Abstract
In the current study, bioelectricity and polyhydroxyalkanoate (PHA) production were integrated by a dual chamber microbial fuel cell (MFC) using Enterobacter sp. TS1L and textile wastewater (TW), and the method was evaluated. The highest open circuit voltage (OCV, 986.50 mV), current density (CD, 6.04 W/m3) and power density (PD, 0.32 W/m3) were observed when sterile TW was used as the substrate. Sterilization had no effect on electricity generation, while the OCV, CD and PD were not significantly different. The prolong MFC was operated by Enterobacter sp. TS1L using non-sterile TW for a period of 7 days. Throughout the 7-day operation, the experiment yielded a consistent CD output, ranging from 1.56 A/m3 to 2.31 A/m3. After MFC treatment, the quality of the effluent surpassed the Thailand water quality standard. A high percentage of decolorization (72.9%) was observed by Enterobacter sp. under TW-fed MFC. Thereafter, biomass was collected, then, PHA was extracted and characterized. The highest biomass (3.5 ± 0.6 g/L) and PHA (56.1 ± 1.5% cell dry mass, CDM) level were observed at 6 days of MFC operation. The isolated polymer from Enterobacter sp. TS1L was confirmed to be medium chain length-co-long chain length PHA (mcl-co-lcl PHA) containing 3-hydroxyhexadecanoate (3HD, C14) and 3-hydroxyoctadecanoate (3HOD, C18).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile industry is a multi-stage operation process that produces significant amount of highly toxic wastewater. It has been reported that textile processes consume 100–350 m3 of water for a ton of textile product, and 17–20% of wastewater is produced [1, 2]. Textile wastewater (TW) contains high amounts of chemicals, dyes and auxiliaries. Approximately 1.5 × 104 tons of dyestuff are lost during different textile process occurs in the TW [1]. Therefore, TW seriously damages the environment [3]. The development of effective, economical, and easy-to handle alternative treatment technology for TW is of significant interest. Various methods can be used to treat TW, such as chemical, physical, physiochemical, oxidation and biological methods [4]. However, many treatment processes, such as ultrafiltration, nanofiltration and reverse osmosis, are expensive and unaffordable for small and medium enterprises. In addition, traditional physiochemical and biological methods are not suitable for TW treatment because this waste contains a lower ratio of biological oxygen demand to chemical oxygen demand (BOD5/COD) [5]. In our previous study, we successfully developed a TW treatment process using Enterobacter sp. This strain could decolorize culture medium supplemented with 50 mg/L methylene blue (MB) and 100% TW at 92.6 and 72.3%, respectively [6]. Interestingly, the value-added polymer polyhydroxyalkanoate (PHA) was produced (88.66% of cell dry mass) by Enterobacter during wastewater treatment process via β-oxidation pathway [7, 8]. PHA is biodegradable polyester produced microorganism. These polymers are potential candidate for substitution of petrochemical plastics. PHA are applied in many fields, such as medicine, agriculture and packaging. However, the process using Enterobacter sp. is limited by a high electric cost. To solve this problem, a method that can produce electricity from TW, such as a microbial fuel cell (MFC) would be very attractive [9,10,11,12]. Shameeda and Rahman [13] reported the effectiveness of MFC for TW treatment. The maximum COD removal of 77.03% was detected at a concentration of 1,350 mg COD/L. The MFC produced a maximum current of 4.8 mA and power density of 16.8 mW/m2. Rezaei et al. [14] found that with cellulose as a substrate, Enterobacter cloacae produces bioelectricity in MFCs with a high efficiency. Enterobacter sp. are attractive bacteria for wastewater treatment because they can use a wide range of substrates to generate bioenergy and can completely degrade complex substrates. A current yield between 0.2 and 4.4 A/m2 (corresponding to 0.005 and 2.0 W/m2) was detected in MFCs using Enterobacter with various substrates, such as glucose, cellulose, acetate and Luria–Bertani (LB) broth [15]. Hsueh et al. [16] reported the decolorization of reactive green 19 (RG19) by Enterobacter cancerogenus BYm30 using a single chamber membrane-less MFC (SCML-MFC). The decolorization rate of RG19 in Enterobacter-MFC was faster than that in non-MFC culture. The treatment of rhodamine B (RB) contaminated wastewater by laccase and manganese peroxide-producing bacteria using MFC was also evaluated by Chaijak et al. [17]. The findings indicated that 4,816 mA/m3 and 2,320.08 mW/m3 were obtained. In addition, RB was removed at 80.56%.
This current study aimed to investigate the ability of Enterobacter sp. to produce bioelectrics and PHA in a dual chamber MFC. The production of electricity and PHA was demonstrated in modified nutrient broth (NB), synthetic wastewater and TW. In addition, the effect of non-sterile TW on electricity was assayed. The characteristics of TW before and after treatment by MFC were determined and compared with the Thailand water quality standard (Pollution Control Department, Thailand). Afterward, Enterobacter cells were harvested, and PHA was extracted and characterized. This is the first reported on PHA recovery from MFC. The majors advantageous of the present study including (1) reduce TW treatment using bioelectricity generate by MFC and (2) the value-added production of PHA. Therefore, it is economically sustainable and industrially very important.
