Abstract
New Zealand mud snails (Potamopyrgus antipodarum) were first introduced to the United States in 1987 and have subsequently invaded water bodies across the United States. At high densities, P. antipodarum can outcompete native fauna, disrupt community interactions, and alter ecosystem function, making them a threat to aquatic habitats worldwide. In this paper, we detail the invasion, population dynamics, and secondary production of P. antipodarum at seven sites in the Green River below Flaming Gorge Dam (0.8–49 km below the dam). New Zealand mud snails were first detected in the Green River in 2001. For more than a decade, densities of P. antipodarum in the Green River remained relatively low (< 1200 individuals m−2) at all sites. However, in 2015 densities started to increase dramatically at three sites below the dam, eventually reaching > 19,000 individuals m−2. Annual secondary production estimates of P. antipodarum for the year 2016 ranged from < 0.1 g m−2 yr−1 to 14.2 g m−2 yr−1. Furthermore, in terms of production, P. antipodarum was one of the dominant invertebrate taxa at six of the seven sites. Why densities dramatically increased after 2015 at only three of the seven sites remains unclear. Moreover, it is currently unknown whether populations of P. antipodarum in the Green River will undergo a “bust” period following their dramatic increase in density like populations of P. antipodarum in other river systems. Nevertheless, given the recent increases in densities of P. antipodarum and the relatively high rates of production at several sites, we recommend continued monitoring and suggest that attention be brought to the potential effects of New Zealand mud snails on the structure and function of the Green River ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasion by non-native species is one of the greatest threats to freshwater biodiversity and ecosystem functioning (Gallardo et al. 2016). The establishment and spread of non-native species can disrupt the structure and functioning of freshwater ecosystems via direct (e.g., predation, competition with native species) and indirect (e.g., changes in habitat structure, water quality) pathways (Crooks 2002; Gallardo et al. 2016). Moreover, human activities have increased the rates of introductions and distances of spread by non-native species in freshwater ecosystems (Kolar and Lodge 2001). One such human activity, dam construction, has facilitated the invasion and spread of non-native species worldwide (Ruhi et al. 2019). By altering flow and temperature regimes of rivers, dams can negatively affect native species adapted to historical conditions and create niche opportunities for non-native species to establish and spread (Bunn and Arthington 2002; Ruhi et al. 2019). Furthermore, many regulated rivers have been exposed to repeated deliberate or accidental introductions of non-native species through recreational activities (e.g., boating, angling), contributing to the proliferation of non-native species below dams.
The New Zealand mud snail (Potamopyrgus antipodarum) has been introduced to freshwater habitats throughout the world, including regulated river systems (Alonso and Castro-Díez 2008). The New Zealand mud snail is a highly fecund, parthenogenic and ovoviviparous snail common in lakes and streams throughout New Zealand, where it is endemic (Winterbourn 1970; Alonso and Castro-Díez 2008). Over the last 150 years, P. antipodarum has become invasive in freshwater ecosystems of Europe, Australia, Asia, South America, and North America (Alonso and Castro-Díez 2008; Collado 2014). New Zealand mud snails first arrived in the United States in 1987 and have since spread rapidly throughout the western United States and the Great Lakes region. To date, all western states have confirmed populations (Benson et al. 2020).
At extremely high densities, P. antipodarum has the ability to profoundly alter freshwater communities and ecosystems. New Zealand mud snails can reach densities of 800,000 individuals m−2 outside of their native range (Dorgelo 1987) and can be responsible for up to 95% of total invertebrate production in streams (Hall et al. 2006). As a result of their high densities and production, P. antipodarum is able to disrupt community interactions by competitively excluding other primary consumers (Kerans et al. 2005; Riley et al. 2008). In addition, they can consume vast quantities of primary production (up to 75% of gross primary production; Hall et al. 2006), and alter biogeochemical cycling in streams (Hall et al. 2003). Although they are often consumed by fish such as trout, New Zealand mud snails can survive passage through the intestinal tracts of native trout (Bruce et al. 2009) and offer very little nutritional value to fish due to their hard, undigestible shells (McCarter 1986; Vinson and Baker 2008; Butkus and Višinskienė 2020). However, like many other non-native species, populations of P. antipodarum often exhibit boom-bust dynamics (i.e. the rapid increase of a population followed by a dramatic decline), suggesting that such strong effects of P. antipodarum on community structure and ecosystem processes may be attenuated over time (Simberloff and Gibbons 2004; Strayer et al. 2017).
