Abstract
The main aim of this study is to investigate the performance of organic oxidation and denitrification of the system under long-term operation. The MFC reactor was operated in continuous mode for 180 days. Nitrate was successfully demonstrated as terminal electron acceptor, where nitrate was reduced at the cathode using electron provided by acetate oxidation at the anode. The removal efficiencies of chemical oxygen demand (COD) and nitrate were higher in the closed circuit system than in open circuit system. Both COD and nitrate reduction improved with the increase of organic loading and subsequently contributed to higher power output. The maximum nitrate removal efficiency was 88 ± 4 % (influent of 141 ± 14 mg/L). The internal resistant was 50 Ω, which was found to be low for a double chambered MFC. The maximum power density was 669 mW/m3 with current density of 3487 mA/m3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cell (MFC) is a new and promising technology that converts the chemical energy contained in the organic matter to electrical energy [1–3]. MFC is also an emerging cost-effective technology for wastewater treatment [4] and the energy recovery from MFC systems can partially offset the operating costs of the treatment processes [5]. MFC has grown tremendously in terms of the number of researchers in the recent years [6]. Nevertheless, MFCs have been mostly operated in laboratory investigation, there were a small number of pilot-scale tests have been conducted, but the results were not up to par [7]. There is still no commercial application of the technology yet due to its challenges in scaling up [8, 9]. More intensive MFC studies are in great need to advance the knowledge in this field, so that this technology can be successfully used in the real-world application in the future.
Most of the MFCs studies have been designed in a half biological manner, where a biological anode and an abiotic cathode were employed in the system. There are several shortcomings in the abiotic cathode as it requires electron mediator, which needs constant replenishment after exhaustion to ensure the efficiency of electron transfer. Consequently, it increases the cost of construction and operations as well as lowers the sustainability of the MFC system [10]. Precious metal such as platinum is commonly used as cathode material in MFC due to its exceptional catalytic ability. However, platinum is high in cost and can be toxic, and possibly affect the growth of microorganism. Therefore, it is less practical to apply in the actual wastewater treatment process.
Microorganisms can also be used as catalysts and mediators in the cathode of a MFC system [11]. Biocathode in MFC system is appearing to be a more sustainable alternative compared to the use of precious metal [12]. Besides that, the cost and properties of biocatalysts and their compatibility with operating conditions tend to be more favourable for MFC applications in the future as compared to metal-based catalysts [11]. Furthermore, the microbes in biocathodes also enable the use of other electron acceptors in wastewater to remove unwanted compounds such as nitrate through denitrification process in MFC [10]. This practice is of great interest because it can improve the MFC systems with respect to economic viability and environmental sustainability. However, biocathode has its shortcoming like low reduction potential of the half-cell, particularly the sulphate reduction (E°′ = −217 mV) [7] compared to oxygen or air cathode [9]. Nitrate has a much higher reduction (E°′ = +760 mV for NO3 −/N2), which demonstrated that nitrate is competitive to oxygen (E°′ = +818 mV for O2/H2O) as an electron acceptor [7, 13]. Even though the redox potential of sulphate is low and its ability to receive electronic receptor is weaker than the nitrate, the researchers are still positive about the potential that sulphate can be employed as the cathode electron acceptor since its reduction does not require strict anaerobic conditions [14]. According to Virdis et al. [15], biocathode may experience some potential loss ascribed to the bacterial energy consumption for growth and maintenance; however, the reduced overpotentials, which are a direct consequence of the biocatalysis will be more than that compensated for this loss. Modified processes for the removal of organic substrates and nitrogen in wastewater using MFCs have been studied [3, 16]. Virdis et al. [15] also demonstrated the operation of a biocathode double chambered MFC using a separate nitrification tank for simultaneous carbon and nitrogen removal.
The stability and treatment efficiencies in long-term operation of MFCs with organic, nutrient removal and simultaneous bioelectricity generation are lack of documentation. The research led by Zhang et al. [17] had demonstrated a biocathode manure fed MFC operation for 171 days, and reported that MFC power started to decline from day 140 as a result of insufficient fuel. Nevertheless, long-term operation studies in MFC systems are very limited and in great need as it will be useful in revealing long-term stability in an operation and longevity of the system. Such studies will be helpful in developing a promising method of energy recovery and make the wastewater treatment infrastructure more sustainable.
