Abstract
Newly isolated Basic Red 9-degrading bacteria were isolated from textile wastewater using a pretreatment method. Nine strains were isolated; however, only five strains accumulated polyhydroxyalkanoates (PHAs). Thereafter, PHA-producing strains were identified through 16S rDNA sequencing analysis and phylogenetic evaluation and were found to belong to Enterobacter with 100% identification. The five isolated strains were incubated with a PHAs-producing medium containing 100 mg/l Basic Red 9 (BR9) to study decolorization efficiency, and PHAs production. Enterobacter sp. strains TS3 and TS1L effectively decolorized the BR9 dye with degradation rates of 63.43% and 79.15%, respectively. PHAs production from TS3 and TS1L was also observed to be 75.34% and 72.32% of dry cell weight (DCW), respectively. Furthermore, Enterobacter sp. strains TS3 and TS1L accumulated medium-co-long-chain-length PHAs (mcl-co-lcl PHAs). This is the first report using Enterobacter strains to degrade BR9 dyes from textile wastewater and to assess their ability to produce mcl-co-lcl PHAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consumption of plastic has been increasing every day. Plastic is a chemical product derived from the petrochemical industry. It is non-biodegradable, with the potential to accumulate and cause environmental problems, therefore biodegradable plastic such as PHAs, would seem to be an appealing alternative [1, 2]. PHAs are a family of polyesters that are synthesized and accumulated intracellulary as a carbon and energy storage materials by various microorganisms [3]. PHAs are produced by using many pure cultures, e.g. Bacillus sp., Pseudomonas sp., Wautersia eutropha and Enterobacter sp. SEL2 as well as consortia [4,5,6,7,8,9]. Biodegradability is not the only beneficial feature of PHAs, as these materials also display high biocompatibility [10].
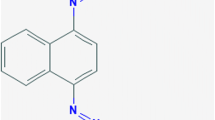
Another problem that concerns environmentalists, perhaps even more than plastic pollution, is pollution caused by the textile industry. In this context, woven bulrush mat, a famous product in Phatthalung (Thailand), is dyed with commercial dye such as BR9. BR9 or pararosaniline hydrochloride (CPR; CAS No. 569-61-9), also known as parafuchsine hydrochloride, C.I. basic red monohydrochloride, C.I. 42500, magenta 0, 4,4′-(4-iminocyclohexa-2,5-dienylidene- methylene)dianilinehydrochloride 9, is a solid with a red color and a green metallic sheen. This dye is slightly soluble in water and ether and soluble in ethanol, methanol, benzyl alcohol, and ethylene glycol methyl ether [11]. BR9 belongs to the triaryl methane class of dyes and is used for coloring various types of materials and products such as paper, leather, textiles, glass, waxes, and cosmetics, as well as in the manufacture of inks and paints [12,13,14,15,16,17]. Results from animal experiments have led researchers to propose that this dye is suspected to be carcinogenic. BR9 induced hepatocellular carcinomas in male and female mice and in male rats; adrenal gland phaeochromocytomas in female mice; benign and malignant skin tumours in male rats; and subcutaneous fibromas, thyroid gland follicular-cell tumours and Zymbal gland carcinomas in male and female rats [54]. Occupational and environmental issues related to the use of BR9 are therefore of concern, especially regarding its treatment and final disposal [18]. The amount of BR9 in the United States was estimated to be greater than 0.9 tonnes in 1972 and 0.5 tonnes in 1975 [17]. After processing, wastewater containing dye is kept in natural ponds and treated through physical methods. These treatment processes are time consuming and there is still some residue in the treated water that is released into natural water sources. To overcome such pollution we should isolate the bacteria that can decompose this dye to remove it from textile wastewater. Moreover, dye-degrading bacteria have been reported to have the ability to produce and accumulate PHAs. Previously, the use of textile dyes such as Orange 3R, Direct Red 5B, and Direct Blue GLL, to produce lcl PHAs has been reported [19].
