Abstract
Ischemic heart disease is a leading cause of death on a global scale, placing major socio-economic burdens on health systems worldwide. Myocardial ischaemia and reperfusion (I/R)-induced tissue injury is associated with alteration in activity of inflammatory system and nitric oxide pathway. Sumatriptan, which is mainly used to relieve migraine headache, has recently been shown to exert anti-inflammatory properties. In this study, we aimed to assess the possible cardioprotective effect of sumatriptan in a rat model of I/R injury. Male Wistar rats were subjected to 30-min ligation of left anterior descending coronary artery and 120-min reperfusion. Animals were randomly divided into five groups: (1) Sham (2) I/R (3) I/R treated with sumatriptan (0.3 mg/kg i.p.) 20 min after induction of I/R rats, (4) GR127935 (a selective antagonist of 5-HT1B/D serotonin receptors; 0.3 mg/kg) 20 min after induction of I/R, and (5) GR127935 (0.3 mg/kg) 15 min before administration of sumatriptan. Post-infarct treatment with sumatriptan increased left ventricular function, which was damaged in I/R animal’s heart. Sumatriptan (0.3 mg/kg) decreased lipid peroxidation, CK-MB and lactate dehydrogenase levels; tumor necrosis factor concentration; and Nf-ҡB’ protein production. Treatment with sumatriptan significantly increased the endothelial nitric oxide synthase (eNOS) expression consequences nitric oxide metabolites’ level in I/R rats. Also, injection of sumatriptan remarkably decreased myocardial tissue injury assessed by histopathological study. These findings suggest that sumatriptan may attenuate I/R injury via modulating the inflammatory responses and endothelial NOS activity. But therapeutic index of sumatriptan is narrow according to the result of this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac ischaemia is common in critically ill patients and accounts for a major increase in mortality and morbidity (Nazari et al. 1999). ischaemia-induced cardiac dysfunction is usually followed by oxygen supply deficiency and formation and rapture of atherosclerosis plaque, and leads to serious complication such as, irreversible myocardial injury (Ambrose and Singh 2015; Grønholdt et al. 1998). A considerable number of studies have applied a pharmacological approach to limit the consequences of ischaemia-induced tissue injury (Zhao et al. 2003). Due to the development of highly successful interventions to recover blood flow by primary percutaneous coronary intervention (PCI) to the ischemic heart tissue, the prognosis of cardiac ischaemia disease has been improved significantly (Rawlins et al. 2014). Paradoxically, while coronary reperfusion enhances the prognosis of patients, it also augments reperfusion injury and myocardial necrosis in the ischemic area (Buja 2005; Turer and Hill 2010). Focusing on animal studies, several approaches have been developed to alleviate reperfusion injury and improve clinical outcomes (Rawlins et al. 2014; Ibáñez et al. 2015). A substantial body of evidence suggests that local and systemic inflammatory mediators are involved in myocardial ischaemia–reperfusion which augment tissue injury and disrupt left ventricular recovery (Oyama et al. 2004). A variety of inflammatory mediators including reactive oxygen species (ROS), cytokine cascade, and neutrophils are considered to be responsible in myocardial tissue injury (Frangogiannis et al. 2002). In this regard, several anti-inflammatory strategies have been implemented in animal models of ischaemia–reperfusion to inhibit the inflammatory response (Yeh et al. 2002). Serotonin (5-HT) is a biogenic amine produced predominantly by intestinal enterochromaffin cells, and stored in platelets; it has a broad spectrum of physiological functions, usually through interaction with specific G protein-coupled receptors. In the heart, serotonin has been involved in regulation of normal cardiac development and homeostasis regulation (Mohammad-zadeh et al. 2008; Vanhoutte 1987). Serotonin effects appear to be mediated through different receptor subtypes as judged by the actions of selective ligands (Barnes and Neumaier 2011). Sumatriptan is a 5HT1B/1D agonist, developed for specific pain-relieving actions in migraine. Both 5-HT1B and 5-HT1D receptors serve as pre-synaptic auto-receptors; so, this anti-migraine drug is thought to block the release of neurotransmitters from the sensory nerve terminals and consequently constrict the smooth muscle of blood vessels (Rutz et al. 2006; Roberts et al. 2001; Araldi et al. 2016). Focusing on animal studies, some previous studies have demonstrated that sumatriptan exerts anti-inflammatory effects in visceral pain, vincristine-induced peripheral neuropathy (Brahadeesh and Suresha 2016), Carrageenan rat paw oedema, Turpentine-induced arthritis and Cotton pellet-induced granuloma model (Khalilzadeh et al. 2018), most likely through peripheral 5HT1B/D receptors (Humphrey and Goadsby 1994; Vera-Portocarrero et al. 2008). However, possible mechanisms involved in anti-inflammatory effects of Sumatriptan have been poorly studied. GR127935 is a selective 5HT1B/1D receptor antagonist and this compound was used to test the role of 5HT1B/1D receptors in protective effects of sumatriptan in this study (Skingle et al. 1995; Haddadi et al. 2018). The aim of this study is to examine the anti-inflammatory effects of sumatriptan via stimulation of 5HT1B/1D receptors in an experimental model of ischaemia–reperfusion injury.
