Abstract
Shortjaw tapertail anchovy, Coilia brachygnathus, has invaded and expanded in the Three Gorges Reservoir (TGR) over the last few years. The population size and biological characteristics related to this invasion are poorly understood. To investigate its invasion mechanisms, we studied spatial and temporal changes in C. brachygnathus biomass and abundance and its reproductive traits and diet. Evidence of progressive directional invasion suggested that C. brachygnathus invaded the TGR from downstream in 2016. The TGR provided an initial blockage and later facilitated C. brachygnathus dispersal. In 2020 and 2021, C. brachygnathus accounted for 11.66% and 16.06% of biomass and abundance, respectively, of the TGR fish community and both biomass and abundance decreased with distance to the dam. The fecundity in the TGR was higher than that in downstream native areas. Although their contributions to diet exhibited spatial differences, C. brachygnathus mainly consumed shrimps (Exopalaemon modestus and Macrobrachium nipponensis) and zooplankton in the TGR. Within a short period, C. brachygnathus has successfully colonized and expanded into the whole reservoir. Its fast maturity and high fecundity, coupled with the lentic habitats and abundant food availability, are probable key factors that explain its invasion. We propose that prevention measures to block the expansion of this species are urgently needed after the impoundment of cascaded reservoirs with navigation passages located upstream of the TGR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, 58 713 large dams (i.e., height > 15 m or height of 5–15 m and impounding > 3 million m3) have been constructed in the world (ICOLD, 2019), and the concomitant river impoundment greatly influences local fish assemblage and biodiversity. Documented detrimental effects include declining migratory and rheophilic fish species (Sá-Oliveira et al., 2015; Loures & Pompeu, 2019) and species invasions. In some cases, dams can hinder the spread rate of invasive aquatic species into new areas (e.g., Dana et al., 2011), but the dam-driven hydrological alterations can often facilitate fish invasion (Júlio Júnior et al., 2009; Casimiro et al., 2017). That is because flooding, impounding, and navigation- and fish passage devices associated with impoundment infrastructure can eliminate natural barriers to fish movement and facilitate the invasion of previously disconnected catchments (e.g., Cooper et al., 2021; Kerr et al., 2021).
Both biological and environmental factors can influence the success of an invasion process. Species traits such as the ability to tolerate novel conditions, superior competitive ability, and a plastic diet are essential contributors to invasion success (Pereira et al., 2015). Moreover, the generation of suitable habitat and food resources after the dam construction expedites invasion success (Liew et al., 2016). Even so, identifying the key species traits and the eco-evolutionary mechanism that determine the success of an invasive process is still challenging due to the complexity of ecological systems (Muniz et al., 2021).
The Three Gorges Reservoir (TGR) is one of the largest reservoirs in the world (Yang et al., 2012), which was created through three filling stages in 2003, 2006, and 2009, raising the water levels to 135 m, 156 m, and 175 m, respectively (Liao et al., 2018). Its impoundment severely transformed the main-channel riverine habitats of the upper Yangtze River, China, with concomitant effects on fish assemblages (Liao et al., 2018). Nowadays, there is an overall decline of lotic fishes (Gao et al., 2010; Liao et al., 2018), whereas long distance migratory fish species are highly endangered (e.g., Acipenser sinensis Gray 1835 (Acipenseridae), CR; IUCN, 2021) due to the double barriers imposed by the Three Gorges Dam and the Gezhouba Dam (Zhang et al., 2020). In terms of fish invasions, a few documented outbreaks occurred after approximately 2003 (i.e., icefish Neosalanx taihuensis Chen 1956 and Protosalanx hyalocranius (Abbott 1901) (Salangidae); Gong et al., 2009). By 2012, 23 non-previously reported fish species had been sampled by different investigations in TGR, and these species were mainly introduced through aquaculture activities. Still, only Megalobrama amblycephala Yih 1955 (Cyprinidae) and N. taihuensis established populations in the reservoir (Ba & Chen, 2012). Between 2015 and 2017, five non-native species were collected in the reservoir, and the icefish (N. taihuuensis and P. hyalocranius) accounted for 1.03%, while Coilia brachygnathus Kreyenberg & Pappenheim 1908 (Engraulidae) accounted for 0.15% of the total fish assemblage biomass (Liao et al., 2018). The population of C. brachygnathus unexpectedly exploded after ≈2016 and dispersed rapidly in the whole reservoir, yet its population status and invasion mechanisms in the TGR are still poorly understood.
Coilia brachygnathus (maximum standard length 354 mm; Qin et al., 2018) is native to the middle and lower Yangtze River basin, China and is widely distributed and an important fishing target therein (Whitehead et al., 1988; Wang et al., 2016; Qin et al., 2018). In these areas, C. brachygnathus is also significant prey for other larger carnivorous fishes and the Yangtze finless porpoise Neophocaena phocaenoides (G. Cuvier 1829) (Phocaenidae). Coilia brachygnathus exclusively inhabit freshwaters and is a sister taxon to a sympatric freshwater-tolerant anchovy, C. nasus Temminck & Schlegel 1846 (Whitehead et al., 1988). The species was first described from the Dongting Lake of the Yangtze River Basin (Kreyenberg & Pappenheim, 1908), which is about 420-km downstream of the Three Gorges Dam. The genetic distance of C. brachygnathus between the native Dongting Lake and the novel TGR populations is close, suggesting that the TGR population is likely derived from the downstream Dongting Lake population (Yang, 2019). Coilia brachygnathus has a broad diet, including zooplankton, shrimp, and small fish (Zhang et al., 2013), and has a relatively high fecundity (Luo, 2006; Liu, 2008; Wang, 2016), high growth rate (Liu, 2008; Qin et al., 2018), and early mature age (one year; Luo, 2006; Wang, 2016) in native habitats. These biological traits are similar to those of the two invasive icefish species in the TGR, indicating that these population attributes are likely related to invasion success. Understanding the invasion process and population characteristics of C. brachygnathus in the TGR is essential from a fisheries management perspective, particularly in response to recent management actions. For instance, it is uncertain whether this species will invade the upstream portion of the reservoir after the creation of a reservoir cascade upstream of the TGR (Cheng et al., 2015) and how will the size of the novel population change following a ten-year fishing ban of the Yangtze River native population (Zhou et al., 2020).