Therefore, this study aimed to isolate and screen dye-degrading bacteria from textile wastewater. The ability of newly isolated bacteria to decolorize BR9 and to produce PHAs were also determined. In addition, PHAs was subjected to a GC-FID for monomer determination and the degradation product of BR9 was also evaluated by ATR-FTIR and PHAs intracellular accumulation by transmission electron microscopy (TEM).
Material and Methods
Sample Collection
Textile wastewater and sediment were collected from the Ban Phraek Weaving Group (Phatthalung, Thailand). The samples were collected in sterile plastic buckets and stored at -20 °C for further analysis. Each waste sample was measured for chemical oxygen demand (COD), temperature, pH, color, and total suspended solids (TSS). Determination of COD was carried out using a Spectroquant® COD Cell Test instrument (Germany). The temperature and pH were determined using a laboratory thermometer and a pH meter, respectively. The sample color was determined using the spectrophotometric method [20]. Finally, to determine the TSS, the wastewater samples were filtered through a standard GF/F glass fiber filter. The residual matter retained on the filter was dried in an oven at 103–105 °C to a constant weight and the increase in the weight of the filter represented the total suspended solids [21].
Isolation and Purification of Newly BR9-Degrading Bacteria and Their Ability to Produce PHAs
One milliliter or 1 g of wastewater or sediment, respectively, was added to 10 ml of Bushnell Haas medium (BHM) containing 0.2 g/l MgSO4, 1.0 g/l K2HPO4, 0.02 g/l CaCl2, 0.05 g/l FeCl3, 1.0 g/l NH4NO3, supplemented with 10 g/l glucose and 100 mg/l BR9 [22]. The final pH of the medium was adjusted to pH 7 using 5 N NaOH and 5 N HCl. Samples were incubated at 35 °C at 150 rpm for 24 h [23]. After incubation overnight, 100 µl of the sample was spread directly onto plates of BHM supplemented with 100 mg/l BR 9 and incubated at 35 °C for 48 h. Thereafter, BHM with 100 mg/l BR 9 was defined as BHM-BR9 medium. After incubation, morphologically distinct bacterial isolates (those showing clear zones around their colonies due to decolorization of the dye) were streaked on BHM-BR9 agar plates to obtain single colonies. The pure culture stocks of these isolates were stored at 4 °C on BHM-BR9 agar and further inoculated in PHAs-producing medium [22].
Nine newly isolated strains of dye-degrading bacteria were transferred to PHAs-producing medium to determine their ability to convert dye to PHAs. The pure newly isolated bacteria were inoculated into 250 ml Erlenmeyer flasks containing 50 ml of PHAs-producing medium [2 g/l (NH4)2SO4, 13.3 g/l KH2PO4, 1.2 g/l MgSO47H2O, 1.7 g/l citric acid, 10 ml/l trace element solution] at pH 7 [23] and incubated at 35 °C for 24 h with agitation at 150 rpm. After overnight incubation, 100 µl of the sample was spread directly on PHAs-detection agar plates (20 g/l glucose, 2 g/l (NH4)2SO4, 13.3 g/l KH2PO4, 1.2 g/l MgSO4·7H2O, 1.7 g/l citric acid, trace 10 ml/l elements solution, and 15 g/l agar containing 0.5 μg/ml Nile Red stain) [24] and incubated at 35 °C for 24 h. After overnight incubation, plates were observed under UV light. Colonies producing fluorescence were purified and streaked on PHAs-detection agar, for further study. Five newly isolated bacteria, which were able to degrade BR9 and produce PHAs, were cultivated in PHAs-producing medium for 18 h at 35 °C and 150 rpm. The new isolates were utilized throughout this study for 16S rDNA, biochemical characterization, dye degradation, and PHAs production.