Methods
Chemicals
The following drugs were used: Sumatriptan (Sigma, St. Louis, MO, USA), GR 127,935 (Sigma, St. Louis, MO, USA), triphenyltetrazolium chloride (TTC) (Sigma, St. Louis, MO, USA).
Animals In the present study, all procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (the Institutional Animal Care and Use Committee (IACUC) at Tehran University of Medical Sciences (Tehran, Iran)). All details of this proposal are approved by the national institute of medical research development (NIMAD), based in Iran on 2017/04/17 and approval number is 958842. Male Wistar rats (weighing 250–300 g at the beginning of surgery) were housed under standardized laboratory conditions (12-h light/dark cycle, temperature 22 ± 2 °C and humidity of 55 ± 2%) with access to tap water and food.
Rat model of myocardial ischaemia and reperfusion
After the induction of anesthesia with sodium thiopental (60 mg/kg i.p), the rats were posed in the supine position and body temperature maintained at 37 °C. Then, animals were intubated and ventilated by room air by a rodent ventilator (tidal volume 2–3 ml, respiratory rate 65–70/min, Harvard rodent ventilator model 683, Holliston, MA, USA). Ensuing a tracheotomy, the heart was exposed, and pericardium was incised. The left anterior descending coronary artery (LAD) was ligated via placing a 0–6 suture close to its origin to achieve myocardial infarction (MI). Both ends of the silk suture were then passed through coronary ligator. Segment of ST elevation in ECG and discolouration of myocardium were used as predominant indices to confirm ischaemia. After 30 min of ischaemia, the occluder was removed and animals were subjected to 120 min of reperfusion.
The sham-operated animals underwent the same procedure of thoracotomy, without the ligation of the coronary artery. Five experimental groups were used in this study; each group consisted of eight rats.
Group I control sham-operated group: treated with normal saline for the duration of the study.
Group II ischaemia /reperfusion (I/R) group.
Group III I/R + sumatriptan-treated group: sumatriptan (0.3 mg/kg i.p) was administered 20 min after induction of MI.
Group IV I/R + GR127935 group: GR127935 (0.3 mg/kg i.p) was administered 20 min after induction of MI.
Group V I/R + GR127935 + sumatriptan group: GR127935 (0.3 mg/kg, i.p) was administered 15 min prior to administration of sumatriptan (0.3 mg/kg, i.p; administered 20 min after induction of MI).
In a pilot study, we used three different doses of sumatriptan (0.1, 0.3, and 3 mg/kg) to determine the most effective doses based on our haemodynamic studies. In this assessment, sumatriptan (0.3 mg/kg) was chosen for the following experiments. No significant effect was detected with 0.1 mg/kg. Non-protective and even destructive effects were noticeable with high dose (3 mg/kg). The most effective and reliable dose was 0.3 mg/kg which showed the highest protective effects.
Haemodynamic studies
We evaluated haemodynamic parameters 120 min after reperfusion. A PE50 catheter, coupled with the PowerLab data acquisition system via pressure transducer (AD Instrument Pty Ltd, Mountain View, CA, USA), was applied to cannulate the right common carotid artery. Left ventricular end diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), and mean arterial pressure (MAP) were recorded and monitored by PowerLab data acquisition system (AD Instrument) via lead II ECG.
Blood sampling
Blood samples were collected from the tail vein for measurement of biochemical parameters (CK-MB, LDH, MDA and nitric oxide metabolites). The samples were centrifuged at 5000 rpm, 4 °C, for 15 min; after that, the separated serum was stored at − 70 °C until biochemical analysis.