This study used C. brachygnathus as a model system to investigate fish invasion mechanisms in the TGR. Specifically, we investigated spatial (i.e., lower, middle, and upper sections) and temporal variations (i.e., 2005–2021) in the relative abundance and biomass of C. brachygnathus in the TGR. We also studied several biological traits that may explain its invasion success, including body size, sex ratio, maturity size, fecundity, and diet. Our study aimed to (1) summarize processes and trends of the invasion of C. brachygnathus based on its current and past abundance; (2) assess the diet of C. brachygnathus in the TGR based on stomach contents and stable isotopes analyses; (3) compare the body size and fecundity of the C. brachygnathus population in the TGR with those of native populations; and (4) discuss possible implications for the management of invasive fish species in large reservoirs.
Methods and materials
Sampling sites
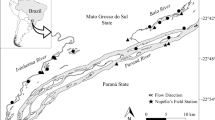
Seasonal sampling was conducted at five mainstem sites (Zigui, Wushan, Yunyang, Zhongxian, and Banan) and four secondary tributary sites (Qinggan River, Xiangxi River, Yuanshui River, Pengxi River) along the TGR (Fig. 1), during the spring (April 2020, April 2021), summer (July 2020), autumn (October 2020), and winter (January 2021) seasons. These nine sampling sites cover the lower, middle, and upper sections of the TGR (Table 1), representing current lentic, transitional, and lotic habitats. We also seasonally measured water transparency (with a Secchi disk) and water quality parameters, including pH, temperature, dissolved oxygen, and specific conductivity (with a YSI Pro Plus multi-parameter water quality analyzer) (Table 1).
Map of detected sites and times of C. brachygnathus at the upper reach of the Yangtze River (A), and the current sampling sites in the mainstem and secondary tributaries of the Three Gorges Reservoir, located in the upper reach of the Yangtze River, China (B). For site codes, capital letter L represents the lower section, M represents the middle section, and U represents the upper section
Fish sampling and data collection
We sampled fishes in benthic and pelagic habitats using experimental multi-panel gillnets. Benthic and pelagic gillnets have different heights (2 and 5 m, respectively) but the same length and mesh size structures. The total length of each gillnet was 30 m and consisted of 12 panels (2.5 m each) of different mesh sizes (10, 16, 20, 25, 31, 39, 48, 58, 70, 86, 110, 125 mm, knot-to-knot). We randomly selected three locations (≈500 m apart) at each sampling site described above and deployed three benthic gillnets and three pelagic gillnets for 12 h (18:00–19:00 to 6:00–7:00) per location. To increase our sample size, we repeated our sampling the next day at each sampling site per season, amounting to an effort of 3780 m2·over 24 h (1890 m2·12 h in one day) of gillnets per season. To shed light on the temporal variations in the abundance of C. brachygnathus, we compared the present relative biomass with historical data (2005–2006, 2013–2014, and 2015–2017). Specifically, we retrieved relative biomass data of C. brachygnathus in 2005–2006 and 2013–2014 from published literatures (Wu et al., 2007; Lian, 2016). Data for 2015–2017 were obtained from our past surveys of fish assemblages in the upper (Banan), middle (Wanzhou), and lower sections (Zigui) of the TGR, using gillnets and trawl nets (Liao et al., 2018).
In the present study, all fishes collected were identified to species, and we randomly chose a subset of individuals and measured their total length (TL), standard length (SL), and body weight (BW) to 0.1 mm, 0.1 mm, and 0.01 g, precision, respectively. We dissected a sub-sample of C. brachygnathus to characterize reproductive traits, including size at first maturity (N = 192; April 2020, July 2020, and April 2021) and diet (April 2021). For each specimen, we identified its sex by visual inspection of the gonads, recorded reproductive stage (in a scale from I to VI, where stage I-immature stage, stage II-quiescent stage, stage III-ripening stage, stage IV-ripeness stage, stage V-reproduction stage, stage VI- spent stage; West, 1990, Yin, 1995) and weighed the eviscerated body to the nearest 0.01 g. For mature female individuals (Stage IV–V), we sampled about 0.1 g of mature ovaries from each ovary lobe’s anterior, middle, and posterior sections and then weighed (Wegg) and preserved the ovaries in a 10% formalin solution. We counted the number of eggs per sub-sample under a light microscope (Leica S8APO), measured the length (DL) and width (DW) of eggs, and calculated egg diameter (DM) from the mean DL and DW values. We removed and preserved the entire stomach in a 10% formalin solution for further dietary analysis. Food contents were removed from stomachs in the laboratory and identified to the lowest taxonomic level possible using a dissecting microscope. We weighed each food type to the nearest 0.01 g. We randomly sampled three to five fish individuals per sampling site and collected muscle samples to analyze stable isotope ratios of C (δ13C) and N (δ15N). Based on the results of stomach content analyses, we also sampled potential food resources for isotope analyses, including zooplankton and two shrimp species (Exopalaemon modestus (Heller, 1862) and Macrobrachium nipponensis (De Haan, 1849)). Tissue samples were analyzed by the Analyses and Testing Center, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China. For some individuals with C:N ratio higher than 3.5, we corrected their δ13C signature to remove the influence of lipids (Skinner et al., 2016), following the method described by McConnaughey & MaRay (1979) and Kiljunen et al. (2006).
