Abstract
A novel aromatic diamine monomer, 4-(4-diethylamino)phenyl-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine (EPAPP), containing pyridine ring units, ether linkage moieties and diethylaminophenyl pendent groups, has been designed and synthesized through three-step methods, and then used to prepare for a series of polyimides with commercial aromatic dianhydrides via two-step solution polycondensation. The resulting polyimides showed good solubility in common polar solvents, such as NMP, DMF, DMSO. They exhibited high thermal stability with the glass transition temperature (Tgs) more than 254 °C, and the temperature of 10% weight loss over 544 °C with more than 64% residue at 800 °C under nitrogen. They presented excellent hydrophobic properties with the contact angle in the range of 81.9–91.9°. In addition, the results of Wide-angle X-ray Diffraction (WAXD) indicated that these polymers revealed an amorphous structure.

A series of containing pyridine ring and diethylaminophenyl polyimides were designed and synthesized from a novel diamine EPAPP with several aromatic dianhydrides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyimides are representative class of high performance polymers due to them excellent overall properties, such as high thermal stability, excellent mechanical properties, good dimensional stability and remarkable insulation performance, which are widely used in microelectronics, optoelectronic, aerospace, automotive, gas separation, polymer memory and other fields [1,2,3,4,5]. It is generally true that molecular structures have a significant influence on the properties of polymers. Most polyimides usually demonstrate low transparency and deep color in view of the charge transfer complex (CTC) formation. Furthermore, aromatic polyimides have high softening temperature and processing temperature which limiting their processability and solubility in that their rigid structure. In order to solving problems and overcoming weaknesses the solubility and processability of traditional aromatic polyimides, many efforts have been carried out by introduction of bulky pendant [6,7,8,9,10,11,12,13,14], fluoridated structure [15,16,17], and flexible linkage [18,19,20,21,22,23], such as -O-, -S-, -CH2-, and -SO2- into the polymer backbones. Liu et al [19] designed and synthesized a series of fluoridated polyimides containing bulky pendent groups and non-coplanar structures, and these polymers presented excellent solubility, high thermal stability and transparency.
However, although the introduction of bulky pendents or flexible chains enhances the solubility of the polymer, there are still some problems. It is well known that flexible linkages could lead lowering glass transition temperatures(Tgs). Therefore, improvement of the solubility and processability without sacrificing the thermal stability of the polymer is a hot spot. The introduction of aromatic rings is a common method to increase the thermal stability of polyimides [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Dong, et al [34] reported a series of polyimides containing pyridine and fluorine has low dielectric, good thermal stability, and the temperatures of 5% weight loss (T5%) located between 468 °C and 499 °C.
The purpose of this research was to design and synthesis of a series of novel diamine monomer through incorporation of ether, diethylaminophenyld and pyridine unit into the polymer skeleton in order to fabricate a series of polyimides with excellent thermal stability, good processing performance and dissolving capacity. All of these polymers were characterized through FI-IR. In addition, the physical properties of the prepared polymers were summarized, such as solubility, thermal stability, hydrophobic properties, aggregate structure and viscosity.
Experimental
Materials
4-Hydroxyacetophenone and 4-chloronitrobenzene (Shanghai Darui Fine Chemicals Co., Ltd., Shanghai, China), 4-diethylaminobenzaldehyde (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), Palladium 10% on carbon (wetted with ca. 55% water) (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), are used without any purification. Commerical pyromellitic dianhydride (PMDA) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), biphenyl tetracarboxylic dianhydride (BPDA) and oxydiphtahalic anhydride (OPDA) (Changzhou linchuan Chemical Co., Ltd., Jiangsu, China), benzophenone tetracarboxylic dianhydiide (BTDA) and 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) were recrystallized form acetic anhydride and then dried in vcauumas at 150 °C overnight prior to use. N-methyl-2-pyrrolidone (NMP) and N,N-dimethylformamide (DMF) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China) were purified by vacuum distillation over calcium hydride prior to use. All of the other reagents, including Tetrahydrofuran (THF) N,N-dimethylacetamide (DMAc), dichloromethane (CH2Cl2), and chloroform (CHCl3), were used without further purification.