Nitrate as terminal electron acceptor in MFCs was demonstrated as an innovative technology for nitrate removal from wastewater. Oxygen is commonly used as electron acceptors in MFC studies, while the study on nitrate removal using double chambered MFC is scarce. The aim of this study was to investigate the performance of double chambered MFCs in terms of wastewater treatment and bioelectricity generation, where organic matter reduction, nitrate (NO3 −), voltage, power density, and current density were evaluated. Nitrate was used as terminal electron acceptor to study the cathodic nitrate reduction in MFC system.
Materials and methods
MFC design and operation
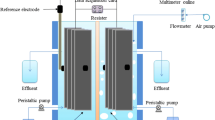
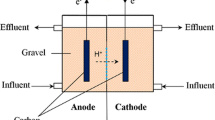
Double chambered MFCs were designed and fabricated by assembling acrylic plates. The MFC reactor consists of two-equal volume chambers with the internal dimensions of 10 × 10 × 20 cm (L × W × H). Both anodic and cathodic chambers were separated by a proton exchange membrane (PEM), i.e., Nafion 117 (Ion Power Inc, USA), which allows protons to move across to the cathode while blocking the diffusion of oxygen into the anode [18].
The activated carbon flakes (ACF) (size 3 ± 2 mm) were inoculated in anaerobic mixed cultures sludge that collected from a glove manufacturing factory’s wastewater treatment plant for a month before it was used as bio-electrodes in MFC setup. The volume of ACF as electrode was 1100 cm3 in each chamber and the average net anodic compartment (NAC) and net cathodic compartment (NCC) were 0.67 L each. Carbon rods were used as contact and were inserted into ACF. Then carbon rods were connected with copper wire to an external resistor of 1000 Ω. Both chambers were sealed to prevent the diffusion of oxygen into the system. The schematic diagram of double chambered ACF MFC reactor is shown in Fig. 1.
The MFC was operated in a continuous mode using a peristaltic pump (Natong BL-100C, China), which was controlled by a timer with 1 h on, followed by 1 h off cycles. Synthetic wastewater was fed at a flow rate of 0.93 mL/min [corresponding to a 1 day hydraulic retention time (HRT)] into the bottom of the MFC reactors. The voltage generated for both MFC reactors was recorded with a data logger (midi LOGGER GL820, China). The MFC reactors were operated under ambient temperature conditions in the laboratory for 180 days.
Synthetic wastewater characteristics
The anodic chamber of ACF MFC was fed with a synthetic wastewater containing CH3COONa (1.569 g/L), NH4Cl (0.31 g/L), MgCl26H2O (0.1 g/L), KCl (0.13 g/L), NaCl (0.116 g/L), K2HPO4 (3.4 g/L) and KH2PO4 (4.0 g/L) unless otherwise specified. Nutrients, phosphate buffer solution and with additional NH4NO3 (0.176 g/L) as the electron acceptors were fed to the cathodic chamber of ACF MFC reactor.
Analyses
Liquid samples were collected from the effluent point for COD and NO3 − analysis. Samples were centrifuged (Cence L500, China) at 4200 rpm for 10 min and samples supernatant were collected for COD analysis. The concentration of COD was analysed using a HACH DR/890 Colorimeter, while NO3 − was analysed using nitrate ion electrode and ammonium ion electrode that connected to pH/ORP meter (Bante Instruments 920, China).
Calculation
The current (I) in amperes (A) was calculated by Ohm’s law using the measured voltage, which was calculated by Eq. (1)
where V (V) is the cell voltage, R (Ω) is the external resistance.
The power, P (W) was calculated by Eq. (2):
where I is in amperes (A) and V is the voltage (V).
