30:317–326
Foo SS, Abott DF, Lawrentschuk N, Scott AM (2004) Functional imaging of intratumoural hypoxia. J Mol Imaging Biol 6:291–305
Lee ST, Scott AM (2007) Hypoxia positron emission tomography imaging with 18F-fluoro misonidazole. Semin Nucl Med 37:451–461
Komar G, Seppänen M, Eskola O, Lindholm P, Grönroos TJ, Forsback S, Sipilä H, Evans SM, Solin O, Minn H (2008) 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J Nucl Med 49:1944–1951
Rajendran JG, Krohn KA (2015) F-18 fluoromisonidazole for imaging tumor hypoxia: imaging the microenvironment for personalized cancer therapy. Semin Nucl Med 45:151–162
Bonnitcha PD, Bayly SR, Theobald MB, Betts HM, Lewis JS, Dilworth JR (2010) Nitroimidazole conjugates of bis(thiosemicarbazonato)64Cu(II)—potential combination agents for the PET imaging of hypoxia. J Inorg Biochem 104:126–135
Zha Z, Zhu L, Liu Y, Du F, Gan H, Qiao J, Kung HF (2011) Synthesis and evaluation of two novel 2-nitroimidazole derivatives as potential PET radioligands for tumor imaging. Nucl Med Biol 38:501–508
Mallia MB, Subramanian S, Mathur A, Sarma HD, Venkatesh M, Banerjee S (2010) Synthesis and evaluation of 2-, 4-,5-substituted nitroimidazole-iminodiacetic acid-99mTc(CO)3 complexes to target hypoxic tumors. J Label Compd Radiopharm 53:535–542
Mallia MB, Kumar C, Mathur A, Sarma HD, Banerjee S (2012) On the structural modification of 2-nitroimidazole-99mTc(CO)3 complex, a hypoxia marker, for improving in vivo pharmacokinetics. Nucl Med Biol 39:1236–1242
Mallia MB, Subramanian S, Mathur A, Sarma HD, Venkatesh M, Banerjee S (2008) On the isolation and evaluation of a novel unsubstituted 5-nitroimidazole derivative as an agent to target tumor hypoxia. Bioorg Med Chem Lett 18:5233–5237
Mallia MB, Subramanian S, Banerjee S, Sarma HD, Venkatesh M (2006) Evaluation of 99mTc(CO)3 complex of 2-methyl-5-nitroimidazole as an agent for targeting tumor hypoxia. Bioorg Med Chem 14:7666–7670
Mallia MB, Subramanian S, Mathur A, Sarma HD, Banerjee S (2014) A study on nitroimidazole-99mTc(CO)3 complexes as hypoxia marker: some observations towards possible improvement in vivo efficacy. Nucl Med Biol 41:600–610
Giglio J, Fernandez S, Pietzsch HJ, Dematteis S, Moreno M, Pacheco JP, Cerecetto H, Rey A (2012) Synthesis, in vitro and in vivo characterization of novel 99mTc-’4+1’-labeled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl Med Biol 39:679–686
Fernandez S, Dematteis S, Giglio J, Cerecetto H, Rey A (2013) Synthesis, in vitro and in vivo characterization of two novel 68Ga-labelled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl Med Biol 40:273–279
Liu S, Edwards DS (1999) 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev 99:2235–2268
Banerjee S, Pillai MRA, Ramamoorthy N (2001) Evolution of Tc-99m in diagnostic radiopharmaceuticals. Semin Nucl Med 4:260–277
Banerjee SR, Maresca KP, Francesconi L, Valliant J, Babich JW, Zubieta J (2005) New directions in the coordination chemistry of 99mTc: a reflection on technetium core structures and a strategy for new chelate design. Nucl Med Biol 32:1–20
Baldas J, Bonnyman J (1985) Substitution reactions of 99mTcNCl4 −—a route to a new class of 99mTc-radiopharmaceuticals. Int J Appl Radiat Isot 36:133–139
Alberto R, Schibli R, Egli A, Schubiger PA, Abram U, Kaden TA (1998) A novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(H2O)3(CO)3]+ from [99mTcO4]− in aqueous solution and its reaction with a bifunctional ligand. J Am Chem Soc 120:7987–7989
Pasqualini R, Comazzi V, Bellande E, Duatti A, Marchi A (1992) A new efficient method for the preparation of 99mTc-radiopharmaceuticals containing the TcN multiple bond. Appl Radiat Isot 43:1329–1333
Marchi A, Rossi R, Marvelli L (1993) Nitrido-technetium (V) complexes with amino acids: preparation and X-ray crystal structure of the l-cysteinate ethyl ester technetium (V) complex. Inorg Chem 32:4673–4674
Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D, Comet M (1994) Bis(dithiocarbamato) nitrido technetium-99m radiopharmaceuticals: a class of neutral myocardial imaging agents. J Nucl Med 35:334–341