Materials and Methods
Microorganism and Culture Conditions
Enterobacter sp. strain TS1L was provided by Rakkan and Sangkharak [18]. This strain was isolated from TW (Phatthalung, Thailand) and deposited in GenBank (No. MN508473). This strain was stored in methylene blue-containing nutrient agar (MB-NA) plates containing peptone 10 g/L, beef extract 1 g/L, yeast extract 2 g/L, NaCl 5 g/L, methylene blue (MB) 50 mg/L and agar 15 g/L at an initial pH of 7 [19]. Afterward, the strain was aerobically cultivated in MB-containing nutrient broth (MB–NB) at 35 °C in an orbital shaker at 150 rpm. The cells were collected after 48 h at the late log-phase by centrifugation at 8,000 × g for 10 min at 4 °C. The cells were washed three times and resuspended in MB–NB. The cell suspension was used as the starter for the MFC experiments.
TW Collection and Characterization
TW was collected from the Ban Phraek Weaving Group (Phatthalung, Thailand). Samples were placed on ice and transferred to the laboratory. The sample was preserved at − 25 °C before use. The characteristics of wastewater were determined following standard methods described by Rakkan and Sangkharak [18] including pH, total dissolved solids (TDS), settleable solids, total suspended solids (TSS), fat, oil and grease (FOG), sulfide, total Kjeldahl nitrogen (TKN), COD and color.
MFC Design and Construction
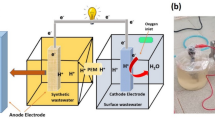
The dual chamber MFC was constructed based on a modified model proposed by Khajeh et al. [20]. The cathodic and anodic chambers were established using a 125 mL suction flask. A silicon tube with a diameter of 1 cm was employed as a salt bridge, which was filled with a gel containing 1 mol/L KCl [21]. For the catholyte, a solution of 400 µmol/L KMnO4 was utilized [22]. The cathodic electrode consisted of a copper (Cu) plate measuring 1 × 2 cm, while the anodic electrode was formed by an aluminum (Al) plate of the same dimensions. The MFC bioreactor and its schematic diagram were illustrated in Fig. 1.
Electricity Generation in MB–NB
First, the potential of electricity generation was evaluated in MB-NB. A 90 mg/L of MB was added to NB and used as the anolyte in the experimental setup. 10 mL of 48 h-old bacterial seed culture were combined with 90 mL of MB-NB. This mixture was then introduced into the anodic chamber of the setup. Simultaneously, a cathodic chamber was filled with 100 mL of a solution containing 400 µM KMnO4. The open-circuit voltage (OCV) was recorded at 10-min intervals over a period of 3 h to monitor any changes in electrical potential. Additionally, measurements of the close-circuit voltage (CCV) were obtained while varying the external resistance within a range of 1 to 5,000 Ω. A polarization curve was constructed, illustrating the relationship between the applied external resistance and the corresponding values of maximum power density and maximum current density. The open-circuit voltage (OCV) and close-circuit voltage (CCV) were monitored and recorded during the observation period. MB-NB without bacterial inoculation was used as a control.
Electricity Generation in MB-synthetic Wastewater and TW
The potential of bioelectric generation in synthetic wastewater and TW was also determined. MB-synthetic wastewater and TW were prepared as follows. Synthetic wastewater was prepared as described by Thipraksa and Chaijak with modifications [22]. The MB-synthetic wastewater contained 255.50 mg/L NaHCO3, 21.70 mg/L KH2PO4, 15.60 mg/L MgSO4, 2.50 mg/L CaCl2, 0.08 mg/L (NH4)2SO4, and 50 mg/L MB. The OCV was recorded at 10-min intervals over a period of 3 h. Additionally, the CCV values were obtained while varying the external resistance within a range of 1 to 5,000 Ω. A polarization curve was constructed.
Effect of Sterile and Non-sterile TW on Electricity Generation
TW was also used as a substrate for electricity in the MFC. TW was centrifuged (3,000 × g, 10 min) to remove contaminated sediment. For the sterile condition, textile wastewater was autoclaved at 121 °C for 15 min. Afterward, the effect of sterile TW and non-sterile TW was studied. The electricity production from MB-NB, MB-synthetic wastewater and TW was compared.
Effect of Prolong Operation on Bioelectric Generation, PHA Production and Decolorization
A total of 90 mL of non-sterile wastewater was carefully combined with 10 mL of bacterial seed to create the mixture, which was subsequently introduced into the anodic chamber. In addition, the effect caused by a prolong operation period on the current output was also investigated. The experiment spanned 7 consecutive days during which the CCV at 1,000 Ω was recorded at 24 h intervals. Systematic data was collected to establish and verify the current output performance under prolonged operating conditions. The bioelectric production of sterile and non-sterile TW was compared. In addition, 5 mL of bacterial culture was harvested every day for 7 days. The sample was centrifuged, and the cell were collected for PHA extraction and characterization. The supernatant was also collected and subjected to characterization. In addition, the percentage of decolorization was determined.