The biological reaction occurs at anode; hence the power output is typically normalized to the projected anode surface area [19]. The power density (P d) was calculated by Eq. (3) on the basis of the effective volume of the anode (m3) or the NCC:
Results and discussion
Wastewater treatment performance
Chemical oxygen demand monitoring
The COD removal in the anodic chamber was monitored to evaluate the potential of MFC as a wastewater treatment unit. COD influent of 1142 ± 48 mg/L was supplied to the MFC system. Figure 2 shows the COD effluent and removal efficiency as a function of time. The COD removal efficiency was relatively higher at the very beginning of the study (up to ∼89 %), which was mainly due to the adsorption of the ACF and partly due to biodegradation by the anaerobic microbes on the organic compounds. It is corresponding to the voltage output as there was a slight decrease in voltage output at the beginning of operation. A fluctuation in COD removal efficiency was observed during the 12th to 42nd day of the operation. The average removal efficiency was only 33 ± 6 %. This result can be due to the adsorption of ACF reached saturation. Therefore, the removal of COD after day 11 was mainly contributed by biodegradation, where the acetate in the anodic chamber was consumed by the microbial oxidation. The fluctuation trend also suggested that acclimation took place in the system. Then, the COD removal efficiency increased to 44 ± 5 % over the operating period of day 43–63, and it showed an increasing trend. The ever increasing COD removal efficiency can be contributed by the large specific surface area of activated carbon flake that allows microbes’ attachment and growth. Fang et al. [20] also explained that the high specific surface area in the granular activated carbon significantly benefited biofilm growth, and the organic matter could be adsorbed onto the biofilm and then be oxidized by microbes on the biofilm.
The system showed a rather stable COD removal after 80 days of operation, and the circuit of MFC system was disconnected from day 101–114 to investigate the MFC performance under open circuit. The COD removal efficiency during open circuit was approximately 9 % lower than the closed circuit. This could be due to the closed circuit MFC enables the system to transfer electrons produced from anode to cathode; the electron transfer can stimulate the bacterial in COD removal. This result was supported by the finding from Sevda and Dominguez-Benetton [21] and Jia et al. [13] that showed COD removal rates were higher in the closed circuit than open circuit. After the MFC system was switched to closed circuit, the COD removal performance resumed to the previous state rapidly. The removal efficiency improved steadily to an average of 82 ± 4 % from day 115–148 of the MFC operation. This may be contributed by the growth of biofilm at the anode over time. The organic loading was increased to 1641 ± 27 mg/L from day 149 onwards. A relatively stable removal efficiency of 76 ± 3 % was observed. As the system was supplied with higher availability of substrate for microbial oxidation in the anodic chamber, the COD reduction improved about 340 mg/L. Overall, the COD removal trend was corresponding to nitrate removal as well as voltage output.
Nitrate removal monitoring
The variation of nitrate removal of the MFC system that operated for 180 days is shown in Fig. 3. Synthetic wastewater containing 141 ± 14 mg/L of nitrate was fed into the cathodic chamber of the MFC. During the first 19 days of operation, the nitrate removal efficiency achieved was up to 96 %. However, this exceptionally high removal during the initiation stage was mainly attributed to the activated carbon adsorption and also partly due to denitrification process. It was noticed that the nitrate reduction decreased and fluctuated after about 19 days of operation. Similar trend also showed in the COD removal, which also indicated that ACF adsorption reached saturation after 19 days. It was suggested that nitrate removal was then due to denitrification process by denitrifying bacteria. There were some decrease and fluctuation in nitrate removal observed during day 20–55. This phenomenon could be due to the bacteria for denitrification in the system was still undergoing acclimation. Since the external carbon source was not supplied to the cathodic chamber, this may lead to a decrease in certain bacterial community such as the heterotrophic bacteria when no external carbon source added into the system to sustain bacterial activities. While autotrophic bacteria could survive in the system even without addition of carbon source, and it was hypothesized that there could be more autotrophic bacteria present in the cathodic chamber, and these species would be the dominant in the MFC system in acclimation.