Instrumental
Fourier transform infrared spectroscopy (FT-IR) was measured with a Thermo Nexus 470 FT-IR infrared spectrometer. Nuclear magnetic resonance (NMR) spectra were given by 500 MHz Bruker nuclear magnetic resonance instruments, with reference to internal standard TMS, with CDCl3 or DMSO-d6 as the solvent. Mass Spectrometry (MS) was tested on the ACQUITYTM UPLC & Q-TOF MS Premier mass spectrometer at Waters, USA. Differential scanning calorimetry (DSC) was run on NETZSCH 204, instrument in nitrogen at a heating rate of 10 °C/min. Thermogravimetric analysis (TGA) was get on a TGA Q500 (US TA) under the protection of 10 °C/min temperature in the nitrogen atmosphere. Determination of solubility: polymer with a weight of 10 mg was dissolved in 1 mL of the organic solvent to observe the dissolution of the polymer at room temperature or on heating conditions. The hydrophobicity of the polymer was characterized by the contact angle, and the value was measured using a contact angle analyzer (JY-PHb). Inherent viscosities were tested by Ubbelohde viscometer, and measured at a concentration of 0.5 g/dL in NMP at 25 °C. Wide angle X-ray diffraction (WAXD): using the Dutch Panako X’Pert PRO type X-ray diffractometer, ray source Cu/K-α, scanning angle of 5 ~ 40°.
Monomer synthesis
Synthesis of 4-(4-nitrophenoxy)acetophenone (NPAP)
4-Hydroxyacetophenone (20.00 g, 0.14 mol), 4-chloronitrobenzene (23.14 g, 0.14 mol) and anhydrous potassium carbonate (42.64 g, 0.31 mol) were dissolved in 150 mL of purified DMF in a 250 mL three-necked round-bottom flask fitted with a magnetic stirring bar, condenser and thermometer. After stirring 30 min at room temperature, the mixture was heated to 145 °C and keeps for 6 h. After the reaction was completed. The resulting suspension was cooled to about 60 °C. The above mixed liquid was poured into ice water to get pale yellow emulsion, and a small amount of NaCl was added to obtain yellow precipitates. The crude product was obtained after filtrating and washing with mother liquor, and then dried in vacuum environment keep for 12 h at 80 °C. After that the resulting solid was recrystallized from a solvent of ethanol/ethyl acetate (1:1, v/v) to give pure white solid 4-(4-nitrophenoxy)acetophenone (NPAP). Yield: 65%. m.p. 83 °C. 1H NMR (DMSO, 500 MHz) δ: 8.25 (d, 2H,), δ: 8.04 (d, 2H J = 8.4 Hz), δ: 7.22 (t, 4H, J = 9.8 Hz), δ: 2.57 (s, 3H, -CH3); 13C NMR (DMSO, 500 MHz) δ: 197.32, 162.14, 159.27, 143.69, 134.19, 131.65, 126.97, 120.31, 119.38, 27.39. FT-IR (KBr, cm−1): 3109 cm−1, 3073 cm−1 (-CH3), 1681 cm−1 (C=O), 1507, 1347 cm−1(-NO2), 1168 cm−1 (C-O-C).