Measurement of LDH and CK-MB
Serum levels of LDH and CK-MB were determined with a commercial kit (Pars Azmoon; Tehran, Iran) based on a colorimetric method according to the manufacturer’s instructions.
Measurement of MDA (malondialdehyde)
Formation of thiobarbituric acid-reactive substances (TBARS) as the index of MDA was measured in heart homogenates (20% w/v), using the Buege and Aust method (Grønholdt et al. 1998). The hearts were separated and homogenized with 10-ml normal saline solution in a manual Teflon homogenizer. The MDA density was expressed as mol/mg protein using the extinction coefficient at 535 nm.
Western blot analysis to determine eNOS and Nf-kB
Specimens were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted to polyvinylidene difluoride (PVDF) membranes, blocked with 3% bovine serum albumin (BSA) for 90 min at room temperature, then incubated with primary antibodies overnight at 4 °C with primary antibodies against Nf-kB (rabbit monoclonal 1:3000; Cell Signalling Technology, Danvers, MA, USA) and eNOS (mouse monoclonal 1:1000; Cell Signalling Technology, Danvers, MA, USA), followed by horse radish peroxidase (HRP)-conjugated secondary antibodies (1:5000) for 1 h at room temperature and visualized with the enhanced chemiluminescence system. β-Actin was applied as a control probe to confirm that equal amount of protein was loaded in each lane. At the next step, immunoblots were analyzed for densitometry using ImageJ software (NIH) (Zhao et al. 2003).
Measurement of TNFα
For the measurement of tissue TNFα, an ELISA kit (ab46070; Abcam) was used following the manufacturer’s recommendation. Specimens were homogenized in ice-cold phosphate-buffered saline (PBS), and then centrifuged at 14,200g for 30 min. During the first incubation, all samples or standards, and a biotinylated anti-TNF-α monoclonal antibody were incubated simultaneously. After washing, the enzyme streptavidin–peroxidase that binds to the aforementioned biotinylated antibody was added to each well. To induce a colored reaction product, a TMB (3, 3′, 5, 5′-Tetramethylbenzidine) substrate solution was added. A linear standard curve was used for determination of TNF-α concentration.
Measurement of nitric oxide (NO) metabolites
Serum NO metabolite, nitrite (NO2−), was measured as an index of NO production, based on the Griess reaction (Azizi et al. 2013). Briefly, samples were separated and mixed with 10-μL vanadium (80 mg/10 ml HCl 1 M) and 50 μL of sulfanilamide solution (dissolved in 5% HCl). 50 μL of N-1-napthylethylenediamine dihydrochloride solution (0.1% in water) was added to each sample, after 5-min incubation at room temperature. After 30-min incubation under reduced light at room temperature, samples were measured at 540-nm wavelength in a spectrophotometer. Nitrite level was calculated against a nitrite standard (0.1 M NaNO2 in water).
Histological examination
The hearts samples were fixed in buffered formaldehyde solution (10%) and embedded in paraffin. Tissue sections (5-μM thickness) on slides were stained with hematoxylin and eosin (H&E). The tissue sections were analyzed by light microscopy and photographed with a digital camera.
Statistical analysis
The results are presented as mean ± SEM. Statistical differences between groups were analyzed via one-way ANOVA followed by the Newman–Keuls multiple comparison tests, using GraphPad Prism 5 (GraphPad Software Inc., CA, USA). The results were considered significant at p < 0.05, and each experiment was repeated three times.
Results
Haemodynamic studies
Haemodynamic data, obtained after reperfusion, are summarized in Fig. 1a–c. There were no significant differences among groups at baseline before treatment. LVSP, and MAP vividly diminished in I/R injury group in comparison with the sham-operated rats, while LVEDP was increased in I/R group. Although sumatriptan did not exert robust effects to normalize LVEDP, LVSP, and MAP in sumatriptan-treated group comparing to sham-operated animals, it prevented the decline in (LVSP and MAP) and enhancement in (LVEDP) at some level (p < 0.001) compared to the I/R group.
