Abstract
A series of novel organo-soluble and optical transparent polyimides were synthesized via conventional one-step polycondensation from a novel diamine, 3,3′-diterbutyl-4,4′-diaminodiphenyl-4″-morpholinophenylmethane (TAMPM) and various commercial aromatic dianhydrides. The structures of monomer and polymers were confirmed by FT-IR and NMR. The solubility, thermal stability, optical transparency, dielectric and hydrophobic properties of polyimides were investigated. The results showed polymers presented excellent solubility in common organic solvents, such as N,N-dimethylformamide (DMF), N,N-dimethyl acetamide (DMAc), dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone (NMP), chloroform (CHCl3), tetrahydrofuran (THF) and dichloromethane (CH2Cl2). The polymers exhibited prominent thermal stability and high optical transparency with the glass transition temperature (T g) in the range of 311–355 °C, and the cutoff wavelengths in the range of 287–344 nm. Furthermore, all of the polymers also presented low dielectric constants in the range of 2.85–3.16 at 1 MHz, and outstanding hydrophobic properties with the contact angle in the rang of 85.1–94.2 o.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that polyimides are a class of representative high-performance polymers because of its excellent integrative properties, such as high thermal stability, excellent mechanical properties, good dimensional stability, remarkable insulation performance and prominent hydrophobic properties, and so on. At present, they are extensively used in various fields of microelectronics, optoelectronics, aerospace, automobile, gas separation, polymer electrolyte fuel cells, polymer memories, etc. [1–5]. However, polyimides often present poor solubility in common solvents and have high melting temperature. It is due to aromatic polyimide have rigid chains and strong interchain interaction. Strong interactions of polymer derive from intra- and interchain charge transfer complex (CTC) formation and electronic polarization, which lead to deep color of polyimides.
In order to overcome these drawbacks of polyimides as mentioned above, there are many efforts to develop solubility and processability of polyimides by modifying the molecular structure of the chain backbones. So far, the major structural modification method has been involved by the introduction of flexible linkages, such as -O-, −S-, −CH2- and -SO2- [6–8], bulky substituents [9–12], bulky pendant group [13–15], non-coplanar [16, 17], unsymmetrical structure, such as ortho- or meta-linked unit [18–20], and acyclic moieties into the polymer backbones [21–23]. The solubility and optical transparency of polyimides could be improved by introduction of flexible ether and acyclic linkages in the polymer backbone. Unfortunately, it will sacrifice some of their advantageous properties by the modification method, such as thermal stability and mechanical property. It must be emphasized that much efforts have been devoted to synthesizing fluorinated polyimide by incorporation of fluorinated substituent, especially the trifluoromethyl group [24–28]. The optical transparency and solubility of fluorinated polyimide has been significantly improved, which resulted from low polarizability of the bond and the increase in free volume. Its application, however, is subject to some limit due to high-cost of the fluoride monomer in many fields. Therefore, it is well recognized the incorporation of bulky substituents, non-coplanar and unsymmetrical structures into main chain may have improved the solubility and processability of polyimide, and not sacrificing their thermal stability and mechanical properties. Such structure type of polyimides can decrease the rigidity of the polymer backbone and loosen close packing of the chains, which reduce the interchain interactions, and then improve the solubility and processability of polyimides.
In comparison with the previous research work, we expect to provide a new approach to design and synthesize a series of novel organo-soluble and transparent polyimides without sacrificing their thermal stability and mechanical properties. In this paper, a series of novel polyimides are prepared by incorporation of the larger bulky groups (tert-butyl) and non-planar steric structures (morpholinyl moieties) in the polymer chain, and utilizing the synergism of tert-butyl and morpholinyl moieties to obtain a series of organo-soluble and transparent polyimides. A novel diamine monomer 3,3′-diterbutyl-4,4′-diaminodiphenyl-4″-morpholinophenylmethane (TAMPM) was designed and synthesized firstly, and then used to obtain a series of polyimides bearing tert-butyl and morpholinyl moieties by one-step polycondensation with various aromatic dianhydrides. All of obtained polymers were characterized and their physical properties, such as solubility behavior, thermal stability, optical transparency, dielectric constant and hydrophobic properties were summarized.