Decolorization Measurement
The decolorization efficiency was measured in a UV–Vis spectrophotometer at 540 nm. The TW after MFC was collected and centrifuged at 8,000 × g for 10 min prior to measurements [23]. The percentage of decolorization was calculated as follows:
Cell Recovery and PHA Extraction
Cells from the non-sterile TW MFC chamber were washed twice with sterile distilled water. The collected cell was dried in a hot air oven (80 °C) until a constant weight was observed. The concentration of cells was calculated using the following formula:
To determine the content of PHA, dried cells were resuspended in 10 mL of 4% HClO and incubated at 37 °C and150 rpm for 2 h. The mixture was then centrifuged (8,000 × g, 10 min), and then, the residue was collected and washed twice with 5 mL of mixed solvent (acetone:ethanol:diethyl ether,1:1:1). PHA was obtained by centrifugation (8,000×g, 10 min) [24]. The PHA content was calculated using the following formula:
Determination of PHA Functional Groups and Composition
The functional groups of PHA were analyzed by Fourier transformed infrared (FTIR) spectrophotometry. The spectra of the PHA were recorded in the wavenumber range from 650 to 4,000 cm−1 (Jasco FTIR-6100, Japan) [25]. The sample was prepared in a 50 mL glass round bottom flask. A 2 mg of PHA was mixed with 2 mL of CHCl3 and 2 mL of CH3OH. Afterward, 0.5 mL of H2SO4 was also added. The sample was refluxed in silicone oil (100 °C, 2 h) and then cooled to room temperature (25 °C). Afterward, distilled water (2 mL) and saturated NaHCO3 (2 mL) were added. The mixture was left to separate, and only the bottom layer (CHCl3) was collected. Residual water was removed using Na2SO4. The sample was filtered through a 0.45 μm nylon membrane. The filtrate was subjected to gas chromatography (GC) analysis [26].
PHA was analyzed by a Hewlett Packard GC-6890 with an HP-INNOWAX capillary (30 m long, 0.25 mm internal diameter, 0.25 μm film thickness). The initial column temperature was set at 65 °C (5 min), and then the temperature was increased at 10 °C/min to 150 °C. Then, the temperature was increased to 200 °C at a rate of 5 °C/min, and the temperature was set at 200 °C for 6.5 min. The injection temperature was set at 250 °C. The temperature of the MS quadrupole (150 °C) and MS source (230 °C) was set. A constant flow of carrier gas (helium) was set at 1 mL/min.
Statistical Analysis
All experiments were run in triplicate. A completely randomized design was used throughout this study. Data were subjected to analysis of variance (ANOVA), and mean comparison was carried out using Duncan’s multiple range test [27]. All analyses were performed using the statistical package for social science, SPSS (SPSS 24 for Windows, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Electricity Generation in MB–NB
First, Enterobacter sp. TS1L was cultivated in NB to evaluate its ability to produce bioelectricity. The results indicate that the bacterium Enterobacter sp. TS1L cultured in NB exhibited a significant increase in the OCV, with a maximum value of 134.87 mV (Fig. 2A). This OCV peak was achieved after an operation time of 170 min. In contrast, the maximal OCV generated by the control (NB without bacteria) was notably (64.20 mV). These findings highlight the superior electrochemical performance of Enterobacter sp. TS1L in NB, suggesting its potential as a promising candidate for applications requiring high OCV output. The polarization curve analysis demonstrated that peak values were attained for the maximal current density (CD) and power density (PD). Specifically, when the bacterium Enterobacter sp. TS1L was cultured in NB under optimal external resistance conditions, a remarkable CD of 3.44 A/m3 and a corresponding PD of 0.04 W/m3 were achieved (Fig. 2B). These results underscore the favorable electrochemical performance of Enterobacter sp. TS1L in the culture medium, emphasizing its potential as an efficient and viable option for applications demanding high current and power densities in microbial electrochemical systems. This study indicated that Enterobacter sp. TS1L can be used as an electrochemically active bacterium. Generally, most microbes are electrochemically inactive and need mediators, such as methyl viologen, thionine and humic acid, to transfer electrons to the electrode. To date, some bacterial genera have been reported as electrochemically active bacteria that can produce electricity by transferring electrons to electrodes without the facilitation of mediators [28]. These electrochemically active bacteria include Geobacter, Enterobacter, Shewanella and Bacillus. Therefore, a pure culture of Enterobacter sp. TS1L was used throughout this study. Compared to using a mixed microbial community, utilizing a single strain in an MFC system may be advantageous, it is easier to control the optimal conditions for one strain and determine the mechanism and physiological details [28].
Moreover, the capability of the bacterium Enterobacter sp. TS1L to degrade textile dye and produce bioelectricity was also demonstrated in NB containing 50 mg/L MB (MB-NB medium). Both the OCV and polarization curve were evaluated as part of the study. The results revealed a highly impressive maximal OCV of 1,138.00 mV (Fig. 3A). Moreover, under these experimental conditions, the bacterium demonstrated a substantial CD of 0.81 A/m3 and an associated PD of 0.11 W/m3 (Fig. 3B). These findings highlight the significant electrochemical potential of Enterobacter sp. TS1L in the presence of MB within the culture medium, suggesting its proficiency in electricity generation. Due to these attributes, the bacteria are a promising candidate for potential applications in microbial electrochemical systems in which higher OCV, CD, and PD are desirable.
The decolorization mechanism of Enterobacter sp. in MFCs involves a similar step as that of other microorganisms. First, color is removed by a biodegradation mechanism rather than biosorption by bacterial cells [29]. At the anode, bacteria produce protons and electrons by oxidizing organic substrates. Then, the product is transported to the cathode by a proton exchange membrane and an external circuit [28]. Azo bonds of MB are broken under anaerobic conditions in the anode compartment, and the dye and intermediates are completely degraded abiotically under aerobic conditions in the cathode chamber [30]. Aerobic treatment results in the complete reduction of the dye. The biological process utilized by Enterobacter sp. to degrade MB was already demonstrated by Rakkan and Sangkharak [18]. These bacteria can degrade MB by oxidative deamination and carboxylation, generating phthalic acid and di(2-propylpentyl) ester, which are transformed into various fatty acids and aldehydes can directly/indirectly enter the oxidation pathway to produce acetyl-CoA, NaDH2 and FADH2.
