Abstract
A new triphenylpyridine-containing aromatic diamine monomer named 4-phenyl-2,6-bis(4-aminophenyl) pyridine was successfully synthesized. A series of polyimides (PIs) with triphenylpyridine moieties were prepared from the newly synthesized diamine monomer via a one-step polymerization with oxydiphthalicanhydride. All the PIs were amorphous and showed excellent solubility in many polar aprotic solvents at room temperature and most of them could afford flexible, transparent, and tough films with good mechanical properties. All the PIs had useful levels of thermal stability associated with high glass-transition temperatures (299–341 °C), 10 % weight-loss temperatures in excess of 537 °C, and char yields at 800 °C in nitrogen higher than 54.9 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyimides (PIs) are well accepted as high-performance materials because of their high-temperature durability, good mechanical properties as well as their chemical resistance, and they have been widely applied in many fields such as proton exchange membranes [1], composites [2], fibers [3, 4], and liquid crystal alignment layers in liquid crystal displays [5, 6]. However, the application of PI is restricted because of insolubility and high softening temperature caused by their rigidity and strong interaction among polymer chains [7, 8]. Based on several modification efforts, it has recently been shown that the introduction of bulky substituents or bulky pendant groups into rigid polymer backbones would be possible, especially the pendant phenyl groups [9].
Incorporation of ether linkages into PI backbone lowers chain rotation barrier and is helpful to improve solubility of PI [10, 11]. Flexible linkages do not destroy thermal stability, but it could damage the rigidity of PI main chains and leads to lower glass-transition temperature (T g). Introduction of the asymmetrical structures into PI is one of the common approaches for increasing solubility and processability of PIs without sacrificing thermal properties [12]. It was found that the presence of bulky substituents on backbones could improve the solubility and maintain high T g of PI [13]. The fluorinated structures lower the intermolecular energy and enhance the solubility of PI [14]. In addition, random copolymerization is another important and effective method for improving the solubility of PI via reducing polymer chains regularity and crystallization capability [15].
In this study, we have attempted to incorporate rigid units and bulky substituted groups into polymer to prepare organo-soluble and thermally stable PIs. The PIs were synthesized from a new rigid diamine with triphenylpyridine units. The rigid and twisted triphenylpyridine groups might improve the solubility and enhance the thermal properties of the PIs. Therefore, these PIs are expected to possess excellent solubility and retain other desirable properties such as thermal properties.
Experimental section
Materials
Benzaldehyde, 4,4′-diaminodiphenyl ether (ODA), 4,4′-diaminodiphenylmethane (MDA), and 4-nitroacetophenone were brought from Aladin Reagents Co. Ltd. 4,4′-oxydiphthalicanhydride (ODPA, >98 %, Shanghai Research Institute of Synthetic Resins) was purified by recrystallization from acetic anhydride. 4,4′-Diamino-3,3′-dimethyldiphenylmethane (DMMDA) and 2,2′-bis(trifluoromethyl)benzidine (BFMB) were obtained from Xiya Reagents Co. Ltd. (Chengdu, China). All the other solvents and chemicals were used as received without further purification, unless otherwise specified.
Measurements
Fourier transform infrared (FTIR) spectra were obtained on a Nicolet 560 Fourier transform spectrophotometer. Nuclear magnetic resonance (NMR) spectra were recorded from a 400 MHz Unity INVOA 400 spectrophotometer. Elemental analysis was made on a EURO EA3000 elemental analyzer (Euro vectro S.P.A, USA). Thermogravimetric analysis (TGA) was performed on a DuPont TGA 2100 at a heating rate of 20 °C/min under nitrogen. Differential scanning calorimetry (DSC) analyses were taken on a NETZSCH DSC 204 at a scan rate of 20 °C/min in flowing nitrogen (20 cm3/min). The wide angle X-ray diffraction (WAXD) measurement was used to investigate the morphology of the PIs by Philips X’Pert PRO MPD (Philips, Netherlands) using Cu-Kα radiation (λ = 1.5418 Å). Inherent viscosities of the PIs were measured at 0.5 g/dL in NMP using an Ubbelohde viscometer. An Instron Universal Tester model 5567 with a load cell of 100 N was used to study the stress–strain behavior of the samples at a crosshead speed of 10 mm/min. Measurements were performed at room temperature with film specimens (0.5 cm wide, and 2-cm gage length), and an average of at least five individual determinations was used.
