Abstract
Microbial fuel cells (MFCs) were constructed using azo dyes as the cathode oxidants to accept the electrons produced from the respiration of Klebsiella pneumoniae strain L17 in the anode. Experimental results showed that a methyl orange (MO)-feeding MFC produced a comparable performance against that of an air-based one at pH 3.0 and that azo dyes including MO, Orange I, and Orange II could be successfully degraded in such cathodes. The reaction rate constant (k) of azo dye reduction was positively correlated with the power output which was highly dependent on the catholyte pH and the dye molecular structure. When pH was varied from 3.0 to 9.0, the k value in relation to MO degradation decreased from 0.298 to 0.016 μmol min−1, and the maximum power density decreased from 34.77 to 1.51 mW m−2. The performances of the MFC fed with different azo dyes can be ranked from good to poor as MO > Orange I > Orange II. Furthermore, the cyclic voltammograms of azo dyes disclosed that the pH and the dye structure determined their redox potentials. A higher redox potential corresponded to a higher reaction rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The microbial fuel cell (MFC), first demonstrated to work by Potter (1912), is a technology using microorganism to catalyze the conversion of organic matters into electricity. A number of recent reports (Liu et al. 2004; Feng et al. 2008; Min and Logan 2004; He et al. 2005) show that electricity can be harvested from wastewater, and simultaneously complex organic pollutants in wastewater can be substantially removed by oxidation with the help of biocatalysts. But there are still some contaminants that cannot be degraded by the anodic oxidation process owing to their positively high redox potentials. To remove them by reduction in an MFC cathode is thus of practical importance regarding the complete treatment of wastewater and further development of the MFC technology.
In line with this, it has been previously reported that microorganisms in biotic cathodes can remove nitrogen (Clauwaert et al. 2007a; Virdis et al. 2008; Jia et al. 2008). However, despite the successful denitrification in the cathode, the denitrifying MFC has its shortcomings (Clauwaert et al. 2007a) of longer start-up time for cathode inoculation and lower power generation in comparison to the oxygen-based system. The reason attributed to such drawbacks is the involvement of microbial catalysts for the bioreduction reaction. Indeed, an abiotic cathode such as the potassium ferricyanide-feeding cathode (Schröder et al. 2003) cannot only offer a faster reaction rate but also produce a much higher power output against the oxygen-feeding counterpart. However, use of potassium ferricyanide will add operational costs of MFCs because recycle of ferricyanide is difficult.
Many pollutants in wastewater with positively high redox potentials might be good alternatives to potassium ferricyanide such as nitro aromatic compounds, chlorinated aromatic compounds, and some metal ions (e.g., chromium(VI) (Wang et al. 2008), manganese(VII) (You et al. 2006), technetium(VI), and uranium (VI)). In this regard, it is unclear which pollutants are suitable as efficient oxidants in an MFC process and what operation conditions such as pH, reactor design, and electrode materials selected are required in the cathode. The aims of this study were to (1) demonstrate the feasibility of direct utilization of toxic azo dyes that are widely used in the textile industry (Xu et al. 2007; Guaratini et al. 2001) as the electron acceptors for the cathode of an MFC and to (2) examine the power performance and dye degradation rate of MFCs that are operated with a few azo dyes with different redox potentials under various experimental conditions such as pH. A dual-chamber MFC was constructed, and the biocatalyst employed was Klebsiella pneumoniae strain L17 (Li et al. 2009) fed with glucose.
Materials and methods
Microorganisms and medium in the anode chamber
The bacteria, K. pneumoniae strain L17 (CCTCC AB 208106) isolated from subterranean forest sediment by our group was inoculated in the anode chamber of an MFC. Before being transferred to the MFC reactor, the frozen bacteria (1 mL) dissolved in 100 mL of medium were allowed to grow overnight (18 h) at 30°C on an orbital shaker incubator at 150 rpm. Glucose (3 g L−1) used as the energy source was added to the anode chamber and was anaerobically oxidized by the microorganism. The microbial growth medium (pH 7.0) in the anode chamber contained 5.84 g L−1 NaCl, 0.10 g L−1 KCl, 0.25 g L−1 NH4Cl, 12.00 g L−1 Na2HPO4·12H2O, 2.57 g L−1 NaH2PO4·2H2O, 10-mL vitamin solution, and 10-mL mineral solution. The required vitamin and mineral solutions were prepared as previously described (Lovley and Phillips 1998). Prior to use, the above medium were sterilized by autoclaving at 121°C for 20 min.