Electricity Generation in Synthetic Wastewater
Bioelectricity production by Enterobacter sp. TS1L in synthetic wastewater containing 50 mg/L MB was also demonstrated. The OCV and polarization curve were recorded. The experimental findings demonstrated a remarkably high maximal OCV of 1,885.00 mV (Fig. 4A). Additionally, during the stationary phase, the bacterium exhibited impressive electrochemical performance, attaining a maximum CD of 0.92 A/m3 and an accompanying PD of 0.15 W/m3 (Fig. 4B). These outcomes underscore the remarkable electrical properties of Enterobacter sp. TS1L when exposed to synthetic wastewater containing MB. Moreover, the observed values of OCV, CD, and PD highlight the considerable potential of this bacterium for generating electricity in microbial electrochemical systems, making it a promising candidate for various practical applications.
Effect of Sterile and Non-sterile TW on Electricity Generation
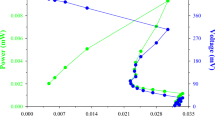
The effect of sterile and non-sterile TW on the electricity-generating capacity of Enterobacter sp. TS1L was evaluated. For sterile TW, the sample was subjected to autoclaving at 121 °C for 15 min before being employed as the anolyte in the experiments. The results revealed an impressive maximal OCV of 986.50 mV (Fig. 5A). Interestingly, Enterobacter sp. TS1L exhibited a remarkable CD of 6.04 A/m3 and a corresponding PD of 0.32 W/m3 using sterile TW (Fig. 5B).
To increase the feasibility and ease of operating MFC for industrial use, the effect caused by non-sterile TW was also determined. The bacterial seed was directly inoculated into the TW without any sterilization processes. The experimental results revealed a notable maximal OCV of 822.50 mV (Fig. 6A). Additionally, the polarization curve analysis demonstrated the electrochemical performance of the bacterium, wherein a maximum CD of 6.04 A/m3 and an accompanying PD of 0.32 W/m3 were produced (Fig. 6B). Similar values for CD and PD were observed in sterile and non-sterile TW. The result may due to the minimal disparity observed in the CD and PD between sterile and non-sterile wastewater samples in our MFC operation study can be attributed to the primary source of electrical energy being derived from textile dye compounds. These textile dyes are inherently resistant to degradation under autoclave conditions. Consequently, this leads to the similarity in CD and PD values between the sterile and non-sterile wastewater samples. The utilization of non-sterile wastewater further emphasizes its practical applicability in sustainable wastewater treatment and energy generation processes. A comparison between bioelectric generation from various dyes using different types of MFCs and inoculum is also provided in Table 1. The data showed that a more desirable PD was obtained from Enterobacter sp. compared with other reports. However, a lower CD was obtained compared with that of Mu et al. [31]. Based on the literature, most MFCs are operated by mixed consortiums. However, the production of electricity from mixed microbial communities and a single strain using Enterobacter sp. were not significantly different. There are too many factors that affect the performance of MFCs, including the type of bacteria and their metabolism, design of the MFC, anodic and cathodic chamber, type and concentration of substrate, operating conditions and type of membrane [28]. Therefore, a study to increase electricity production from Enterobacter sp. using TW is needed in the future.
Effect of Prolong Operation on Electricity Generation
The prolong electricity-generating capability of Enterobacter sp. TS1L using TW was also studied. The bacterial seed was directly inoculated into non-sterile TW, and the system was operated continuously for a period of 7 days. Throughout the 7-day operation, the experiment yielded a consistent CD output, ranging from 1.56 to 2.31 A/m3 (Fig. 7). It is essential to consider the relationship between the operating time and the growth of microorganisms or exoelectrogens, as this directly influences the electrical output. Specifically, when the MFC operates during the logarithmic growth phase of the bacterial population, we observe higher electrical energy generation. Conversely, if the operating time corresponds to the bacterial death phase, the electrical energy output decreases. This phenomenon aligns with findings from the study conducted by Akman et al. [37] as referenced in the link provided. Lower CD (2.31 A/m3) was obtained under prolong condition compared with the result from 3-h operation. it is important to recognize that power generation under prolong operation may depend on various parameters such as the control of operating conditions, MFC stability, the accumulation of inhibitors etc. Therefore, all variations are major barrier as it exerts a direct influence on the electrical energy generated.
Zhang et al. [38] reported an MFC operated with a biocathode manure for 171 days. The electricity decreased from Day 140 results from a lack of fuel. However, long-term MFCs are very limited and greatly needed, as they will be helpful for achieving the long-period stability and longevity of the process. These investigations will be useful in gaining a reliable method of recovery electricity and increasing the stability of the TW treatment infrastructure. These results demonstrate the sustained and reliable electricity generation potential of Enterobacter sp. TS1L when utilizing TW as a renewable and economically feasible substrate. The ability of the bacterium to consistently produce electricity over an extended duration underscores its potential applicability for prolong and practical use in microbial electrochemical systems. However, information on the effect caused by prolong operation of MFCs on stability, treatment efficiency, organic and nutrient removal and electricity generation is lacking [39]. Therefore, for future applications, a longer operation period will be employed and the overall performance of MFCs using Enterobacter sp. and TW will be studied in more detail to increase the potential of MFC as an applicable technology.
