Abstract
The dimercaptosuccinic acid metronidazole ester (DMSAMe) was synthesized and radiolabeled with 99mTc to form the 99mTc-DMSAMe complex in high yield. The radiochemical purity of the 99mTc-DMSAMe complex was over 90%, as measured by TLC and by HPLC, without any notable decomposition at room temperature over a period of 6 h. Its partition coefficient indicated that it was a lipophilic complex. The tumor cell experiment and the biodistribution in mice bearing S 180 tumor showed that the 99mTc-DMSAMe complex had a certain hypoxic selectivity and accumulated in the tumor with high uptake and good retention. The tumor/blood and tumor/muscle ratios increased with time, suggesting it would be a possible tumor hypoxia imaging agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For several years, it has been known that hypoxia plays an important role in the resistance of cancers to treatment by radiotherapy. A number of invasive methods have been developed for the measurement of hypoxia, but none of these is in routine clinical use because of their invasive nature, inconvenience, and inability to acquire repeated measures. The limitations of these techniques stimulate the development of noninvasive imaging with a hypoxia targeted radiopharmaceutical. In the development of hypoxia imaging agent, nitroimidazole derivatives are enzymatically reduced and accumulated in hypoxic regions, therefore labeled nitroimidazole analogues have received great attention. Currently, the positron emission tomography (PET) tracer [18F]Fluoromisonidazole ([18F]FMISO) has been used to evaluate tumor hypoxia [1]. However, the short half life and high cost of the [18F] isotope restrict its wide application in clinical nuclear medicine. Given the favorable characteristics of technetium-99m (short half-life, optimal γ-energy, inexpensive cost), research has focused on 99mTc labeled radiopharmaceutical for targeting hypoxia. Recently, several 99mTc labeled nitroimidazole analogues have been reported [2–7]. However, the relative lower tumor uptakes and slow clearance from blood observed for these hypoxia imaging agents are their common defects. There still exists great interest in the search for an ideal hypoxia imaging agent.
It is known that dimercaptosuccinic acid (DMSA) ligand can be used to form stable 99mTc(V)O complex on the basis of efficient binding of the oxotechnetium group to four thiolsulphur atoms. 99mTc(V)O-DMSA has been widely used for imaging medullary carcinoma of the thyroid and other soft tissue tumors [8]. Bearing in mind metronidazole has the potential to target hypoxia tumors and 99mTc(V)O-DMSA is a tumor imaging agent, we present the synthesis of metronidazole ester using DMSA as a chelator and evaluate its 99mTc labeled complex as a potential tumor hypoxic imaging agent. To the best of our knowledge, this report constitutes the first of its kind in using the DMSAMe in the preparation of 99mTcO complex as targeted agent for hypoxia imaging.
Experimental
Materials
Metronidazole and meso-2,3-dimercaptosuccinic acid (DMSA) were purchased from Fluka, Germany. All other chemicals were of reagent grade and were used without further purification. 99Mo/99mTc generator was obtained from the China Institute of Atomic Energy (CIAE). HPLC analysis was carried out with a reversed-phase column (Kromasil 100-5C, 250 × 4.6 mm), Shimadzu SCL-10AVP series. 1H-NMR and 13C-NMR spectra were recorded on a 400 MHz BRUKER AVANCE spectrophotometer.
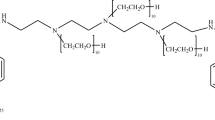
Synthesis of DMSAMe (Compound 1)
About 0.36 g of DMSA and 1.10 g of metronidazole were stirred vigorously in 15 mL of anhydrous pyridine solution. To the above solution, 0.5 g of N,N′-dicyclohexylcarbodiimide (DCC) dissolved in anhydrous pyridine was then added in an ice bath. The mixture was stirred for 24 h at room temperature. The solvent was removed under reduced pressure and the residue was dissolved in 20 mL of CH2Cl2 and filtered off. The filtrate was washed by 5% NaHCO3, saturated NaCl solution and water. The organic phase was dried with anhydrous Na2SO4 and the solvent was removed under vacuum. The yellow crude product was recrystallized from ethyl acetate to give DMSAMe (compound 1, 0.28 g, 29%).
Preparation of 99mTc-DMSAMe
1 mL of saline containing [99mTcO4]− (15 MBq) was added to a glucoheptonate (GH) kit containing 0.1 mg of stannous chloride dihydrate, 20.0 mg of GH. The mixture was kept at room temperature for 20 min. Successively, 1.0 mg of compound 1 dissolved in 1.0 mL ethanol was then added and the resulting solution was heated at 100 °C for 30 min. The TLC was performed on a polyamide strip and eluted with saline. HPLC analysis was carried out with a reversed-phase column, working at a flow rate of 1.0 mL/min. Water (A) and acetonitrile (B) mixtures with 0.1% trifluoroacetic acid were used as the mobile phase and the following gradient elution technique was adopted for the preparation (0–10 min 80%B, 10–40 min 100%B).