Synthesis of 4-(4-diethylamino)phenyl-2,6-bis(4-(4-nitrophenoxy)phenyl)pyridine (EPNPP)
NPAP (29.02 g, 0.11 mol) and 4-diethylaminobenzaldehyde (10.00 g, 0.56 mol) were added into a 500 mL three-necked flask equipped with a magnetic force stirrer, reflux condenser, and then placed into ammonium acetate (52.18 g) and 130 mL glacial acetic acid. After stirring for 30 min at room temperature, and then heat to 125 °C for 8 h. The resulting suspension was cooled to about 60 °C after the reaction, and then subjected to a rotary evaporator to remove most of the acetic acid. The yellow precipitate was collected by filtering and overnight drying under vacuum at 120 °C. The crude product was recrystallized from DMF to obtain light-yellow solid EPNPP. Yield: 61%. 1H NMR (DMSO, 500 MHz) δ: 8.25 (d, 4H, J = 8.4 Hz), δ: 8.19 (d, 4H, J = 8.8 Hz), δ: 7.61 (m, 4H), δ: 7.26 (d, 4H, J = 8.8 Hz), δ: 7.22 (d, 4H, J = 8.8 Hz), δ: 6.66 (d, 2H, J = 8.0 Hz), δ: 3.39 (s, 4H, -CH2-), δ: 1.07 (t, 6H, -CH3); 13C NMR(DMSO, 500 MHz) δ: 187.79, 162.36, 158.68, 150.19, 146.05, 143.58, 135.86, 131.92, 131.54, 126.92, 121.85, 120.37, 119.22, 115.79, 111.78, 44.50, 13.13; FT-IR (KBr, cm−1): 2830 ~ 2960 cm−1 (diethyl), 1593 cm−1, 1437 cm−1 (pyridine), 1519 cm−1, 1340 cm−1 (-NO2), 1184 cm−1 (C-O-C).
Synthesis of 4-(4-diethylamino)phenyl-2,6-bis(4-(4-aminophenoxy)phenyl) pyridine (EPAPP)
In a 500 mL three-necked flask equipped with a dropping funnel and a reflux condenser, EPNPP (24.32 g, 0.37 mol), palladium on activated carbon (Pd/C, 10%) and 300 mL anhydrous ethanol were added, and then heated to 85 °C with stirring. The hydrazine monohydrate (90 mL) was drop-wise into reaction bottle and keep for 1 h. After the addition of hydrazine monohydrate was finished, the mixture was refluxed for an additional 8 h. The above mixture was added to a large amount of hot ethanol, and then the hot solution filtered to remove Pd/C to give a clear pale yellow solution. The resulting solution was removed with a rotary evaporator and then dried in a vacuum oven overnight to give white solid. The crude product was purified by the method of silica gel column chromatography in the mixed solvent of ethyl acetate/hexane (10:13, V:V) to give white solid power EPAPP. Yield: 63%. 1H NMR (DMSO, 500 MHz) δ: 8.19 (d, 4H, J = 8.0 Hz), δ: 7.92 (S, 2H), δ: 7.80 (d, 2H, J = 8.0 Hz), δ: 6.95 (d, 4H, J = 8.0 Hz), δ: 6.81 (d, 4H, J = 8.4 Hz), δ: 6.74 (d, 2H, J = 8.8 Hz), δ: 6.60 (d, 4H, J = 8.0 Hz), δ: 5.04 (s, 4H, -NH2), δ: 3.36 (m, 4H, -CH2-), δ: 1.08 (tri, 6H, -CH3, J = 6.2 Hz); 13C NMR (DMSO, 500 MHz) δ: 160.50, 156.37, 149.91, 148.84, 146.33, 145.92, 133.58, 129.01, 128.78, 124.06, 121.73, 117.01, 115.57, 114.34, 112.15, 44.39, 13.14; FT-IR (KBr, cm−1): 3381 ~ 3445 cm−1 (-NH2), 2845 ~ 2965 cm−1 (diethyl), 1596 cm−1, 1428 cm−1 (pyridine), 1168 cm−1 (C-O-C); MS (m/z): 593.29 ([M + H]+).
Polymer synthesis and film preparation
PI-2 was given as an example, and the experimental procedure was described as follows: A mixture of EPAPP (0.6176 g, 1.042 mmol, BPDA (0.3066 g, 1.042 mmol), and NMP (5 mL) were added into a 25 mL round-bottom flask equipped with stirred under nitrogen for 1 h, and then ice bath for 4 h. After the reaction was further stirred at room temperature and kept for 24 h to obtain a viscous PAA poly(amic acid) solution. The PAA precursor was then transformed into polyimide by the thermal imidization process at 80 °C 12 h, 120 °C, 150 °C, 180 °C, 200 °C, 250 °C, 300 for 1 h. After cooling, the glass sheet carrying the polymer was removed from the muffle furnace and placed into a petri dish with deionized water to obtain a polyimide films, and then dried overnight to obtain PI-2 (EPAPP and BPDA). Simultaneously, PI-1 (EPAPP and PMDA), PI-3 (EPAPP and ODPA), PI-4 (EPAPP and BTDA), PI-5 (EPAPP and 6FDA) were prepared according to the similar method as mentioned above.
