Abstract
Densities (ρ) have been measured from 298.15 to 313.15 K at atmospheric pressure over the entire composition range for binary mixtures of benzyl alcohol with 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane, trichloroethylene and tetrachloroethylene. Further, the speeds of sound in these mixtures were also measured from 303.15 to 313.15 K. The experimental density data were used to compute excess molar volumes (V E) which were compared with the Redlich–Kister and Hwang equations. Excess speeds of sound (u E), isentropic compressibilities (κ S ) and excess isentropic compressibilities (\( \kappa_{S}^{\text{E}} \)) were evaluated from experimental speed of sound and density data. Moreover, the experimental speeds of sound were compared in terms of theoretical models proposed by Schaaff’s collision factor theory and Jacobson’s free length theory. The experimental and derived properties are discussed in terms of intermolecular interactions between component molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thermodynamic properties of non electrolyte solutions have proved to be a useful tool in elucidating the structural interactions among component molecules [1]. For example, excess volume and density data can be used to study solvent–solvent specific interactions as a function of temperature, while the composition dependence provides valuable un-substitutable information about the presence and the stoichiometry of complex adducts. The intermolecular interactions influence the structural arrangement along with the shape of the molecules. The sign and magnitude of these properties guide us to understand possible interactions between the component molecules. The knowledge of physicochemical properties of non-aqueous binary liquid mixtures has relevance in theoretical and applied areas of research, and such results are frequently used in the design process (flow, mass transfer or heat transfer calculations) in many chemical and industrial processes. The excess properties derived from these physical property data reflect the physicochemical behavior of the liquid mixtures with respect to the solution structure and intermolecular interactions between the component molecules of the mixture [2, 3]. In continuation of our studies of acoustic and volumetric properties of non-electrolyte liquid mixtures [4], the present study reports densities (ρ) at 298.15, 303.15, 308.15 and 313.15 K and speed of sound data at 303.15 and 313.15 K for binary mixtures of benzylalcohol with 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane, trichloroethylene and tetrachloroethylene over the entire composition range. From these data, excess molar volumes (V E), excess speed of sound (u E) and excess isentropic compressibility (\( \kappa_{S}^{\text{E}} \)) were calculated. Further, the experimental speed of sound data were compared with Schaaff’s collision factor theory (CFT) and Jacobson’s free length theory (FLT).
Liquids that were chosen in the present investigation are of much interest due to their various industrial and consumer applications. Aromatic alcohols show a large variety of relevant technical applications including as an antimicrobial agent. In addition, they are good solvents for gelatin, cellulose acetate and shellac, encountered in perfumery, veterinary science, or in microscopy as an embedding material. Benzylalcohol is also used as an additive for synthetic fuels, derived from the Fischer–Tropsch process for usage in ground and air vehicles. Further, it significantly retards the thermal degradation of jet fuels at high temperatures and is used as a dielectric solvent for the dielectrophoretic reconfiguration of nano wires [5]. Chloroalkanes and alkenes have many industrial application [6, 7], the most common use of 1,2-dichloroethane is in the production of vinyl chloride and vinyl products including polyvinyl chloride (PVC); it is also used as a solvent that is added to leaded gasoline to remove lead. 1,1,2,2-tetrachloroethane and trichloroethylene are nonflammable solvents, traditionally used in fats, waxes, resins, oils, rubber, paints, varnishes, natural resins, and alkaloids. The applications of tetrachloroethylene are in dry cleaning, textile processing, degreasing metals, insulating fluid, and cooling gas in electrical transformers.
A survey literature of binary mixtures containing benzylalcohol shows that excess volume data of benzylalcohol with benzene and substituted benzenes [8], benzylalcohol with ethanol [9], chloroalkanes and chloroalkenes with alcohol [10], benzylalcohol with 1-alcohols [11] and enthalpy data of benzylalcohol with 1-alkanols [12] and benzylalcohol with chloroalkanes and chloroalkenes [13] were reported. To the best of our knowledge, acoustic and volumetric properties of binary mixtures of benzylalcohol with the chloroalkanes and alkenes considered in this work are not reported in literature.