Potamopyrgus antipodarum was first discovered in the Green River in 2001, approximately 36 km below Flaming Gorge Dam (Utah, USA). Here, we present a time series of invasion by P. antipodarum and examine changes in its abundance over time at seven sites in the Green River below Flaming Gorge Dam. In addition, we estimate annual secondary production of P. antipodarum from monthly samples collected from 2015–2016 at the same seven sites below the dam. One of our goals was to bring attention to the invasion, spread, and potential impacts of P. antipodarum in the Green River and other rivers in the western United States. Improved documentation and monitoring of the distribution and densities of P. antipodarum in western rivers will enable scientists and managers to better understand what drives New Zealand mud snail population dynamics and their potential impacts on freshwater communities and ecosystem processes. We compare the invasion timeline and production estimates from the Green River with results of other studies from rivers in the western United States.
Methods
Study site
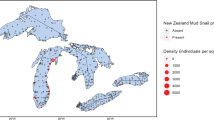
We studied seven sites along a 49 km reach of the Green River below Flaming Gorge Dam (40°54′54.0"N, 109°25′18.4"W) near Dutch John, Utah, USA (Fig. 1). The Green River is the largest tributary of the Colorado River, originating in the Wind River Range in Wyoming and flowing south to its confluence with the Colorado River in southern Utah. Construction of Flaming Gorge Dam began in 1959 and was completed in 1962. The dam is used primarily for hydroelectric power generation and flood control. Two small perennial tributaries and a larger intermittent tributary (Red Creek) enter the Green River 2.4, 12, and 18 km downstream from the dam. Upstream of Red Creek, the Green River flows through a deep bed-rock canyon and substrate consists of coarse gravel, cobbles and boulders (Vinson 2001). In this section of the Green River above Red Creek, channel width ranges from 70 to 150 m while water depth ranges from 1 to 10 m (Vinson 2001). The bryophyte Amblystegium and the green algae Chara and Cladophora are the dominant primary producers covering most cobbles and boulders while the macrophytes Elodea and Potamogeton are common in sand deposits and eddies. Downstream of Red Creek, the Green River leaves the canyon and substrate consists primarily of gravel and cobble overlain by deposits of sand and silt (Vinson 2001). River width increases to 200 m and depth decreases from 0.5 to 2 m (Vinson 2001). In this section, macrophytes and bryophytes are less abundant and primary producers are dominated by Chara (Vinson 2001).
River temperature and discharge data were obtained from USGS stream gage 09234500 (Green River near Greendale, UT). This gage is near site GR1 (~ 0.8 km below the dam) and best characterizes the temperature and flow regimes at that site. Small tributary inputs along the length of our study reach likely attenuate the influence of the dam on the temperature and flow regimes at sites further downstream. The tailwater section of the Green River can be characterized by cold (dam release temperatures of 2–14 °C), clear water with deep runs and pools. Mean annual temperature at the gage during our study was 8.8 °C and ranged from 7.8 to 12.4 °C (Fig. 2a). Over the course of our study, mean annual discharge below the dam ranged from 26.9 m3/s to 116.8 m3/s with a mean of 53.8 m3/s (Fig. 2b). Mean annual discharge has not changed appreciably since dam closure. However, dam regulation has resulted in a reduction of flows from April through July and an increase in flows from August through March (Vinson 2001). To characterize the annual thermal regimes at each site, water temperature was measured at each site from 28 April 2015 to 10 May 2016 at 30 min intervals using HOBO Pendant Temperature Data Loggers (Hobo UA-001–64, Onset Computer Corporation, Bourne, MA USA). Temperature data for each site are presented in the Supplementary Material (Table S1). The fish community below the dam is dominated by brown (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) and trout biomass can exceed 800 kg wet mass ha−1 (Filbert and Hawkins 1995). The tailwater section of Flaming Gorge Dam is one of the most productive and popular trout fisheries in North America.