Results and discussion
Monomer synthesis
The diamine monomer EPAPP was derived from 4-hydroxyacetophenone, 4-Chloronitrobenzene and 4-diethylaminobenzaldehyde through a three-step synthetic as shown in Scheme 1. The structure of NPAP, EPNPP and EPAPP was confirmed by FT-IR, NMR. In addition, EPAPP was also characterized by MS. The FT-IR spectrum of NPAP was shown in Fig. S1. The value of 3109 cm−1 and 3073 cm−1 were the characteristic absorption peak of methyl (-CH3). The characteristic absorption peaks at 1681 cm−1 belong to (C=O). The ether bond C-O-C absorption peak at 1168 cm−1, the value of 1347 cm−1 was the characteristic absorption peak of nitro group. The 1H-NMR and 13C-NMR spectrum of presoma NPAP were given in Fig. S4. As shown at 1H-NMR, the precursor NPAP five kinds of proton hydrogen, the value of 2.57 ppm attributable to methyl groups. However, the proton H2 H3 on the benzene ring was similar to the chemical environment, so it was coupled to the 7.22 ppm. However, the chemical shifts of H5 and H4 were at 8.25 ppm and 8.04 ppm, respectively. The carbon spectrum of NPAP shows there were a total of 10 different peaks, 197.32, 162.14, 159.27, 143.69, 134.19, 131.65, 126.97, 120.31, 119.38, 27.39, respectively. These data show that the obtained results are consistent with the molecular design results.
The infrared spectrum of EPNPP was shown as Fig. S2, the strong absorption at 2830 ~ 2960 cm−1 belonged to diethyl, the value of 1593 cm−1, 1437 cm−1 were pyridine absorption peak, the characteristic absorption peak of nitro group were in 1519 cm−1, 1340 cm−1, and the characteristics of the ether bond C-O-C absorption peak at 1184 cm−1. As shown Fig. S5, the nitro compounds EPNPP have nine different proton hydrogens. Because of the value of H2 was the proton hydrogen on the alkyl which similar to the chemical shift of water. Thus, they are coupled together. H3-H9 attributable to the benzene ring and the chemical shifts were 6.66, 7.22, 7.26, 7.61, 8.19 and 8.25 ppm, respectively. Proton H8 and H9 formation of a proton peak with double, due to the influence of the adjacent hydrogen atoms. The 13C-NMR spectrum shown that compound EPNPP have 17 carbon atoms, 187.79, 162.36, 158.68, 150.19, 146.05, 143.58, 135.86, 131.92, 131.54, 126.92, 121.85, 120.37, 119.22, 115.79, 111.78, 44.50, 13.13.
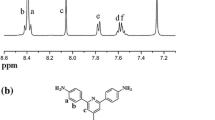
However, in the infrared spectrum of the two amine monomer EPAPP, the characteristic absorption peak of the nitro group disappeared, a characteristic strong absorption band for the amino group present to at 3445 ~ 3381 cm−1, and strong absorption at the range of 2965 ~ 2845 cm−1 belonged to diethyl, the absorption peak at 1596 cm−1, and 1428 cm−1 was pyridine characteristic absorption peak, and the characteristics of the ether bond C-O-C absorption peak at 1168 cm−1. The 1H-NMR and 13C-NMR spectrum of EPAPP were given in Fig. 1. In the 1H-NMR spectroscopy of Fig. 1, the value of 1.08 and 6.65 ppm were the proton on the alkyl because of alkyl proton chemical shift of H2 similar to the chemical shift of water, so they are coupled together, and the benzene ring of protons H4-H10 chemical shift were 6.60, 6.74, 6.81, 6.95, 7.80, 7.92, 8.19 ppm, the aromatic rings of the -NH2 protons at 5.38 ppm. The 13C-NMR spectrum shown that diamine monomer EPAPP have 17 carbon atoms, 160.50, 156.37, 149.91, 148.84, 146.33, 145.92, 133.58, 129.01, 128.78, 124.06, 121.73, 117.01, 115.57, 114.34, 11,215, 44.39, 13.14 ppm, respectively. Therefore, the FT-IR, NMR and MS determination of diamine monomer structure that the result is consistent with the designed structure.