Haemodynamic parameters. a: left ventricular systolic blood pressure (LVSP; mmHg); b: left ventricular end diastolic blood pressure (LVEDP; mmHg); c: mean arterial pressure (MAP; mmHg); ischaemia/reperfusion (I/R) injury; data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared to sham group; $p < 0.05, $$p < 0.01, $$$p < 0.001 compared to I/R group; #p < 0.05, ###p < 0.001 compared to sumatriptan (0.3 mg/kg)
GR-127935 had no significant effects on haemodynamic factors in comparison with I/R injury group. However, co-administration of GR-127935 with sumatriptan slightly reversed the protective effects of sumatriptan comparing to sumatriptan-treated animals.
Effect of sumatriptan on LDH and CK-MB level
As demonstrated in Fig. 2, induction of I/R substantially increased the plasma levels of CK-MB and LDH in rats. Compared to I/R group, treatment with sumatriptan prevented elevation of LDH activity in plasma after induction of I/R injury (p < 0.001). Also, administration of sumatriptan significantly attenuated the increase in CK-MB levels in comparison with I/R group (P < 0.001). Similar to haemodynamic results, GR-127935 diminished the effects of sumatriptan on LDH and CK-MB levels.
Effect of sumatriptan on lipid per oxidation level
MDA plasma level, indicating the extent of lipid per oxidation, was consequently increased after I/R injury. However, MDA level significantly declined after treatment with sumatriptan compared to I/R injury group. On the other hand, anti-lipid per oxidation effects of sumatriptan was faded away when co-administered with GR-127935 (Fig. 3).
Effect of sumatriptan on the protein expression of Nf-ҡB
In this study, we investigate the inflammatory pathway mediated by Nf-ҡB via screening the protein expression. Figure 4a, b shows that administration of sumatriptan significantly decreased Nf-ҡB expression in rats in comparison with I/R injury group. These results suggest that sumatriptan prevents inflammatory-induced injuries by regulating the expression of Nf-ҡB. Reversely, co-administration of GR-127935 with sumatriptan impeded the anti-inflammatory effects of sumatriptan.
Western blotting results for protein expression (a) of Nf-ҡB (b) and eNOS (c); ischaemia/reperfusion (I/R) injury; data are presented as means ± SEM. p < 0.05, **p < 0.01, ***p < 0.001 compared to sham group; $p < 0.05, $$p < 0.01, $$$p < 0.001 compared to I/R group; #p < 0.05, ###p < 0.001 compared to sumatriptan (0.3 mg/kg)
Effect of sumatriptan on the protein expression of eNOS
As shown in Fig. 4c, the level of eNOS protein increased significantly in sumatriptan-treated group compared to I/R injury rats, whereas co-administration of GR-127935 clearly blocked this effect of sumatriptan. These data support the hypothesis that sumatriptan may exert cardio-protective roles via increasing NO level subsequent to eNOS expression.
Effect of sumatriptan on TNF-α level
A significant increase in level of TNF-α was observed after induction of I/R injury compared to the sham-operated animals. Administration of sumatriptan led to a significant decrease in the amount of TNF-α, as compared to I/R injury group. As predicted, GR-127935 prevented the effect of sumatriptan on TNF-α level (Fig. 5).
Effect of sumatriptan on NO metabolites level
As shown in Fig. 6, post-infarct treatment with sumatriptan substantially increased serum level of NO metabolites in comparison with I/R injury group. These data are in line with the theory that sumatriptan can attenuate the debilitating effects of I/R injury through increasing NO formation.
Level of nitrite as a nitric oxide (NO) metabolite in heart tissue of rat; ischaemia/reperfusion (I/R) injury; data are presented as means ± SEM. p < 0.05, **p < 0.01, ***p < 0.001 compared to sham group; $p < 0.05, $$p < 0.01, $$$p < 0.001 compared to I/R group; #p < 0.05 compared to sumatriptan (0.3 mg/kg)
Effect of sumatriptan on myocardial remodeling
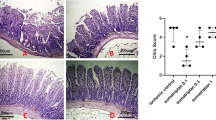
When compared to sham-operated group, histological examinations of hearts obtained from control rats subjected to I/R injury demonstrated a significant degree of tissue injury, including eosinophilic and leucocyte infiltration, increased spaces between cells, marked intracellular oedema, changes in cardiomyocytes structure and shape, and tissue disarrangement. Heart sections obtained from rats treated with sumatriptan demonstrated a significant reduction in the severity of these histologic features of myocardial injury, when compared with animals from control group subjected to I/R only. As hypothesized previously, GR-127935 hindered sumatriptan effects on histologic indexes (Fig. 7).