DNA Extraction, PCR Amplification of the 16S rDNA Gene, Bioinformatics Analysis, and Phylogenetic Tree Construction
Isolated bacteria were cultivated, cells were harvested by 15 min centrifugation at 10,000 rpm and genomic DNA was extracted. The 16S rDNA gene was amplified using universal primers 20F (5′-AGAGTTTGATCATGGCT CAG-3′) and 1500R (5′-GGTTACC TTGTTACG ACTT-3′) [25]. The reaction mixture contained 5 μl template DNA, 7.5 μl of PCR Master Mix (2 ×) and 2 μl of each primer (20 μM) and the final volume was 15 μl. The thermal PCR profile was as follows: initial denaturation at 94 °C for 3 min 30 s., primer annealing at 57 °C for 1 min, extension at 72 °C for 1 min 30 s, and elongation for 7 min (Lorenz, 2012). The PCR products were purified using GFX PCR DNA and gel band purification kits (GE Healthcare, Little Chalfont, UK). In addition, 16S rDNA gene sequencing was carried out on a 3730 series genetic analyzer at maximum throughput and scalability. The consensus sequences were aligned with previously-published sequences of bacterial strains using the BLAST function available in the National Centre for Biotechnology Information (NCBI) database [26]. Phylogenetic and molecular evolutionary analyses were conducted using the software MEGA X [27].
Biochemical characterization
Newly isolated bacteria were tested for biochemical characterization with KB009 HiCarbohydrate™ kits (KB009A/KB009B/KB009C) as described by the manufacturer. KB009 is a comprehensive test system that can be used to study the biochemical profile of a wide variety of organisms and can also be used for validating known laboratory strains. This test kit provides a combination of 35 tests for the utilization of carbohydrates. The kit contains Part A and Part B, each having 12 carbohydrate utilization tests, and Part C, containing 11 sugars and a control [28].
Decolorization and PHAs Production in Shake-Flask Culture
Newly isolated bacteria were cultivated in PHAs-producing medium containing 20 g/l of glucose as a carbon source and 100 mg/l BR9, which was defined as PHAs-BR9 medium, where the initial pH was adjusted to pH 7. Each culture was incubated at 35 °C at 150 rpm for 60 h. After incubation, the samples were analyzed for decolorization, biomass, PHAs intracellular accumulation, content and composition. The results reported were the averages of three replicates for all experiments.
Cell Growth Measurement
Ten milliliters of newly isolated bacteria was centrifuged at 13,000 rpm for 10 min at 4 °C. The pellet was washed twice with distilled water and then suspended in 10 ml of distilled water. After mixing, growth was monitored by measuring absorbance at 660 nm [29].
Biomass Analysis
The total cell concentration was determined by weighing the dry cell mass obtained as follows. Ten milliliters of culture samples were centrifuged at 13,000 rpm for 15 min at 4 °C. The pellet was resuspended in distilled water (10 ml) and centrifuged again for washing. The washed cells were dried at 105 °C for 24 h in a hot air oven then cooled down in desiccators [30]. The drying process was repeated until a constant weight was obtained.
Determination of PHAs Intracellular Accumulation by TEM
One milliliter after incubation 60 h was collected for PHAs intracellular accumulation using TEM analysis. The culture was centrifuged in a microcentrifuge at 10,000 rpm for 10 min at 25 °C. Pellets were fixed with 2.5% of glutaraldehyde and then stained with osmium tetroxide and uranyl acetate. Samples were viewed in a Leica TEM.
Extraction of PHAs
A sample of 0.4 g of dye biomass was dissolved in 10 ml of methanol at room temperature without agitation for 1 h. After 1 h, the sample was collected and centrifuged at 12,000 rpm for 5 min, and the precipitate was transferred to a cellulose extraction thimble and inserted into a Soxhlet extractor containing 150 ml of chloroform, which was used as the extracting solvent. After 5 h of extraction, the solvent was evaporated under vacuum to approximately about 1 ml of residue. Then, 2 ml of cold methanol was added to reprecipitate and to obtain a dull white solid which was then filtered, dried, weighed, and subjected to further analysis by GC-FID [31].