Synthesis of polyimides
A sort of polyimides has been prepared via two-step solution polycondensation method as showed in Scheme 2. First, EPAPP and dianhydrides were reacted at room temperature for 24 h to obtain poly(amic acid) (PAA) solution in nitrogen environment, and then the inherent viscosity of obtained PAA was measured by an Ubbelohde viscometer and the value in the range of 0.33–0.97 g/dL that indicates the polymer with high molecular weight. After thermal imidization, the PPA has been fully converted to polyimides and the representative FT-IR spectrum of the polymers was given in Fig. 2. The strong absorption at 3381–3415 cm−1 was disappeared corresponding to amino group that indicated complete thermal imidization. The characteristic absorption peak at 3000~3119 cm−1 assigned to diethyl. The absorption peak at 1783 cm−1 and 1731 cm−1 were imide C=O symmetric stretching. The value of 1379 cm−1 belonged to imide C-N stretching vibration peak. Imide ring bending vibration absorption peak at 723 cm−1 which was indicated the formation of imide ring.
Thermal properties
Thermal stability of polymers was characterized by DSC and TGA which curves as shown in Figs. 3 and 4, and the results were summarized in Table 1. The glass transition temperature (Tg) of polyimides was evaluated from Fig. 3. Generally, Tg was contacted with the rigidity and conformation of polymer backbone. The Tg of obtained polymers was located in 254 ~ 305 °C. The possible reason is that the rigidity of the polymer was greatly enhanced by introducing pyridine in diamine monomer and a large number of benzene rings in dianhydride into the polymer backbone. On the other hand, the introduction of large side groups and flexible groups into the polyimides main chain would decrease the packing denisity which may lower the melting temperature and improve the solubility. Thus, the resulting polymers have good processability. As shown in Fig. 4, the polyimides exhibited high thermal stability in nitrogen atmospheres. The values of thermal onset decomposition temperature (Td) of polyimides were in the range of 499 ~ 521 °C. It is noticeable PI-1 derived from PMDA was shown the highest glass transition temperature. The reason is that the polyimides skeleton introduces a large amount of benzene ring and aromatic heterocyclic pyridine, which leads to the rigidity of the molecular chain segment and makes the glass transition temperature increase. However, PI-3 derived from ODPA showed the lowest Td owing to the presence of flexible ether linkage moieties in the polymer. In addition, the temperature of 5% and 10% weight loss were in the range of 493 ~ 525 °C and 544 ~ 567 °C in nitrogen atmosphere, respectively. Moreover, the char yield at 800 °C of the resulting polymers was all above 64%. Thereof, these characteristics indicate all the polyimides possess excellent thermal stability and good processibility which should be due to heterocyclic pyridine provides a rigid structure, whereas large side groups and flexible groups of ether linkage increase the molecular chain space and reduce the force between the molecular chains. Thus, that will be well balancing the thermal stability and processability of polymers.