2 Experimental Section
2.1 Chemicals Used
All the chemicals used in the present work were of analytical reagent grade procured from S.D. Fine chemicals Ltd., India and Merck and their purities were as follows: benzylalcohol (99.5 %), 1,2-dichloroethane (99.0 %), 1,1,1-trichloroethane (99.0 %), 1,1,2,2-tetrachloroethane (99.5 %), trichloroethylene (99.0 %) and tetrachloroethylene (99.0 %) Prior to experimental measurements, all the liquids were purified as described in the literature [7, 14, 15].
2.2 Analysis of Water Content in Chemicals
The water content of solvents used in this work was measured using an Analab (Micro Aqua Cal 100) Karl Fischer Titrator and Karl Fisher reagent from Merck. It can detect water content from less than 10 ppm to 100 % by conductometric titration with dual platinum electrodes.
The water contents are given in Table 1 along with their CAS number, supplier and manufacturer’s stated purities.
The purities of chemicals, after distillation, were checked by comparing the measured densities and speeds of sound, which are in good agreement with literature values [4, 6, 10, 16–21] and these are given in Table 2.
2.3 Measurements
All binary liquid mixtures were prepared by weighing appropriate amounts of pure liquids on an electronic balance (Afoset, ER-120A, India) with a precision of ±0.1 mg, by syringing each component into airtight stopper bottles to minimize evaporation losses. The uncertainty of the mole fraction was ±1 × 10−4. After mixing, a bubble free homogenous sample was transferred into the U-tube of the densimeter through a syringe. The density measurements were performed with a Rudolph Research Analytical digital densimeter (Model DDM-2911), equipped with a built-in solid state thermostat and a resident program, with an accuracy of temperature of ±0.03 K. The uncertainty in the density measurements is ±2 × 10−5 g·cm−3. Calibration of the densimeter, at each temperature, was with doubly distilled, deionized water and with air as standards. The ultrasonic speeds in the pure liquids and in their mixtures were measured by using a multi frequency ultrasonic interferometer (M-82 Model, Mittal Enterprise, New Delhi, India) single-crystal variable-path at 303.15 and 313.15 K. The uncertainty in the measurement of ultrasonic sound velocity is ±0.3 %. The temperature stability is maintained within ±0.01 K by a circulating thermostatic water bath around the cell with a circulating pump. The present investigation has been devoted to the study of densities, speed of sounds of binary liquid mixtures at different temperatures and at a pressure of 0.1 MPa.
3 Results and Discussion
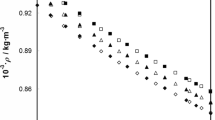
The measured densities (ρ) and excess volume data of the binary liquid mixtures of benzylalcohol with 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane, trichloroethylene and tetrachloroethylene at the temperature range from 298.15 to 313.15 K are given in Table 3 along with graphical representation in Figs. 1, 2, 3, 4 and 5. The excess molar volume (V E) of all the binary mixtures were calculated from the measured densities by using the following equation:
where x 1, x 2, M 1, M 2, ρ 1, ρ 2 and ρ m are the mole fraction, molar mass, and the density of pure components 1 and 2 and mixture, respectively. Moreover, the experimental excess volumes were also analyzed in terms of Redlich–Kister [22] and Hwang et al. [23] equations and these are also included in Table 3.
The empirical relation proposed by Redlich–Kister is given as follows:
where a i , are adjustable parameters and x 1 is the mole fraction of benzylalcohol. The values of these parameters were obtained by the least-squares method.
The Hwang et al. equation takes the following form:
where x 1 and x 2 represent the mole fractions of benzylalcohol and chloroalkanes and alkenes, respectively, and the b i are constants. The computation of b i coefficients in the above equation was described earlier [24, 25]. The values of the two sets of constants are given in Tables 4 and 5 along with standard deviation (σV E) and these values point out that equations of Redlich–Kister and Hwang et al. also precisely represent the experimental excess volume data.