Determination of PHAs Composition by GC-FID
PHAs were analyzed in whole-cell samples or after extraction with chloroform and purification by repeated precipitation from a chloroform solution with ethanol. The PHAs content and composition were determined by subjecting 8 mg of lyophilized cells or 2 mg of isolated PHAs, respectively, to methanolysis which was performed in a mixture of chloroform and methanol containing 15% (v/v) sulfuric acid [32]. The resulting hydroxyacyl methyl esters were analyzed with a gas chromatograph [33]. Compared to those of authentic standards. The authentic standards are consisting of methyl butyrate (C4:0), methyl pentanoate (C5:0), methyl hexanoate (C6:0), methyl heptanoate (C7:0), methyl octanoate (C8:0), methyl nonanoate (C9:0), methyl decanoate (C10:0), methyl undecanoate (C11:0), methyl laurate (C12:0), methyl tridecanoate (C13:0), methyl myristate (C14:0), methyl pentadecanoate (C15:0), methyl palmitate (C16:0), methyl heptadecanoate (C17:0), methyl stearate (C18:0), methyl oleate (C18:1), methyl linoleate (C18:2), and methyl linolenate (C18:3) from NU-CHEK-PREP, Inc, U.S.A.
The initial structural assignments of the methyl esters analyzed were based on their retention times. Gas chromatographic analysis was performed on a Hewlett Packard GC-6890 system equipped with an HP-INNOWAX capillary column (length, 30 m; internal diameter, 0.25 mm; film thickness, 0.25 μm) and an FID.
Decolorization and Biodegradation Studies
The samples were centrifuged at 10,000 rpm for 10 min. Decolorization efficiency was analyzed by measuring the absorbance of the culture supernatant at 540 nm [22]. The decolorizing efficiency was expressed as the percentage of decolorization.
The metabolites obtained after degradation of Basic Red 9 were determined using a Cary 630 FTIR spectrometer in the mid-IR region of 4000–650 cm−1 with 32 sample scans [34].
Statistical Analysis
All experiments were run in triplicate. A completely randomized design was used throughout this study. Data were subjected to analysis of variance and mean comparisons were carried out using Duncan’s multiple range test [35]. Statistical analysis was performed using the SPSS package (SPSS 24 for Windows, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Isolation and Purification of Newly Isolated BR9-Degrading Bacteria and Their Ability to Produce PHAs from Textile Wastewater
Textile wastewater from the Ban Phraek Weaving Group (Phatthalung, Thailand) was utilized as a source for dye-degrading bacteria. This wastewater comes from the textile industry after dyeing and weaving bulrush mat products. The partial characterization of textile wastewater is shown in Table 1. Textile wastewater was characterized as 5600 mg/l COD, at pH 7.45, and temperature 29.25 °C, which are conditions where Enterobacteriaceae can live and grow. There has been no previous report of the newly isolated bacteria from contaminated wastewater resulting from the use of BR9 to dye woven bulrush mat products in Thailand. Nine strains of newly isolated dye-degrading bacteria were isolated from textile wastewater using the BHM-BR9 medium and pretreatment. Among the strains investigated, five isolates were identified as newly isolated PHAs-producing strains, based on the strong bright fluorescence that appeared after using the Nile Red staining method. All isolated strains were strongly fluorescent, as depicted in Fig. 1. The five newly isolated strains that were able to degrade dye and produce PHAs were TS3, TS1L, TS1P, TW1P, and TW1L. Thus, the strains where fluorescent behaviour was detected could be considered PHAs-accumulating bacteria because of the fluorescent dye binding to polymer granules within the cell [24, 36]. On the other hand, no fluorescence was observed in the non-PHAs producing isolates after Nile Red staining determination. All the fluorescent isolates were selected and used in this study. This study thus reveals that bacteria producing PHAs utilize dyes from textile wastewater as their source of carbon. A high COD value (5600 mg/l) of textile wastewater, mostly rich in organic contents and lower in nitrogen and phosphorus [37], was suitable for PHAs production. These unbalanced nutrient states (especially the carbon to nitrogen ratio) create selective pressures [38, 39] which may play a major role in the accumulation of PHAs granules in the cytosol of the microbes. Many bacteria have been isolated from dye wastewater, as reported in previous studies, such as Aeromonas hydrophila, Halomonas, Ralstonia sp. strain PBA and Hydrogenophaga sp. strain PBC, Pseudomonas sp. SUK1, a newly isolated bacterial consortium RVM 11.1, and Enterobacter hormaechei strain CUIZ which produced from reactive Black 5, azo dyes, 4-aminobenzenesulfonate, reactive textile dye red BLI, reactive violet 5 and azo dye methyl red, respectively [40,41,42,43,44,45]. However, only some of them show the ability to convert dye and to produce PHAs.