It was noticed that the nitrate removal was relatively stable from day 56–100 with the removal efficiency of 75 ± 3 %. The result could be contributed by the bacteria metabolism over time, which enhanced the electron transfer and consequently, facilitated nitrate reduction. The nitrate removal dropped about 11 % when the MFC circuit was disconnected. Under an open circuit system, the electrons generated at the anodic chamber were not able to be transferred to cathode; hence nitrate utilization was lower compared to the nitrate reduced under the closed circuit system. After 14 days of disconnection, the system was switched back to closed circuit for further investigation. The cathodic nitrate reduction improved gradually over time and 78 ± 4 % of nitrate removal efficiency was obtained during day 115–148. Higher organic loading was supplied to the MFC system from day 149–180. The reduction of nitrate requires electrons; the increased in organic loading led to the more electrons produced from the oxidation of organic matter at the anode. Thus, more electrons will be transferred to the cathode, and more nitrate can be reduced in the cathodic chamber. The nitrate removal efficiency was increased to 88 ± 4 % during that operation period (influent of 141 ± 14 mg/L). Moreover, the result of nitrate removal was also in accordance with the COD removal and voltage output of the system. In the denitrification process, the complete reduction of nitrate (NO3 −) into nitrogen gas can occur via the following pathway,
where nitrate was transformed into nitrite (NO2 −), nitric oxide (NO), and lastly resulting in the production of nitrogen gas (N2) [22]. In this study, there was a slight increase of ammonium (NH4 +) (∼9 %) in the cathodic compartment (data not shown); it could be due to production of ammonium via dissimilatory nitrate reduction to ammonium (DNRA). Nitrate can be biologically reduced through DNRA process to ammonium as follows [23],
Zhu et al. [24] also reported that the increased of NH4 + along the cathode compartment of MFC system could be due to DNRA, where DNRA bacteria could be acted as the biocatalyst for such reaction. In this study, it was assumed that the most of the nitrate transformed to nitrogen gas and partially transformed to ammonium. The MFC system in this study achieved higher nitrate removal than some of the double chambered MFC systems reported previously [25, 26]. A 36.7 % (influent 32.4 mg/L of NO3–N) of nitrate removal was reported by Zhu et al. [24], which denitrification achieved in the cathodic chamber of a membrane-less MFC with biocathode.
Bioelectricity generation performance
Voltage output monitoring
The variation of voltage output in the ACF MFC continuous flow system is shown in Fig. 4. The voltage output decreased slightly during the first 4 days of operation. This was because the COD reduction was mainly due to adsorption, and partial biodegradation. Therefore, only a small amount of electrons produced that can be used for nitrate reduction to generate electricity and caused a low-voltage output. The overall voltage output of the ACF MFC showed an increasing trend throughout the study (except during the circuit disconnection period) which was from ~10 to ~370 mV. The voltage output was low, but it increased rapidly after the system was continuously fed with synthetic wastewater during the initial stage of operation. From day 20–37, the voltage increased at a lower rate because during this period, the removal of nitrate and COD started to decrease and fluctuate. As a result, fewer electrons and protons generated for electricity generation. After the system operated for approximately 3 months, the voltage output increased and stabilized at 343 ± 2 mV (day 90–100). The increase of voltage output can be ascribed to the growth of microbial community and formation of biofilm at anode and then accelerated the transfer of electron to electron acceptor as the operation proceeded. Venkata Mohan et al. [27] also reported the positive effect of biofilm formation on the anode surface for bioelectricity production.
The MFC circuit was disconnected (open circuit) from day 101–114, so no voltage output was recorded. It was noticed that when the circuit was switched from open to closed circuit, the voltage output was only 290 mV, which was relatively lower than voltage produced prior circuit disconnected. This scenario can be caused by the decrease in electrochemical-active bacterial under the open circuit condition since electron transfer was unavailable in open circuit system [13]. Nevertheless, when the system was reconnected to closed circuit, the voltage could resume to the potential before circuit disconnection within 6 days. The electrochemical-active bacterial could have grown better under the closed circuit system. After that, stable voltage output (358 ± 6 mV) was observed from day 121–148. The average organic loading of 1641 ± 27 mg/L was fed to the anodic chamber from day 149 onwards; consequently, the voltage output increased and stabilized at 370 ± 2 mV. The highest voltage output of the system was also observed during this period. Due to the higher concentration of acetate presented to the system, more acetate was oxidized by the microbes and subsequently produced higher protons and electrons to the electron acceptor in the cathodic chamber, and higher voltage was generated. These results were consistent with the COD removal and nitrate removal of the MFC system during the respective phases.