Data analysis
To assess the spatial changes in population status of C. brachygnathus in 2020–2021 across the TGR, we implemented linear mixed-effects modeling (LMM) with relative abundance or biomass (e.g., abundance of C. brachygnathus / total abundance per sampling site per season) as a function of distance from the dam. We included sampling site as a random factor to control for the lack of independence among sites sampled during multiple seasons (Bates et al., 2015). Prior to model implementation, we logit transformed proportional data (p′) (logit transformation = log [p′/(1 − p′)] (Warton & Hui, 2011), using the R package car (Fox & Weisberg, 2011). We visually inspected model fit based on residuals vs. fitted value plots and standardized residuals vs. theoretical quantile plots and used the Shapiro test to check data normality. We also calculated catch per unit effort (CPUE, g/m2/24 h) of gillnet type (benthic gillnet or pelagic gillnet) per sampling event to estimate the absolute biomass of C. brachygnathus (Emmrich et al., 2012). We then implemented linear mixed-effects modeling (LMM) to compare the CPUE among sections and gillnet types. We included sampling site and sampling season as random factors to account for the lack of independence between multiple sampling events conducted at the same site and during the same season (Bates et al., 2015). We then performed within-section pairwise comparisons once we identified a significant difference, using the R package emmeans (Lenth et al., 2022). We also assessed spatial and seasonal differences in body size (i.e., standard length-SL, measured from the tip of the snout to the base of the caudal fin) of C. brachygnathus via LMM. We modeled SL as a function of sampling site and season and included sampling site as a random factor. We also retrieved SL data of C. brachygnathus populations from six native locations in the middle and lower reaches of the Yangtze River (downstream from TGR), including Kuilei Lake, Zilang Lake, Poyang Lake, Dongting Lake, Changhu Lake, and Tianezhou Oxbow from published studies and our unpublished data (Wu et al., 2015; Wang et al., 2016; Gong et al., 2018; Qin et al., 2018). We used Wilcoxon tests to compare the mean SL between the TGR and native locations.
We retrieved data on the relative fecundity of native C. brachygnathus from Poyang Lake, Changhu Lake, and Swan Oxbow from the literature (Luo, 2006; Liu, 2008; Wang, 2016). We used a one-sample t test to compare relative fecundity between the TGR and native locations. We used the Chi-square test (Χ2 test) to test the deviation of the sex ratio of C. brachygnathus from the expected 1:1 ratio. Lastly, we used Bayesian stable isotope mixing models to estimate the contribution of different food sources to C. brachygnathus biomass (Parnell et al., 2013). We conducted all statistical analyses in R (v.4.0.2, R Foundation for Statistical Computing, Vienna, Austria). The regression models and main effects estimation (Type II and III Wald’s Χ2 test) were conducted using the packages lme4 (Bates et al., 2015) and car (Fox & Weisberg, 2011), respectively. The Bayesian stable isotope mixing model was performed with the package siar (Parnell et al., 2013).
Results
Relative abundance, biomass, and CPUE
In 2020–2021, the relative biomass of Coilia brachygnathus accounted for 11.66% ± 2.91% (mean ± S.E.), and the relative abundance accounted for 16.06% ± 3.80% of the TGR fish community. The highest mean values were both recorded toward the dam at site L1 (biomass: 31.95%, abundance: 37.83%), while the lowest values were both found at the most upper-sampled site U1 (biomass: 4.07%, abundance: 3.42%; Fig. 2). Both relative biomass and relative abundance decreased with distance to the dam (LMM, biomass: Χ2 = 6.46, P < 0.01; abundance: Χ2 = 10.83, P < 0.001; Fig. 3). Based on our past sampling, individuals of C. brachygnathus were first recorded in 2016, right above the dam (site L1), while in 2017, individuals were recorded at the middle (site M1) and upper (site U1) sections of the TGR. Relative biomass in 2017 was low in all sections, and it had a fourfold to 40-fold increase by 2020–2021 (Table 2). The CPUE of C. brachygnathus was non-significantly different among sections (LMM, section: Wald’s Χ2 = 2.86, P = 0.24; net type: Wald’s Χ2 = 0.01, P = 0.91), neither was the interaction between section and net type (Wald’s Χ2 = 0.57, P = 0.75). Specifically, the averaged CPUEs were 4.75 ± 0.05, 1.54 ± 0.03, and 1.70 ± 0.10 g/m2/24 h in the lower, middle, and upper sections, respectively.
Body size
The mean standard length (SL) of C. brachygnathus was 203.66 ± 0.99 mm (mean ± S.E.) in the TGR. Individually, site or season did not seem to influence SL (LMM, sampling site, Wald's Χ2 = 13.89, P = 0.08; season, Wald’s Χ2 = 4.92, P = 0.18). Instead, SL changed spatially across seasons, as indicated by a significant interaction between sampling site and season (LMM: sampling site * season, Wald's Χ2 = 145.11, P < 0.01; Fig. 4). Specifically, the smallest mean SL was recorded at the lower section (site L5) in winter (164.47 ± 14.04 mm) and the largest mean SL at the upper section (site U1) in autumn (259.33 ± 7.38 mm). The mean SL of C. brachygnathus from the TGR was significantly larger than those of native fish from lakes located in the middle and lower reaches of the Yangtze River, such as Poyang Lake, Dongting Lake, Changhu Lake, Tianezhou Oxbow, Kuilei Lake, and Zilang Lake (Wilcoxon test, all P < 0.01; SL at the TGR and native locations is summarized in Table 3).