Catholytes
The azo dye characterized by the –N=N– bond was used as the electron acceptor in the cathode. The catholyte was continuously sparged with pure N2 (90 mL/min) to avoid O2 competition with azo dyes for electrons. The reduction reactions that may be involved in the cathode chamber are described as follows(Goyal and Minocha 1985; Menek and Karaman 2005), in which the –N=N– double bond was reduced to hydrazo (1) or amine (2), via the consumption of two or four electrons.
To assess the pH impact on MFC performance and degradation rate of the investigated azo dye, a precise control of the catholyte pH was required and was achieved in a phosphate buffer solution. The pH was varied between 3.0 and 9.0 and adjusted by manipulating the composition of H3PO4, NaH2PO4, and NaH2PO4 used. Furthermore, three sets of experiments were conducted to evaluate the effect of dye structure on the power output. Three azo dyes of Orange I, Orange II, and MO (Fig. 1) were examined in this investigation. For comparison, air as an oxidant was also bubbled (90 mL/min) through the cathode chamber as an oxygen-fed MFC process.
MFC construction and operation
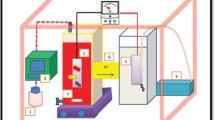
The MFCs consisted of two identical chambers separated by a cation exchange membrane (Electrolytica Corporation). Each cell chamber had an effective volume of 75 mL. Both electrodes were made of carbon felt (4.5 × 4.5 cm each, Panex 33 160 K, Zoltek), and Ti wire was inserted inside the carbon felt to connect the circuit. The cation exchange membrane was boiled in a 80°C H2O2 (3% V/V) solution and was then soaked in deionized water for 1 day for cleaning. To operate the MFC, the microorganism growth medium containing glucose was added to the anode chamber, and the catholyte containing azo dyes was added to the cathode chamber. All the experiments were conducted at a controlled temperature of 30°C and atmospheric pressure. Unless otherwise stated, a 2,000 Ω resistor was used as the external load for the MFC.
Electrochemical tests
Cyclic voltammetry (CV) tests were carried out in a conventional three-electrode electrochemical cell using an Autolab potentiostat (PGSTAT 30, EcoChemie), in which the working electrode was the carbon felt with the same size (4.5 × 4.5 cm) of that used in MFCs. A saturated calomel electrode (SCE) was used as the reference electrode, and a platinum mesh served as the counter electrode. All the potentials reported throughout this study were referred to SCE. Prior to each test, the electrolyte was deoxygenated by bubbling pure nitrogen gas for 30 min, and all experiments were performed while nitrogen gas was continuously bubbled over the surface of the electrolyte.
Calculation and analyses
All the data recorded according to the following methods were subjected to triplicate measurements. To quantify the cell performance, the resulting voltage (V) throughout the experiments was recorded every 2 min by using a data acquisition system (AD 8223 type voltage collector with 16 channels, Rui Bo Hua Technology Control Corporation of Beijing, China) with a PC. Then, the current density (I) and power density (P) normalized to the projected surface area of the cathode can be calculated according to I = V R −1 and P =V 2R −1, respectively, where R is the external resistance. A polarization curve was obtained by adjusting the external resistor from 20 to 50,000 Ω. At each fixed resistance, voltage of the tenth minute was collected when a pseudo-steady state was approached.