Power output monitoring
Polarization curves were obtained by varying different resistance (20,000–32 Ω) to determine the maximum power density and current density. The power densities increased with the increase of current densities until it reached its maximum value [3]. The performance of power density and current density of the system is shown in Fig. 5. The power density of the MFC system increased rapidly during the first 2 months of operation. The power density increased about 151 % from day 25 to day 38 from 75 to 188 mW/m3. Meanwhile, the power density increased steadily as the operation proceeded and reached the maximum of 613 mW/m3 during day 125 when 1142 mg/L of organic loading was supplied to the system. Organic loading was increased to 1641 mg/L after the system operated for 148 days. The power density of ACF MFC system showed an increment during that period as the nitrate reduction in the MFC system increased accordingly. The bioelectricity was generated in the MFC as a result of nitrate reduction in the cathodic chamber [28]. The highest power density was 669 mW/m3 (1006 mW/m3 NCC) at current density of 3487 mA/m3 (4982 mA/m3 NCC) during day 172. The power output was corresponding to the voltage output of the system. The power output in this study was lower than that obtained with a two chambers MFC using nitrate as electron acceptor with power density of 3.9 W/m3 NCC [28]. However, the power density obtained in this study was relatively higher compared to the study of Zhu et al. [24], where power density of 0.0712 W/m3 was generated with nitrate as electron acceptor by a membrane-less MFC. In addition, power density obtained in this study was also higher than that in Wei et al. [29], which obtained 66.83 mW/m3 of maximum power density using sucrose (3.5 g/L), in which oxygen was used as electron acceptor. While much higher power output (24.3 W/m3) was generated from the reduction of oxygen by biocathode with a double chambered flat plat MFC by Wei et al. [30]. In Guo et al. [31], 310.08 mW/m3 was generated from a double chambered MFC system, where refinery wastewater was used as fuel and Fe(III)-EDTA was utilized to facilitate the electron transfer in the cathodic chamber. By comparing with other studies, the differences in power densities could be due to the difference in reduction activity of biocathode and the electrons produced from the oxidation of carbon sources. The power performance of MFC can also be influenced by various factors, including the operating conditions (such as organic loading, feed rate and shear stress, pH and temperature) [32], electrode surface area, type of electrodes, different microorganisms involved [6], and configuration of MFCs [18].
The internal resistance of the MFC system was 50 Ω, which was determined from the polarization curves. This internal resistance of this system was much lower than many double chambered MFCs that reported previously such as Wei et al. [29] and Liu et al. [33] with 700 and 220 Ω of internal resistant, respectively. The vast difference of internal resistance in these studies can be due to the different electrode material used in the system. Oon et al. [34] reported that the internal resistance could be influenced by the electrode material used in the system i.e., activated carbon (200–430 Ω) compared to carbon felt (820–4300 Ω) in Oon et al. [35]. The high specific surface of activated carbon is a good medium for the attachment of microorganisms, the good biocompatibility and moderate electrical conductivity of activated carbon also make it an effective electrode material [30], hence contributed to the low internal resistance. Moreover, Zhao et al. [36] found that the MFC performance improved with the development of biofilm, the internal resistance of the system reduced by 66.5 % to 80.3 Ω. By comparing numerous MFCs studies, it is worth noting that the internal resistance of this study was relatively lower than most of double chambered MFC systems reported in the literature. The internal resistance of a MFC system can be influenced by the electrodes’ spacing, operation mode, ionic strength, substrate properties [37], and the size and type of membrane [15].