Reproductive traits
The overall sex ratio of C. brachygnathus was 1.49 (188 females, 126 males) in the TGR, and it was significantly different from 1 (X2 = 5.79, P = 0.02). The SL at 50% maturity (L50) of C. brachygnathus was 224.23 mm in the TGR population. The absolute and relative fecundities in the TGR population averaged 25,914.18 ± 2,465.76 eggs and 778.6 ± 63.2 eggs/g (mean ± S.E., n = 32), respectively, and its mature egg size averaged 0.40 ± 0.001 mm (mean ± S.E., n = 4586). The relative fecundity of C. brachygnathus in the TGR was significantly higher than that in the native populations, including the Swan Oxbow population (RF = 487.65 ± 59.57 egg/g; One sample t test, t = 4.68, P < 0.001) and the Changhu Lake population (RF = 487.65 egg/g; P < 0.001), and was similar with that in the native Poyang Lake population (RF = 765.83 ± 182 egg/g; P = 0.86).
Diet traits
Stomach content analyses revealed that zooplankton and shrimp (Exopalaemon modestus and Macrobrachium nipponensis) were the primary food types consistently consumed by C. brachygnathus across sampling sites, which contributed on average (± SE) 65.71% ± 9.03% (zooplankton), 24.78% ± 3.40% (E. modestus), and 9.51% ± 1.31% (M. nipponensis) to the overall diets of C. brachygnathus in the TGR (Table 4). The δ13C of C. brachygnathus ranged from -28.84‰ to -21.87‰, and the δ15N ranged from 12.04‰ to 16.60‰. Bayesian stable isotope mixing models suggested that E. modestus contributed the highest proportion to C. brachygnathus biomass in four out of seven sampling sites (i.e., lower end of the credibility intervals > 0% for sites L5, M1, M2, and U1). Zooplankton contributed the most to three out of seven sites (i.e., lower end of the credibility intervals > 0% for sites L1, L2, and L3); M. nipponensis contributed the lowest (i.e., lower end of the credibility intervals = 0% for six out of seven sites; Table 4; Fig. 5).
Discussion
Role of the dam in the invasion of C. brachygnathus
The relationship between dam construction and fish invasions is highly debated (Krieg et al., 2020; Kerr et al., 2021). Dams can prevent fishes from spreading between downstream and upstream reaches of a dammed river by limiting aquatic connectivity and fragmenting the river network (Frings et al., 2013). However, the processes of creating a reservoir, flooding, and impounding a river can facilitate invasion by eliminating natural barriers that prevent fishes from dispersing among catchments disconnected before the impoundment (Júlio Júnior et al., 2009). Our study supported both arguments: the dam’s initial blockage and later dispersal facilitation. Between 2010 and 2016, the Three Gorges Dam appeared to have blocked the upstream invasion of C. brachygnathus from the Gezhouba reservoir into the TGR. Our reasoning is based on earlier detection of non-native C. brachygnathus populations downstream of the TGR. The species was detected during 2004 and 2009 at the Yichang section, downstream of the Gezhouba Dam (Ma et al., 2014), and during 2009 and 2010 at the Gezhouba Reservoir, immediately downstream of the TGR (Liu et al., 2012). However, C. brachygnathus was not detected at five sampling sites (Low-1, Low-5, Middle-1, Middle-3, and Upper-1; downstream to upstream, respectively) within the TGR from 2013 to 2014 (Lian, 2016). Such observation suggests that the abundance of this species was below the detectability threshold at that stage of the invasion. Our past work demonstrated that C. brachygnathus was first detected in the lower section of the TGR during 2016 and expanded its distribution toward the upper sections in 2017. Although there is no direct evidence of how this species expanded its distribution into the TGR, the observed progressive directional invasion suggests that C. brachygnathus invaded the TGR from downstream. Thus, we argue that the navigation lock should be the most likely path for the fish invasion, given that the Three Gorges project did not build a fish passage mechanism (Shi et al., 2015).
Since C. brachygnathus was first detected in the lower section of the TGR in 2016, it has rapidly expanded its distribution to the entire reservoir and spread into tributaries, becoming a dominant fish species in the lower and middle sections (lentic habitats) of TGR. Such rapid expansions of invasive fishes have been observed in other systems, such as Neogobius melanostomus (Pallas 1814) (Gobiidae) in the Laurentian Great Lakes, USA (Raad et al., 2018) and armored catfish Loricariichthys platymetopon Isbrücker & Nijssen 1979 (Loricariidae) in the Itaipu reservoir, Brazil (Casimiro et al., 2017). The invasion of C. brachygnathus likely resulted as the impoundment of the TGR eliminated the natural barriers to dispersal formed by the natural “Three Gorges” with its 120-m drop from Chongqing to Yichang and that between the Yangtze River main stem and its numerous tributaries (Zolotov & Shaitanov, 2000).
Meanwhile, longitudinally abiotic gradients created from impoundment also are likely to affect the invasion and dispersal progression (Raad et al., 2018). In our study, the lower density (i.e., relative biomass, relative abundance, but not CPUE) of C. brachygnathus with distance from the dam indicated that C. brachygnathus has different abilities to live in the longitudinal gradient of lentic, transitional, and riverine habitats along the TGR (Cheng et al., 2015). Such differential adaptations allow C. brachygnathus to progressively invade the lotic upper section of TGR. Coilia brachygnathus is an anchovy species exclusive to freshwater lakes and reservoirs (Whitehead et al., 1988), which usually inhabits pelagic waters and produces pelagic eggs in lentic habitats (Qin et al., 2018). The upper section of the TGR maintains a lotic habitat with an average water velocity of 1.28 m/s after its final impoundment in 2009 (MEP, 2017). The spatially different abundance of C. brachygnathus suggests that higher water velocity may create environmental restrictions making the C. brachygnathus expansion toward the upstream riverine areas of the TGR difficult. The persistence of fluvial-adapted species supports this argument, because lentic-adapted species do not dominate these lotic habitats after damming. For instance, the abundance of invasive lentic-adapted species N. melanostomus declined from the reservoir to upstream of the Grand River (Ontario, Canada; Raab et al., 2018).