Experimental
Materials
2-tert-butylaniline (Wuxi TPW Pharmaceutical Technology Co. Ltd.) and 4-morpholinobenzaldenhyde (Accela ChemBio Co. Ltd.), Pyromellitic dianhydride (PMDA) (Shanghai Guoyao Chemical Co. Ltd.), 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA), 4,4′-oxydiphthalic anhydride (ODPA) (Changzhou Linchuan Chemical Co. Ltd.), 3,3,4,4′-benzophenone tetracarboxylic dianhydride (BTDA) and (4,4-hexafluoroisopropylidene)-diphthalic anhydride (6FDA) (Tokyo Chemical Industry Co. Ltd.) were recrystallized from acetic anhydride and then dried in vacuums at 120 °C overnight before use. N, N-dimethyl formamide (DMF) and m-Cresol (Shanghai Guoyao Chemical Co. Ltd.) were purified by vacuum distillation over calcium hydride prior to use. All the other solvents were analytical-grade and used without further purification.
Monomer synthesis
3,3′-diterbutyl-4,4′-diaminodiphenyl-4″-morpholinophenylmethane (TAMPM)
In a 100 mL three-necked round-bottomed flask equipped with a condenser, dropping funnel and nitrogen inlet, 4-morpholinobenzaldenhyde (12.0 g, 0.063 mol) and 2-tert-butylaniline (23.41 g, 0.16 mol) was added and heated 120 °C under nitrogen atmosphere. 2.5 mL hydrochloric acid (12 N) was dropped in the mixture of 4-morpholinobenzaldenhyde and 2-tert-butylaniline about 1 h, then the mixture was heated 150 °C about 10 h under nitrogen atmosphere. When the reaction was completed, the reaction mixture was cooled to 60 °C, and 10 % aqueous solution of NaOH (12.0 mL) was added to the mixture. After fully neutralized, the mixture was poured into separating funnel and extracted by 100 mL CH2Cl2, then washed by appropriate water three times until PH was about 7. The obtained mixture solution was dried over anhydrous MgSO4, filtered and CH2Cl2 evaporated to obtain brown powder, then the monomer compound was purified by column chromatography on silica gel [V(ethyl acetate) : V(hexane) = 2:5] to give white solid production (19.32 g, 65 % yield); m.p. 155.2 °C (by DSC at a scan rate of 5 °C/min)
1H NMR (400 MHz, DMSO) δ: 6.91 (d, 2H, J = 8.0 Hz), δ: 6.86 (s, 2H), δ: 6.81 (d, 2H, J = 8.8 Hz), δ: 6.59 (d, 2H, J = 7.6 Hz), δ: 6.52 (d, 2H, J = 8.0 Hz), δ: 5.04 (s, 1H), δ: 4.56 (s, 4H -NH2), δ: 3.68 (t, 4H, J = 4.8 Hz), δ: 3.00 (t, 4H J = 4.8 Hz), δ: 1.23 (s, 18H, −CH3); 13C NMR (400 MHz, DMSO) δ: 149.29, 144.09, 137.37, 133.20, 131.98, 129.70, 127.30, 126.99, 117.22, 115.23, 66.60, 54.99, 49.14, 34.41, 29.73. FT-IR (KBr, cm−1): 3500 ~ 3300 cm−1 (−NH2), 3000 ~ 2800 cm−1 (C-H), 1120 cm−1 (C-O-C); MS (m/z): 472.3 ([M + H]+).
Polymer synthesis
The general procedure for the preparation of the polyimides was given in Scheme 2. All polyimides were prepared by one-step procedure. The mixture of TAMPM (0.8873 g, 1.88 mmol) and BPDA (0.5535 g, 1.88 mmol), 15.0 mL m-Cresol and 5 drops of isoquinoline were added to in 50 mL three-necked round-bottomed flask equipped with a mechanical stirrer, nitrogen inlet and a reflux condenser. The mixture was stirred at room temperature for 1 h in nitrogen atmosphere, and then heated to 50 °C until the solid completely dissolved. Then the reaction was heated to 120 °C for 4 h, and for another 12 h at 200 °C. After the reaction was cooled, the viscosity mixture solution was slowly poured into 400 mL alcohol to obtain fibrous precipitate. The precipitate was filtered, and washed with alcohol several times, and then dried for 12 h by vacuum drying oven in 120 °C. At last the polymer PI-2 (TAMPM-BPDA) was obtained by re-precipitating at least twice. The PI-1 (TAMPM -PMDA), PI-3 (TAMPM -ODPA), PI-4 (TAMPM -BTDA) and PI-5 (TAMPM -6FDA) were also prepared according to a similar method as mentioned above.