To determine the kinetics of the azo dye degradation, the dye concentrations were determined by a UV-vis spectrophotometry (TU1800-PC, Beijing China) at different reaction times. All the samples were measured as they were taken from the cathode compartment. Additional identification of the degradation products was also performed by high-performance liquid chromatography (HPLC)–mass spectra (MS) using a reverse-phase Hypersil GOLD column of 150 × 2.1 mm, equipped with a silica precolumn guard. The reverse-phase column was coupled with Finnigan-MAT LCQ mass spectra via the Finnigan electrospray interface (Finnigan-MAT, San Jose, CA, USA) operating in the selected ion monitoring scan type. The injection volume was 10 μL. For the detection of MO degradation products, acetonitrile and 0.1 mol L−1 ammonium acetate filtered through a 0.2-μm filter were used as the mobile phase. For the detection of Orange I and Orange II degradation products, acetonitrile and 0.1% acetic acid was used as the mobile phase. The flow rate of mobile phase was 0.1 mL min−1.
Results
The electricity generation and azo dye degradation
An MFC system consisting of two electrodes was established: an anaerobic respiration anode and an MO-dye-fed cathode. Upon addition of 0.05 mM of MO solution (pH 3.0) to the cathode chamber, the open-circuit voltage of MFC, fed with 3 g L−1 of glucose to the bacteria-inoculated anode chamber, rapidly increased to 710 mV (data not shown), indicating a redox potential difference between anode and cathode. Repeatable voltage generation of MFC was obtained in eight cycles with the fresh MO azo dye feeding to the cathode, as shown in Fig. 2a. In the first cycle, a maximum voltage of 259 mV was observed across the external resistance of 2,000 Ω. Then, the MFC produced a relatively stable voltage of 250 ± 15 mV for about 2.7 h. Beyond this period, a sharp drop of voltage to 75 ± 10 mV was found, attributed to the lack of MO in the cathode. This pattern of voltage output variation was reproducible when the fresh MO was added. These results suggest that the MO-fed MFC is able to continuously generate electricity by replacing the MO dye.
a Cell voltage as a function of time of the microbial fuel cell operation with the MO-fed cathode (0.05 mM of MO, pH 3.0, and temperature of 30°C). Arrows indicate the MO electrolyte replenishment. bk value as a function of MO-feeding cycle. The inset shows the degradation (filled squares) and adsorption (empty circles) of MO as a function of time upon first-cycle feeding
To investigate the kinetics of MO degradation in the cathode chamber, the concentration of MO was analyzed against reaction time. The variation of MO concentration with respect to the first feeding cycle revealed that there was a linear relationship between C t/C 0 (the ratio of remaining MO concentration to initial MO concentration) and t (the reaction time), as shown in the inset of Fig. 2b. This observation indicates that MO degradation follows the zero-order kinetics at 30°C under atmospheric pressure and that the kinetic rate constant can thus be obtained. In such a case, the MO dye was completely removed within 3 h, while the MO concentration was approaching to zero and the voltage output was declining to the minimum point, as shown in Fig. 2a. It was also found from Fig. 2b that the k value for each feeding cycle was approximately constant, confirming the same pattern of pollutant transformation in the MFC process. Another important observation from the inset of Fig. 2b was that there was 15.5% MO adsorbed on the carbon felt electrode after 3 h. This indicates that only 84.5% of MO contributed to the electricity generation.
UV-vis absorption spectra of the reaction solutions further proved the successful degradation of the azo dye in the cathode chamber. The reaction products were also detected by HPLC–MS. As shown in Fig. 3a, the absorbance band of MO at 500 nm can be ascribed to its double bond; meanwhile, the band at 247 nm was possibly ascribed to sulfanilic acid, in line with the data reported (Fan et al. 2009). The decreased absorbance at 500 nm should result from the reduction of the –N=N– double bond of MO cathodically to amine, and the increased absorbance at 247 nm should result from the formation of sulfanilic acid as a final product, indicating that the overall reduction follows the pathway described by Eq. 2. Further UV-vis studies with the Orange-I-based and Orange-II-based cathodes were then performed. Similar behaviors of dye degradation were observed in these two systems (Fig. 3b, c). In each case, there was also a gradual increase in the intensity of the absorbance at 247 nm. These results suggest that the identical reduction product (sulfanilic acid) exist with respect to the breakdown of three azo dyes (MO, Orange I, and Orange II). The reaction products detected by HPLC–MS were listed in Table 1. Clearly, in addition to sulfanilic acid, the second product of N,N-dimethyl-p-phenylenediamine, 1-amino-4-naphthol, and 1-amino-2-naphthol were also revealed corresponding to the reduction of MO, Orange I, and Orange II, respectively. Based on the data collected from UV-vis and HPLC–MS techniques, it can be concluded that these three dyes have been completely reduced to amines in the cathode of an MFC.