Fish communities upstream of the Yangtze River is the most species rich in China and includes numerous rare and endemic species (Liu et al., 2020). Coilia brachygnathus are yet to be reported in the natural Yangtze River, upstream from the TGR, and the upper cascade reservoirs (Li et al., 2020; Qu et al., 2020). However, assuming that C. brachygnathus can colonize the upstream cascading reservoirs through the navigation locks, it can be expected that it can differently impact its competitor and prey populations. In that case, the repeating lentic habitats formed by their impoundments are likely suitable for the colonization of C. brachygnathus. Monitoring and prevention measures are therefore needed. Based on the aforementioned spatial selectivity, to prevent the expansion of this invasive species, we propose that new hydropower projects should be forbidden between the tail of the TGR and the Xiangjiaba Dam to protect the remaining > 300 km of lotic stretches therein (Cheng et al., 2015). This management strategy would hinder the further upstream expansion of C. brachygnathus.
Biological traits related to the establishment and spread of C. brachygnathus
In the present study, both stable isotope and stomach content analyses suggest that C. brachygnathus mainly preyed on zooplankton and shrimps (especially E. modestus) in the TGR. These patterns are similar to the diet of native C. brachygnathus populations, such as those in the Hongze Lake, the Gaoyou Lake, the Luoma Lake (Gu et al., 2019), and the Poyang Lake (Zhang et al., 2013), where zooplankton and shrimps also contributed the highest to their diet. Shrimps were available to C. brachygnathus at each section of the TGR (2.51, 2.65, and 2.28 kg/boat/24 h in the lower, middle, and upper sections, respectively, during 2015–2017; Liao, C., unpublished data). The densities of zooplankton were 6.09 × 104, 5.07 × 104, and 5.40 × 104 ind./L in the lower, middle, and upper sections, respectively, during 2016–2017 (Zhang & Huang, 1995; Wu et al., 2021), which indicates that zooplankton was also relatively abundant across the entire reservoir. The diet similarity between the native and invasive populations indicates that abundant and widely distributed zooplankton and shrimps may satisfy the food resource requirements of C. brachygnathus, which should be considered a key factor explaining their successful invasion, especially at the initial stage (Blumenthal et al., 2006). This argument is consistent with the resource hypothesis, which proposes that good resource availability leads to low defense investment and high enemy damage; therefore, invasive fishes under high resource availability obtain sufficient energy to become established (Blumenthal et al., 2006).
The TGR had another invasion after its first filling, two icefish species (N. taihuensis and P. hyalocranius) were intentionally introduced to the lower section after approximately 2003 and posteriorly colonized the lower section of the reservoir (Gong et al., 2009; Ba & Chen, 2012). Considering these successful invasions and the ecological characteristics of these species, the results of our work and previous successful invasions suggest that the TGR is susceptible to invasion by exotic carnivorous and invertivorous fishes. It is worth noting that we also caught individuals of other non-native predatory species in the TGR, such as Odontobutis obscurus (Temminck & Schlegel 1845) (Odontobutidae), which is native to the middle and lower reaches of the Yangtze River basin. Further monitoring is needed to assess whether these species will flourish and expand their distribution in the future.
Invasive fish species usually share similar life history characteristics making them successful invaders, such as rapid growth, high fecundity, and early reproduction (Agostinho et al., 2015). Coilia brachygnathus has not been reported to invade other freshwater systems in China. Our study, however, demonstrated that C. brachygnathus shares some of these reproductive traits. TGR C. brachygnathus can reach 200 mm within six months of age, which indicates that females in the TGR can achieve 50% maturity (SL = 224.23 mm) within the first year of life and then spawn once a year (Tang et al., 1987; Liu, 2008). The TGR C. brachygnathus population has higher fecundity than some native populations, such as the Swan Oxbow and the Changhu Lake populations (Luo, 2006; Wang, 2016). Previous studies on other invasive species also found that non-native populations have higher fecundity than the native population (e.g., round goby N. melanostomus, Horkova & Kováč, 2014). The TGR and the native habitats are all located in the Yangtze River basin with similar latitude, and it seems unlikely that differences in temperature and other climate conditions were large enough to explain spatial variation in fecundity. Instead, we argue that the abundant foods and low intra- and inter-specific competition at this stage of the invasion may explain such variation in fecundity (Wooton & Smith, 2015).
Invasive freshwater fishes in newly occupied areas usually allocate more energy to reproduction and offspring maintenance resulting in smaller body sizes than populations from native areas (Záhorská et al., 2009; Novomeska et al., 2013). In the present study, the body size of the population of C. brachygnathus examined is considerably larger than those of six native populations from the middle and lower Yangtze River (Wu et al., 2015; Wang et al., 2016; Gong et al., 2018; Qin et al., 2018). It was only five years since C. brachygnathus invaded the TGR. The differences in the body size between the TGR and native habitats observed in this study were unlikely to result from genetic differences but from phenotypic plasticity (Iguchi et al., 2019). We also found that standard length tends to increase from the lower to the upper section of the TGR, although the trend was statistically non-significant. Bigger invaders likely possess better adaptive and competitive abilities. Over time, the body size of the TGR population may gradually lead back to the body size typical of individuals from native populations (Novomeska et al., 2013). Such fast maturity and short generation time, large body size, and high fecundity, coupled with abundant food availability, likely formed the critical factors of the successful invasion of C. brachygnathus (Agostinho et al., 2015). It is no longer realistic to eliminate the C. brachygnathus population from the Three Gorges Reservoir, and developing mechanisms to control its abundance should be a priority. We suggest strengthening targeted fishing to increase catching effort on C. brachygnathus (Giakoumi et al., 2019).