PI-1
The PI-1 was prepared from diamine (TAMPM) and PMDA. The yield was 94 %. 1H NMR (400 MHz, DMSO) δ: 8.44 (d, 2H, ArH), δ: 7.42 (d, 2H, ArH), δ: 7.36 (s, 2H, ArH), δ: 7.16 (d, 2H, ArH), δ: 7.11 (d, 2H, ArH), δ: 6.95 (s, 2H, ArH), δ: 5.70 (s, H, CH), δ: 3.70 (s, 4H, CH2), δ: 3.07 (s, 4H, CH2), δ: 1.17 (s, 18H, CH3). FT-IR (KBr, cm−1): 3031 ~ 2872 (alkyl), 1779 and 1731 (C = O, imide, stretching vibration), 1374 (C-N), 732 (C = O, imide, bending vibration). Elemental analysis calculated for (C41H43N3O7)n: C, 71.39 %; N, 6.09 %. Found: C, 71.16 %; N, 6.12 %.
PI-2
The PI-2 was prepared from diamine (TAMPM) and BPDA. The yield was 92 %. 1H NMR (400 MHz, DMSO) δ: 8.44 (s, 2H, ArH), δ: 8.36 (d, 2H, ArH), δ: 8.09 (d, 2H, ArH), δ: 7.42 (d, 2H, ArH), δ: 7.28 (d, 2H, ArH), δ: 7.11 (d, 2H, ArH), δ: 7.00 (d, 2H, ArH), δ: 6.94 (d, 2H, ArH), δ: 5.68 (s, H, CH), δ: 3.70 (m, 4H, CH2), δ: 3.06 (m, 4H, CH2), δ: 1.17 (s, 18H, CH3). FT-IR (KBr, cm−1): 3031 ~ 2870 (alkyl), 1777 and 1727 (C = O, imide, stretching vibration), 1371 (C-N), 746 (C = O, imide, bending vibration). Elemental analysis calculated for (C47H43N3O5)n: C, 77.34 %; N, 5.76 %. Found: C, 78.29 %; N, 5.68 %.
PI-3
The PI-3 was prepared from diamine (TAMPM) and ODPA. The yield was 90 %. 1H NMR (400 MHz, DMSO) δ: 8.04 (d, 2H, ArH), δ: 7.66 (d, 2H, ArH), δ: 7.63 (d, 2H), δ: 7.39 (d, 2H, ArH), δ: 7.22 (s, 2H, ArH), δ: 7.08 (m, 4H, ArH), δ: 6.92 (d, 2H, ArH), δ: 5.65 (s, H, CH), δ: 3.69 (m, 4H, CH2), δ: 3.05 (m, 4H, CH2), δ: 1.14 (s, 18H, CH3). FT-IR (KBr, cm−1): 3031 ~ 2871 (alkyl), 1779 and 1726 (C = O, imide, stretching vibration), 1374 (C-N), 750 (C = O, imide, bending vibration). Elemental analysis calculated for (C47H43N3O6)n: C,75.68 %; N, 5.63 %. Found: C, 76.43 %; N, 5.58 %.
PI-4
The PI-4 was prepared from diamine (TAMPM) and BTDA. The yield was 95 %. 1H NMR (400 MHz, DMSO) δ: 8.25 (d, 2H, ArH), δ: 8.20 (d, 2H, ArH), δ: 7.93 (d, 2H, ArH), δ: 7.63 (d, 2H, ArH), δ: 7.40 (d, 2H, ArH), δ: 7.29 (d, 2H, ArH), δ: 7.08 (d, 2H, ArH), δ: 6.92 (d, 2H, ArH), δ: 5.67 (s, H, CH), δ: 3.69 (m, 4H, CH2), δ: 3.05 (m, 4H, CH2) δ: 1.15 (s, 18H, CH3). FT-IR (KBr, cm−1): 3031 ~ 2870 (alkyl), 1779 and 1727 (C = O, imide, stretching vibration), 1374 (C-N), 727 (C = O, imide, bending vibration). Elemental analysis calculated for (C48H43N3O6)n: C, 76.07 %; N, 5.54 %. Found: C, 75.88 %; N, 5.61 %.