Conclusions
This study demonstrates that nitrate was successfully used as terminal electron acceptor in the double chambered biocathode MFC and generates bioelectricity simultaneously in long-term operation. The performances of COD and nitrate removal efficiencies were better in the closed circuit than open circuit. Reduction of COD and nitrate improved with the increase of organic loading and subsequently contributed to higher power output. The internal resistance was found to be low for a double chambered MFC, which was only 50 Ω. The MFC system achieved a maximum power density of 669 mW/m3 with current density of 3487 mA/m3.
References
Rabaey K, Verstraete W (2005) Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 23:291–298. doi:10.1016/j.tibtech.2005.04.008
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. doi:10.1021/es0605016
Lefebvre O, Al-Mamun A, Ooi WK et al (2008) An insight into cathode options for microbial fuel cells. Water Sci Technol 57:2031–2037. doi:10.2166/wst.2008.611
Aelterman P, Rabaey K, Clauwaert P, Verstraete W (2006) Microbial fuel cells for wastewater treatment. Water Sci Technol 54:9–15. doi:10.2166/wst.2006.702
Wang H, Lu L, Cui F, Liu D, Zhao Z, Xu Y (2012) Simultaneous bioelectrochemical degradation of algae sludge and energy recovery in microbial fuel cells. RSC Adv 2:7228–7234. doi:10.1039/c2ra20631e
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543. doi:10.1016/j.biortech.2009.10.017
Zhou M, Gu T (2013) The next breakthrough in microbial fuel cells and microbial electrolysis cells for bioenergy and bioproducts. J Microb Biochem Technol 01:10–13. doi:10.4172/1948-5948.S12-003
Logan BE, Wallack MJ, Kim K et al (2015) Assessment of microbial fuel cell configurations and power densities. Environ Sci Technol Lett 2:206–214. doi:10.1021/acs.estlett.5b00180
Zhou M, Wang H, Hassett DJ, Gu T (2013) Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J Chem Technol Biotechnol 88:508–518. doi:10.1002/jctb.4004
He Z, Angenent LT (2006) Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 18:2009–2015. doi:10.1002/elan.200603628
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells: an overview. J Power Sources 180:683–694. doi:10.1016/j.jpowsour.2008.02.074
Rozendal RA, Hamelers HVM, Rabaey K et al (2008) Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol 26:450–459. doi:10.1016/j.tibtech.2008.04.008
Jia YH, Tran HT, Kim DH et al (2008) Simultaneous organics removal and bio-electrochemical denitrification in microbial fuel cells. Bioprocess Biosyst Eng 31:315–321. doi:10.1007/s00449-007-0164-6
Song H, Zhu Y, Li J (2015) Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells—a mini review. Arab J Chem. doi:10.1016/j.arabjc.2015.01.008
Virdis B, Rabaey K, Yuan Z, Keller J (2008) Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res 42:3013–3024. doi:10.1016/j.watres.2008.03.017
Kim J, Kim B, Kim H, Yun Z (2014) Effects of ammonium ions from the anolyte within bio-cathode microbial fuel cells on nitrate reduction and current density. Int Biodeterior Biodegrad 95:122–126. doi:10.1016/j.ibiod.2014.04.015
Zhang G, Zhao Q, Jiao Y, Lee D-J (2015) Long-term operation of manure-microbial fuel cell. Bioresour Technol 180:365–369. doi:10.1016/j.biortech.2015.01.002
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25:464–482. doi:10.1016/j.biotechadv.2007.05.004
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285. doi:10.1021/es034923g
Fang Z, Song HL, Cang N, Li XN (2013) Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour Technol 144:165–171. doi:10.1016/j.biortech.2013.06.073
Sevda S, Dominguez-Benetton X (2014) Evaluation and enhanced operational performance of microbial fuel cells under alternating anodic open circuit and closed circuit modes with different substrates. Biochem Eng J 90:294–300. doi:10.1016/j.bej.2014.06.024