In conclusion, the findings from this study revealed when C. brachygnathus entered the TGR and how its abundance changed spatially and temporally. Our study also highlighted biological traits of C. brachygnathus that facilitated their successful invasion, such as early maturity, high fecundity, and an invertivorous diet. Our results improve scientific understanding of why C. brachygnathus successfully colonized and expanded into the whole reservoir within four to five years after first recorded. It is critical to further assess the potential impacts of C. brachygnathus on native pelagic and zooplanktivorous fishes therein, such as Hemiculter leucisculus (Basilewsky 1855), H. tchangi Fang 1942, Pseudolaubuca sinensis Bleeker 1864, and P. engraulis (Nichols 1925), as well as the potential impacts on the two native shrimp populations. The present study also issued a risk alarm that the lentic habitats formed by cascaded reservoirs in the upper reaches of the Yangtze River may facilitate C. brachygnathus to continue expanding upstream. Based on our findings, this invasive species could be managed through habitat conservation and fishing regulation.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used in this study are available from the corresponding author on reasonable request.
References
Agostinho, A. A., H. I. Suzuki, R. Fugi, D. C. Alves, L. H. Tonella & L. A. Espindola, 2015. Ecological and life history traits of Hemiodus orthonops in the invasion process: looking for clues at home. Hydrobiologia 746: 415–430.
Ba, J. W. & D. Q. Chen, 2012. Invasive fishes in Three Gorges Reservoir area and preliminary study on effects of fish invasion owing to impoundment. Journal of Lake Sciences 24: 185–189 (In Chinese with English abstract).
Bates, D., M. Maechler, B. M. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48.
Blumenthal, D. M., 2006. Interactions between resource availability and enemy release in plant invasion. Ecology Letters 9: 887–895.
Casimiro, A. C. R., D. A. Z. Garcia, A. D. A. Costa, J. R. Britton & M. L. Orsi, 2017. Impoundments facilitate a biological invasion: dispersal and establishment of non-native armoured catfish Loricariichthys platymetopon (Isbrückler & Nijssen, 1979) in a Neotropical River. Limnologica 62: 34–37.
Cheng, F., W. Li, L. Castello, B. R. Murphy & S. G. Xie, 2015. Potential effects of dam cascade on fish: lessons from the Yangtze River. Reviews in Fish Biology and Fisheries 25: 569–585.
Cooper, A. R., D. M. Infante, J. R. O’Hanley, H. Yu, T. M. Neeson & K. J. Brumm, 2021. Prioritizing native migratory fish passage restoration while limiting the spread of invasive species: a case study in the Upper Mississippi River. Science of the Total Environment 2021: 148317.
Dana, E. D., J. García-de-Lomas, R. González & F. Ortega, 2011. Effectiveness of dam construction to contain the invasive crayfish Procambarus clarkii in a Mediterranean mountain stream. Ecological Engineering 37: 1607–1613.
Emmrich, M., I. J. Winfield, J. Guillard, A. Rustadbakken, C. Vergès, P. Volta, E. Jeppesen, T. L. Lauridsen, S. Brucet, K. Holmgren, C. Argillier & T. Mehner, 2012. Strong correspondence between gillnet catch per unit effort and hydroacoustically derived fish biomass in stratified lakes. Freshwater Biology 57: 2436–2448.
Fox, J. & S. Weisberg, 2011. An R Companion to Applied Regression, 2nd ed. Thousand Oaks, CA, USA, Sage:
Frings, R. M., S. C. K. Vaeßen, H. Groß, S. Roger, H. Schüttrumpf & H. Hollert, 2013. A fish-passable barrier to stop the invasion of non-indigenous crayfish. Biological Conservation 159: 521–529.
Gao, X., Y. Zeng, J. W. Wang & H. Z. Liu, 2010. Immediate impacts of the second impoundment on fish communities in the Three Gorges Reservoir. Environmental Biology of Fishes 87: 163–173.
Giakoumi, S., A. Pey, A. Di Franco, P. Francour, Z. Kizilkaya, Y. Arda, V. Raybaud & P. Guidetti, 2019. Exploring the relationships between marine protected areas and invasive fish in the world’s most invaded sea. Ecological Applications 29: e01809.
Gong, J., T. Wang, D. Huang, J. Z. Shen & C. Gong, 2018. Interannual variation of the fish community structure in the Tian-e-zhou Oxbow of Yangtze River. Journal of Hydroecology 39: 46–53 (In Chinese with English abstract).
Gong, W. B., L. Wu, J. S. Liu, S. Q. Xie, Z. J. Li, B. R. Murphy & S. G. Xie, 2009. Variation in reproductive traits between populations of Neosalanx taihuensis above and below the Three-Gorges Dam. Journal of Freshwater Ecology 24: 529–533.
Gu, X. K., T. T. Zhang, D. M. Li, X. W. Liu, Y. S. Liu, D. D. Shen, F. Xu & H. Wang, 2019. Feeding characteristics of Coilia nasus taihuensis in typical waters of Jiangsu Province. Chinese Journal of Ecology 38: 3115–3122 (In Chinese with English Abstract).
Horkova, K. & V. Kováč, 2014. Different life-histories of native and invasive Neogobius melanostomus and the possible role of phenotypic plasticity in the species’ invasion success. Knowledge and Management of Aquatic Ecosystems 412: 1–11.
Iguchi, K., Y. Matsumoto, Y. Kurita & K. Watanabe, 2019. Adaptive downsizing in the piscivorous cyprinid fish, Opsariichthys uncirostris, facilitates rapid establishment after introduction to a small-scale habitat in Japan. Biological Invasion 21: 2059–2066.
ICOLD (International Commission on Large Dams), 2019. Number of Dams by Country Members. http://www.icold-cigb.org/.
IUCN (International Union for Conservation of Nature), 2021. A Global Standard for the Identification of Key Biodiversity Areas. Version 2021–1. IUCN, Gland, Switzerland.
Júlio Júnior, H. F., C. D. Tós, Â. A. Agostinho & C. S. Pavanelli, 2009. A massive invasion of fish species after eliminating a natural barrier in the upper Rio Paraná basin. Neotropical Ichthyology 7: 709–718.