PI-5
The PI-5 was prepared from diamine (TAMPM) and 6FDA. The yield was 91 %. 1H NMR (400 MHz, DMSO) δ: 8.13 (d, 2H, ArH), δ: 7.93 (d, 2H, ArH), δ: 7.83 (d, 2H, ArH), δ: 7.37 (d, 2H, ArH), δ: 7.29 (d, 2H, ArH), δ: 7.07 (m, 4H, ArH), δ: 6.91 (d, 2H, ArH), δ: 5.64 (s, H, CH), δ: 3.68 (m, 4H, CH2), δ: 3.04 (m, 4H, CH2), δ: 1.13 (s, 18H, CH3). FT-IR (KBr, cm−1): 3032 ~ 2871 (alkyl), 1786 and 1728 (C = O, imide, stretching vibration), 1372 (C-N), 724 (C = O, imide, bending vibration). Elemental analysis calculated for (C50H43F6N3O5)n: C, 68.65 %; N, 4.78 %. Found: C, 69.46 %; N, 4.70 %.
Preparation of membranes
The transparent and flexible polyimide films were obtained by the method of solution casting. The polyimide was dissolved in DMF with a concentration of approach 2 wt.% or 10 wt.%. Then the solution of polymer was poured into dry-clean glass plate and solvent DMF was disappeared at 60 °C for 24 h. When the most of the DMF was evaporated, the polymer film was formed and then was heated at 180 °C in vacuum drying oven for 12 h to evaporate the residual DMF completely. Then the polyimide films was cooled to room temperature, moved to water, peeled off from the glass substrate, and removed water in a vacuum drying oven for 120 °C for 12 h.
Characterization
NMR spectrum was recorded using an Avance III 400 instrument with deuterated dimethylsulfoxide (DMSO-d 6) as solvent and tetramethylsilane (TMS) as an internal standard. Fourier transform infrared (FT-IR) spectrum was studied on a Thermo Nexus 470 FTIR spectrometer. UV-visible spectrum (UV–vis) was performed on a UV-3600 UV-visible spectrophotometer. Thermogravimetric analysis (TGA) was carried out on a TGA Q500 analyzer at a heating rate of 10 °C · min−1 in nitrogen. Differential scanning calorimetry (DSC) was evaluated on a DSC-204 phoenix thermal analyzer instrument at a heating rate of 20 °C · min−1 in a nitrogen atmosphere. Elemental analysis was investigated by a Perkin-Elmer model 2400 II instrument. Mass spectrometry was performed an Elementar Vario EL III/Isoprime. Gel permeation chromatography (GPC) was measured on a Waters 1515 analyzer relative to polystyrene standard using DMF as the eluent. Dielectric constant was determined by the parallel plate capacitor method using a dielectric analyzer (Agilent 4294A) on thin film. Gold electrode was vacuum deposited coating on both surfaces of film, and these films were tested in a dry chamber at different frequency. The contact angle on the film for water was tested by a JY-PHb contact angle analysis instrument in the room temperature.
Results and discussion
Monomer synthesis
As shown in Scheme 1, the aromatic diamine monomer, 3,3′-diterbuty-4,4′-diaminodiphenyl-4″-morpholinophenylmethane (TAMPM) was synthesized by the 2-tert-butylaniline with 4-morpholinobenzaldenhyde in the presence of catalyst hydrochloric acid. After reaction, the crude product was purified by silica gel chromatography, and then obtained pure diamine monomer TAMPM. The chemical structures of the diamine TAMPM were confirmed by FT-IR, NMR and MS spectrometry.