Dependence of the power performance on catholyte pH and dye structure
Using a phosphate buffer in the cathode chamber is necessary for pH control as the power performance is heavily sensitive to the catholyte pH. Figure 4a shows the effect of pH on the polarization curves and the power density curves obtained by varying the external resistance from 20 to 50,000 Ω. It can be clearly seen that the best cell performance was achieved at pH 3 in the whole investigated range of pH. Raising the pH resulted in a decrease of power density. The maximum power density reached 34.77 mW m−2 at a corresponding current density of 154.78 mA m−2 at pH 3.0, whereas the maximum power density was only 1.51 mW m−2 at pH 9.0.
Figure 4b shows the power performance of aerated MFCs as a function of pH in the cathode. The comparison of Fig. 4a, b indicates that the MO-feeding MFC produced a comparable cell performance to that of an air-feeding MFC at pH 3.0. This suggests that the azo dye, enriched from contaminated environments, could be an alternative to air serving as the cathode oxidant in an MFC. However, the MO-feeding MFC operated under circumneutral and alkalic conditions generated slightly lower power than the air-feeding MFC.
The power output was also highly dependent on the dye selection. In addition to the MO dye, two additional dyes (Orange I and Orange II) were also tested as electron acceptors in the cathode to study the effect of dye structure. Figure 5 clearly shows the differences of power densities resulting from these three dyes with different molecule structures, which can be ordered as MO > Orange I > Orange II at all pH tested. For example, the Orange-II-feeding MFC at pH 3.0 produced a maximum power density of 18.51 mW m−2, which was 90% of that produced from the Orange I-feeding cathode MFCs and 52% of that produced from the MO-feeding cathode MFCs.
Owing to the identical anode used, the different cathode systems predominantly contribute to the difference in power performance (Figs. 4a and 5). Figure 6 shows the anode and cathode polarization curves of MO-feeding MFCs at different pH. Clearly, the anode polarization curves exhibit negligible difference, but the cathode polarization curves are highly dependent on pH in the cathode. Such dependence is in agreement with the relationship between the power performance and catholyte pH, indicating that the cathode is the limiting factor in this study.
Dependence of the dye degradation rate on catholyte pH and dye structure
The kinetic experiments of dye degradation were conducted to quantify the relative reactivity of different types of azo dyes at different catholyte pH. Figure 7 shows the effect of a variation of pH on electrochemical reduction of MO (Fig. 7a), Orange I (Fig. 7b), and Orange II (Fig. 7c), in the cathode of MFCs, respectively. Similar trends of the pH-dependent degradation rate were observed for three cases, that is, the low value of pH led to a faster dye degradation rate than at high pH. For example, the zero-order reaction rate constant at pH 3.0 in buffered solution was found to be 0.298 μmol min−1 and that at pH 9.0 it was only 0.016 μmol min−1 (calculated from Fig. 7a).
A careful comparison of the time required for the complete reduction of each dye reveals that the effect of dye structure on the reaction rate was also pronounced. Typically, at pH 3.0, MO can be completely reduced within 2.5 h, slightly less than 3.0 h for Orange I and less than 5.0 h for Orange II. These results indicate that MO is more subject to being electrochemically reduced than other dyes under MFC conditions, in accordance with the results obtained from the chemical reduction (Hou et al. 2007).