P. antipodarum sampling
We compiled data on the abundance of P. antipodarum from seven locations downstream of Flaming Gorge Dam (Fig. 1) between 1994 and 2020 from a database at the National Aquatic Monitoring Center, Utah State University. Benthic macroinvertebrates were collected as part of a long-term monitoring project evaluating the effects of flow releases on macroinvertebrate assemblages in the Green River below Flaming Gorge Dam. Quantitative macroinvertebrate samples were collected quarterly using a Hess sampler (area = 0.086 m2, 250 μm mesh) or D-frame net coupled with a sampling square (area = 0.090 m2, 250 μm mesh), depending on flow. In general, the Hess sampler was used at sites GR1–GR6 where flow is faster while the D-frame net was commonly used at site GR7 where flow is slower. On each date, eight random samples were collected from a 100 m long riffle section of the river and combined to form one composite sample. Samples were preserved in 70% ethanol in the field and returned to the laboratory where they were processed following protocols of the National Aquatic Monitoring Center (usu.edu/buglab/). Briefly, invertebrates were separated from organic matter using a dissecting microscope (10 × magnification) and samples were subsampled using a Caton tray (Caton 1991) to obtain a minimum of 300 random individuals. Invertebrates were identified to the lowest practical taxonomic level (genus in most cases) and enumerated.
Secondary production
We calculated annual secondary production of P. antipodarum and other common invertebrates at all seven sites (Table S2). From December 2015 through November 2016, we sampled invertebrates from each site approximately monthly using methods outlined above. The body length of invertebrates was measured to the nearest 0.1 mm using an ocular micrometer and biomass (dry mass) was estimated using published length-mass regressions (Towers et al. 1994 for P. antipodarum; Benke et al. 1999 for other invertebrates). Biomass of P. antipodarum and other snails (e.g., Physa) included organic tissue in the shell, but not the shell itself. We estimated invertebrate secondary production using methods most appropriate for each taxon at a given site. We estimated production using the size-frequency method, corrected for cohort production interval (CPI), or from the product of mean annual biomass and the appropriate P:B (5 for assumed univoltine taxa, 10 for bivoltine taxa, and 2.5 for semivoltine taxa with life cycles > 1 year; Benke and Huryn 2017). While most methods for calculating secondary production require identifying and tracking distinct cohorts from field data, the size-frequency and P:B methods can be used when a population cannot be followed as a cohort (Benke and Huryn 2017). We used the size-frequency method to estimate production of P. antipodarum at six of the seven sites. At site GR1, production of P. antipodarum was estimated using the P:B method due to a low number of detections throughout the year. The size-frequency method is an appropriate method for estimating the production of P. antipodarum in the Green River as reproduction within populations occurs throughout the year and cohorts cannot be easily followed. Additionally, growth rates of P. antipodarum were not empirically measured, thus precluding the use of the instantaneous growth rate method. The size-frequency method assumes that the mean size-frequency distribution of a population throughout the year approximates the mortality curve of an average cohort (Benke and Huryn 2017). Briefly, the decrease in abundance from one size class to the next is multiplied by the mean mass between the two size classes. These products are then multiplied by the total number of size classes, as it is assumed that there is the same number of cohorts during a year as there are size classes (Benke and Huryn 2017). Production is calculated as the sum of these products. If development time is greater than or less than one year, production is corrected by multiplying it by 365/CPI, where CPI is the development time in days from eggs hatching to final size (Benke 1979). We estimated CPIs by examining size-frequency histograms of taxa over time (Benke and Huryn 2017). To account for uncertainty in CPI estimates, we randomly select CPI values within a restricted range and used bootstrapping to generate 100 estimates of CPI-corrected secondary production. Production estimates using the size-frequency method were calculated as the mean of the 100 bootstrapped estimates. Production estimates, the method used to calculate production, and the range in CPI values used for each taxon can be found in the Supplementary Material (Table S2). A more detailed description of secondary production methods can be found in Benke and Huryn (2017). All analyses and secondary production calculations were performed using R statistical software version 4.1.0 (R Core Team 2021).