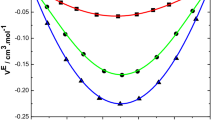
An examination of V E data in Figs. 1, 2, 3, 4 and 5 for the binary mixtures of benzylalcohol with 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane are positive, while for trichloroethylene and tetrachloroethylene systems they are negative, over the entire mole fraction range at all temperatures. Desnoyers and Perron [26] suggested that representing the nonideality as excess quantities can be quite misleading, specifically for mixtures having strong interactions at low concentrations, and it is better to plot V E/x 2(1 − x 2) against x 2, which gives nearly straight line for binary liquid mixtures of similar size and polarity. This function V E/x 2(1 − x 2) is equivalent to an apparent molar quantity over the whole mole fraction range, and its extrapolation to x 2 = 0 and x 2 = 1 will give the two excess partial molar quantities, which are important to understand the solute–solvent interactions. For the binary mixtures of benzylalcohol with 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane, the plots of V E/x 2(1 − x 2) against x 2 are shown in supporting information (Figs. S1–S5). Comparisons of Figs. 1, 2, 3, 4 and 5 with Figs. S1–S5, show that V E/x 2(1 − x 2) is more sensitive than V E to interactions which occur at low concentrations.
The variation of excess volume with mole fraction, for all the binary mixtures under the present investigation, may be explained by the following factors: (i) contraction of volume due to dipole–dipole and dipole-induced dipole interactions; (ii) complex formation between benzylalcohol with chloroethanes [27]. A perusal of the V E data in Figs. 1, 2, and 3 reveals that the former factor, which contributes to positive excess volumes is dominant in the binary mixtures of benzylalcohol and chloroalkanes(1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane).
The excess volumes for mixtures of chloroalkanes show the following order: 1,2-dichloroethane > 1,1,1-trichloroethane > 1,1,2,2-tetrachloroethane
This order suggests an absence of H-bonding in these systems, since the interactions are maximum in the system of 1,1,2,2-tetrachloroethane. Further, the presence of one more chlorine atom in 1,1,1-trichloroethane increases it’s electron accepting capacity and therefore it reacts more strongly towards a benzylalcohol molecule when compared to 1,2-dichloroethane, making less positive values for benzylalcohol with 1,1,1-trichloroethane than for benzylalcohol with 1,2-dichloroethane. Similarly, in the case of the benzylalcohol with 1,1,2,2-tetrachloroethane system, less positive values are observed when compared to benzylalcohol with the 1,2-dichloroethane and 1,1,1-trichloroethane systems. This suggests that as the number of chlorine atoms in an alkane molecule increases, the intermolecular interactions increase, thereby less positive V E values are observed in the system of 1,1,2,2-tetrachloroethane than in the cases of 1,2-dichloroethane and 1,1,1-trichloroethane, molecules containing two and three chlorine atoms, respectively. Thus, the fewer chlorine atoms in the alkane molecule, the more positive is the observed value of V E [28].
The V E data of the system containing tetrachloroethylene are more negative than for the benzylalcohol with trichloroethylene mixtures. This result likely reflects the double bond character of chloroethylenes, shielding of the ethylenic double bond by chlorine atoms and partial saturation of the electron accepting nature of chlorine atoms by π-electrons of the ethylenic double bond [6, 17, 29].
The excess volume data for mixtures of chloroalkanes and chloroalkenes shows the following order: tetrachloroethylene > trichloroethylene
The more negative V E data for the system of benzylalcohol with tetrachloroethylene may be ascribed to π-π interactions between the unlike molecules, but π-electrons of the tetrachloroethylene donor are involved because of partial shielding by the chlorine atoms [30]; also, the shielding capacity of double bond character is observed to a greater extent in tetrachloroethylene, because of the presence of four chlorine atoms, when compared with trichloroethylene.