taken from a sham-operated rat (a); a heart tissue of rat subjected to myocardial ischaemia/reperfusion (I/R) (b); a heart section taken from a rat subjected to myocardial I/R administered sumatriptan (c); a heart section taken from a rat subjected to myocardial I/R administered GR-127935 (d); and I/R co-administered GR-127935 with sumatriptan (e). Hematoxylin and eosin, original magnification × 150
Histologic assessment: a heart section
Discussion
The present study revealed that sumatriptan significantly restricts the progression of myocardial injuries via improving haemodynamic parameters and changing the level of biochemical metabolites in cardiac tissue. Pathological study showed protective effects after induction of cardiac I/R injury. Animals with I/R injury showed LVEDP, LVSP, and MAP alteration, but sumatriptan injection resulted in a significant improvement in these parameters. Biochemical parameters including CK-MB and lactate dehydrogenase levels were diminished after sumatriptan treatment in I/R rats, whereas nitric oxide quantity showed an increase after treatment. Sumatriptan reduces TNF-α level and Nf-ҡB expression as inflammatory responses, as well as MDA level as oxidative stress function. In addition, the expression of eNOS increased after sumatriptan treatment in cardiac tissue of I/R rats. Moreover, sumatriptan improves histopathological marker including hemorrhage, neutrophil infiltration, and intracellular oedema of I/R injury. Overall, the present study showed beneficial effects for sumatriptan in a rat model of I/R.
Oxidative stress pathways have long been acknowledged to play pivotal roles in I/R injury by causing debilitating interactions with proteins, membrane lipids, and nucleic acids (Kaminski et al. 2002). The principal index of lipid per oxidation, MDA, is commonly employed to investigate oxidative stress-induced cellular injury. Previous literature supports the profitable effects of antioxidants, since these components exert cardioprotective effects against I/R damages. Ikeda et al. (2002) has demonstrated the free radical scavenging property of sumatriptan against superoxide, hydroxyl, and NO radicals. In this study, sumatriptan lowered the level of MDA, suggesting that antioxidant properties of sumatriptan may play a robust role in attenuation of I/R-induced myocardial injury, which is in accordance with prior investigations. In addition, sumatriptan reduced the amount of CK-MB and LDH as an index for identifying cell injury in the current study. CK-MB and LDH are principal metabolic myocardial enzymes which are leaked out of cells consequent to ischaemia cell destruction and necrosis (Vera-Portocarrero et al. 2008; Dhalla et al. 1999; yang et al. 2009; Yin et al. 2013). In fact, our results support the perception that sumatriptan inserts cardioprotective effects against I/R-induced injuries via lowering the level of MDA, CK-MB, and LDH in injured myocardium.
The protective roles of NO in I/R-induced injuries, including the attenuation of myocardial remodeling and regulation of heart rate (Azizi et al. 2013) are demonstrated. An increased level of ROS resulting from ischaemia–reperfusion can exhaust bioactivity of NO, particularly through inactivation of NO via ROS superoxide and inhibiting eNOS function (Forstermann 2010). Endothelial NOS is the main isoform of NOS in endothelial cells which represents anti-inflammatory properties, as well as the mediating roles in regulation of vascular tone and structure (Dobutović et al. 2011). Beneficial contributions of medications that can improve the release of NO, including statins, calcium antagonists, and dexamethasone have been extensively explored and demonstrated in a considerable number of previous studies (Siegfried et al. 1992). Gao et al. have reported that NO substantially facilitates insulin-induced anti-apoptotic effects in myocardial ischaemia–reperfusion (Schulz et al. 2004). In the present study, sumatriptan significantly increased NO levels in the heart tissue of subjects. Moreover, according to the results of western blotting, administration of sumatriptan led to a substantial increase in eNOS protein expression in cardiac tissue. Several studies have delved into the interactions between sumatriptan and NO, particularly in migraine pathology, and revealed the stimulatory effects of sumatriptan in production of NO (De Felice et al. 2010). Therefore, we can propose that sumatriptan applies significant cardioprotective effects via promoting NO formation in I/R-induced myocardial injuries.