Synthesis of monomer
4-Phenyl-2,6-bis(4-nitrophenyl)pyridine (1)
A 500-mL three-neck round-bottomed flask was charged with 2.12 g (20 mmol) of benzaldehyde, 6.60 g (40 mmol) of 4-nitroacetophenone, 20 g of ammonium acetate, and 100 mL of glacial acetic acid was refluxed for 8 h. Upon cooling, the precipitated brown solid was collected by filtration and washed with ethanol. The crude product was recrystallized from N,N-dimethylformamide/ethanol to afford 5.39 g (68 %) of light yellow solid 1. FTIR (KBr): 1514 and 1347 cm−1 (NO2), 1550 and 1593 cm−1 (pyridine). 1H NMR (400 MHz, CDCl3-d): δ = 7.548–7.607 (m, 3H, aromatic C–H), 7.763–7.780 (d, 2H, aromatic C–H), 8.058 (s, 2H, aromatic C–H), 8.366–8.420 (d, 8H, aromatic C–H).
4-Phenyl-2,6-bis(4-aminophenyl) pyridine (2)
In a 250-mL round-bottomed flask, a mixture of 5 g (14.7 mmol) of 1, 0.3 g of Pd/C and 50 mL of ethanol was heated to reflux, and 10 mL of hydrazine hydroxide was added dropwise to the mixture over 1 h. After 12 h, the ethanol was removed. Tetrahydrofuran (THF) was added to the mixture, filtered to remove Pd/C. The solvent was removed by using rotation vapor. The crude product was separated out by column chromatography (v/v, dichloromethane: petroleum ether = 5:3). Finally, 3.86 g (91 %) of light yellow solid 2 was obtained. FTIR (KBr): 3432 and 3375 cm−1 (NH2). 1H NMR (400 MHz, DMSO-d6): δ = 5.437 (s, 4H, NH2), 6.665–6.686 (d, 4H, aromatic C–H), 7.463–7.559 (m, 3H, aromatic C–H), 7.793 (s, 2H, aromatic C–H), 7.932–7.951 (d, 2H, aromatic C–H), 7.999–8.020 (d, 4H, aromatic C–H).
Synthesis of polyimides
The synthesis of PIb was used as an example to illustrate the general synthetic route to the PIs. In a 100-mL three-necked flask equipped with a magnetic stirrer, a nitrogen inlet, and a distillation head, diamine 2 (0.217 g, 0.644 mmol) was dissolved in 8 mL of m-cresol. After the diamine was dissolved, ODPA (0.400 g, 1.288 mmol) and isoquinoline (five drops) were added. Then the reaction mixture was stirred 1 h at ambient temperature and then heated to 80 °C for 2 h. When the mixture was cooled to room temperature, ODA (0.129 g, 0.644 mmol) was added. The mixture was reacted at 80 °C for 2 h and then at 180 °C for 24 h. After cooling, the solution was poured into a large amount of methanol under vigorous stirring to form fibrous polymer precipitate. The obtained polymers were filtered off and washed thoroughly with hot methanol, extracted with ethanol in a Soxhlet apparatus, and finally dried in a vacuum oven at 80 °C overnight.
Results and discussion
Monomer synthesis
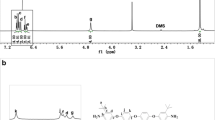
The diamine 2 was synthesized via a two-step synthetic route according to Scheme 1. The nitro compound 1 was synthesized with a modified Chichibabin reaction. The FTIR spectrum of 1 showed characteristic bands of the nitro groups at 1518 and 1343 cm−1. The C=N bands of the pyridine rings were at 1550 and 1593 cm−1. The target diamine monomer 2 was reduced from 1 by means of Pd/C and hydrazine hydroxide. The FTIR spectrum of 2 displayed that the bands of the nitro groups had disappeared and the characteristic bands of the amino groups were at 3432 and 3375 cm−1. The 1H NMR spectra indicated the formation of the pyridine heterocyclic group present at 8.058 ppm for the nitro compound and 7.793 ppm for the diamine. A new signal at 5.437 ppm appeared in the 1H NMR spectrum of compound 2 (Fig. 1b), which was characteristic of amino groups. The FTIR and 1H NMR spectra clearly confirmed that the diamine monomer was completely consistent with the proposed structure.