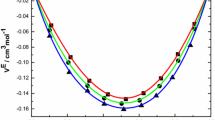
Data for mole fraction (x 1) of benzylalcohol, density (ρ) of pure liquids and their liquid mixtures, and experimental sound velocities (u), isentropic compressibilities (\( \kappa_{S} \)) and excess isentropic compressibilities (\( \kappa_{S}^{\text{E}} \)) data are presented Table 6. The excess isentropic compressibility data are also represented graphically in Figs. 6 and 7.
Variation of excess isentropic compressibility (\( \kappa_{S}^{\text{E}} \)) of the binary liquid mixtures of benzylalcohol (1) with 1,2-dichloroethane (square), 1,1,1-trichloroethane (circle), 1,1,2,2-tetrachloroethane (triangle), trichloroethelene (inverted triangle) and tetrachloroethelene (filled square) at 303.15 K
Variation of excess isentropic compressibility (\( \kappa_{S}^{\text{E}} \)) of the binary liquid mixtures of benzylalcohol (1) with 1,2-dichloroethane (square), 1,1,1-trichloroethane (circle), 1,1,2,2-tetrachloroethane (triangle), trichloroethelene (inverted triangle) and tetrachloroethelene (filled square) at 313.15 K
From the result of speed of sound (u), densities (ρ), excess isentropic compresibilities (\( \kappa_{S}^{\text{E}} \)) was calculated as [31]:
Here, C p i and α i are the molar heat capacity and the thermal expansion coefficient of the ith component respectively. The value of C p i and α i were obtained and evaluated from the literature [4, 19, 20].
The excess speed of sound (u E) data of the binary mixtures under the investigation [31] were calculated from the following equations, and are given in Table 6.
An examination of \( \kappa_{S}^{\text{E}} \) data in Table 6, for the mixtures of benzylalcohol with chloroalkanes and chloroalkenes, shows they are negative over the entire composition range at 303.15 and 313.15 K. This may be attributed to the relative strength of effects which influence the free space between component molecules. The negative \( \kappa_{S}^{\text{E}} \) values arise from changes of free volume in the real mixture and presence of π-electrons in benzylalcohol resulting in the formation of weak intermolecular complexes leading to negative excess isentropic compressibility [32, 33].
Perusal of \( \kappa_{S}^{\text{E}} \) data in Table 6 suggests that the factors that are responsible for negative \( \kappa_{S}^{\text{E}} \) are dominate in all the binary mixtures.
The excess volume data for mixtures of chloroalkanes and chloroalkenes follows the order:
1,2-dichloroethane < 1,1,1-trichloroethane < 1,1,2,2-tetrachloroethanetrichloroethylene < tetrachloroethylene
This order suggests that as the number of chlorine atoms in alkane and alkene molecules increase the weak dipolar interactions between unlike molecules, which leads to decreases in free space, thereby an increase in the speed of sound and negative \( \kappa_{S}^{\text{E}} \) values are observed [34].
The effect of increasing the temperature appears to increase the magnitude of excess volume, suggesting of the existence weak dipolar interactions and complex formation. Further, as the temperature increases, \( \kappa_{S}^{\text{E}} \) becomes more negative, which may be due to the thermal dissociation of hetero-aggregates in liquid mixtures and more interstitial accommodation of the components [35].
Experimental ultrasonic sound velocities were analyzed in terms of collision factor theory (CFT) [36], free length theory (FLT) [37, 38] and these are also included in Table 6 along with experimental ultrasonic sound velocities. The pure component data namely, the molar volume (V m ), molar volume at absolute zero (V 0), molar available volume (V a), free length (L f), surface area (Y), collision factor (S), average molecular radius (r m ), actual volume of molecules per mole (B) and molecular sound velocity (R) that were used in the theoretical calculations were collected from the literature [39]. The methods and details of calculation were discussed earlier [33]; these values are given in Table 6.