Stability study
The stability of the complex was assayed by measuring the radiochemical purity by TLC at different times (3 and 6 h) after preparation.
Determination of the partition coefficient for the complex
The partition coefficient was determined by mixing the complex with an equal volume of 1-octanol and phosphate buffer (0.025 M, pH 7.4) in a centrifuge tube. The mixture was vortexed at room temperature for one min and then centrifuged at 5,000 r/min for 5 min. From each phase 0.1 mL of the aliquot was pipetted and counted in a well γ-counter. Each measurement was repeated three times. Care was taken to avoid cross contamination between the phases. The partition coefficient, P, was calculated using the following equation:
Usually the final partition coefficient value was expressed as log P.
Cellular accumulation
The complex was in vitro evaluated following the previous method [7]. S180 cells were suspended in fresh DMEM growth medium at a cell concentration of 2 × 106 cells/mL. Aliquots of 20 mL were placed in glass vials and incubated at 37 °C with gentle stirring under an atmosphere of 95% air plus 5% carbon dioxide (aerobic exposure) or 95% nitrogen plus 5% carbon dioxide (hypoxic exposure, oxygen concentration < 10 ppm). After a 30–45-min equilibration period, 0.3 mL of 99mTc-DMSAMe was added to each vial at a final activity of approximately 0.25 MBq/mL and a drug concentration of approximately 0.7 μg/mL. The 0.9-mL samples were removed at various time intervals. From each sample, the 0.2-mL sample was centrifuged at 1,500 r/min for 5 min. A 90 μL aliquot of the supernatant was removed for counting, and the left sample containing cells and 110 μL medium was also counted. The ratio of the radioactivity in the cell pellet to that in the supernatant medium (Cin/Cout) was calculated as (residue counts -supernatant counts)/supernatant counts. At each time point, five samples were determined. During the whole procedure, the cells’ viability was more than 90%.
Biodistribution study
Biodistribution studies were performed in Kunming male mice (weighing 18–20 g) bearing S 180 tumor, which grew to a leg diameter of 10–15 mm. 0.1 mL of 99mTc-DMSAMe (7.4 × 105 Bq) was injected via a tail vein and the injected radioactivity was measured with a well-type NaI(Tl) detector. The mice were sacrificed at 0.5, 2 and 4 h post-injection. The tumor, other organs of interest and blood were collected, weighed and measured for radioactivity. The results were expressed as the percent uptake of injected dose per gram of tissue (%ID/g). All biodistribution studies were carried out in compliance with the national laws related to the conduct of animal experimentation.
Results and discussion
Synthesis
The dimercaptosuccinic acid metronidazole ester (DMSAMe)(compound 1) was prepared by reacting DMSA with metronidazole in the presence of N,N′-dicyclohexylcarbodiimide (DCC) and anhydrous pyridine [9]. The reaction is schematically shown in Scheme 1.
The compound 1 was characterized by 1H-NMR and 13C-NMR. 1H-NMR(Methanol-d4)δ : 2.47(s, 3H, CH3), 2.89 (s, 1H, CHCOOC), 3.75(t, 2H, CH2), 4.40(t, 2H, CH2), 7.96 (s, 1H, CH). 13C-NMR (Methanol-d4)δ : 152.67, 147.98, 143.24, 130.87, 128.69, 61.50, 50.02, 13.97.
Preparation of 99mTc-DMSAMe
The preparation of 99mTc-DMSAMe was carried out using the following procedure in Scheme 2.
For labelling, 99mTc-DMSAMe was prepared by ligand-exchange reaction with 99mTc-glucoheptonate (GH). 99mTc-GH is a suitable substrate for the substitution reaction with the compound 1 to give the final complex 99mTc-DMSAMe.
The radiochemical purity of the complex was routinely checked by TLC and HPLC. By TLC, in saline, 99mTcO4 −, 99mTcO2·nH2O and 99mTc-DMSAMe remained at the origin while 99mTc-GH migrated with the front. The HPLC pattern of 99mTc-DMSAMe is shown in Fig. 1. It was observed that the retention time of 99mTcO4 − was 6.4 min and 99mTc-GH was 6.8 min, while that of 99mTc-DMSAMe was found to be 24.0 min. Single peak suggested only one product (99mTc-DMSAMe) was formed. The mean radiochemical purity of the product was over 90% immediately after the preparation.