Sotres A, Cerrillo M, Viñas M, Bonmatí A (2016) Nitrogen removal in a two-chambered microbial fuel cell: establishment of a nitrifying–denitrifying microbial community on an intermittent aerated cathode. Chem Eng J 284:905–916. doi:10.1016/j.cej.2015.08.100
Zhang B, Liu Y, Tong S et al (2014) Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. J Power Sources 268:423–429. doi:10.1016/j.jpowsour.2014.06.076
Zhu G, Onodera T, Tandukar M, Pavlostathis SG (2013) Simultaneous carbon removal, denitrification and power generation in a membrane-less microbial fuel cell. Bioresour Technol 146:1–6. doi:10.1016/j.biortech.2013.07.032
Fang C, Min B, Angelidaki I (2011) Nitrate as an oxidant in the cathode chamber of a microbial fuel cell for both power generation and nutrient removal purposes. Appl Biochem Biotechnol 164:464–474. doi:10.1007/s12010-010-9148-0
Cai J, Zheng P, Xing Y, Qaisar M (2015) Effect of electricity on microbial community of microbial fuel cell simultaneously treating sulfide and nitrate. J Power Sources 281:27–33. doi:10.1016/j.jpowsour.2015.01.165
Venkata Mohan S, Veer Raghavulu S, Sarma PN (2008) Influence of anodic biofilm growth on bioelectricity production in single chambered mediatorless microbial fuel cell using mixed anaerobic consortia. Biosens Bioelectron 24:41–47. doi:10.1016/j.bios.2008.03.010
Puig S, Serra M, Vilar-Sanz A et al (2011) Autotrophic nitrite removal in the cathode of microbial fuel cells. Bioresour Technol 102:4462–4467. doi:10.1016/j.biortech.2010.12.100
Wei L, Yuan Z, Cui M et al (2012) Study on electricity-generation characteristic of two-chambered microbial fuel cell in continuous flow mode. Int J Hydrogen Energy 37:1067–1073. doi:10.1016/j.ijhydene.2011.02.120
Wei J, Liang P, Cao X, Huang X (2011) Use of inexpensive semicoke and activated carbon as biocathode in microbial fuel cells. Bioresour Technol 102:10431–10435. doi:10.1016/j.biortech.2011.08.088
Guo X, Zhan Y, Chen C et al (2015) Simultaneous bioelectricity generation and biodegradability improvement of refinery wastewater using microbial fuel cell technology. Desalin Water Treat 53:2740–2745. doi:10.1080/19443994.2014.931535
Oliveira VB, Simões M, Melo LF, Pinto AMFR (2013) Overview on the developments of microbial fuel cells. Biochem Eng J 73:53–64. doi:10.1016/j.bej.2013.01.012
Liu L, Tsyganova O, Lee D-J et al (2013) Double-chamber microbial fuel cells started up under room and low temperatures. Int J Hydrogen Energy 38:15574–15579. doi:10.1016/j.ijhydene.2013.02.090
Oon YL, Ong SA, Ho LN et al (2016) Synergistic effect of up-flow constructed wetland and microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour Technol 203:190–197. doi:10.1016/j.biortech.2015.12.011
Oon YL, Ong SA, Ho LN et al (2015) Hybrid system up-flow constructed wetland integrated with microbial fuel cell for simultaneous wastewater treatment and electricity generation. Bioresour Technol 186:270–275. doi:10.1016/j.biortech.2015.03.014
Zhao Y, Li P, Wang X, Sun Y (2012) Influence of initial biofilm growth on electrochemical behavior in dual-chambered mediator microbial fuel cell. J Fuel Chem Technol 40:967–972. doi:10.1016/S1872-5813(12)60034-6
Liang P, Huang X, Fan M-Z et al (2007) Composition and distribution of internal resistance in three types of microbial fuel cells. Appl Microbiol Biotechnol 77:551–558. doi:10.1007/s00253-007-1193-4
Acknowledgments
The authors gratefully acknowledge the financial support of ScienceFund (Grant No. 02-01-15-SF0201) provided by Ministry of Science, Technology and Innovation (MOSTI), Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oon, YS., Ong, SA., Ho, LN. et al. Long-term operation of double chambered microbial fuel cell for bio-electro denitrification. Bioprocess Biosyst Eng 39, 893–900 (2016). https://doi.org/10.1007/s00449-016-1568-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1568-y