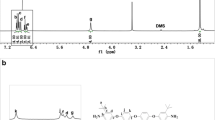
The FT-IR spectrum of diamine TAMPM showed the characteristic absorptions of the N-H stretching bands of the amino groups at 3500 ~ 3300 cm−1, the C-H stretching bands of the alkyl groups at 3000 ~ 2800 cm−1, and the characteristic absorption bands appeared at 1120 cm−1 corresponding to C-O-C stretching. The 1H NMR spectrum of diamine TAMPM was shown in Fig. 1a. The signal at δ 4.56 ppm was special amino groups. The double of aromatic protons appeared in range of δ 6.50–6.91 ppm. The characteristic resonance signals at δ 5.04 ppm, δ 3.68 ppm, δ 3.00 ppm and δ 1.23 ppm corresponded to alkyl -CH-(H5), −CH2- (H3), −CH2- (H2), and –C(CH3)3- (H1) groups. Obviously, the protons of H1, H2, H3, H4 and H5 resonated at higher field than other aromatic protons because of the electron-donating property of the amino and alkyl groups. Furthermore, the peaks at δ 2.48 ppm and at δ 3.33 ppm were assigned to DMSO and water in DMSO, respectively. The 13C NMR spectrum was shown in Fig. 1b, all the carbon atoms resonated in the region of δ 29.73-149.30 ppm, and all the spectrum spectrometry were in good agreement with the propose molecular structure. In addition, MS spectrometry analysis agreed well with the molecular structure. The melting point of monomer was measured by DSC, and the melting peak appeared at 155.2 °C.
Polyimide synthesis
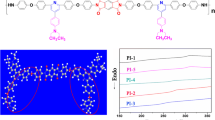
Generally, the methods of preparation of polyimides have one-step and two-step polymerization. As shown in Scheme 2, the polyimides were prepared from diamine monomer TAMPM and various aromatic dianhydrides (PMDA, BPDA, ODPA, BTDA and 6FDA) in the solvent of m-Cresol with the catalyst of isoquinoline under nitrogen protection by one-step procedure. After fully polymerization, the fibrous polyimides were obtained by dumping the polymer solution into an excess of alcohol and re-precipitating using DMF at least twice.
The molecular weight and polydispersity index (M w/M n) of the polyimides were characterized by GPC, and the results were summarized in the Table 1. The M w (weight average molecular weight) and M w/M n (polydispersity) were in the range of 10 × 104 ~ 38.6 × 104 g · mol−1 and 1.85 ~ 5.51, respectively. The results showed that polyimides have high molecular weight and low polydispersity index, which were used for obtaining flexible and tough films. In addition, the elemental analysis results of polyimides were also listed in Table 1, and the measurement of polymers were in complete agreement with the theoretically calculated values. The FT-IR spectrum of polyimides was also confirmed. As shown in Fig. 2, the characteristic absorptions of imide at 1779 cm−1 and 1728 cm−1 attributed to the typical of imide carbonyl asymmetrical and symmetrical stretches. The characteristic absorptions of C-N stretch were around 1374 cm−1, and the characteristic absorption at 1109 cm−1 and 732 cm−1 were attributed to imide ring deformation. Moreover, the spectra in the range of 2870 ~ 3030 cm−1 were confirmed corresponding to alkyl groups. At the same time, the disappearance of N-H characteristic absorption bands identified that diamine monomer was completely transformed into polyimide. The 1H NMR spectrum of polyimide was in complete agreement with the proposed polymer structure (Fig. 3).