After MFC treatment, treated TW was collected. The supernatant and bacterial cells were separated. Cell-free TW was characterized and compared with the Thailand water quality standard. Microbial cells were collected and subjected to PHA accumulation.
Characterization of TW and Decolorization Efficiency
The characteristics of TW before and after MFC were determined (Table 2). Interestingly, the water quality of the textile effluent after the MFC passed the Thailand water quality standard. The initial characteristics of TW include pH 7.9, 27.5 °C, TDS 1,564 mg/L, SS 194 mg/L, TSS 340 mg/L, FOG 2 mg/L, sulfide 0.02 mg/L, TKN 42 mg/L, COD 2,995 mg/L and dark blue color. However, after the MFC process, the pH was reduced to 6.0 and 30 °C. A similar trend was also observed in other parameters. All parameters correlated with the Thailand water quality standard were significantly reduced. The final characteristics of TW after MFC were TDS 200 mg/L, SS < 0.1 mg/L, TSS 10 mg/L, FOG 1 mg/L, sulfide 0.01 mg/L, TKN 35 mg/L, COD 50 mg/L and light blue color. This result indicated that MFC could improve the quality of TW.
The decolorization efficiency of TW by the MFC was also investigated (Table 1). In this study, the MFC performance was different with the different substrate. The highest current density was obtained from TW. The result may due to the bioelectric generation is primarily attributed to the organic substrate used, whether it be wastewater or microbial medium. It is noteworthy that a higher organic content within the system leads to an increase in electrical energy production. This observation aligns with the findings of the study conducted by Zhao et al. [40]. In addition, the rationale behind the observed difference in electrical energy generation between TW and synthetic wastewater lies in the higher organic matter content present in TW. This organic matter serves as a richer bacterial feedstock compared to synthetic wastewater, consequently leading to increased microbial activity and enhanced electrical energy production. The power generation was related with decolorization efficiency. High percentage of decolorization lead to high power production. A high percentage of decolorization at 72.9% was observed by Enterobacter sp. in the TW-fed MFC. Interestingly, the decolorization efficiency of Enterobacter sp. TS1L was higher than that produced by two identical chamber MFCs containing 100 mg/L Congo red and a dual chamber MFC containing 10–20 mg/L methyl orange, which reached only 4.8 and 16.2% decolorization, respectively [34, 39]. However, the highest decolorization efficiency was observed in two equal rectangular Perspex frames containing 0.06 mg/L acid orange 7, which yielded 77.4% decolorization [31]. The structure of synthetic dyes mainly affects the decolorization efficiency. It has been reported that monoazodyes are more easily biodegraded than polyazo dyes [41]. The hydroxyl groups of azo dyes ortho to the azo bond exhibit different decolorization rates. The ranking of the biodecolorization rate was as follows: para > ortho > meta. The electrons at the para position are easily withdrawn. On the other hand, steric hindrance at the ortho and meta positions results in slower color removal [28].
Production of PHA by Enterobacter sp. TS1L During Prolong MFC
The functional groups of the isolated polymer from TW-fed MCF were first characterized by FTIR. The spectra of the isolated polymer demonstrated hydroxyl and methyl groups at 3,316 and 2,945 cm−1, respectively. Asymmetric CH2 and CH3 were detected at 2,850–2,920 cm−1. In addition, a PHA marker peak at 1,730 cm−1 corresponding to the carbonyl ester bond was also recorded. Absorption at 1,370 and 1,300–700 cm−1 was assigned to CH3 groups and the C–O–C and C–C linkages of PHA. This result indicated that the isolated polymer from Enterobacter sp. TS1L after MFC operation is PHA (Table 3).
Afterward, the PHA content and monomer composition were analyzed by GC–MS (Table 4). Table 4 indicates that bacterial biomass and PHA increased with increasing operation time. The highest biomass (3.5 ± 0.6 g/L) and PHA (56.1 ± 1.5% CDM) were observed at 6 days of MFC operation. Afterward, biomass and PHA production maintained constant in 7 days of MFC operation due to the reason of the substrate was limited, bacteria enter stationary phase and PHA accumulation reached a maximum. The GC results indicated that Enterobacter sp. TS1L produced medium-co-long chain length (mcl-co-lcl) PHA containing 3-hydroxyhexadecanoate (3HD, C14) and 3-hydroxyoctadecanoate (3HOD, C18). 3HD showed a molecular ion of m/z 270 with a structural formula of C16H31O2 and 3HOD had a molecular ion of m/z 296 with a structural formula of C18H35O2. At 0–5 days of MFC operation, 100 mol% 3HD was detected. However, 3HD (56.3–80.5 mol%) and 3HOD (19.5–43.7 mol%) were observed after 6 and 7 days (Table 4). A similar trend was also observed in Enterobacter sp. TS1L cultivated in PHA-producing medium containing 50 mg/L MB. The highest PHA (56.0% CDM) with 92.6% decolorization efficiency was reported after 72 h of cultivation [6]. The mcl-co-lcl PHA was detected by strain TS1L using PHA producing medium containing MB as the substrate. However, three monomers were detected, including 3-hydroxytetradecanoate (3HTD), 3HD and 3HOD, at 39.5, 50.4 and 9.9 mol%, respectively [6]. The mechanism for PHA production from dye by Enterobacter sp. was already evaluated in our former study [25]. The first mechanism, dye was changed to oxime-, methoxy-phenyl by oxidoreductase. Afterward, oxime-, methoxy-phenyl changed to pyruvate which can be converted into acetyl-CoA by dearomatization and amino acid. The second mechanism, dye degradation, oxidative deamination and carboxylation lead to produce phthalic acid, di(2-propylpentyl) ester, which can be transformed into different fatty acids and aldehydes. The phthalate, fatty acids and aldehydes can directly/indirectly enter into fatty acid oxidation reactions (β-oxidation). When the nitrogen and phosphorus in the substrate are limited, the tricarboxylic acid cycle (TCA) process would be delayed, limiting the biological process of bacterial synthesis of cytoplasm and genetic materials, and leading to the slow growth rate of bacteria. As a result, the remaining acyl-CoA molecules that cannot be completely used in the TCA progress enter the PHA pathway. Afterward, the mcl-co-lcl PHA was accumulated.