Kerr, J. R., A. S. Vowles, M. C. Crabb & P. S. Kemp, 2021. Selective fish passage: restoring habitat connectivity without facilitating the spread of a non-native species. Journal of Environmental Management 279: 110908.
Kiljunen, M., J. Grey, T. Sinisalo, C. Harrod, H. Immonen & R. I. Jones, 2006. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. Journal of Applied Ecology 43: 1213–1222.
Kreyenberg, W. & P. Pappenheim, 1908. Ein Beitrag zur Kenntnis der Fische der Jangtze und seiner Zuflüsse. Sitzungsber. Ges. Naturf. Freunde Berlin, 95–109.
Krieg, R. & A. Zenker, 2020. A review of the use of physical barriers to stop the spread of non-indigenous crayfish species. Reviews in Fish Biology and Fisheries 30: 423–435.
Lenth, R. V., P. Buerkner, M. Herve, J. Love, F. Miguez, H. Riebl & H. Singmann, 2022. emmeans: estimated marginal means, aka least-squares mean. R Package Version 1(7): 3.
Li, T., L. Tang, L. Wang, L. An, J. Wang, K. L. Mo & Q. W. Chen, 2020. Distribution characteristics and ecological types changes in fish communities under hydropower development from Xiluodu to Xiangjiaba reach. Acta Ecology Sinica 40: 1473–1485.
Lian, Y. X., 2016. Spatio-temporal distribution patterns of fishes as related to ecological factors in the Three Gorges Reservoir. PhD Dissertation, University of Chinese Academy of Science. (In Chinese with English Abstract)
Liao, C. S., S. B. Chen, S. S. De Silva, S. B. Correa, J. Yuan, T. L. Zhang, Z. J. Li & J. S. Liu, 2018. Spatial changes of fish assemblages in relation to filling stages of the Three Gorges Reservoir, China. Journal of Applied Ichthyology 34: 1293–1303.
Liew, J. H., H. H. Tan & D. C. J. Yeo, 2016. Dammed rivers: impoundments facilitate fish invasions. Freshwater Biology 61: 1421–1429.
Liu, Y. L., 2008. Species identification and biological characteristics of Coilia brachynathus in the Poyang Lake. Master Dissertation, Nanchang University. (In Chinese with English Abstract)
Liu, C. C., X. Gao, P. C. Lin, S. R. Yang, H. Z. Liu & W. X. Cao, 2012. Fish community structure in Gezhouba Reservoir. Resources and Environment in the Yangtze Basin 21: 843–849 (In Chinese with English Abstract).
Liu, H., C. B. Guo, X. Qu, F. Y. Xiong, C. P. Paukert, Y. S. Chen & W. Su, 2020. Fish diversity, endemism, threats, and conservation in the Jinsha River Basin (upper Yangtze River), China. North American Journal of Fisheries Management 41: 967–984.
Loures, R. C. & P. S. Pompeu, 2019. Temporal changes in fish diversity in lotic and lentic environments along a reservoir cascade. Freshwater Biology 64: 1806–1820.
Luo, H. B., 2006. Age, growth and gonadal development of Coilia brachygnathus in Swan Oxbow of the Yangtze River. Master Dissertation, Southwest University. (In Chinese with English Abstract)
Ma, Q., P. C. Lin, H. Z. Liu, W. X. Cao & X. Gao, 2014. Effects of the gillnets on fish resources in the Yichang reaches of the Yangtze River. Sichuan Journal of Zoology 33: 762–767 (in Chinese with English abstract).
McConnaughey, T. & C. P. McRoy, 1979. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Marine Biology 53: 257–262.
MEP (Ministry of Environmental Protection of People's Republic of China), 2017. Ecological and environmental bulletin of Three Gorges Project. http://www.cnemc.cn. [in Chinese]
Muniz, C. M., E. García-Berthou, M. J. M. Ganassin, A. A. Agostinho & L. C. Gomes, 2021. Alien fish in Neotropical reservoirs: assessing multiple hypotheses in invasion biology. Ecological Indicators 121: 107034.
Novomeská, A., S. Katina, G. H. Copp, G. Pedicillo, M. Lorenzoni, L. Pompei, J. Cucherousset & V. Kováč, 2013. Morphological variability of black bullhead Ameiurus melas in four non-native European populations. Journal of Fish Biology 82: 1103–1118..
Parnell, A. C., D. L. Phillips, S. Bearhop, B. X. Semmens, E. J. Ward, J. W. Moore, A. L. Jackson & R. Inger, 2013. Bayesian stable isotope mixing models. Environmetrics 24: 387–399.
Pereira, L. S., A. A. Agostinho & L. C. Gomes, 2015. Eating the competitor: a mechanism of invasion. Hydrobiologia 746: 223–231.
Qin, X., T. Wang, P. Lin, X. Wang & H. Liu, 2018. Age, growth, mortality and movement patterns of shortjaw tapertail anchovy, Coilia brachygnathus, in the channel connecting Dongting Lake and the Yangtze River in central China. Aquatic Living Resources 31: 1–9.
Qu, X., C. B. Guo, F. Y. Xiong, W. Xin, Y. S. Chen & W. Su, 2020. Characterization of the fish community and environmental driving factors during development of cascaded dams in the lower Jinsha River. Journal of Hydroecology 41: 46–56 (in Chinese with English abstract).
Raab, D., N. E. Mandrak & A. Ricciardi, 2018. Low-head dams facilitate Round Goby Neogobius melanostomus invasion. Biological Invasions 20: 757–776.
Sá-Oliveira, J. C., J. E. Hawes, V. J. Isaac-Nahum & C. A. Peres, 2015. Upstream and downstream responses of fish assemblages to an eastern Amazonian hydroelectric dam. Freshwater Biology 60: 2037–2050.