Polymer solubility
The solubility of polyimides was tested in various organic solvent with 10 mg polymer in 1 mL solvent, and the results were displayed in Table 2. As is known to all, the traditional polyimide has poor solubility in most organic solvents because of their stiff chain characteristic and strong interchain interactions, which limited the application of polyimides in certain areas. In this work, in order to improve the solubility of polyimides, various structural modifications have been employed and verified. Obviously, the resulting polyimides presented good solubility in various solvents, such as DMF, DMSO, NMP, CHCl3, THF and CH2Cl2 under heating conditions owe to introduction flexible ether bond and large suspension structure diethylaminophenyl into the polymer backbone increasing the polymers solubility. It was obvious that, PI-3 received from EPAPP and ODPA and PI-5 obtained from EPAPP and 6FDA which exhibited the best solubility. They can dissolve polar solvents at room temperature, for instance DMF, DMSO and NMP. The solubility of PI-3 in that introduction of flexible chain ether bond (C-O-C) on the main chain of polyimides, increasing the flexibility and free volume of the PI segment, making the solvent easier to enter the macromolecular chain. Nevertheless, PI-5 as a result of -C(CF3)2- was introduced into the polymers main chain enhance the free volume, leading to less efficient chain packing and increase the solubility of the polymer. Furthermore, PI-4 was obtained by EPAPP and BTDA which have good solubility in polar solvents under the heating condition. However, PI-1 gained from EPAPP and PMDA and PI-2 obtained from EPAPP and BPDA demonstrate relatively poor solubility of the polymers, which can dissolved in NMP under heating conditions yet they were partially dissolved in DMSO and DMF on heating, the reason is that most of benzene ring and pyridine in the molecular chain greatly improved the density of the polymer.
Hydrophobic properties
As shown in Fig. 5, the hydrophobic properties of polyimide films were investigated, and the values were outlined in Table 1. In generally, the hydrophobic property of the polymer is evaluated by measuring its contact angel for water. Usually, the higher contact angle polymer has, and the better hydrophobic property it will take on. [33] The contact angles of these polyimide films were in the range of 81.9 ~ 91.9°, which higher than that of commercial Kapton (81.6°). [38] These results indicated that polymers presented excellent hydrophobicity due to the introduction of hydrophobic units, such as pyridine and bulky pendent groups in the polymer chains. Among these polymers, PI-5 derived from 6FDA showed the best hydrophobicity (θw: 91.9°). This is mainly due to the incorporation of fluoridated -C(CF3)2- groups in the main chain, which reduced greatly the surface energy of polymer owing to the unique characteristic of the fluorine atom.
X-ray diffraction
All of these polyimide films were examined for crystallinity by using WAXD analysis with graphite monochromatized Cu Kα radiation and with 2θ ranging from 5° to 40°. As shown in Fig. 6, the representative X-ray diffractograms indicated that all the polymers were amorphous in nature as broad peaks. It is obvious that the formation of amorphous structure was great extent due to the introduction of flexible ether linkage moieties, bulky pendent groups, and non-coplanar structures in the polymer backbones, which increased the space of molecular chain and reduced the intermolecular and intramolecular interactions, and then resulting in loose-packed polymer molecular and the formation of amorphous structures. In addition, no melting peak in Fig. 3 and the dissolution behaviors in organic solvents in Table 2, which was also in agreement with the analysis results of WAXD.
Conclusions
In this study, a series of polyimides containing rigid pyridine ring units, flexible ether linkage moieties, and bulky pendent groups were successfully designed and synthesized via two-step solution polymerization. All polyimides presented high thermal stability, good solubility, and prominent hydrophobic properties. In addition, all of the polymers have potential applications in the areas of microelectronic, electronic packaging, flexible printed circuit boards, and so on.