Results
The first confirmed specimens of P. antipodarum in the Green River below Flaming Gorge Dam were collected on 18 September 2001 from GR6 (36 km below the dam), near Swallow Canyon (Fig. 3, Table S3). By July 2005, P. antipodarum was detected at all seven study sites (Fig. 3, Table S3). Densities of P. antipodarum remained relatively low (< 1,200 individuals m−2) at all sites until 2015, when densities at three sites (GR4, GR5, GR6) began to increase (Fig. 3). Maximum densities at these three sites reached > 19,000 individuals m−2 and occurred after September 2019 (Fig. 3). Maximum densities at the other four sites (GR1, GR2, GR3, and GR7) remained < 1200 individuals m−2 (Fig. 3). Mean annual densities of P. antipodarum ranged from 0 to 10,132 individuals m−2 and varied significantly among sites (Figure S1, Table S4).
Mean annual biomass, secondary production, and biomass turnover rates (P:B) of P. antipodarum varied among sites. Mean annual biomass ranged from < 0.1 g dry mass m−2 at site GR1 to 2.1 g dry mass m−2 at site GR4 (Table 1). Annual secondary production of P. antipodarum ranged from < 0.1 g dry mass m−2 yr−1 to 14.2 g dry mass m−2 yr−1 and was highest at GR4 (Table 1). Production:biomass ratios (P:B) ranged from 5.0 yr−1 at site GR1 to 10.0 yr−1 at site GR3 (Table 1). The percent contribution of P. antipodarum to total invertebrate production also varied among sites. The contribution of P. antipodarum to total production ranged from < 0.1% at GR1 to 25.6% at GR4 (Table 1). In general, P. antipodarum ranked high in terms of production compared to other members of the invertebrate assemblage, but there were differences among sites (Fig. 4, Table S4). Rank production of P. antipodarum ranged from the most productive taxon at site GR4 to the least productive taxon at site GR1 (Fig. 4).
Discussion
We documented the invasion and potential start of the “boom” period of an invasive freshwater gastropod, P. antipodarum, in the Green River below Flaming Gorge Dam. Many invasive species undergo boom-bust dynamics in which they rapidly become numerically dominant after establishment (i.e., the “boom”), only to decline dramatically a short time after (i.e., the “bust”; Simberloff and Gibbons 2004; Strayer et al. 2017). Populations of New Zealand mud snails exhibit such boom-bust dynamics. For example, over a 7-year period, the abundance of P. antipodarum increased from fewer than 500 individuals m−2 to ~ 100,000 individuals m−2 in a California stream (Moore et al. 2012). Following this “boom” period was a “bust” period for New Zealand mud snails in which their abundance rapidly decreased to fewer than 1,000 individuals m−2 (Moore et al. 2012). Likewise, between the years 2000 and 2001 the biomass of P. antipodarum in Polecat Creek (Wyoming, USA) was approximately 25 g AFDM m−2 (Hall et al. 2006; Greenwood et al. 2020). However, after approximately 10 years, biomass of P. antipodarum decreased to less than 2 g AFDM m−2 (Greenwood et al. 2020). What causes such boom-bust dynamics in populations of New Zealand mud snails is largely unknown. Exploitation of disturbed habitats and release from natural predators, parasites, or pathogens are likely explanations for rapid population growth (Strayer et al. 2017). Increased mortality due to predation or parasitism, environmental change leading to unfavorable conditions, or an overconsumption of their food resources have been proposed as possible mechanisms for the “bust” period of the cycle (Moore et al. 2012; Greenwood et al. 2020). Regardless of the actual mechanisms facilitating boom-bust cycles of P. antipodarum, continued monitoring of New Zealand mud snail populations in the Green River will contribute towards an improved understanding of P. antipodarum population cycles in large regulated rivers and their potential effects on the Green River ecosystem.