Solubility
The solubility of polyimides was tested in various organic solvent, and the results were listed in Table 2. As is well known, the polyimide usually shows poor solubility in organic solvents due to their stiff chain characteristic and strong inter-chain interactions, which caused limitation on their applications in extensive fields. Comparing to these insoluble polyimide, the prepared polyimides presented excellent solubility in the high boiling point solvents such as DMF, DMAc, DMSO, NMP and m-Cresol at room temperature. Moreover, these obtained polyimides also exhibited outstanding solubility in the low boiling point solvents such as CHCl3, CH2Cl2, THF, acetone and ethyl acetate. Obviously, the excellent solubility of these polyimides could be partly due to the introduction of bulky tert-butyl groups, bulky pendant 4-morpholinophenyl groups, flexible linkages and non-coplanars in the polymer main, which loosen the packing density of polymer chain, decrease the intermolecule interactions, and increase the fractional free volume. The PI-3 based on ODPA exhibited good solubility behavior in common organic solvents due to the introduction of ether linkage which increased flexibility of polymer. Moreover, the PI-5 could be soluble in acetone, it is due to the introduction of trifuoromenthyl derived from the 6FDA in the polymer backbone. It must be underlined that the outstanding solubility of the polyimides in low boiling point solvent can reduce the processing temperature of the polymer film. The excellent solubility of the polyimides may have the potential application prospects in many high-tech fields, such as coating materials for advanced microelectronics manufacturing, flexible organic solar cells, flexible printed circuit board (FPCP), and flexible displays [23, 29–31].
Thermal properties
The thermal properties of polyimides were evaluated by TGA and DSC in nitrogen atmosphere, and the corresponding results were listed in Table 3. In the Fig. 4, the glass-transition temperatures (T g) of the polyimides were in the range from 311 °C to 355 °C. Generally, T g is related to the rigidity and conformation of polymer backbone. Moreover, the introduction of bulky tert-butyl group into the ortho-position of the imide nitrogen impeded the rotation of the two aromatic rings around the C-N bond, which improved the stiffness of backbone and glass transition temperature of polyimide [32, 33]. Furthermore, the incorporation of non-coplanar in the main chains also improved the rigidity of polymer. The PI-1 originated from PMDA presented the highest T g due to the characteristic structure of the rigidity of wholly aromatic dianhydride PMDA units. However, PI-3 derived from ODPA showed the lowest T g because of the presence of flexible ether linkages moieties in the polymer backbone.
The curves of TGA of polyimides were listed in Fig. 5. All the polyimides exhibited high thermal stability. The values of thermal onset decomposition temperature (T d) of polyimides were in the range of 456–468 °C in the Table 3. The 5 % and 10 % weight loss temperature were also listed in Table 3 which based on the original TGA curves. In the nitrogen atmospheres, the T 5% and T 10% weight loss of polyimides were in range of 425–473 °C and 472–494 °C, respectively. Furthermore, char yields of the polyimides at 800 °C was in range from 55 to 67 %. The polyimides showed outstanding thermal stability due to the rigid structure of imides and stiff morpholinophenyl moieties in polymer backbone. The PI-5 based on 6FDA showed comparatively higher thermal stability which can be attributed to strong C-F bond present in -C(CF3)2- units in the polymer chain.
Optical properties
The UV–vis spectra of the polyimide films were performed with the thickness of ca. 10–15 μm, and results were listed in Table 3. As shown in Fig. 6, the polyimide exhibited lower cutoff wavelength and higher optical transparency. The cutoff wavelength of polyimide films was in the range of 287–344 nm. The transparency of polymer films in the visible region was investigated by averaging the transmittances in the range from 400 to 700 nm. The results showed that the average transmittance of films were above 78 % in the wavelength range of 400–700 nm. According to the literature [34], the obtained polymer films presented better optical transparency than that of commercial Kapton film with similar thickness. Moreover, as shown in Fig. 7, compared with a photograph of the commercial Kapton film with the thickness of ca. 45 μm in visible light, it was obviously that the color of polyimide films is more shallow and transparency than that of Kapton film. The polyimide films presented outstanding optical properties due to the introduction of bulky tert-butyl group, bulky pendant 4-morpholinophenyl group, flexible linkage and non-coplanar moieties in the polymer backbone, which can loosen the density of polymer chain packing and decrease the intermolecular interaction, and then reduce the formation of intermolecular charge-transfer complex (CTC) between the alternating electron-donar (diamine) and electron-acceptor (dianhydride) moieties [35]. Obviously, PI-5 presented lowest cutoff wavelength and the highest optical transpatency which attributed to the flexible linkage of fluorinated bulky -C(CF3)2-, which might reduce the intermolecular cohesive force because of the lower polarizability of the C-F bond. Furthermore, PI-3 exhibited excellent optical transparency due to the presence of the flexible ether linkages in the polymer chain, which could weaken the formation of the inter- and intro-molecular CTC effectively. Unexpectedly, PI-2 originated from BPDA also showed lighter color in the visible light, it may be the result of the twist of biphenyl moieties in the polymer backbone, which could helpful to decrease the polymer conjugation effect and inhibit the formation of CTC.