Identification of Newly Isolated PHAs-Producing Isolate Based on the 16S rDNA Gene Sequence
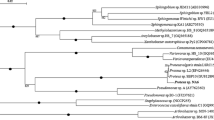
All newly isolated PHAs-producing bacterial strains were submitted to 16S rDNA sequencing analysis. The data bank search and alignment suggested the bacterial strains shared the highest similarity to the Enterobacter genus, overall (Fig. 2). Five strains of bacterial isolates were found belonging to Enterobacter with 100% identification. All sequences of isolates (TS1L, TS1P, TS3, TW1L, and TW1P) were submitted to GenBank with the accession numbers MN508471, MN508472, MN508473, MN508474, and MN508475, respectively.
Phylogenetic tree derived from neighbor-joining analysis showing phylogenetic position of newly isolated TW1L, TS1P, TS1L, TS3, and TW1P based on comparison of 16S rRNA gene sequences. Numbers above nodes represent bootstrap confidence values above 70% from 1000 resamplings. Bar scales indicate 0.02% sequence dissimilarity
Biochemical Characterization of Bacterial Isolates
Newly isolated Enterobacter are gram-negative (gram-negative bacteria are more resistant to textile dyes than Gram-positive bacteria, because they have a largely impermeable cell wall), rod-shaped, and nonspore-forming bacteria. The five strains of Enterobacter isolated from wastewater containing BR9 were tested for biochemical characteristics using a kit containing 12 carbohydrates and 11 sugars. The results showed that the isolated strains could use various sugars, such as C5–C18, therefore the strains can accumulate PHAs with different monomers. The isolated strains could not use α-methyl-d-glucoside or sorbose as a carbon source. It has been reported that the alpha-methyl-d-glucoside and sorbose was interfered with the growth of bacteria strains when used as a sole carbon source. Moreover, those substrates were inhibited the activity of enzyme which involve in the degradation of glucoside and sorbose [55]. Similar biochemical activities were observed in all the isolated strains. The biochemical and physiological characterization showed similarities with Enterobacter species [46, 47]. Interestingly, this study indicated that all Enterobacter strains were able to use glycerol and cellulose (Table 2) which means that they can be used to produce PHAs from waste in various industries such as the biodiesel, oil palm, and paper industries. Wang et al., found that Enterobacter sp. EC3 has a decolorization efficiency for Reactive Black 5 of approximately 92.56% [48].