Shi, X. T., B. Kynard, D. F. Liu, Y. Qiao & Q. W. Chen, 2015. Development of fish passage in China. Fisheries 40: 161–169.
Skinner, M. M., A. A. Martin & B. C. Moore, 2016. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Communications in Mass Spectrometry 30: 881–889.
Tang, Y., 1987. On the population dynamics of lake anchovy in Taihu Lake and its rational exploitation. Journal of Fisheries of China 11: 61–72. (in Chinese with English abstract)
Wang, X. G., 2016. Population ecology and genetic diversity of Coilia brachygnathus in Lake Changhu. Master Dissertation, Shanghai Ocean University. (in Chinese with English abstract)
Wang, X. G., Y. F. He, H. C. Li, W. B. Yan & D. G. Yang, 2016. Study on the age and growth characteristics of Coilia brachygnathus in Lake Changhu. Freshwater Fisheries 46: 29–33. (in Chinese with English abstract)
Warton, D. I. & F. K. Hui, 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92: 3–10.
West, G., 1990. Methods of assessing ovarian development in fishes: a review. Marine and Freshwater Research 41: 199–222.
Whitehead, P. J. P., G. J. Nelson & T. Wongratana, 1988. FAO Species Catalogue. Vol. 7 Clupeoid Fishes of the World (Suborder Clupeoidei). An Annotated and Illustrated Catalogue of the Herrings, Sardines, Pilchards, Sprats, Anchovies and Wolfherrings. Part 2: Engraulididae. FAO Fisheries Synopsis (7–2).
Wooton, R. J., C. Smith, 2015. Reproductive Biology of Teleost Fishes. John Wiley & Sons, Ltd.
Wu, B., C. L. Fang, P. F. Fu, X. Y. Xiong, Y. P. Zhang, H. M. Zhou, G. He, S. Wang & Q. P. Wang, 2015. Growth characteristic of Coilia brachygnathus in the Poyang Lake-Yangtze River water way. Journal of Hydroecology 36: 51–55 (in Chinese with English abstract).
Wu, L., H. Y. Tang, Y. Gong, Z. Yang, Z. Q. Zhu & X. J. Chen, 2021. Temporal-spatial distribution of zooplankton community in the main stem of Three Gorges Reservoir under normal operation. Journal of Hydroecology 42: 58–65 (in Chinese with English abstract).
Wu, Q., X. B. Duan, S. Y. Xu, C. X. Xiong & D. Q. Chen, 2007. Studies on fishery resources in the Three Gorges Reservoir of the Yangtze River. Freshwater Fisheries 37: 70–75 (in Chinese with English Abstract).
Yang, F., 2019. Genetic diversity comparison of Coilia brachygnathus and Neosalanx taihuensis between populations in the Three Gorges Reservoir and Dongting Lake. Master Dissertation, Southwest University. in Chinese with English abstract
Yang, S. R., X. Gao, M. Z. Li, B. S. Ma & H. Z. Liu, 2012. Interannual variations of the fish assemblage in the transitional zone of the Three Gorges Reservoir: persistence and stability. Environmental Biology of Fishes 93: 295–304.
Yin, M. C., 1995. Fish Ecology, China Agriculture Press, Beijing: (in Chinese).
Záhorská, E., V. Kováč, I. Falka, K. Beyer, S. Katina, G. H. Copp & R. E. Gozlan, 2009. Morphological variability of the Asiatic cyprinid, topmouth gudgeon Pseudorasbora parva, in its introduced European range. Journal of Fish Biology 74: 167–185.
Zhang, H., G. G. Wu, H. Zhang, P. Xie, J. Xu & Q. Zhou, 2013. Role of body size and temporal hydrology in the dietary shifts of shortjaw tapertail anchovy Coilia brachygnathus (Actinopterygii, Engraulidae) in a large floodplain lake. Hydrobiologia 703: 247–256.
Zhang, H., M. Kang, L. Shen, J. M. Wu, Y. J. Li, H. Du, C. Y. Wang, H. L. Yang, Q. Zhou, Z. G. Liu & H. Gorfine, 2020. Rapid change in Yangtze fisheries and its implications for global freshwater ecosystem management. Fish and Fisheries 21: 601–620.
Zhang, Z. S. & X. F. Huang, 1995. Study Methods of Freshwater Plankton, China Scienc Publishing & Media Ltd, Beijing:
Zhou, H. H., C. Li, H. T. Deng, H. W. Tian, Y. B. Chen, X. Gao, B. Zhu & D. Q. Chen, 2020. Research on status and dynamic varietal trends of rare and unique fish stocks in the upper reaches of Yangtze River. Freshwater Fisheries 50: 3–14 (in Chinese with English abstract).
Zolotov, L. A. & V. Y. Shaitanov, 2000. Construction of navigation structures for the “Three Gorges” hydroproject. Hydrotechnical Construction 34: 62–64.
Acknowledgements
This study was funded by the National Key Research and Development Program of China (2020YFD0900504), the China Postdoctoral Science Foundation (2020M672448), the National Natural Science Foundation of China (32102798), and the Earmarked Fund for China Agriculture Research System (CARS-45). S.B. Correa was supported by the Forest and Wildlife Research Center of Mississippi State University, USA (MISZ-081700).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by [LC], [YJ], [WJ], [SBC], [XF], [YS], and [LJ]. The first draft of the manuscript was written by [LC] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to this article.
Ethical approval
The manuscript has not been published or is under consideration for publication elsewhere, in whole or in part.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling Editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, C., Yu, J., Wang, J. et al. Trends and mechanisms behind the invasion of Coilia brachygnathus (Actinopterygii, Engraulidae) in one of the world’s largest reservoirs. Hydrobiologia 849, 2919–2932 (2022). https://doi.org/10.1007/s10750-022-04896-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04896-8