Dielectric properties
It is well known that polymers have been widely used in dielectric material fields due to its lower dielectric constant, easy preparation and excellent properties. In particular, polyimides as dielectric materials have been attracted much more attention in microelectronic fields in recent years. It is attributed to its excellent comprehensive properties, such as heat resistance, ultraviolet radiation resistant, weathering resistance, high modulus and low dielectric constant as compared with other polymers.
The dielectric constants of polyimide films were determined by the parallel plate capacitor method using a dielectric analyzer (Agilent 4294A) on thin film, and all of the film samples were tested under the same conditions. The results were summarized in Table 4. The obtained polyimide films with a thickness of 50–60 μm were used in this study. The dielectric constants of all the polyimides were in the range of 2.97-3.33 at 1 kHz, 2.92–3.28 at 10 kHz and 2.85–3.16 at 1 MHz, respectively, which were lower than those of the commercial polyimide such as Kapton (3.48 at 1 MHz). The lower dielectric constants may be attributed to the introduction of the bulky side group (tert-butyl) and the pendant group (morpholinophenyl), which loosened polymer packing density and increased free volume. Furthermore, PI-5 originated from diamine TAMPM and dianhydride 6FDA exhibited lowest dielectric constant, which may be due to the result of the strong eletronegativity of the fluorine atoms, which resulted in very low polarizability of the C-F bonds and then reduced the dielectric constant.
Hydrophobic properties
The contact angle of polyimides PI 1–5 and Kapton for water (θw) were listed in Table 4. Figure 8 depicted the profiles of droplet on the film surfaces. It is well known that the higher contact angle polymer has, the hydrophobic property of the polymer will become better. Among all the polyimides, the contact angles of obtained polyimides (85.1–94.2°) were higher than that of commercial Kapton (81.6°) due to the incorporation of the hydrophobic groups, such as tert-butyl and morpholinophenyl moieties in the polymer main, and the PI-5 derived from 6FDA presented the best hydrophobic property. It is because of the introduction of 6FDA in the backbone, which reduced the surface energy of polymer, and then presented excellent hydrophobic property. The contact angle of PI-3 was 85.1°, and it was a little lower than that of other obtained polyimides. It may be due to the ether linkage of PI-3 originated from ODPA form hydrogen bond with water, and then lead to relatively poorly hydrophobic property.
Conclusions
A novel aromatic diamine TAMPM containing tert-butyl and morpholinyl units was synthesized by reasonable molecular design, and then a series of new polyimides were synthesized by polycondensation via the conventional one-step method. All of the polyimide presented excellent solubility, good thermal stability, high optical transparency, low dielectric constants and outstanding hydrophobic properties. These polymers could be considered as potential applications for high performance polymer materials, flexible solar cells, flexible displays, flexible printed circuit board, microelectronic devices, and so on.