Discussion
Here, we demonstrated the utilization of toxic azo dyes as the cathodes for the anaerobic respiration of K. pneumoniae strain L17 in the MFC technology. The electrons donated through the respiration of a Fe(III)-reducing microorganism can be successfully transferred to the target pollutant, leading to the degradation of azo dyes via a reduction pathway. In such an MFC process, the strategy of separating the bacterial-involved oxidation reaction from the pollutant-involved reduction reaction with a solid cation exchange membrane can simultaneously enable energy harvesting and pollutant degradation.
By combining the data from Figs. 4, 5, and 7, it is evident that the power output is positively correlated to the reaction rate of azo dyes. Taking into consideration the identical anode employed in all experiments, the difference in the electron transfer rate is mainly related to the performance of the cathode which can be highly dependent on the experimental conditions such as pH variation and dye structure. Due to the involvement of protons in the reactions, pH is a crucial factor to determine the redox potential of an azo dye (Gupta et al. 2007). The azo dye having a high redox potential is thermodynamically preferable to be reduced, thus corresponding to a high reaction rate. Further analysis of the relationship between the redox potential and pH was performed by using the CV techniques. Voltammograms of the investigated MO on the carbon felt electrode at different pH provided a direct evidence of its redox potential change with various pH values, as illustrated in Fig. 8a. Apparently, all MO dyes exhibited similar electrochemical characteristics and a pair of well-defined redox peaks. The cathodic peaks observed in the range of −0.25 to 0.15 V could be associated with the breakdown of –N=N– double bond to the corresponding amine (Pinheiro et al. 2004). The anodic peaks observed in the range of 0.15 to 0.65 V could be relevant to the oxidation of amine. A remarkable shift of both peaks to the negative side due to the increase of pH was observed in Figure 8a, suggesting that the reduction of MO species occurs more easily at low pH rather than at high pH. From a thermodynamic standpoint, the low catholyte pH is beneficial to increase the MFC potential (potential difference between cathode and anode) and thus to magnify the power output. Good linear correlation between the cathodic peak potential (E P) and pH was obtained (Fig. 8b). The slope of the straight line was found to be 60 mV per pH unit, indicating that four electrons and four protons were involved in the reaction of MO reduction electrochemically (Mandic et al. 2004; Ghoneim et al. 2008).
a Representative cyclic voltammograms of the MO dye on carbon felt electrodes at 30°C in pH values of 3.0, 5.0, 7.0, and 9.0. The data were recorded at a scan rate of 50 mV s−1, and the scan range was between +0.8 and −0.9 V. b The relationship between the cathodic peak potentials of different azo dyes as a function of pH
Another important observation from Fig. 8a was that the ratio of anodic current peak (I a) to cathodic current peak (I c) heavily relied on pH. In accordance with the literature (Mandic et al. 2004), increasing pH can increase the ratio of I a/I c. Typically, at pH 3.0, the ratio of I a/I c for the –N=N– double bond/amine couple was 0.62 which was significantly lower than 0.95 at pH 9.0. This observation indicates that the electron transfer is more kinetically favored at pH 3.0 than at pH 9.0 (Mandic et al. 2004).
The substituent group on azo bridge also plays an important role in determining the redox potential of an azo dye. For example, Ghoneim et al. (2008) reported that the reduction of –N=N– double bond was enhanced by the electron-withdrawing substituent, whereas the electron-donating substituent prevented its reduction. The difference in the reduction potential obtained from CV, due to the difference in the dye structure, was also observed in Fig. 8b. The E P was highly sensitive to the type of azo dye. At pH 3.0, 5.0, and 7.0, the peak reduction potentials of MO were 0.106, 0.03, and −0.195 V; the peak reduction potentials of Orange I were −0.155, −0.282, and −0.371 V; and the peak reduction potentials of Orange II were −0.172, −0.334, and −0.460 V, respectively. At the fixed pH, the cathodic peak potentials of three dyes were in an order of MO > Orange I > Orange II, as same as the trend of their power generation performance and degradation rate. This order can be understood based on that fact that MO has an electron-withdrawing p-dimethylamino group attached to the benzene ring, while Orange I and Orange II have an electron-donating hydroxyl group conjugated with the azo bridge. The electro-withdrawing group increases the acidity of the unprotonated nitrogen atom and hence promotes the proton-involved reduction reaction (Ghoneim et al. 2008). Further, our observation that Orange I performs better than Orange II is in agreement with the result of the previous report (Eriksson and Nyholm 2001) which demonstrated that the electron-donating hydroxyl group in the para position to the bridge favors the breakdown of azo bond as compared to that in the ortho position.