References
Liaw DJ, Wang KL, Huang YC, Lee KR, Lai JY, Ha CS (2012) Advanced polyimide materials: Syntheses, physical properties and applications. Prog Polym Sci 37:907–974
Ding MX (2007) Isomeric polyimides. Prog Polym Sci 32:623–668
Dhara MG, Banerjee S (2010) Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog Polym Sci 35:1022–1077
Yi L, Huang W, Yan DY (2017) Polyimides with side groups: Synthesis and effects of side groups on their properties. J Polym Sci Part A Polym Chem 55:533–559
Kurosawa T, Higashihara T, Ueda M (2013) Polyimide memory: a pithy guideline for future applications. Polym Chem 4:16–30
Xing Y, Wang D, Gao H, Jiang ZH (2011) Synthesis and properties of novel polyimide optical materials with different haloid pendant. J Appl Polym Sci 122:738–747
Yi L, Li CY, Huang W, Yan DY (2014) Soluble aromatic polyimides with high glass transition temperature from benzidine containing tert-butyl groups. J Polym Res 21:572
Li Y, Fu LW, Chen YJ, Tian HF, Xiang AM, Rajulu AV (2017) Improved thermal stability and flame resistance of flexible polyimide foams by vermiculite reinforcement. J Appl Polym Sci 134:44828
Liu CJ, Mei M, Pei XL, Huang XH, Wei C (2015) Aromatic polyimides with tertbutyl-substituted and pendent naphthalene units: synthesis and soluble, transparent properties. Chinese J Polym Sci 33:1074–1085
Zhang SJ, Bu QQ, Li YF, Gong CL, Xu XY, Li H (2011) High organosolubility and optical transparency of novel polyimides derived from 2′,7′-bis(4-amino-2-trifluoromethylphenoxy)-spiro (fluorene-9,9′-xanthene). Mater Chem Phys 128:392–399
Huang XH, Mei M, Liu CJ, Pei XL, Wei C (2015) Synthesis and characterization of novel highly soluble and optical transparent polyimides containing tert-butyl and morpholinyl moieties. J Polym Res 22:169
Zhao J, Peng L, Zhu YL, Song YJ, Wang LJ, Shen YZ (2016) Synthesis and memory characteristics of novel soluble polyimides based on asymmetrical diamines containing carbazole. Polymer 91:118–127
Javadi A, Shockravi A, Shourkaei FA, Koohgard M, Malek A (2018) Highly refractive thiazole-containing polyimides: a structural property comparison. J Polym Res 25:99
Hsiao SH, Liao YC (2018) Synthesis and properties of novel organosoluble and light-colored poly(ester-amide)s and poly(ester-imide)s with triptycene moiety. J Polym Res 25(2):52
Zhang XM, Song YZ, Liu JG, Yang SY (2016) Synthesis and properties of cost-effective light-color and highly transparent polyimide films from fluorine-containing tetralin dianhydride and aromatic diamines. J Photopolym Sci Tec 29:31–38
Wozniak AI, Yegorov AS, Ivanov VS, Igumnov SM, Tcarkova KV (2015) Recent progress in synthesis of fluorine containing monomers for polyimides. J Fluor Chem 180:45–54
Wang CY, Zhao HP, Li G, Jiang JM (2011) Novel fluorinated polyimides derived from an unsymmetrical diamine containing trifluoromethyl and methyl pendant groups. Polym Adv Technol 22:1816–1823
Tundidor-Camba A, Terraza CA, Tagle LH, Coll D, Ortiz P, Pérez G, Jessop IA (2017) Aromatic polyimides containing cyclopropylamide fragment as pendant group. A study of the balance between solubility and structural rigidity. Macromol Res 25:276–281
Liu CJ, Pei XL, Mei M, Chou GQ, Huang XH, Wei C (2016) Synthesis and characterization of organosoluble, transparent, and hydrophobic fluorinated polyimides derived from 3,3′-diisopropyl-4,4′-diaminodiphenyl-4′′-trifluoromethyltoluene. High Perform Polym 28:1114–1123
Wu F, Zhou X, Yu X (2017) Synthesis and characterization of novel star-branched polyimides derived from 2,2-bis[4-(2,4-diaminophenoxy)phenyl]hexafluoropropane. RSC Adv 7:35786–35794
Yagci H, Ostrowski C, Mathias L (1999) Synthesis and characterization of novel aromatic polyimides from 4,4-bis(p-aminophenoxymethyl)- 1-cyclohexene. J Polym Sci Part A Polym Chem 37:1189–1197
