Abstract
Densities (ρ), speeds of sound (u) and viscosities (η) are reported for binary mixtures of 2-chloroaniline (CA) with chlorotoluenes [o-chlorotoluene (o-CT), m-chlorotoluene (m-CT), and p-chlorotoluene (p-CT)] over the entire range of mole fraction at 303.15, 308.15, 313.15 and 318.15 K and atmospheric pressure. By using this data, the excess molar volumes, excess isentropic compressibilities, and deviation in viscosity for the binary systems were calculated and fitted to the Redlich–Kister equation to determine the fitting parameters and the root-mean-square deviations. The excess molar volumes, excess isentropic compressibilities, deviations in viscosity and excess Gibbs energy of activation of viscous flow have been analyzed in terms of charge-transfer complexes, and dipole–dipole interactions between unlike molecules in the mixtures. The viscosity data have been correlated using three equations: the Grunberg–Nissan, Katti–Chaudhri and Hind et al.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The thermodynamic and transport properties of liquid mixtures have attracted much attention from the point of view of both theoretical and engineering applications. Many engineering applications require data on the density, speed of sound and viscosity of liquid mixtures. They also provide information about the nature and molecular interactions between liquid mixture components.
The liquids were chosen in the present study on the basis of their industrial importance. 2-Chloroaniline is used as parent substance in the production of antioxidants, agricultural, pharmaceutical and rubber chemicals. It is also used in manufacture of intermediates for synthetic dyes, and organic pigments, especially for red color. Chlorotoluenes are used as intermediates in the pesticide, pharmaceutical and dye industries. A fundamental understanding of the mixture behavior of 2-chloroaniline with chlorotoluenes is therefore important from the technical and engineering standpoint.
The present investigation is a continuation of our earlier research [1–6] on thermodynamic properties of binary liquid mixtures. In this paper we report measurements of densities, speeds of sound and viscosities for three binary systems, 2-chloroaniline + o-chlorotoluene, 2-chloroaniline + m-chlorotoluene and 2-chloroaniline + p-chlorotoluene at 303.15, 308.15, 313.15 and 318.15 K and atmospheric pressure. The aim of this work is to investigate the influence on both the sign and magnitude of excess/deviation properties by the introduction of a chlorogroup into the toluene molecule in mixtures with 2-chloroaniline.
Several researchers investigated density, speed of sound, and viscosity of binary mixtures of dimethylformamide with chlorotoluenes [7], tetrahydrofuran with chlorotoluenes [8], dimethylsulfoxide with chlorotoluenes [9], and benzyl alcohol with chlorotoluenes [10]. However, no attempt has been made to measure excess/deviation properties of binary mixtures of 2-chloroaniline with chlorotoluenes. We report here excess volume (V E), excess isentropic compressibility (\( \kappa_{S}^{\text{E}} \)), deviation in viscosity (∆η) and excess Gibbs energy of activation of viscous flow (G *E) for the above said binary systems. The variations of these properties with composition of the binary mixtures are discussed in terms of molecular interactions between components and structural effects.
2 Experimental
The mass fraction purity of the liquids obtained from Merck, and S.D. Fine Chemicals Ltd., India are as follows: 2-chloroaniline (0.995), o-chlorotoluene (0.995), m-chlorotoluene (0.995), and p-chlorotoluene (0.996). Prior to experimental measurements, all the liquids were used after double distillations and partially degassed with a vacuum pump under an inert atmosphere. The purity of these solvents was ascertained by comparing the measured density, speed of sound, and viscosity of the pure components with available literature values [11–16] as shown in Table 1. The binary mixtures of 2-chloroaniline with o-chlorotoluene, m-chlorotoluene, and p-chlorotoluene were prepared in glass bottles with air-tight stoppers, and adequate precautions were taken to minimize losses through evaporation. The weighing of solutions was made using Afoset ER-120A electronic balance with a precision of ±0.1 mg. The uncertainty in solution composition expressed in mole fraction was found to be less than 1 × 10−4. After mixing, the bubble-free homogeneous sample was transferred into the U-tube of the densimeter using a syringe. The density measurements were performed with a Rudolph Research Analytical digital densimeter (DDH-2911 Model), equipped with a built-in solid-state thermostat and a resident program with giving temperature control to ± 0.03 K. The uncertainty in the density measurements was found to be less than ±4 × 10−5 g·cm−3. Proper calibration at each temperature was achieved with doubly distilled, deionized water and air as standards. A multi–frequency ultrasonic interferometer (M-82 Model, Mittal Enterprise, New Delhi, India) operated at 2 MHz was used to measure the ultrasonic velocities in the binary liquid mixtures; the temperature was controlled by a digital, constant temperature water bath. The uncertainty in the measurement of speed sound is ±0.2 %.