Dye Decolorization and PHAs Accumulation
The five newly isolated strains of bacterial isolates were incubated with PHAs-BR9 to study decolorization efficiency and PHAs accumulation. The results showed that newly isolated Enterobacter sp. strains TS3, TW1P, TW1L, TS1L, and TS1P effectively decolorized the BR9 dye with a degradation rate of 16.86% to 79.15% (Table 3). The most effective dye decolorization was obtained from strain TS1L (79.15% degradation rate). Observation of the culture media showed a significant decrease in color (Fig. 3). Nadaroglu et al., reported using immobilized magnetite nanoparticles and their apolaccase-modified counterparts, which showed decolorization efficiencies of 81.01% and 88.6% [16], respectively, for the removal of BR9 from industrial wastewater by way of Fenton and Fenton-like processes. However, there has been no report that Enterobacter can remove BR9 before. This research is the first to use newly isolated Enterobacter strains to degrade BR9 dyes from textile wastewater although Enterobacter are used to decolorize other types of dyes, such as azo dye methyl red by Enterobacter hormaechei strain CUIZ and Enterobacter agglomerans [49]. Furthermore, it has been reported that C. I. Reactive Red 195 and Reactive Black 5 can be decolorized by Enterobacter sp. and Enterobacter sp. EC3 [48, 50].
The metabolites of BR9 after degradation were studied using ATR-FTIR. The peaks in the FTIR spectrum of BR9 were found to be the N–H stretching vibration of 1°amines at 3365 cm−1 and the N–H bending vibration of 1°amines at 1638 cm−1 [11]. The metabolites (product after degradation) of the dye after degradation showed peaks at 1078 cm−1 for C–N stretching of aliphatic amines after degradation of the benzene ring (aromatic amines) in BR9 (Fig. 4). This means that the newly isolated Enterobacter sp. strains changed the aromatic amines of BR9 to aliphatic amines after degradation.
All the newly isolated strains were also harvested and analyzed for PHAs production. The results showed that all the newly isolated Enterobacter sp. in this study accumulated PHAs using 100 mg/l of BR9. The PHAs content was in the range of 52.37–75.34% of DCW (Table 3). The highest PHAs content was observed in strain TS3, at 75.34% of DCW, and the strain TS3 has demonstrated the ability to form the PHAs polymer in the cell (Fig. 5). To date, to the best of our knowledge, only four publications deal with PHAs-producing Enterobacter strains using sugar cane molasses, glucose, lactose, butyric acid and Calophyllum inophyllum oil cake as the substrates [8, 47, 51, 52]. This study found that the newly isolated strains of Enterobacter sp. were able to produce more PHAs after degradation of BR9.
Monomer Composition of PHAs
The five newly isolated strains of Enterobacter that had been isolated were analyzed by GC-FID to confirm and to characterize the PHAs monomers they synthesized. It was found that newly Enterobacter sp. TS3 accumulated mcl-co-lcl PHAs consisting of hydroxy dodecanoate (HDD, C = 12:0), hydroxy pentadecanoate (HPD, C15:0), hydroxy hexadecanoate (HHD, C = 16:0), hydroxy octadecanoate (HOD, C = 18:0), and hydroxy linoleate (HLI, C = 18:2) was also observed to be 3.25, 15.00, 4.27, 67.22, and 10.26 mol%, respectively. PHAs monomers of Enterobacter sp. strains TS1L, TS1P, TW1P, and TW1L consisted of HPD, HOD, and HLI (10.39–18.92, 73.83–78.42, and 7.25–13.70 mol%, respectively). Interestingly, a different monomer was observed in strain TS3 (Table 4). A bacterial strain designated Enterobacter FAK 1384 was isolated from a shark jaw that, when grown on copra oil, produced mcl-PHAs specifically of 62 mol% 3HD, 12 mol% 3HDD and 7.6 mol% 3HDD [53]. Samrot et al., showed that Enterobacter cloacae SU-1 produced mcl-PHAs when grown on glucose or lactose [47]. In this current study, mcl- and lcl-PHAs was produced by Enterobacter sp. TS3 using BR9 as a substrate. In addition, isolated strains can produce more than 67 mol% of the HOD monomer. Therefore, textile wastewater was found to be an attractive material for utilization as a substrate for mcl- and lcl-PHAs production.