Reactive oxygen species and cytokines aggravate I/R-induced injuries urging scientists to focus on anti-inflammatory based therapeutic approaches (Yeh et al. 2002; Murphy and Steenbergen 2008). In addition, NF-ƙB activation increases the expression of pro-inflammatory genes during I/R-induced inflammation (Frangogiannis et al. 2002). Our study confirmed that ischaemia–reperfusion led to NF-ƙB activation and increased the plasma level of TNFα resulting in ischemic damages in I/R in vivo model. Focusing on the role of inflammation in the pathophysiology of ischaemia–reperfusion injury, several scientists have employed anti-inflammatory agents to restrict cardiac injury (Yeh et al. 2002). Sumatriptan has long been recognized to exert anti-inflammatory properties in neuropathic pain (Vera-Portocarrero et al. 2008; Khalilzadeh et al. 2018). Nikai et al. investigated the sumatriptan effects in somatic and visceral pain and showed that sumatriptan could suppress pain via inhibiting both peripheral and systemic inflammations (Nikai et al. 2008). The anti-inflammatory effects of sumatriptan appear to be facilitated indirectly through stimulation of 5HT 1B/1D auto-receptors, since these effects were blocked by the mixed 5HT 1B/1D receptor antagonist, GR-127935. GR-127935 is considered as a precious tool to block the receptor-related effects of sumatriptan. Thus, we suggest that treatment with sumatriptan may exhibit an anti-inflammatory effect through stimulating 5HT 1B/1D auto-receptors, and inhibit the activation of NF-ƙB and production of and TNF-α.
To summarize, these findings demonstrate that sumatriptan (0.3 mg/kg) treatment could attenuate I/R-induced cardiac injury via lipid per oxidation inhibition, NO formation increase, and inflammatory pathways modulating. Since other experimental doses (0.1, 1.0, and 3.0 mg/kg) were not protective, the present study highlights the notion that sumatriptan is a compound with narrow therapeutic index in this model and may require dose monitoring to achieve the protective effect.
References
Ambrose JA, Singh M (2015) Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep 7:08
Araldi D, Ferrari LF, Levine JD (2016) Gi-protein coupled 5-HT1B/D receptor agonist sumatriptan induces type I hyperalgesic priming. Pain 157(8):1773
Azizi Y, Faghihi M, Imani A et al (2013) Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides 46:76–82
Barnes NM, Neumaier JF (2011) Neuronal 5-HT receptors and SERT. Tocris Biosci Sci Rev Ser 34:1–16
Brahadeesh M, Suresha RN (2016) Screening of the drug amiodarone for its antiinflammatory potential in albino rats. IJPLS:7(5):5042–5046
Buja LM (2005) Myocardial ischaemia and re perfusion injury. Cardiovasc Pathol 14(4):170–175
De Felice M, Ossipov MH, Wang R et al (2010) Triptan-induced enhancement of neuronal nitric oxide synthase in trigeminal ganglion dural afferents underlies increased responsiveness to potential migraine triggers. Brain 133(8):2475–2488
Dhalla NS, Golfman L, Takeda S et al (1999) Evidence for the role of oxidative stress in acute ischaemic heart disease: a brief review. Can J Cardiol 15(5):587–593
Dobutović B, Smiljanić K, Soskić S et al (2011) Nitric oxide and its role in cardiovascular diseases. Open Nitric Oxide J 3(3):65–71
Förstermann U (2010) Nitric oxide and oxidative stress in vascular disease. Pflugers Arch 459(6):923–939
Frangogiannis NG, Smith CW, Entman ML (2002) The inflammatory response in myocardial infarction. Cardiovascular res 53(1):31–47
Grønholdt M, Dalager-Pedersen S, Falk E (1998) Coronary atherosclerosis: determinants of plaque rupture. Eur Heart J 19:C24–C29
Haddadi NS, Foroutan A, Shakiba S et al (2018) Attenuation of serotonin-induced itch by sumatriptan: possible involvement of endogenous opioids. Arch Dermatol Res 310(2):165–172
Humphrey P, Goadsby P (1994) The mode of action of sumatriptan is vascular? A debate. Cephalalgia 14(6):401–410
Ibáñez B, Heusch G, Ovize M et al (2015) Evolving therapies for myocardial ischaemia/re perfusion injury. J Am Coll Cardiol 65(14):1454–1471