The viscosities of the pure liquids and their mixtures were determined at atmospheric pressure, at 303.15, 308.15, 313.15 and 318.15 K, using an Ubbelohde viscometer that was calibrated with benzene and doubly distilled water. The Ubbelohde viscometer bulb has a capacity of 15 mL and the capillary tube with a length of about 90 mm with 0.5 mm internal diameter. The viscometer was thoroughly cleaned and dried, and was filled with the sample liquid and its limbs were closed with Teflon caps to avoid evaporation. The viscometer was kept in a transparent walled bath with a thermal stability of ±0.01 K for about 20 min to obtain thermal equilibrium. An electronic digital stopwatch with an uncertainty ±0.01 s was used for flow time measurements. The viscosity values of pure liquids and mixtures are calculated using the relation:
where, a and b are the characteristic constants of the viscometer, ρ is the density, and t represents the flow time. The uncertainty of viscosity thus estimated was found to be ±0.005 mPa·s.
3 Calculations
From these experimental values of densities (ρ) viscosity (η) and sound speed (u) data were used to calculate excess molar volumes (V E), excess isentropic compressibility (\( \kappa_{S}^{\text{E}} \)) deviation in viscosity (∆η) and excess Gibbs energy of activation of viscous flow (G *E) for binary mixtures of 2-chloroaniline with chlorotoluene at 303.15, 308.15, 313.15, 318.15 K are shown in Table 2 and also excess properties are graphically represented in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, and 16, respectively \( V^{\text{E}} \), ∆η, \( G^{{*{\text{E}}}} \) and \( \kappa_{S}^{\text{E}} \) are calculated from the experimental measurements by Eqs. 2–9:
where for each equation, ρ, V, and η are the density, the molar volume and the dynamic viscosity of the mixtures and x i , V i , M i , and η i (i = 1, 2) are the mole fraction, molar volume, the molar mass and the dynamic viscosity of the components 2-chloroaniline (1) with chlorotoluene (2), respectively. R is the gas constant and T the absolute temperature
where \( \kappa_{S}^{\text{id}} \) is the ideal value of the isentropic compressibility and was calculated from the following equation [17]:
where φ i is ideal state volume fraction of component i in mixture and is defined by relation:
T is temperature and \( \kappa_{{{S}i}} \), \( V_{i}^{\text{o}} \), \( \alpha_{i}^{\text{o}} \) and \( C_{pi} \) are isentropic compressibility, molar volume, coefficent of isobaric thermal expansion and molar heat capacity, respectively, for pure component I; \( \alpha_{i}^{\text{o}} \) was calculated from measured densities by relation:
3.1 Theory
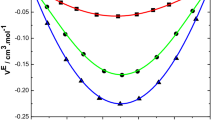
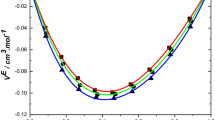
The observed \( V^{\text{E}} \) values are the resultant of physical and chemical forces and they may be recognized as: (i) the breaking of liquid order on mixing with the second component; (ii) non-specific physical interactions and unfavorable interactions between unlike molecules; (iii) specific interactions appearing in the mixture between dissimilar molecules by hydrogen bond formation; and (iv) specific dipole–dipole interactions in the mixture between solvent and co-solvent molecules. The first two factors contribute for the expansion of volume and the latter two factors contribute to the reduction of the volume. From the \( V^{\text{E}} \) curves shown in Figs. 1, 2, 3, and 4, it is clear that the volume reducing factors are dominant over the expansion factors in the present systems. The negative excess molar volumes show that the volume reduction factors play an important role between unlike molecules. This indicates the formation of hydrogen bond complexes. Thus, the observed negative \( V^{\text{E}} \) indicate the predominance of formation of (–NH····π) bonds over the rupture of bonds present in pure 2-chloroaniline molecules. 2-Chloroaniline and chlorotoluenes are polar solvents; their high dipole moments favor dipole–dipole interactions. Hence, there will be dipole–dipole interactions between unlike molecules of the three systems, contributing to the reduction in the volume.