Conclusion
Textile industries consume large volumes of water and chemicals during wet processing. The main visible pollutant from textile wastewater is the dye, which is aesthetically unpleasant and hazardous to human and aquatic life; the use of biodegrading bacteria to treat textile wastewater is a good solution to this problem. In this study, wastewater containing BR9 was used as the source of dye-degrading bacteria. Nine newly isolated strains were obtained from wastewater and further studied for their ability to convert waste into PHAs; however, there were only five strains accumulated that PHAs. Newly isolated Enterobacter sp. strain TS1L was shown to decolorize BR9 dye effectively with a degradation rate of 79.15%. PHAs production from newly isolated TS1L was also observed as 72.32% of DCW and it can accumulate lcl-PHAs with high a HOD monomer content of 79.15 (mol%). This is the first report of lcl-PHAs from Enterobacter using BR9 with 67 mol% of the HOD monomer. Therefore, further work is required to characterize lcl-PHAs, to describe its application, and to study optimal conditions for the production of PHAs. Further, it is necessary to study in greater detail the increase in of PHA products by the by newly isolated strain Enterobacter.
References
Kemavongse K, Prasertsan P, Upaichit A, Methacanon P (2008) J Microbiol Biotechnol 24:2073
Sangkharak K, Prasertsan P (2011) Afr J Biotechnol 10:17812
Anderson AJ, Dawes EA (1990) Microbiol Rev 54:450
Gouda MK, Swellam AE, Omar SH (2001) Microbiol Res 156:20
Kim Y, Kim DY, Rhee YH (1999) Macromolecules 32:6058
Sandoval A, Arias-Barrau E, Arcos M, Naharro G, Olivera ER, Luengo JM (2007) Environ Microbiol 9:737
Loo CY, Lee WH, Tsuge T, Doi Y, Sudesh K (2005) Biotechnol Lett 27:1405
Naheed N, Jamil N (2014) Braz J Microbiol 45:417
Patnaik PR (2005) Crit Rev Biotechnol 25:153
Koller M (2018) Molecules 23:362
Duman O, Tunc S, Polat TG (2015) Microporous Mesoporous Mater 210:17
Martins AO, Canalli VM, Azevedo CMN, Pires M (2006) Dyes Pigm 68:227
Zargar B, Parham H, Hatamie A (2009) Talanta 77:1328
IARC (2010) Some aromatic amines, organic dyes, and related exposures. Lyon, Franc
Perdih F, Perdih A (2011) Cellulose 18:1139
Nadaroglu H, Gungor AA, Celebi N (2015) Int J Environ Res Public Health 9:991
US national library of medicine (1992) Hazardous substances data bank (HSDB record nos. 2952 and 6192), Bethesda, MD
Scaringelli FP, Saltzman BE, Frey SA (1967) Anal Chem 39:1709
Tamboli DP, Kurade MB, Waghmode TR, Joshi SM, Govindwar SP (2010) J Hazard Mater 182:169
Anouzla A, Abrouki Y, Souabi S, Safi M, Rhbal H (2009) J Hazard Mater 166:1302
APHA (2005) Standard methods for the examination of water and wastewater. Washington, DC
Moosvi S, Kher X, Madamwar D (2007) Dyes Pigm 74:723
Sangkharak K, Prasertsan P (2012) Biotechnol Bioprocess Eng 18:272
Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbüchel A (1999) Arch Microbiol 171:3
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) Arch Microbiol 171:697
Aremu BR, Babalola OO (2015) Int J Environ Res 12:12356
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) Mol Biol Evol 35:1547
Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Kumar S, Patel S, ChandraKalia V (2008) Bioresour Technol 99:5444