Ikeda Y, Jimbo H, Shimazu M et al (2002) Sumatriptan scavenges superoxide, hydroxyl, and nitric oxide radicals: in vitro electron spin resonance study. Headache 42(9):888–892
Oyama J-i, Blais C, Liu X et al (2004) Reduced myocardial ischaemia–re perfusion injury in toll-like receptor 4-deficient mice. Circulation 109(6):784–789
Kaminski KA, Bonda TA, Korecki J et al (2002) Oxidative stress and neutrophil activation—the two keystones of ischaemia /re perfusion injury. Int J Cardiol 86(1):41–59
Khalilzadeh M, Panahi G, Rashidian A et al (2018) The protective effects of sumatriptan on vincristine-induced peripheral neuropathy in a rat model. Neurotoxicology 67:279–286
Mohammad-Zadeh L, Moses L, Gwaltney-Brant S (2008) Serotonin: a review. J Vet Pharmacol Ther 31(3):187–199
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischaemia -re perfusion injury. Physiol Rev 88(2):581–609
Nazari A, Sadr SS, Faghihi M, Azizi Y et al (1999) Evidence for the role of oxidative stress in acute ischaemic heart disease: a brief review. Can J Cardiol 15(5):587–593
Nikai T, Basbaum AI, Ahn AH (2008) Profound reduction of somatic and visceral pain in mice by intrathecal administration of the anti-migraine drug, sumatriptan. Pain 139(3):533–540
Rawlins J, Wilkinson J, Curzen N (2014) Evidence for benefit of percutaneous coronary intervention for chronically occluded coronary arteries (CTO)–clinical and health economic outcomes. Interv Cardiol 9(3):190
Roberts C, Price GW, Middlemiss DN (2001) Ligands for the investigation of 5-HT autoreceptor function. Brain Res Bull 56(5):463–469
Rutz S, Riegert C, Rothmaier AK et al (2006) Presynaptic serotonergic modulation of 5-HT and acetylcholine release in the hippocampus and the cortex of 5-HT1B-receptor knockout mice. Brain Res Bull 70(1):81–93
Schulz R, Kelm M, Heusch G (2004) Nitric oxide in myocardial ischaemia /re perfusion injury. Send to Cardiovasc Res 61(3):402–413
Siegfried MR, Erhardt J, Rider T et al (1992) Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischaemia-re perfusion. J Pharmacol Exp Ther 260(2):668–675
Skingle M, Beattie DT, Scopes DI et al (1995) GR127935: a potent and selective 5-HT1D receptor antagonist. Behav Brain Res 73(1–2):157–161
Turer AT, Hill JA (2010) Pathogenesis of myocardial ischaemia-re perfusion injury and rationale for therapy. Am J Cardiol 106(3):360–368
Vanhoutte PM (1987) Serotonin and the vascular wall. Int J Cardiol 14(2):189–203
Vera-Portocarrero LP, Ossipov MH, King T et al (2008) Reversal of inflammatory and noninflammatory visceral pain by central or peripheral actions of sumatriptan. Gastroenterology 135(4):1369–1378
Wang J, Huang W, Xu R et al (2012) MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med 16(9):2150–2160
Yang J, Jiang H, Yang J et al (2009) Valsartan preconditioning protects against myocardial ischaemia-re perfusion injury through TLR4/NF-κB signalling pathway. Mol Cell Biochem 330(1–2):39
Yeh C-H, Chen T-P, Wu Y-C et al (2002) The inflammatory response in myocardial infarction. Cardiovascular Res 53(1):31–47
Yin Y, Guan Y, Duan J et al (2013) Cardioprotective effect of Danshensu against myocardial ischaemia/re perfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur J Pharmacol 699(1–3):219–226
Zhao Z-Q, Corvera JS, Halkos ME et al (2003) Inhibition of myocardial injury by ischaemic postconditioning during re perfusion: comparison with ischaemic preconditioning. Am J Physiol Heart Circ Physiol 285(2):H579–H588
Acknowledgements
This study was funded by Experimental Medicine Research Center, Tehran University of Medical Sciences, Tehran, Iran; grant no. 97-01-158-37318, and by a grant (96002757) from Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheibani, M., Faghir-Ghanesefat, H., Dehpour, S. et al. Sumatriptan protects against myocardial ischaemia–reperfusion injury by inhibition of inflammation in rat model. Inflammopharmacol 27, 1071–1080 (2019). https://doi.org/10.1007/s10787-019-00586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00586-5