An examination of curves in Figs. 1, 2, 3, and 4 indicates that the excess molar volumes for all of the binary systems are negative over the entire composition range at 303.15, 308.15, 313.15 and 318.15 K.
The algebraic values of \( V^{\text{E}} \) for the binary mixtures of 2-chloroaniline with chlorotoluenes fall in the following order: o-chlorotoluene < m-chlorotoluene < p-chlorotoluene.
The above order suggests that the dipole moments of the pure solvents are influencing the \( V^{\text{E}} \) data of the binary liquid mixtures. The dipole moments are 2-chloroaniline (1.78 D), o-chlorotoluene (1.56 D), m-chlorotoluene (1.82 D) and p-chlorotoluene (2.21 D). This type of behavior was observed earlier [8, 9]. The more negative \( V^{\text{E}} \) data of p-chlorotoluene when compared with other chlorotoluenes are due to its high dipole moment, which leads to stronger dipole–dipole interactions. Further, introduction of a chloro atom into the toluene molecule influences the magnitude of \( V^{\text{E}} \) to a considerable extent. This type of behavior was reported earlier [18].
An examination of plots in Figs. 5, 6, 7, and 8 reveal that \( \kappa_{S}^{\text{E}} \) is negative over the entire composition range for the binary mixtures of 2-chloroaniline with chlorotoluenes. This may be attributed to the relative strength of the following effects which influences the free spaces between component molecules [19, 20].
-
(a)
Loss of dipolar association and difference in size and shape of component molecules leads to decrease in speed of sound and increase in isentropic compressibility.
-
(b)
Dipole–dipole interactions, electron donor–acceptor interactions or charge-transfer complexes between unlike molecules lead to an increase in speed of sound and decrease in isentropic compressibility. If the strength of the interaction between the components increases, the magnitudes of the parameters become greater. In the present investigation, there is a possibility of electron donor–acceptor type or charge transfer interactions between the nitrogen atom of the amino group of 2-chloroaniline molecule, which acts as donor and the π-electron of the benzene ring of aromatic hydrocarbons, which act as acceptors (chlorotoluenes due to +M effect) resulting in negative \( \kappa_{S}^{\text{E}} \) values [21].
The algebraic values of \( \kappa_{S}^{\text{E}} \) for the binary mixtures of 2-chloroaniline with chlorotoluene are in the following order: o-chlorotoluene < m-chlorotoluene < p-chlorotoluene.
Viscosities of the binary mixtures of 2-chloroaniline with the chlorotoluenes are given in Figs. 9, 10, 11, and 12, which indicate that the deviations in viscosity (∆η) of all the binary mixtures are positive over the entire composition range at 303.15, 308.15, 313.15 and 318.15 K. Fort and Moore [22] observed that the positive deviations in viscosity indicate the specific interactions involving the formation of hetero-molecular complexes. The deviation in viscosity variation gives a qualitative estimation of the strength of the intermolecular interactions. The deviation in viscosity [23] may be generally explained by considering the following factors:
-
(i)
The difference in size and shape of the component molecules and the loss of dipolar association in pure component may contribute to a decrease in viscosity.
-
(ii)
Specific interactions between unlike components such as dipole–dipole interactions and charge transfer complexes may cause increases in viscosity in mixtures compared to the pure components. The former effect produces negative deviations in viscosity and the latter effect produces positive deviations in viscosity.