Preparation of polyimides
The PIs are synthesized via one-step method in m-cresol with isoquinoline as catalyst (Scheme 2). The molar ratio of dianhydride:assisted diamine (ODA, MDA, DMMDA or BFMB):diamine 2 is 100:50:50. The chemical structures of all PIs were confirmed by FTIR and 1H NMR spectra. As shown in Fig. 2, the FTIR spectra of the PIs displayed that the characteristic bands of the imide ring were at 1777–1779, 1722–1729, 1367–1372, and 744–748 cm−1. The representative 1H NMR spectrum of PIe is shown in Fig. 3, where all the signals could be readily ascribed to the protons of the polymer. In addition to the FTIR and 1H NMR spectra, the elemental analysis values of the polymers generally agreed well with the calculated values for the proposed structures. The yields and elemental analyses of the PIs have been listed in Table 1. The inherent viscosities of the PIs were between 0.41 and 1.06 dL/g in NMP solution at 30 °C, and the results are shown in Table 2. All the polymers except PIa could be solution cast into flexible and tough films, indicative of the formation of high molecular weight polymers.
Polymer properties
Solubility property
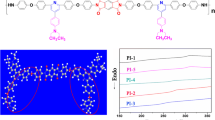
The WAXD measurements of the PI powders indicated that all the polymers were mainly amorphous except a small amount of crystallization, as shown in Fig. 4. The amorphous nature could be attributed to the introduction of bulk, twisted, three-dimensional triphenylpyridine units along the polymer backbone. In addition, the copolymerization would decrease the regularity of the polymer chain, which inhibited the polymer close packing and reduced the crystallization capacity. Thus, all of the PIs were amorphous.
The solubility behavior of the PIs obtained by the one-step procedure was studied qualitatively, and the results are shown in Table 2. The solubility behavior of the polymers depended on their chain packing ability and intermolecular interactions that was affected by the rigidity, symmetry, and regularity of the molecular backbone. In general, these polymers revealed an enhanced solubility as compared with conventional aromatic PIs. This can be attributed to the incorporation of bulky, three-dimensional triphenylpyridine groups, and the random structure of the polymers, which retarded dense chain packing and led to a decreased chain–chain interaction, resulting in loose polymer chain packaging and aggregates. This was in good agreement with the results of WAXD. PIe and PId exhibited better solubility than the other PIs. The trifluoromethyls placed in BFMB and the methyls placed in DMMDA twist the aromatic rings, resulting in a noncoplanar and contorted conformation that is capable of efficiently hindering the chain packing, which may increase the solubility of the PIe and PId.
Thermal property
DSC and TGA techniques were used to investigate the thermal properties of the polymers. The thermal behavior data of all the polymers are summarized in Table 3. The T g of the PIs was found to be 299–341 °C, depending on the structure of assisted diamine component. The high T g values of PIs mainly originate from the backbones stiffness, which can effectively inhibit polymer chains mobility. As shown in Table 3, the T g values of the PIs were improved with an increase of the stiffness of polymer backbones according to the following order: PIc < PIb < PId < PIe < PIa. The homopolymer (PIa) has the highest T g (341 °C), which was attributed to the PI with the maximum rigidity. These results indicated that incorporation of triphenylpyridine units into PI’s backbones could effectively improve T g values (Fig. 5).
Typical TGA curves for PIa, PIc, and PIe are shown in Fig. 6. All of the PIs exhibited a similar TGA pattern with no significant weight loss below 500 °C in nitrogen atmosphere. The 10 % weight-loss temperatures of the PIs in nitrogen were recorded in the range of 537–591 °C. The amount of carbonized residue (char yield) of these polymers in nitrogen atmosphere was more than 54.9 % at 800 °C. The high char yields of these polymers can be ascribed to their high aromatic content. The relatively lower stability of PId was reasonable when considering its less stable aliphatic segments. The results indicated that the presence of triphenylpyridine units and the absence of weak bonds were the primary reasons for good thermal stability of the obtained PIs (PIa and PIe).
Optical property
The transparency of PI was an important parameter for application in photonics materials, thermostable wave guide, materials for liquid crystal display devices, and so on. As shown in Fig. 7, the transmittances of the PI films (20–30 µm in thickness) were investigated by UV–Vis spectrophotometer at room temperature. The PI films exhibited high transparency in the visible region, the maximum transmittances were over 80 %, and cut-off wavelengths were around 370 nm. PIe had the best optical transparency, which could be explained from the decreased intermolecular interactions. The bulky and electron-withdrawing CF3 group in BFMB was effective in decreasing charge-transfer complex (CTC) formation between polymer chains through the inductive effect (by decreasing the electron-donating property of diamine moieties). Thus, PIe exhibited the highest transparency.
Mechanical property
As previously mentioned, except PIa, all PIs could be solution cast into smooth, flexible, and tough films. These films were subjected to tensile testing, and the results are also given in Table 4. The data showed that films had tensile strengths of 119–129 MPa, elongations to break values of 13–23 %, and initial modulus of 2.1–2.4 GPa, which indicated that all copolymer films behaved as ductile materials with good tensile strengths and moderate elongations to break.