Abbreviations
- TAMPM:
-
3,3′-diterbutyl-4,4′-diaminodiphenyl-4″-morpholinophenylmethane
- PMDA:
-
Pyromellitic dianhydride
- BPDA:
-
3,3′,4,4′-biphenyltetracarboxylic dianhydride
- ODPA:
-
4,4′-oxydiphthalic anhydride
- BTDA:
-
3,3,4,4′-benzophenone tetracarboxylic dianhydride
- 6FDA:
-
(4,4-hexafluoroisopropylidene)-diphthalic anhydride
References
Ding MX (2007) Prog Polym Sci 32:623–668
Jeon YW, Lee DH (2015) Environ Eng Sci 32:71–85
Liaw DJ, Wang KL, Huang YC, Lee KR, Lai JY, Ha CS (2012) Prog Polym Sci 37:907–974
Kurosawa T, Higashihara T, Ueda M (2013) Polym Chem 4:16–30
Ghosh A, Sen SK, Banerjee S, Voit B (2012) RSC Adv 2:5900–5926
Huang XH, Huang W, Zhou YF, Yan DY (2011) Chin J Polym Sci 29:506–512
Zhao JJ, Gong CL, Zhang SJ, Shao Y, Li YF (2010) Chin Chem Lett 21:277
Mehdipour-Ataei S, Sarrafi Y, Hatami M (2004) Eur Polym J 40:2009–2015
Yi L, Li CY, Huang W, Yan DY (2014) J Polym Res 21:572
Huang XH, Huang W, Liu JY, Meng LL, Yan DY (2012) Polym Int 61:1503–1509
Zhang SJ, Bu QQ, Li YF, Gong CL, Xu XY, Li H (2011) Mater Chem Phys 128:392–399
Zhang SJ, Li YF, Ma T, Zhao JJ, Xu XY, Yang FC, Xiang XY (2010) Polym Chem 1:485–493
Xia SL, Yi LF, Sun Z, Wang YH (2013) J Polym Res 20:219
Ghaemy M, Khajeh S (2011) Chin J Polym Sci 29:465–474
Li YQ, Chu YY, Fang RC, Ding SJ, Wang YL, Shen YZ, Zheng AM (2012) Polymer 53:229–240
Gong SM, Liu M, Xia SL, Wang YH (2014) J Polym Res 21:542
Liu CJ, Pei XL, Huang XH, Wei C, Sun XY (2015) Chin J Chem 33:277–284
Thiruvasagam P (2012) J Polym Res 19:9965
Huang YC, Wang KL, Lee WY, Liao YA, Liaw DJ, Lee KR, Lai JY (2015) J Polym Sci A Polym Chem 53:405–412
Liaw DJ, Wang KL, Chang FC, Lee KR, Lai JY (2007) J Polym Sci A Polym Chem 45:2367–2374
Barzic AI, Stoica I, Fifere N, Vlad CD, Hulubei C (2013) J Polym Res 20:130
Chen GF, Pei XL, Liu JT, Fang XZJ (2013) J Polym Res 20:159
Guo YZ, Shen DX, Ni HJ, Liu JG, Yang SY (2013) Prog Org Coat 76:768–777
Li H, Zhang SJ, Gong CL, Liang Y, Qi ZG, Li YF (2015) Polym Int 64:352–360
Tapaswi PK, Choi MC, Nagappan S, Ha CS (2015) J Polym Sci A Polym Chem 53:479–488
Gao YF, Zhou YM, He M, Wang HY, Cui YP, Zhang T (2014) Des Monomers Polym 17:590–600
Wang XM, Liu F, Lai JC, Fu ZQ, You XZ (2014) J Fluor Chem 164:27–37
Guan Y, Wang DM, Song GL, Dang GD, Chen CH, Zhou HW, Zhao XG (2014) Polymer 55:3634–3641
Wang JY, Liu C, Su GX, Jian XG (2012) High Perform Polym 24:356–365
Ishii J, Yokotsuka H, Saito T, Hasegawa M (2011) J Photopolym Sci Technol 24:287–291
Pakhuruddin MZ, Ibrahim K, Aziz AA (2013) J Optoelectron Adv Mat 7:377–380
Zhao XJ, Liu JG, Rui JM, Fan L, Yang SY (2007) J Appl Polym Sci 103:1442–1449
Qiu ZM, Wang JH, Zhang QY, Zhang SB, Ding MX, Gao LX (2006) Polymer 47:8444–8452
Matsumoto T, Kurosaki T (1997) Macromolecules 30:993–1000
Dine-Hart RA, Wright WW (1971) Makromol Chem 143:189–192
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51163003 and 21264005), the fund of Guangxi Natural Science Foundation (Nos. 2014GXNSFAA118040 and 2013GXNSFDA019008), Guangxi Ministry-Province Jointly-Constructed Cultivation Base for State Key Laboratory of Processing for Non-ferrous Metal and Featured Materials (13KF-3), and Guangxi Funds for Specially-appointed Expert.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, X., Mei, M., Liu, C. et al. Synthesis and characterization of novel highly soluble and optical transparent polyimides containing tert-butyl and morpholinyl moieties. J Polym Res 22, 169 (2015). https://doi.org/10.1007/s10965-015-0820-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0820-5