The positive values of viscosity deviation for the binary systems investigated suggest that the viscosities of associates formed between unlike molecules are relatively greater than those of the pure components. The deviations in viscosity are found to be opposite in sign to the excess molar volumes for all binary mixtures, which is in agreement with the view proposed by Brocos et al. [24, 25].
According to Reed and Taylor [26] and Palepu et al. [27], the excess Gibbs energy of activation of viscous flow (\( G^{{*{\text{E}}}} \)) may be considered as a reliable criterion to detect or exclude the presence of interaction between unlike molecules. According to these authors, the magnitude of the positive \( G^{{*{\text{E}}}} \) values is an excellent indicator of the strength of specific interactions. The results presented in Figs. 13, 14, 15, and 16 indicate that excess Gibbs energies of activation of viscous flow are positive for three binary mixtures over the entire composition range at all investigated temperatures. The positive values of excess Gibbs energy of activation of viscous flow for the binary systems investigated suggest that the specific interaction between 2-chloroaniline and chlorotoluene molecules takes place through dipole–dipole interaction. Thus, the values of deviation in viscosity and excess Gibbs energy of activation of viscous flow are dependent on the position of the –Cl group in the toluene molecule, indicating a different extent of molecular interactions in chlorotoluene.
The experimental values of density (ρ) and speed of sound (u) were used to calculate the values of acoustic impedance, Z, intermolecular free length (L f). The values of ∆u, ΔZ, and \( L_{\text{f}}^{\text{E}} \) were evaluated with help of the following relation:
where Y E is ∆u, ∆Z, and \( L_{\text{f}}^{\text{E}} \).
The sign and magnitude of the observed values of ∆u, ∆Z, and \( L_{\text{f}}^{\text{E}} \) on mixing were found to depend upon several contributions, which are physical or/and chemical in nature. The physical contributions comprise the dispersion forces and non-specific physical weak interactions that lead to negative values in ∆u, ∆Z or positive \( L_{\text{f}}^{\text{E}} \), hence in order (intermolecular interaction) in the system. Chemical contributions involve breaking up of H-bonded structures, if any, resulting in positive \( L_{\text{f}}^{\text{E}} \) or negative values in ∆u, and ∆Z and specific interactions such as formation of new H-bonds, charge transfer complex and strong dipole–dipole interactions between component molecules result in positive ∆u, and ∆Z and negative \( L_{\text{f}}^{\text{E}} \) values, making the system more ordered due to increased intermolecular interactions (Table 3).
An examination of data in the Table 4 shows that ∆Z values are positive for all binary systems over the entire composition range at 303.15, 308.15, 313.15 and 318.15 K. The positive values of deviation in acoustic impedance for the binary systems investigated suggest that the specific interaction between unlike molecules in mixtures are dominant over the breaking of dipolar interactions between like molecules in the mixture.
The variation of V E, \( \kappa_{S}^{\text{E}} \) and ∆η with mole fraction were fitted to the Redlich–Kister polynomial equation [28] of the type:
The values of a 0, a 1 and a 2 are the coefficients of the polynomial equation and the corresponding standard deviations, with σ obtained by the method of least-squares with equal weights assigned to each point. The standard deviation (σ) is defined as:
where n is the total number of experimental points and m is the number of coefficients. The values of a 0, a 1 and a 2 are determined by a multiple-regression analysis using the least-squares method and summarized along with the standard deviations between the experimental and fitted values of V E, \( \kappa_{S}^{\text{E}} \), and ∆η in Table 5.
Knowledge of the viscosities of the pure liquids and respective mixtures and study of the viscosity calculation methods are important for practical and theoretical purposes. Over the last years, numerous equations for liquid mixture viscosity have been proposed. Methods concerning viscosity modeling can be found in the literature [29, 30]. In the present work, three typical semi-empirical relations are used to correlate the experimental viscosity data of the investigated binary systems.
Grunberg–Nissan provided the following empirical equation containing one adjustable parameter [31]:
where d 12 may be regarded as a parameter proportional to the interchange energy, also an approximate measure of the strength of the interaction between the components.