Conclusions
A novel triphenylpyridine-containing diamine, 4-phenyl-2,6-bis(4-aminophenyl) pyridine, was successfully synthesized and used in a preparation of PIs via a one-step polymerization with ODPA. Because of the presence of the bulky, three-dimensional triphenylamine unit, all the polymers were amorphous, had good solubility in many polar aprotic solvents, and most of them could afford flexible, transparent, and tough films with good mechanical properties. They had useful levels of thermal stability associated with high T gs (299–341 °C), 10 % weight-loss temperatures in excess of 537 °C, and char yields at 800 °C in nitrogen higher than 54.9 %.
References
Li W, Guo X, Fang J (2014) Synthesis and properties of sulfonated polyimide–polybenzimidazole copolymers as proton exchange membranes. J Mater Sci 49:2745–2753. doi:10.1007/s10853-013-7977-2
Standage AE, Turner WN (1967) A high-temperature polyimide reinforced with silica fibre. J Mater Sci 2:103–111. doi:10.1007/BF00549568
Xu Y, Wang S, Li Z, Xu Q, Zhang Q (2013) Polyimide fibers prepared by dry-spinning process: imidization degree and mechanical properties. J Mater Sci 48:7863–7868. doi:10.1007/s10853-013-7310-0
Li W, Wu Z, Jiang H, Eashoo M, Harris FW, Cheng SZD (1996) High-performance aromatic polyimide fibres: part V compressive properties of BPDA-DMB fibre. J Mater Sci 31:4423–4431. doi:10.1007/BF00356470
Barzic AI, Rusu RD, Stoica I, Damaceanu MD (2014) Chain flexibility versus molecular entanglement response to rubbing deformation in designing poly(oxadiazolenaphthylimide)s as liquid crystal orientation layers. J Mater Sci 49:3080–3098. doi:10.1007/s10853-013-8010-5
Gong S, Liu M, Sun Z, Xia S, Wang Y (2014) Synthesis and characterisation of novel soluble polyimides for the application as liquid crystal vertical alignment layers. Liq Cryst 41:1831–1842
Behniafar H, Hosseinpour M (2013) Novel ortho-linked fluorinated poly(ether-imide)s based on 2,2′-substituted 1,1′-binaphthyl units. Polym Adv Technol 24:118–125
Chung IS, Park CE, Ree M, Kim SY (2001) Soluble polyimides containing benzimidazole rings for interlevel dielectrics. Chem Mater 13:2801–2806
Liaw DJ, Liaw BY, Yang CM (1999) Synthesis and properties of new polyamides based on bis[4-(4-aminophenoxy)phenyl]diphenylmethane. Macromolecules 32:7248–7250
Salbeck J, Bauer J, Weissörtel F (1998) Spiro linked compounds for use as active materials in organic light emitting diodes. Macromol Symp 125:121–132
Liaw DJ, Chang FC (2004) Highly organosoluble and flexible polyimides with color lightness and transparency based on 2,2-bis[4-(2-trifluoromethyl-4-aminophenoxy)-3,5-dimethylphenyl] propane. J Polym Sci A 42:5766–5774
Chern YT, Tsai JY (2008) Low dielectric constant and high organosolubility of novel polyimide derived from unsymmetric 1,4-bis(4-aminophenoxy)-2,6-di-tert-butylbenzene. Macromolecules 41:9556–9564
Calle M, LozanoÁE Campa JGDL, Abajo JD (2010) Novel aromatic polyimides derived from 5′-t-butyl-2′-pivaloylimino-3,4,3′′,4′′-m-terphenyltetracarboxylic dianhydride with potential application on gas separation processes. Macromolecules 43:2268–2275
Tao LM, Yang HX, Liu JG, Fan L, Yang SY (2009) Synthesis and characterization of highly optical transparent and low dielectric constant fluorinated polyimides. Polymer 50:6009–6018
Dong J, Yin C, Luo W, Zhang Q (2013) Synthesis of organ-soluble copolyimides by one-step polymerization and fabrication of high performance fibers. J Mater Sci 48:7594–7602. doi:10.1007/s10853-013-7576-2
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51173115), the Ministry of Education (the Foundation for Ph.D. training, Grant No. 20110181110030) of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, Y., Sun, Z., Gong, S. et al. Synthesis and characterization of soluble and thermally stable triphenylpyridine-containing aromatic polyimides. J Mater Sci 50, 6552–6558 (2015). https://doi.org/10.1007/s10853-015-9186-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9186-7