Shimizu H, Shioya S, Suga KI (1990) Eur J Appl Microbiol Biotechnol 7:1
Sangkharak K, Prasertsan P (2008) Electron J Biotechnol 11:173
Manangan T, Shawaphun S (2010) Sci Asia 36:199
Steinbüchel A, Wiese S (1992) Appl Microbiol Biotechnol 37:691
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Appl Environ Microbiol 54:1977
Tamboli DP, Gomare SS, Kalme SS, Jadhav UU, Govindwar SP (2010) Int Biodeterior Biodegradation 64:755
Steel RGD, Torrie JH (1980) Principles and procedures of statistics. McGraw-Hill Book Company, New York
Amara AA, Steinbüchel A, Rehm BHA (2002) Appl Microbiol Biotechnol 59:477
Dircks K, Beun JJ, van Loosdrecht M, Heijnen JJ, Henze M (2001) Biotechnol Bioeng 73:85
Wang JG, Bakken LR (1998) Microb Ecol 35:94
de Lima TCS, Grisi BM, Bonato MCM (1999) Rev Microbiol 30:214
Bouraie ME, Din WS (2016) Sustain Environ Res 26:206
Asad S, Amoozegar MA, Pourbabaee AA, Sarbolouki MN, Dastgheib SM (2007) Bioresour Technol 98:2082
Gan HM, Shahir S, Ibrahim Z, Yahya A (2011) Chemosphere 82:507
Kalyani DC, Patil PS, Jadhav JP, Govindwar SP (2008) Bioresour Technol 99:4635
Moosvi S, Keharia H, Madamwar D (2005) World J Microbiol Biotechnol 21:667
Asma T, Askarne L, Addi EA, Assabbane A, Boubaker H (2018) J Mater Environ Sci 9:2822
Ceyhan N, Ozdemir G (2011) Afr J Microbiol Res 5:690
Samrot AV, Avinesh RB, Sukeetha SD, Senthilkumar P (2011) Appl Biochem Biotechnol 163:195
Wang H, Zheng XW, Su JQ, Tian Y, Xiong XJ, Zheng TL (2009) J Hazard Mater 171:654
Moutaouakkil A, Zeroual Y, Dzayri FZ, Talbi M, Lee K, Blaghen M (2003) Ann Microbiol 53:161
Jirasripongpun K, Nasanit R, Niruntasook J, Chotikasatian B (2007) Thammasat Int J Sci Tech 12:6
Chen ZQ, Li YB, Wen QX (2010) Huan Jing Ke Xue 31:828
Arumugam A, Sandhya M, Ponnusami V (2014) Bioresour Technol 164:170
Wecker P, Moppert X, Simon-Colin C, Costa C, Berteaux-Lecellier V (2015) AMB Express 5:1
IARC (2010) Magenta and CI Basic Red 9. Lyon, Franc
Moreira F, Lenartovicz V, Souza CGM, Ramos EP, Peralta RM (2001) Braz J Micriobiol 32:15
Acknowledgements
The authors would like to acknowledge support of the Thailand Science Research and Innovation (TSRI) through the Royal Golden Jubilee Ph.D. (RGJ-PHD) Program through grant number PHD/00073/2559 for RGJ-PHD. Acknowledgement are also made to the Faculty of Agriculture, Rajamangala University of Technology Srivijaya, Saiyai Campus, Thailand, and the Department of Chemistry, Faculty of Science, Thaksin University, Phatthalung Campus, Thailand. Ken’ichiro MATSUMOTO.
Author information
Authors and Affiliations
Contributions
Miss TR performed the experiments and wrote the original manuscript; Assistant Prof. Dr. NC analyzed the statistic; and Dr. NC helped with the experiments. Dr.KU helped with the experiments. Associate Prof. Dr. KS participated in the design of this work, reviewed, the data analysis and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakkan, T., Chana, N., Chirapongsatonkul, N. et al. Screening and Identification of Newly Isolated Basic Red 9-Degrading Bacteria from Textile Wastewater and Their Ability to Produce Medium-Co-Long-Chain-Length Polyhydroxyalkanoates. J Polym Environ 30, 415–423 (2022). https://doi.org/10.1007/s10924-021-02206-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02206-2