Katti and Chaudhri [32] proposed the following equation:
where W vis /RT is an interaction term.
Hind et al. [33] suggested an equation for the viscosity of binary liquid mixtures as:
where H 12 is Hind’s interaction parameter and is attributed to unlike pair interactions.
Heric and Brewer [34] proposed the following equation:
where Δ12 is the interaction term and other symbols have their usual meaning.
The one-parameter equation due to Tamura and Kurata [35] has the form:
where Φ1 and Φ2 are the volume fractions of components 1 and 2, respectively, T 12 is Tamura and Kurata constant.
The interaction parameter d 12 is positive for binary systems. Nigam and Mahl [36] concluded from the study of binary mixtures that (i) if ∆η > 0, d 12 > 0 and magnitude of both are large then strong specific interaction, (ii) if ∆η < 0, d 12 > 0 then weak specific interaction, and (iii) if ∆η < 0, d 12 < 0 magnitude of both are large then the dispersion force will be dominant. Fort and Moore [19] reported that for any binary liquid mixture, a positive value of d 12 indicates the presence of specific interactions and a negative value of d 12 indicates the presence of weak interactions between the unlike molecules. On this basis, we can say that there is a strong interaction in the binary systems studied.
The interaction parameter W vis/RT shows almost the same trend as that of d 12. In fact, one could say that the parameters d 12 and W vis/RT exhibit almost similar behavior, which is not unlikely in view of logarithmic nature of both equations. The values of interaction parameters of Tamara and Kurata (T 12) and Hind et al. (H 12) do not differ appreciably from each other. This is in agreement with the view put forward by Fort and Moore [19] in regard to the nature of parameters T 12 and H 12. The experimental and theoretical values of viscosity of the liquid mixtures calculated using Eqs. 13–16, including standard deviation, are presented in Table 3. The Grunberg–Nissan relation gives better results than the comparison to other theoretical relations. The differences between experimental and theoretical values are greater for other relations studied here.
4 Conclusions
Experimental data of viscosity, speed of sound, and density are reported for binary mixtures of 2-chloroaniline with o-chlorotoluene, m-chlorotoluene, p-chlorotoluene over the entire range of mole fraction at 303.15, 308.15, 313.15 and 318.15 K. Calculated excess molar volume, excess isentropic compressibility, deviation in viscosity, and excess Gibbs energy of activation of viscous flow are fitted with Redlich–Kister type polynomial equation. Positive values of the deviation in viscosity and excess Gibbs energy of activation of viscous flow, and negative values of excess molar volume and excess isentropic compressibility over the whole composition range are observed for all the investigated binary systems. Thermodynamic functions of activation have been estimated for each binary mixture. The viscosity data have been correlated with several semi-empirical equations (Grunberg–Nissan, Katti–Chaudhri, and Hind et al.). The Grunberg–Nissan relation gives better results than the other theoretical relations. The excess/deviation properties and positive values of viscosity interaction parameter can be interpreted by considering the intermolecular charge transfer complexes, molecular size and shapes of the components. The strong intermolecular interactions have significant effect on the thermodynamic and transport properties of the investigated binary mixtures.