Bolzati C, Boschi A, Uccelli L, Tisato F, Refesco F, Cagnolini A, Duatti A, Prakash S, Bandoli G, Vittadini A (2002) Chemistry of the strong electrophilic metal fragment [99Tc(N)(PXP)]2+ (PXP = diphosphine ligand). A novel tool for the selective labeling of small molecules. J Am Chem Soc 124:11468–11479
Bolzati C, Mahmood A, Malago E, Uccelli L, Boschi A, Jones AG, Refesco F, Duatti A, Tisato F (2003) The [99mTc(N)(PNP)]2+ metal fragment: a technetium-nitrido synthon for use with biologically active molecules. The N-(2-methoxy phenyl) piperazyl-cysteine analogues as examples. Bioconjug Chem 14:1231–1242
Bolzati C, Benini E, Cazzola E, Jung C, Tisato F, Refesco F, Pietzsch HJ, Spies H, Uccelli L, Duatti A (2004) Synthesis, characterization and biological evaluation of neutral nitride technetium (V) mixed ligand complexes containing dithiolates and aminodiphosphines. A novel system for linking technetium to biomolecules. Bioconjug Chem. 15:628–637
Mathur A, Mallia MB, Banerjee S, Sarma HD, Pillai MRA (2013) Preparation and evaluation of a 99mTcN-PNP complex of sanazole analogue for detecting tumor hypoxia. Bioorg Med Chem Lett 23:1394–1397
Bolzati C, Cavazza-Ceccato M, Agostini S, Tokunaga S, Casara D, Bandoli G (2008) Subcellular distribution and metabolism studies of the potential myocardial imaging agent [99mTc(N)(DBODC)(PNP5)]+. J Nucl Med 49:1336–1344
Taillefer R, Laflamme L, Dupras G, Picard M, Phaneuf DC, Leveille J (1988) Myocardial perfusion imaging with 99mTc-methoxy-isobutyl-isonitrile (MIBI): comparison of short and long time intervals between rest and stress injections. Eur J Nucl Med 13:515–522
Baker RJ, Bellen JC, Fornasiero D, Penglis S (1986) The preparation of 99mTc-tertiarybutylisonitrile (99mTc-TBI) by a method suitable for routine clinical use. Int J Radiat Appl Instrum B 13:527–532
Kothari KK, Satpati D, Joshi S, Venkatesh M, Ramamoorthy N, Pillai MR (2005) 99mTc carbonyl t-butyl isonitrile: a potential new agent for myocardial perfusion imaging. Nucl Med Comm. 26:155–161
Zhang XZ, Wang XB, Jia F, Tang ZG, Zhang JB, Liu BL (2002) Preparation, characterization and biodistribution of a new technetium-99m nitrido complex with 2-methoxyisobutylisomtrile and comparison with Tc-99m-MIBI. J Label Compd Radiopharm 45:1029–1043
Zhang XZ, Wang XB, Zhou JM (2001) Preparation, characterization and biodistribution of a new technetium-99m nitrido complex with cyclohexylisonitrile. J Label Compd Radiopharm. 44:S651–S653
Zhang XZ, Wang XB, Zhou JM (2003) A new technetium-99m labeled isonitrile complex 99mTc-TMCHI as a potential blood pool imaging agent. Appl Radiat Isot 59:231–235
Dhake KP, Tambade PJ, Singhal RS, Bhanage BM (2011) An efficient, catalyst and solvent free N-formylation of aromatic and aliphatic amines. Green Chem Lett Rev 4:151–157
Kobayashi G, Saito T, Kitano Y (2011) A novel method for preparing isocyanides from N-substituted formamides with chlorophosphate compounds. Synthesis 20:3225–3234
Mallia MB, Mathur A, Subramanian S, Banerjee S, Sarma HD, Venkatesh M (2005) A novel [99mTcN]2+ complex of metronidazole xanthate as a potential agent for targeting hypoxia. Bioorg Med Chem Lett 15:3398–3401
Giglio J, Fernandez S, Rey A, Cerecetto H (2011) Synthesis and biological characterization of novel dithiocarbamate containing 5-nitroimidazole 99mTc-complexes as potential agents for targeting hypoxia. Bioorg Med Chem Lett 21:394–397
Acknowledgments
The authors gratefully acknowledge the support and encouragement provided by Dr. K. L. Ramakmar, Director Radiochemistry & Isotope Group, Bhabha Atomic Research Centre. The authors acknowledge the supply of Molybdenum-99 by Radiochemicals Section, Isotope Production and Application Division. The help rendered by the staff members of the animal house facility of Radiation Biology and Health Sciences Division, is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vats, K., Mallia, M.B., Mathur, A. et al. Synthesis and evaluation of a novel 99mTcN(PNP)-complex with metronidazole isocyanide ligand as a marker for tumor hypoxia. J Radioanal Nucl Chem 308, 363–369 (2016). https://doi.org/10.1007/s10967-015-4526-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4526-2