Based on the analysis illustrated above, it can be concluded that that the redox potential is the primary factor to determine the rate of electron transfer in the cathode of MFC. This gives insights into the design the azo dye-based cathode of MFC, that is, controlling the redox potentials of pollutant-containing catholyte is important for the control of power output as well as the degradation rate.
Theoretically, not only the azo dye containing –N=N– double bond but also many soluble organic/inorganic contaminants such as nitro aromatic compounds, chlorinated aromatic compounds, and metal ions (e.g., chromium(VI), technetium(VI), and uranium(VI)) can be used as an additional cathode reactant to the commonly used O2, given that their redox potentials are sufficiently high to initiate the microbial reaction in the anode. Despite being thermodynamically favorable, another consideration is that the reduction reactions of these pollutants should be kinetically fast. To overcome kinetic limits of some cathodic reactions, one of the effective ways is to build up a biocathode of MFC (Clauwaert et al. 2007b; Rabaey et al. 2008) in which electrochemically active microorganisms catalyze the reduction of some unfavorable ions in wastewaters. For example, a denitrifying MFC with a biotic cathode to remove nitrate was developed (Clauwaert et al. 2007a). However, the electricity output reported is lower than the oxygen-driven counterpart (Clauwaert et al. 2007a), although the redox potential of NO −3 /N2 is comparable to that of O2/H2O (He and Angenent 2006). In contrast with these reports, our study treated a pollutant in the cathode by the direct chemical reduction rather than the bioreduction. We have achieved competitive power densities of azo dye-based cathode compared to air-based cathode, when other operational conditions in the two systems were identical. The strategy of using azo dyes instead of air as cathode reactants is environmentally sustainable because the concentration of oxygen dissolved in industrial wastewaters is very low.
In addition to the benefit of power production, the electrochemical reduction of azo dyes can be accomplished without the application of an external power source. This saves energy with respect to the electrochemical reactions occurring in the electrolysis cell (Martinez-Huitle and Brillas 2008). Traditional electrolysis of organic pollutants has been extensively studied as a niche technology due to the advantages of low cost and easy handling. As a result, the method demonstrated in this study adds more value to the electrolysis way for treating wastes. It should be noted that the reduction products (i.e., amines) are still toxic, but they can find their uses in many applications. The MO, for example, is reduced to sulfanilic acid and N,N-dimethyl-p-phenylenediamine. Although N,N-dimethyl-p-phenylenediamine is not friendly to environment, it has been widely used for H2S removal from streams (Lu et al. 2006). This implies that this technology should be promising from a practical perspective.