References
Lakshmi, B.J., Sankar, M.G., Rambabu, C., Ramachandran, D.: Volumetric, ultrasonic and viscometric studies of binary liquid mixures of N-ethylaniline + chlorobenzene, + bromobeneze, + 1,2-dichlorobenzene + 1,3-dichlorobenzene + 1,2,4-trichlorobenzene at 303.15 and 308.15 K. Kor J. Chem. Eng. 31, 881–895 (2014)
Jareena, S., Sankar, M.G., Ramachandran, D., Rambabu, C.: Orientation effect on sign and magnitude of excess thermodynamic functions of non electrolyte solutions at different temperatures (303.15 K, 308.15 K, and 313.15 K) Kor. J. Chem. Eng. (2014). doi:10.1007/s11814-014-0088-1
Gowrisankar, M., Venkateswarlu, P., Siva Kumar, K., Sivarambabu, S.: Density, ultrasonic velocity, viscosity and their excess parameters of the binary mixtures of N, N-dimethylaniline + 1-alkanols (C3–C5), + 2-alkanols (C3–C4), + 2-methyl-1-propanol, + 2-methyl-2-propanol at 303.15 K. Kor. J Chem. Eng. 30, 1131–1141 (2013)
Gowrisankar, M., Venkateswarlu, P., Siva Kumar, K., Sivarambabu, S.: Ultrasonic studies on molecular interactions in binary mixtures of n-methyl aniline with methyl isobutylketone, + 3-pentanone, and + cycloalkanones at 303.15 K. J. Solution Chem. 42, 916–935 (2013)
Gowrisankar, M., Venkateswarlu, P., Siva Kumar, K., Sivarambabu, S.: Thermodynamics of amine + ketone mixtures: volumetric, speed of sound data and viscosity at 303.15 K and 308.15 K for the binary mixtures of N,N-diethylaniline + aliphatic ketones (C3–C5) + 4-methyl-2-pentanone. Arab. J. Chem. (2013). doi:10.1016/j.arabjc2013.09.042
M, Gowri Sankar, Venkateswarlu, P., Siva Kumar, K., Sivarambabu, S.: Volumetric, speed of sound data and viscosity at (303.15 and 308.15)K for the binary mixtures of N,N-dimethylaniline + aliphatic ketones (C3–C5), + 4-methyl-2-pentanone, + acetophenone, + cyclicketones. J. Ind. Eng. Chem. 20, 405–418 (2014)
Syamala, V., Raja Sekhar, D., Siva Kumar, K., Venkateswarlu, P.: Volumetric, ultrasonic and transport properties of binary liquid mixtures containing dimethylformamide at 303.15 K. Chin. J. Chem. 25, 32–43 (2007)
Madhusundhana Reddy, P., Sivakumar, K., Venkatesu, P.: Densities and ultrasonic studies for binary mixtures of tetrahydrofuran with chlorobenzenes, chlorotoluenes and nitrotoluenes at 298.15 K. Fluid Phase Equilibr. 310, 74–81 (2011)
Syamala, V., Siva Kumar, K., Venkateswarlu, P.: Volumetric, ultrasonic and viscometric studies of binary mixtures of dimethyl sulphoxide with chloro and nitro substituted aromatic hydrocarbons at T = 303.15 K. Chem. Thermodyn. 38, 1553–1562 (2006)
Venkatramana, L., Sivakumar, K., Gardas, R.L., Dayananda Reddy, K.: Effect of chain length of alcohol on thermodynamics properties their binary mixtures of benzyl alcohol. Thermochim. Acta 581, 123–132 (2014)
Jeevanandham, P., Kumar, S., Periyasamy, P.: Densities, viscosities, refractive indices and excess properties of ortho- and meta-chloroaniline with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 188, 203–209 (2013)
Schaaffs, W.: In: Hellwege, K.H. (ed.) Molekularakustik. Springer, Berlin (1975)
Bhatia, S.C., Rani, R., Sangwan, J., Bhatia, R.: Densities, viscosities, speeds of sound, and refractive indices of binary mixtures of 1-decanol with isomeric chlorotoluenes. Int. J. Thermophys. 32, 1163–1174 (2011)
Jovanovic, J., Knezevic-Stevanovic, A., Grozdanic, D.: Prediction of high pressure liquid heat capacities of organic compounds by a group contribution method. J. Serb. Chem. Soc. 76, 417–423 (2011)
Shaw, R.: Heat capacity of liquids. Estimation of heat capacity at constant pressure and 25 °C using additivity rules. J. Chem. Eng. Data 14, 461–465 (1969)