A comparison between experimental sound velocities and theoretical values suggest that the model proposed by Schaaff’s CFT gives better estimation of sound velocity data. The methods of calculation of these theories were described earlier (Tables 7, 8). The merits of these theories were compared in terms of relative root mean deviation by using the following formula [40]:
The RMSDs for all the binary systems values, given in Table 9, show that Schaaff’s CFT model gives a better estimation in speed of sound for the binary mixtures under the investigation.
The experimental V E, u E and \( \kappa_{S}^{\text{E}} \) data were fitted to the Redlich–Kister Eq. 2 and Hwang Eq. 3 type polynomial equations and the adjustable parameters of the function and are determined using the least-squares method. The corresponding standard deviations σ(Y E) have been computed using the relation:
where m is the total number of experimental points and n is the number of coefficients; the standard deviations for all the binary mixtures are presented in Tables 4, 5, 7 and 8.
4 Conclusions
In the present work excess volume data of binary mixtures of benzylalcohol with 1,2-dichloroethane, 1,1,1-trichloroethane and 1,1,2,2-tetrachloroethane are positive and trichloroethylene and tetrachloroethylene are negative over the entire composition range at 298.15–313.15 K. Further, negative \( \kappa_{S}^{\text{E}} \) values in all the binary mixtures arise due to changes of free volume in the real mixtures and presence of π-electrons in benzylalcohol and result in the formation of strong intermolecular complexes leading to negative excess isentropic compressibility.
References
Wilhelm, E.: Chemical thermodynamics: a journey of many vistas. J. Solution Chem. 43, 525–576 (2014)
Rowlinson, J.S., Swinton, F.L.: Liquid and Liquid Mixtures. Butterworths, London (1982)
Gardas, R.L., Oswal, S.L.: Volumetric and transport properties of ternary mixtures containing 1-butanol or 1-pentanol, triethylamine and cyclohexane at 303.15 K: experimental data, correlation and prediction by the ERAS nodel. J. Solution Chem. 37, 1449–1470 (2008)
Venkatramana, L., Gardas, R.L., Sivakumar, K., Dayananda Reddy, K.: Thermodynamics of binary mixtures: the effect of substituents in aromatics on their excess properties with benzylalcohol. Fluid Phase Equilib. 367, 7–21 (2014)
Gonzalez, J.A., Alonso-Tristan, C., Garcia de la fuente, I., Cobos, J.C.: Thermodynamics of mixtures containing aromatic alcohols. 1. Liquid–liquid equilibria for (phenylmethanol + alkane) systems. J. Chem. Eng. Data 57, 1186–1191 (2012)
Asra Banu, S., Amara Jyothi, K., Sathyanarayana, B., Satyanarayana, N.: Excess molar volumes and sound speed in (phenylacetonitrile + 1,2-dichloroethane), (phenylacetonitrile + 1,1,2-trichloroethane), (phenylacetonitrile + 1,1,2,2-tetrachloroethane), (phenylacetonitrile + trichloroethene), and (phenylacetonitrile + tetrachloroethene) at temperatures of (303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 55, 1405–1410 (2010)
Riddick, J., Bunger, W.B., Sakano, T.K.: Techniques of Chemistry, Organic Solvents, Physical Properties and Methods of Purifications, 4th edn. Wiley Interscience, New York (1986)
Ali, A., Tariq, M.: Thermodynamic and transport behavior of binary liquid mixtures of benzylalcohol with monocyclic aromatics at 303.15 K. J. Mol. Liq. 128, 50–55 (2006)