In addition, one point should be emphasized that the fatty acids composition plays a more vital role in regulating PHA monomer composition and content [42]. As mention above, Enterobacter sp. able to use dye as substrate for PHA production by convert dye into fatty acids such as hexadecenoic acid and octadecanoic acid [6]. The acid production after fermentation stage of dye correlated with PHA monomer observed in this study. Type of fatty acids were also determined. In day 1–5 of operation only hexadecenoic acid was detected which 100% of HD was observed. Afterward, the percentage of hexadecenoic acid decreased on day 6–7 with an increase of octadecanoic acid. The results related with the mol% of PHA presented (Table 4). The production of PHA with medium- and long-chain-length monomers exhibits advantages over scl PHA because it possesses much better thermal and mechanical properties (soft, less crystalline, elastomeric, lower melting point and glass transition temperature) [43]. Currently, little information on PHA accumulation in MFCs has been reported. Srikanth et al. [44] showed that 19% of PHA was produced using treated wastewater after 48 h of cultivation. The organic content was reduced by 59.5 and 76.5% in the anodic and cathodic chambers, respectively. The highest PD (15.2 mW/m2) was detected after 144 h of cultivation. Therefore, a higher content of PHA and COD removal was obtained by Enterobacter sp. TS1L using TW fed-MFC.
Conclusion
In the present study, the performance of MFC for power generation, wastewater treatment and PHA production was investigated. Bioelectricity was successfully generated using Enterobacter sp. TS1L and TW. A maximum OCV, CD and PD at 986.50 mV, 6.04 W/m3 and 0.32 W/m3, respectively. Prolong MFC was also determined using non-sterile TW. A CD output of 1.56 to 2.31 A/m3 was observed. Prolong operating MFC was also determined using non-sterile TW. A CD output of 1.56 to 2.31 A/m3 was observed without any exogenous medium supplement for microbial growth. After 6 days of prolong MFC, the quality of textile effluent was improved and passed the water quality standard (Thailand). In addition, 3.5 g/L of mcl-co-lcl PHA was produced under conditioning. The potential of bioelectricity and simultaneous waste treatment and valuable bioproduct recovery employing MFC are highlighted due to their low cost and environmentally friendly. The limitation and challenges of this process is low energy generation. The power production may be sensitive to operation conditions. Therefore, some parameters need to be studied in future work, such as the factors that affect bioelectric generation and longer operation times. In addition, the improvement of output power and percent decolorization will be determined. Moreover, the use of Enterobacter sp. may involve with regulatory challenges and safety concerns. Therefore, the microbial management should be restricted.
References
Holkar CR, Arora H, Halder D, Pinjari D (2018) Biodegradation of reactive blue 19 with simultaneous electricity generation by the newly isolated electrogenic Klebsiella sp. CNCIM 5546 bacterium in a microbial fuel cell. Int Biodeterior Biodegrad 133:194–201. https://doi.org/10.1016/j.ibiod.2018.07.011
Mahapatra NN (2016) Textile dyes. Woodhead Publishing India Pvt. CRC Press; New Delhi, Boca Raton
Serejo ML, Morgado MF, García D, González-Sánchez A, Méndez-Acosta HO, Toledo-Cervantes A (2020) Environmental resilience by microalgae. Microalgae cultivation for biofuels production. Springer, Heidelberg, pp 293–315. https://doi.org/10.1016/B978-0-12-817536-1.00019-9
Halepoto H, Gong T, Memon H (2022) Current status and research trends of textile wastewater treatments – A bibliometric-based study. Front Environ Sci. https://doi.org/10.3389/fenvs.2022.1042256
Bilińska L, Gmurek M, Ledakowicz S (2016) Comparison between industrial and simulated textile wastewater treatment by AOPs – biodegradability, toxicity and cost assessment. Chem Eng J 306:550–559. https://doi.org/10.1016/j.cej.2016.07.100
Rakkan T, Paichid N, Sangkharak K (2023) The integration of methylene blue decolorization with polyhydroxyalkanoate (PHA) production using Enterobacter strain TS1L. J Polym Environ 31:1202–1208. https://doi.org/10.1007/s10924-022-02692-y
Rakkan T, Chana N, Chirapongsatonkul N, U-taynapun K, Sangkharak K (2022) Screening and identification of newly isolated basic red 9-degrading bacteria from textile wastewater and their ability to produce medium-co-long-chain-length polyhydroxyalkanoates. J Polym Environ 30:415–423. https://doi.org/10.1007/s10924-021-02206-2