Syamala, V., Venkateswarlu, P., Sivakumar, K.: Excess volumes, speeds of sound, isentropic compressibilities, and viscosities of binary mixtures of acetophenone with chlorotoluenes and nitrotoluenes at 303.15 K. J. Chem. Eng. Data 51, 928–934 (2006)
Benson, G.C., Kiyohara, O.: Evaluation of excess isentropic compressibilities and isochoric heat capacities. J. Chem. Thermodyn. 11, 1061–1064 (1979)
Palaiologou, M.M., Molinou, I.E.: Excess volumes of ethyl acetate + toluene, + o-chlorotoluene, and + p-chlorotoluene from 283.15 to 303.15 K. J. Chem. Eng. Data 40, 880–882 (1995)
Jacobson, B.: Intermolecular free lengths in the liquid state. I. Adiabatic and isothermal compressibilities. Acta. Chem. Scand. 8, 1485–1498 (1952)
Jacobson, B.: Ultrasonic velocity in liquids and liquid mixtures. J. Chem. Phys. 20, 927–928 (1952)
Rathnam, M.V., Sudhir, M., Kumar, M.S.S.: Density, excess volume, and viscosity of vinyl acetate or benzyl acetate with (o-, m-, p-)xylenes and ethylbenzene at T = (303.15 and 313.15) K. J. Solution Chem. 39, 1735–1748 (2010)
Fort, R.J., Moore, W.R.: Adiabatic compressibilities of binary liquid mixtures. Trans. Faraday Soc. 61, 2102–2111 (1965)
Joshi, S.S., Aminabhavi, T.M., Balundgi, R.H.: Excess properties of binary liquid mixtures of nitrobenzene with aliphatic liquids in the temperature range of 298.15 to 313.15 K. Ind. J. Technol. 29, 541–544 (1991)
Brocos, P., Pineiro, A., Bravo, R., Amigo, A.: Refractive indices, molar volumes and molar refractions of binary liquid mixtures: concepts and correlations. Phys. Chem. Chem. Phys. 5, 550–557 (2003)
Pineiro, A., Brocos, P., Amigo, A., Pintos, M., Bravo, R.: Prediction of excess volumes and excess surface tensions from experimental refractive indices. Chem. Phys. Liq. 38, 251–260 (2000)
Reed, T.M., Taylor, T.E.: Viscosities of liquid mixtures. J. Phys. Chem. 63, 58–62 (1959)
Palepu, R., Oliver, J., Mackinnom, B.: Viscosities and densities of binary liquid mixtures of m-cresol with substituted anilines. Can. J. Chem. 63, 1024–1030 (1985)
Redlich, O., Kister, A.T.: Thermodynamics of non electrolytic solutions. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Lei, Q., Hou, Y.: Correlation of viscosity of binary liquid mixtures. Fluid Phase Equilib. 154, 153–163 (1999)
Lei, Q., Hou, Y., Lin, R.: Correlation of viscosity of pure liquids in a wide temperature range. Fluid Phase Equilib. 140, 221–231 (1997)
Grunberg, L., Nissan, A.H.: Vaporisation, viscosity, cohesion and structure of the liquids. Nature 164, 799–800 (1949)
Katti, P.K., Chaudhri, M.H.: Viscosities of binary mixtures benzyl acetate with dioxane, aniline and m-cresol. J. Chem. Eng. Data 9, 442–443 (1964)
Hind, R.K., McLaughlin, E., Ubbelohde, A.: Structure and viscosity of liquid camphor and pyrene mixtures. Trans. Faraday Soc. 56, 328–330 (1960)
Heric, E.L., Brewer, J.G.: Viscosity of some binary liquid nonelectrolyte mixtures. J. Chem. Eng. Data 12, 574–583 (1967)
Tamura, M., Kurata, M.: Viscosity of a binary mixture of liquids. Bull. Chem. Soc. Jpn. 25, 32–37 (1952)
Nigam, R.K., Mahl, B.S.: Excess enthalpies and weak interactions in liquid mixtures of methylene chloride with benzene, toluene and xylenes. J. Chem. Soc. Faraday Trans. 68, 1508–1512 (1972)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chand, G.P., Sankar, M.G., Ramachandran, D. et al. Densities, Viscosities and Speeds of Sound of Binary Mixtures of 2-Chloroaniline with o-Chlorotoluene, m-Chlorotoluene and p-Chlorotoluene at Different Temperatures. J Solution Chem 45, 153–187 (2016). https://doi.org/10.1007/s10953-016-0439-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0439-0