References
Clauwaert P, Van Der Ha D, Boon N, Verbeken K, Verhaege M, Rabaey K, Verstraete W (2007a) An open air biocathode enables effective electricity generation with microbial fuel cells. Environ Sci Technol 41:7564–7569
Clauwaert P, Rabaey K, Aelterman P, De Schamphelaire L, Pham TH, Boeckx P, Boon N, Verstraete W (2007b) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360
Eriksson A, Nyholm L (2001) Coulometric and spectroscopic investigations of the oxidation and reduction of some azosalicylic acids at glassy carbon electrodes. Electrochim Acta 46:1113–1129
Fan J, Guo YH, Wang JJ, Fan MH (2009) Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles. J Hazard Mater 166:904–910
Feng YJ, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880
Ghoneim MM, El-Desoky HS, Amer SA, Rizk HF, Habazy AD (2008) Electroreduction and spectrophotometric studies of some pyrazolyl-azo dyes derived from 3-acetylamino-phenyl-5-pyrazolone in buffered solutions. Dyes Pigm 77:493–501
Goyal RN, Minocha A (1985) Electrochemical behaviour of the bisazo dye, direct red-81. J Electroanal Chem 193:231–240
Guaratini CCI, Fogg AG, Zanoni MVB (2001) Studies of the voltammetric behavior and determination of diazo reactive dyes at mercury electrode. Electroanalysis 13:1535–1543
Gupta VK, Jain R, Varshney S (2007) Electrochemical removal of the hazardous dye reactofix red 3 BFN from industrial effluents. J Colloid Interf Sci 312:292–296
He Z, Angenent LT (2006) Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 18:2009–2015
He Z, Minteer SD, Angenent LT (2005) Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ Sci Technol 39:5262–5267
Hou MF, Li FB, Liu XM, Wang XG, Wan HF (2007) The effect of substituent groups on the reductive degradation of azo dyes by zerovalent iron. J Hazard Mater 145:305–314
Jia YH, Tran HT, Kim DH, Oh SJ, Park DH, Zhang RH, Ahn DH (2008) Simultaneous organics removal and bio-electrochemical denitrification in microbial fuel cells. Bioproc Biosyst Eng 31:315–321
Li XM, Zhou SG, Li FB, Wu CY, Zhuang L, Xu W, Liu L (2009) Fe(III) oxide reduction and carbon tetrachloride dechlorination by a newly isolated Klebsiella pneumoniae strain L17. J Appl Microbiol 106:130–139
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285
Lovley DR, Phillips EJP (1998) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lu J, Zheng Y, He D (2006) Selective absorption of H2S from gas mixtures into aqueous solutions of blended amines of methyldiethanolamine and 2-tertiarybutylamino-2-ethoxyethanol in a packed column. Sep Purif Technol 52:209–217
Mandic Z, Nigovic B, Simunic B (2004) The mechanism and kinetics of the electrochemical cleavage of azo bond of 2-hydroxy-5-sulfophenyl-azo-benzoic acids. Electrochim Acta 49:607–615
Martinez-Huitle CA, Brillas E (2008) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87:105–145. doi:https://doi.org/10.1016/j.apcatb.2008.09.017
Menek N, Karaman Y (2005) Polarographic and voltammetric investigation of 8-hydroxy-7-(4-sulfo-1-naphthylazo)-5-quinoline sulfonic acid. Dyes Pigm 67:9–14
Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38:5809–5814
Pinheiro HM, Touraud E, Thomas O (2004) Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigm 61:121–139
Potter MC (1912) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc Lond B 84:260–276
Rabaey K, Read ST, Clauwaert P, Freguia S, Bond PL, Blackall LL, Keller J (2008) Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J 2:519–527
Schröder U, Nieβen J, Scholz F (2003) A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angew Chem Int ed 42:2880–2883
Virdis B, Rabaey K, Yuan ZG, Keller J (2008) Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res 42:3013–3024
Wang G, Huang LP, Zhang YF (2008) Cathodic reduction of hexavalent chromium [Cr (VI)] coupled with electricity generation in microbial fuel cells. Biotechnol Lett 30:1959–1966
Xu MY, Guo J, Sun GP (2007) Biodegradation of textile azo dye by Shewanella decolorationis S12 under microaerophilic conditions. Appl Microbiol Biotechnol 76:719–726
You SJ, Zhao QL, Zhang JN, Jiang JQ, Zhao SQ (2006) A microbial fuel cell using permanganate as the cathodic electron acceptor. J Power Sources 162:1409–1415
Acknowledgements
The authors appreciate the financial supports by the National Science Foundation of China (no. 40771105). We are grateful to the anonymous reviewers for their constructive and helpful comments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, L., Li, Fb., Feng, Ch. et al. Microbial fuel cell with an azo-dye-feeding cathode. Appl Microbiol Biotechnol 85, 175–183 (2009). https://doi.org/10.1007/s00253-009-2147-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2147-9