Chen, K.-D., Lin, Y.-F., Tu, C.-H.: Densities, viscosities, refractive indexes, and surface tensions for mixtures of ethanol, benzylacetate and benzyl alcohol. J. Chem. Eng. Data 57, 1118–1127 (2012)
Vijaya Kumar, R., Viswanathan, S., Anand Rao, M.: Excess volumes, speeds of sound, and isentropic compressibilities of 2-propyn-1-ol + 1,2-dichloroethane, +1,1,1-trichloroethane, +1,1,2,2-tetrachloroethane, and +trichloroethylene at 303.15 K. J. Chem. Eng. Data 41, 755–757 (1996)
Ali, A., Soghra Hyder, A., Anil Kumar, N.: molecular interaction in binary mixtures of benzyl alcohol with ethanol, propan-1-ol and octan-1-ol at 303 K: an ultrasonic and viscometric study. Collect. Czech. Chem. Commun. 67, 125–1140 (2002)
Zarei, H.A.: Excess molar enthalpies of benzyl alcohol + alkanols (C1–C6) and their correlations at 298.15 K and ambient pressure. J. Chem. Eng. Data 55, 4021–4024 (2010)
Vijayakumar, R., Anand Rao, M.: Excess molar enthalpies of chloroalkanes or chloroalkenes + benzyl alcohol at 298.15 K. J. Chem. Eng. Data 40, 99–101 (1995)
Timmermans, J.: Physico-Chemical Constants of Pure Organic Compounds. Elsevier, Amsterdam (1950)
Ciocirlan, O., Teodorescu, M., Dragoescu, D., Iulian, O., Barhala, A.: Densities and excess molar volumes of the binary mixtures of cyclopentanone with chloroalkanes at T = (288.15, 298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 55, 3891–3895 (2010)
Sivaramprasad, G., Venkateshwara Rao, M.: Density and viscosity of ethanol + 1,2-dichloroethane, ethanol + 1,1,1-trichloroethane, and ethanol + 1,1,2,2-tetrachloroethane binary mixtures. J. Chem. Eng. Data 35, 122–124 (1990)
Reddy, D.V.B., Krishnaiah, A., Ramanjaneyulu, K.: Ultrasonic velocities and isentropic compressibilities of acetophenone with some chloroethanes and chloroethenes at 303.15 K. J Phys. Chem. Liq. 20, 221–226 (1989)
Sathyanarayana, B., Savitha Jyostna, T., Sathyanarayana, N.: Acoustic studies of binary mixtures of N-methylacetamide with some chloroethanes and chloroethenes at 308.15 K. Ind. J. Pure Appl. Phys. 44, 587–591 (2006)
Oswal, S.L., Patel, I.N.: Speed of sound, isentropic compressibility and refractive index of binary mixtures of alkyl ethanoates with chloroalkanes at 303.15 K. J. Mol. Liq. 116, 99–107 (2005)
Jovanovic, J., Knezevic-Stevanovic, A., Grozdanic, D.: Prediction of high pressure liquid heat capacities of organic compounds by a group contribution method. Serb. J. Chem. Soc. 76, 417–423 (2011)
Jagadish, G.B., Mrityunjaya, I.A., Tejraj, M.A., Mahadevappa, Y.K., Arjumand, S.K.: Density, viscosity, refractive index, and speed of sound for binary mixtures of anisole with 2-chloroethanol, 1,4-dioxane, tetrachloroethylene, tetrachloroethane, DMF, DMSO, and diethyl oxalate at (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 50, 910–916 (2005)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification solutions. Ind. Eng. Chem. Res. 40, 345–448 (1948)
Hwang, C.A., Holste, J.C., Hall, K.R., Mansoori, G.A.: A simple relation to predict or to correlate the excess functions of multicomponent mixtures. Fluid phase Equib. 62, 173–189 (1991)
Acree Jr, W.E., Zvaizene, A.I., Naidu, P.R.: A new predictive relation for ternary excess volumes. Phys. Chem. Liq. 27, 69–75 (1994)
Sivakumar, K., Naidu, P.R.: Excess volumes of ternary mixtures containing p-chlorotoluene and octane with 1-alkanols at 303.15 K. J. Chem. Eng. Data 39, 2–4 (1994)