Based on a previous characterization of the molecular structure of 99mTc(V)O-DMSA [10], it seems reasonable to presume that the structure of 99mTc-DMSAMe is similar to that of 99mTc(V)O-DMSA, possessing a square pyramidal geometry with an apical Tc = O bond and two DMSAMe ligands spanning the four positions in the basal plane through the four sulfur atoms. Clearly, further studies should be performed, using macroscopic levels of the long-lived 99Tc, to determine and characterize the structure of 99mTc-DMSAMe.
Stability study
No decomposition of the complex occured over 6 h at room temperature, suggesting that the complex was stable in the reaction mixture at room temperature.
Partition coefficient
The partition coefficient (log P) value of 99mTc-DMSAMe was 0.30, suggesting it was lipophilic. This value is less than that of 99mTc-BMS181321 (log P: 1.60) and higher than that of 99mTc-N2IPA (log P: −1.40) [2].
Cellular accumulation
The effect of anoxic or normoxic conditions on the accumulation of 99mTc-DMSAMe in S180 cells as a function of time is illustrated in Fig. 2. From Fig. 2, it is shown that the uptake of 99mTc-DMSAMe in anoxic cells is constantly more than that in normoxic cells, suggesting 99mTc-DMSAMe has a certain hypoxic selectivity.
Biodistribution of the complex in mice
The results of biodistribution of 99mTc-DMSAMe are shown in Table 1. As described in Table 1, 99mTc-DMSAMe has a high uptake at the site of tumor and good target/non-target ratio. The tumor/blood and tumor/muscle ratios increase with time. The kidney uptake is much higher than the hepatic. Early renal activity reflects urinary elimination of this complex. As seen from Table 2, this is comparable to the results of some radiolabeled hypoxia imaging agents in mice bearing tumor. Among the four complexes, 99mTc-DMSAMe exhibits the highest tumor uptake and this may attribute to its four metronidazoles to possess affinity for hypoxic tumors. 99mTc-DMSAMe has a good T/N ratio (2.49 at 2 h p. i.), but it is lower than that of the other three complexes. A decrease of T/B ratio in the order is observed: 99mTc-N2IPA > 99mTc-DMSAme = 99mTc-MNZ-xanthate > 99mTc-BMS181321.
Conclusion
In summary, novel DMSAMe ligand was successfully synthesized and 99mTc-DMSAMe was prepared in high yields through a ligand-exchange reaction, which can be easily used for the preparation of a radiopharmaceutical through a freeze-dried kit formulation. 99mTc-DMSAMe showed a certain hypoxic selectivity and a high tumor uptake. However, the tumor/blood ratio of 99mTc-DMSAMe is not high, thus restricting its potential usefulness as a better tumor hypoxia imaging agent.
References
Yang DJ, Wallace S, Cherif A, Li C, Gretzer MB, Kim EE, Podoloff DA (1995) Radiology 194:795
Chu TW, Li RJ, Hu SW, Liu XQ, Wang XY (2004) Nucl Med Biol 31:199
Kong DJ, Lu J, Ye SZ, Wang XB (2007) J Label Compd Radiopharm 50:1137
Mallia MB, Mathur A, Subramanian S, Banerjee S, Sarma HD, Venkatesh M (2005) Bioorg Med Chem Lett 15:3398
Mallia MB, Subramanian S, Banerjee S, Sarma HD, Venkatesh M (2006) Bioorg Med Chem 14:7666
Mallia MB, Subramanian S, Mathur A, Sarma HD, Venkatesh M, Banerjee S (2008) Bioorg Med Chem Lett 18:5233
Zhang Y, Chu TW, Gao XG, Liu XQ, Yang Z, Guo ZQ, Wang XY (2006) Bioorg Med Chem Lett 16:1831
Ohta H, Yamamoto K, Endo K, Mori T, Hamanaka D, Shimazu A, Lkekubo K, Makimoto K, Lida Y, Konishi J, Morita R, Hata N, Horiuchi K, Yokoyama A, Torizuka K, Kuma K (1984) J Nucl Med 25:323
Bundgaard H, Larsen C, Thorbek P (1984) Int J Pharm 18:67
Blower PJ, Singh J, Clarke SEM (1991) J Nucl Med 32:845
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (20771018) and by Beijing Normal University. The assistance of Ms. Huafan Huang and Prof. Taiwei Chu, College of Chemistry and Molecular Engineering, Peking University, in cellular accumulation study is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Yu, Q., Huo, J. et al. Synthesis and biodistribution of a novel 99mTc-DMSA-metronidazole ester as a potential tumor hypoxia imaging agent. J Radioanal Nucl Chem 283, 481–485 (2010). https://doi.org/10.1007/s10967-010-0455-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0455-2