Li CY, Yi L, Xu ST, Wu XM, Huang W, Yan DY (2017) Synthesis and characterization of polyimides from 4,4′-(3-(tert-butyl)-4-aminophenoxy)diphenyl ether. J Polym Res 24(1):7
Tang HL, Huang B, Xie XJ, Yan T, Cai MZ (2018) Synthesis and properties of novel soluble fluorinated aromatic polyamides containing 4-benzoyl-2,3,5,6-tetrafluorophenoxy pendant groups. J Polym Res 25(2):51
Wang CY, Cao SJ, Chen WT, Xu C, Zhao XY, Li J, Ren Q (2017) Synthesis and properties of fluorinated polyimides with multi-bulky pendant groups. RSC Adv 7:26420–26427
Liu S, Zhang Y, Wang X, Tan H, Song N, Guan S (2015) Synthesis and properties of hyperbranched polyimides derived from tetra-amine and long-chain aromatic dianhydrides. RSC Adv 5:107793–107803
An HY, Zhan MS, Wang K (2009) Synthesis and characterization of soluble poly(ether imide)s containing fluorenyl cardo groups. J Appl Polym Sci 114:3987–3993
Wang CY, Zhao XY, Li G (2012) Synthesis and properties of new fluorinated polyimides derived from an unsymmetrical and noncoplanar diamine. Chinese J Chem 30:2466–2472
Huang XH, Huang W, Liu JY, Meng LL, Yan DY (2012) Synthesis of highly soluble and transparent polyimides. Polym Int 61:1503–1509
Liu CJ, Pei XL, Huang XH, Wei C, Sun XY (2015) Novel non-coplanar and tertbutyl-substituted polyimides: Solubility, optical, thermal and dielectric properties. Chinese J Chem 33:277–284
Wang CY, Zhao XY, Li G (2012) New soluble polyimides with high optical transparency and light color containing pendant trifluoromethyl and methyl groups. Chinese J Chem 30:1555–1560
Liaw DJ, Wang KL, Chang FC, Lee KR, Lai JY (2007) Novel poly(pyridine imide) with pendent naphthalene groups: Synthesis and thermal, optical, electrochemical, electrochromic, and protonation characterization. J Polym Sci Part A Polym Chem 45:2367–2374
Mehdipour-Ataei S, Bahri-Laleh N (2008) Synthesis and properties of polyimides and copolyimides containing pyridine units: A review. Iran Polym J 17:95–124
Huang XH, Chen BC, Mei M, Li H, Liu CJ, Wei C (2017) Synthesis and characterization of organosoluble, thermal stable and hydrophobic polyimides derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine. Polymers 9:484–496
Dong W, Guan Y, Shang D (2016) Novel soluble polyimides containing pyridine and fluorinated units: preparation, characterization, and optical and dielectric properties. RSC Adv 6:21662–21671
Guan Y, Dong W, Wang C, Shang D (2017) Highly refractive polyimides containing pyridine and sulfur units: synthesis and thermal, mechanical, solubility and optical properties. Polym Int 66:1044–1054
Wang KL, Liou WT, Liaw DJ, Huang ST (2008) High glass transition and thermal stability of new pyridine-containing polyimides: Effect of protonation on fluorescence. Polymer 49:1538–1546
Xu X, Chen BC, Li H, Huang XH, Wei C (2017) High glass transition of fluorinated polyimides derived from 4-(3,4-Difluorophenyl)-2,6-bis(4-aminophenyl)pyridine. Chinese J Chem 35:341–346
Xiong L, Wang X, Qi H, Liu F (2013) Synthesis of a new siloxane-containing alicyclic dianhydride and the derived polyimides with improved solubility and hydrophobicity. J Appl Polym Sci 127:1493–1501
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51563005; 51605109), and supported by Opening Project of Guangxi Key Laboratory of Calcium Carbonate Resources Comprehensive Utilization (No. HZXYKFKT201806).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 615 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Li, H., Mei, M. et al. Thermal, soluble, and hydrophobic properties of polyimides derived from 4-(4-diethylamino)phenyl-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine. J Polym Res 26, 100 (2019). https://doi.org/10.1007/s10965-019-1759-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1759-8