Desnoyers, J.E., Perron, G.: Treatment of thermodynamic quantities for liquid mixtures. J. Solution Chem. 26, 749–755 (1997)
Reddy, K.D., Iloukhani, H., Rao, M.V.P.: Excess volume of chlorobenzene and bromobenzene with some chloroethanes at 303.15 K and 313.15 K. Fluid Phase Equib. 17, 123–130 (1984)
Reddy, D.V.B., Ramanjaneyulu, K., Krishnaiah, A.: Volumetric and ultrasonic behavior of ethylacetate with some chloroethanes and chloroethenes. Indian J. Pure. Appl. Phys. 28, 107–110 (1990)
Handa, Y.P., Benson, G.C.: Thermodynamic properties of binary liquid mixtures involving weak specific interactions Part III. Excess enthalpies of binary mixtures of tetrachloromethane and of tetrachloroethene with some alicyclic, pseudoaromatic and aromatic hydrocarbons at 298.15 K. Fluid Phase Equilib. 4, 277–285 (1980)
Reddy, D.V.B., Ramanjaneyulu, K., Krishnaiah, A.: Excess volumes of binary liquid mixtures of acetophenone with some chloroethanes and chloroethenes. Indian J. Tech. 27, 303–307 (1989)
Douheret, G., Davis, M.I., Reis, J.C.R., Blandamer, M.J.: Isentropic compressibilities experimental origin and the quest for their rigorous estimation in thermodynamically ideal liquid mixtures. Chem. Phys. Chem. 2, 148–161 (2001)
Narayanaswami, G., Dharmaraju, G., Raman, G.K.: Excess volumes of toluene mixtures with 1-alkanol at 303.15K. Can. J. Chem. 58, 229–230 (1980)
Syamala, V., Venkateswrlu, P., Sivakumar, K.: Excess volumes, speeds of sound, isentropic compressibilities and viscosities of binary mixtures of acetophenone with chlorotoluenes and nitrotoluenes at 303.15 K. J. Chem. Eng. Data 51, 928–934 (2006)
Rambabu, K., Venkateswarlu, P., Raman, G.K.: Isentropic compressibilities from ultrasonic studies on binary mixtures of γ-butyrolactone with aliphatic and isomeric alcohols. Acoustics Lett. 13, 87–91 (1989)
Oswal, S.L., Oswal, P., Gardas, R.L., Patel, S.G., Shinde, R.G.: Acoustic, volumetric, compressibility and refractivity properties and reduction parameters for the ERAS and Flory models of some homologous series of amines from 298.15 to 328.15K. Fluid Phase Equilib. 216, 33–45 (2004)
Schaffs, W.: Computation of molecular radius from molar volume and velocity of sound. Z. Med. Phys. 115, 69–75 (1940)
Jacobson, B.: Intermolecular free lengths in the liquid state. Acta Chem. Scand. 8, 1485–1498 (1952)
Jacobson, B.: Intermolecular free length in liquids in relation to sound velocity. J. Chem. Phys. 20, 927–928 (1952)
Syamala, V., Rajasekhar, D., Sivakumar, K., Venkateswarlu, P.: Volumetric, ultrasonic and transport properties of binary liquid mixtures containing dimethylformamide at 303.15 K. Chin. J. Chem. 25, 32–43 (2007)
Mohammad, V., AlTuwaim, H.A.E., Alkhaldi, K., Abubaker, A.: Comparative study of physico-chemical properties of binary mixtures of N, N-dimethylformamide with 1-alkanols at different temperatures. J. Chem. Thermodyn. 48, 39–47 (2012)
Acknowledgments
The author (LV) expresses his sincere thanks to Prof. P. Venkateswarlu, Department of Chemistry, S.V. University, Tirupati for providing facilities to carry out part of the present work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Venkatramana, L., Gardas, R.L., Narasimha Rao, C. et al. A Study of the Excess Properties of Aliphatic Chlorinated Compounds with Benzylalcohol at Various Temperatures. J Solution Chem 44, 327–359 (2015). https://doi.org/10.1007/s10953-015-0309-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0309-1