Rakkan T, Chana N, Sangkharak K (2023) Utilization of textile wastewater as a substrate for polyhydroxyalkanoate (PHA) and enhanced production by mutant. J Polym Environ 31:677–687. https://doi.org/10.1007/s10924-022-02563-6
Rahimnejad M, Adhami A, Darvari S, Zirepour A, Oh S-E (2015) Microbial fuel cell as new technology for bioelectricity generation: a review. Alex Eng J 54:745–756. https://doi.org/10.1016/j.aej.2015.03.031
Mohapatra T, Ghosh P (2023) Photo-Fenton remediation of textile wastewater in fluidized-bed reactor using modified laterite: hydrodynamic study and effect of operating parameters. Chem Eng J 473:145324. https://doi.org/10.1016/j.cej.2023.145324
Sikder S, Rahman MM (2023) Efficiency of microbial fuel cell in wastewater (municipal, textile and tannery) treatment and bioelectricity production. Case Stud Chem Environ Eng 8:100421. https://doi.org/10.1016/j.cscee.2023.100421
Peighambardoust S, Rowshanzamir S, Amjadi M (2010) Review of the proton exchange membranes for fuel cell application. Int J Hydrog Energy 35:9349–9384. https://doi.org/10.1016/j.ijhydene.2010.05.017
Shameeda Nk, Rahman R (2020) Microbial fuel cell an alternative for treatment of textile wastewater. Int Res J Eng Tech 07:3962–3967
Razaei F, Xing D, Wagner R, Regan JM, Richard TL, Logan BE (2009) Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl Environ Microbiol 75:3673–3678. https://doi.org/10.1128/AEM.02600-08
Lim SS, Chong PS, Jong BC, Bakar MHA, Daud WRW, Jahim JMd, Salehmin MNI (2022) Microbial fuel cell-based sensor for Enterobacter sp. KBH6958 activity monitoring during hydrogen production: the effects of pH and glucose concentration. J Appl Electrochem 52:1327–1342. https://doi.org/10.1007/s10800-022-01719-5
Hsueh CC, Wang YM, Chen BY (2014) Metabolite analysis on reductive biodegradation of reactive green 19 in Enterobacter cancerogenes bearing microbial fuel cell (MFC) and non-MFC cultures. J Taiwan Inst Chem Eng 45:436–443. https://doi.org/10.1016/j.jtice.2013.05.003
Chaijak P, Sinkan P, Madloh A, Chumkong A (2023) A new report of rhodamine B contaminated wastewater decolorization and electricity generation by laccase based microbial fuel cell. Biointerface Res Appl Chem 13:33. https://doi.org/10.33263/BRIAC131.033
Rakkan T, Sangkharak K (2020) Enhanced decolorization and biodegradation of textile wastewater using single and mixed cultures of a newly isolated Enterobacter strain. Curr Microbiol 77:4085–4094. https://doi.org/10.1007/s00284-020-02246-2
Sangkharak K, Prasertsan P (2012) The production of polyhydroxyalkanoate by Bacillus licheniformis using sequential mutagenesis and optimization. Biotechnol Bioprocess Eng 18:272–279.
Khajeh RT, Aber S, Zarei M (2020) Comparison of NiCo2O4, CoNiAl-LDH, and CoNiAl-LDH@NiCo2O4 performances as ORR catalysis in MFC cathode. Renew Energ 154:1263–1271. https://doi.org/10.1016/j.renene.2020.03.091
Kaoplod W, Chaijak P (2022) Electricity generation in cellulose-fed microbial fuel cell using thermophilic bacterium, Bacillus sp. WK21. Microbiol Biotechnol Lett 50:122–125. https://doi.org/10.48022/mbl.2201.01001
Thipraksa J, Chaijak P (2022) Improved the coconut shell biochar properties for bio-electricity generation of microbial fuel cells from synthetic wastewater. J Degrade Min Land Manage 9:3613–3619. https://doi.org/10.15243/jdmin.2022.094.3613
Zhu Y, Ai M, Jia X (2022) Optimization of a two-species microbial consortium for improved mcl-PHA production from glucose-xylose mixture. Front Bioeng Biotechnol 9:2021. https://doi.org/10.3389/fbioe.202.794331
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9:509. https://doi.org/10.1186/s13104-016-2321-y
Rakkan T, Chana N, Sangkharak K (2022) The integration of textile wastewater treatment with polyhydroxyalkanoate production using newly isolated Enterobacter strain TS3. Waste Biomass Valorization 13:571–582. https://doi.org/10.1007/s12649-021-01504-z
Pinyaphong P, Sriburi P (2022) Optimum condition for polyhydroxyalkanoate production from crude glycerol by Bacillus sp. isolated from liquid-containing wastewater. Trends Sci 19:2588. https://doi.org/10.48048/tis.2022.2588
Abbas SZ, Riaz M, Ramzan N, Zahid MT, Shakoori FR, Rafatullah M (2014) Isolation and characterization of arsenic resistant bacteria from wastewater. Braz J Microbiol 45:1309–1315. https://doi.org/10.1590/s1517-83822014000400022
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresour Technol 131:564–571. https://doi.org/10.1016/j.biortech.2012.12.063
Sun J, Hu Y, Bi Z, Cao Y (2009) Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour Technol 100:3185–3192. https://doi.org/10.1016/j.biortech.2009.02.002
Li Z, Zhang X, Lin J, Han S, Lei L (2010) Azo dye treatment with simultaneous electricity production in an anaerobic-aerobic sequential reactor and microbial fuel cell coupled system. Bioresour Technol 101:4440–4445. https://doi.org/10.1016/j.biortech.2010.01.114
Mu Y, Rabaey K, Rozendal R, Yuan Z, Keller J (2009) Decolorization of azo dyes in bioelectrochemical systems. Environ Sci Technol 43:5137–5143. https://doi.org/10.1021/es90057f
Liu L, Li FB, Feng CH, LI XZ (2009) Microbial fuel cell with an azo-dye-feeding cathode. Appl Microbiol Biotechnol 85:175–183. https://doi.org/10.1007/s00253-009-2147-9
Cao Y, Hu Y, Sun J, Hou B (2010) Explore various co-substrates for simultaneous electricity generation and Congo red degradation in air-cathode single-chamber microbial fuel cell. Bioelectrochemistry 79:71–76. https://doi.org/10.1016/j.bioelechem.2009.12.001
Fu L, You SJ, Zhang GQ, Yang FL, Fang XH (2010) Degradation of azo dyes using in-situ Fenton reaction incorporated into H2O2-producing microbial fuel cell. Chem Eng J 160:164–169. https://doi.org/10.1016/j.cej.2010.03.032
Ding H, Li Y, Lu A, Jin S, Quan C, Wang C, Wang Z, Zeng C, Yan Y (2010) Photo catalytically improved azo dye reduction in a microbial fuel cell with rutile-cathode. Bioresour Technol 101:3350–3505. https://doi.org/10.1016/j.biortech.2009.11.107
Sun J, Bi Z, Hou B, Cao Y, Hu Y (2011) Further treatment of decolorization liquid of azo dye coupled with increased power production using microbial fuel cell equipped with an aerobic biocathode. Water Res 45:283–291. https://doi.org/10.1016/j.watres.2010.07.059
Akman D, Cirik K, Ozdemir S, Ozkaya B, Cinar O (2013) Bioelectricity generation in continuously-fed microbial fuel cell: effects of Anode electrode material and hydraulic retention time. Bioresour Technol 149:459–464. https://doi.org/10.1016/j.biortech.2013.09.102
Zhang G, Zhao Q, Jiao Y, Lee D-J (2015) Long-term operation of manure-microbial fuel cell. Bioresour Technol 180:365–369. https://doi.org/10.1016/j.biortech.2015.01.002
Oon YS, Ong SA, HO LN, Wong YS, Oon YL, Lehl HK, Thung WE (2016) Long-term operation of double chambered microbial fuel cell for bio-electro denitrification. Bioprocess Biosyst Eng 39:893–900. https://doi.org/10.1007/s00449-016-1568-y
Zhao Q, Li R, Ji M, Ren ZJ (2016) Organic content influences sediment microbial fuel cell performance and community structure. Bioresour Technol 220:549–556. https://doi.org/10.1016/j.biortech.2016.09.005
Hsueh CC, Chen BY, Yen CY (2009) Understanding effects of chemical structure on azo dye decolorization characteristics by Aeromonas Hydrophilia. J Hazrd Mater 167:995. https://doi.org/10.1016/j.jhazmat.2009.01.07
Wei T, Fang Q (2022) Regulating the monomer of polyhydroxyalkanoate from mixed microbial culture: with particular emphasis on substrate composition: a review. Environ Eng Res. https://doi.org/10.4491/eer.2021.333. 27.210333
Zhuo XZ, Chou SC, Li SY (2022) Production medium-chain-length polyhydroxyalkanoate from diverse feedstocks by deregulating unsaturated fatty acid biosynthesis in Escherichia coli. Bioresour Technol 365:128078. https://doi.org/10.1016/j.biortech.2022.128078
Srikanth S, Reddy MV, Mohan SV (2012) Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour Technol 125:291–295. https://doi.org/10.1016/j.biortech.2012.08.060
Acknowledgements
The authors wish to acknowledge Department of Chemistry, Faculty of Science, Thaksin University for providing necessary facilities to carry out this research work. This work was supported by the Research and Development Institute Thaksin University (66A105000002) and Research Grant for Talented Mid-Career Researchers (N42A650241) from National Research Council of Thailand (NRCT).
Funding
This work was supported by the Research and Development Institute Thaksin University (66A105000002) and Research Grant for Talented Mid-Career Researchers (N42A650241) from National Research Council of Thailand (NRCT).
Author information
Authors and Affiliations
Contributions
Conceptualization: KS, PC; Methodology: PC, TR and NP; Formal analysis and investigation: TR, JT, PM and NP; Writing—original draft preparation: PC and KS; Writing—review and editing: KS; Supervision: KS, Projection administration: KS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaijak, P., Rakkan, T., Paichaid, N. et al. Exploring Potential Aspect of Microbial Fuel Cell (MFC) for Simultaneous Energy, Polyhydroxyalkanoate (PHA) Production and Textile Wastewater (TW) Treatment. J Polym Environ 32, 3104–3118 (2024). https://doi.org/10.1007/s10924-023-03141-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-03141-0