Why densities dramatically increased after 2015 at only three of the six sites remains unclear. However, three sites at which densities remained relatively low are within 12 km of the dam and above two small tributaries. One of these tributaries, Pipe Creek, contains populations of brown and rainbow trout and is popular with anglers, a potential source of transport and invasion (Schisler et al. 2008). While Pipe Creek and other small tributaries have not been surveyed yet for P. antipodarum, it is likely that P. antipodarum is present. Thus, Pipe Creek and other tributaries may act as a source of P. antipodarum for the Green River. Although P. antipodarum can travel upstream at a rate of 1 km yr−1 (Loo et al. 2007), downstream dispersal via drift is likely faster (Kerans et al. 2005). In fact, New Zealand mud snails have been observed drifting downstream on mats of floating algae in the Madison River and other systems (Kerans et al. 2005; Ribi 1986). Thus, higher densities at sites below these tributaries may result from colonization via downstream drift.
Differences in site characteristics may have also been responsible for differences in P. antipodarum population growth among sites. Sites closer to the dam (e.g., sites GR1, GR2, GR3) are likely more influenced by dam operations than sites further downstream. The effects of dams tend to attenuate with distance from the dam, such that sites further downstream experience more natural thermal and hydrological regimes (Ward and Stanford 1983). Temperature in particular can significantly affect New Zealand mud snail survival, growth, and reproduction (Dybdahl and Kane 2005; Gust et al. 2011; McKenzie et al. 2013). For example, P. antipodarum growth rates increase with increasing temperature (at least up to ~ 24 °C) and population growth rates are highest ~ 18 °C (Dybdahl and Kane 2005). Furthermore, temperature has a clear effect on both the duration of embryonic development and on the quantity of embryos (Gust et al. 2011). Sites closer to Flaming Gorge dam experience lower mean annual temperatures and lower maximum annual temperatures than sites further downstream from the dam (Table S1). Thus, the number of degree days within a year varies among sites and may be a limiting factor for P. antipodarum population growth directly below Flaming Gorge Dam. Relatively low mean and maximum annual temperatures at sites closest to the dam may explain the low densities/production of P. antipodarum at those sites.
Densities of P. antipodarum in the Green River are relatively low compared to several streams and rivers in the western United States and other parts of their invaded range. For example, Hall et al. (2003) reported densities > 500,000 individuals m−2 in streams of the Yellowstone region. In invaded streams in California, densities almost reaching 100,000 individuals m−2 have been reported (Moore et al. 2012). Although densities in the Green River are low relative to other river systems, this may be due to a sampling bias. Samples from this study were collected primarily from riffle habitats. However, in the Green River, evidence suggests that P. antipodarum prefers habitats with lower velocities and abundant vegetation. Densities are often highest within beds of the macrophyte Potamogeton (Mark Vinson, unpublished data). Therefore, further monitoring of P. antipodarum in the Green River should include all potential habitats within the river channel.
The invasion timeline of P. antipodarum in the Green River is also in contrast with the invasion timeline in other streams and rivers. It took approximately 14 years for densities to reach > 1200 individuals m−2 at three of our study sites in the Green River. In the other four study sites, densities have remained < 1200 individuals m−2. In the Colorado River below Glen Canyon Dam, P. antipodarum first appeared in 1995 at relatively low densities (Cross et al. 2010). However, by 1997 densities quickly reached > 30,000 individuals m−2 and remained high through 2006 (Cross et al. 2010). In 2000, densities even reached > 200,000 individuals m−2 (Cross et al. 2010). At one site in the Upper Owens River in California, USA, New Zealand mud snail densities went from fewer than 500 individuals m−2 to almost 100,000 individuals m−2 in just seven years (Moore et al. 2012). Why it took so long for densities of P. antipodarum in the Green River to increase is unclear. There have been no appreciable changes in temperature (Fig. 2a) over the last 20 years but maximum discharge at the USGS stream gage has increased from ~ 5000 to ~ 8000 m3/s since 2010 (Fig. 2b). Seeing as flow conditions can influence the distribution and survival of P. antipodarum (Holomuzki and Biggs 2000, 2007; Schreiber et al. 2003), it is possible that alterations to flow regulation below the dam are responsible for changes in P. antipodarum population dynamics. However, this still does not explain the differences in population growth among sites. Other factors not measured (e.g., recreational use, water quality) may be responsible for the spatial differences in P. antipodarum densities and population growth below the dam.
Our estimates of annual production are relatively low compared to those reported from other studies in the United States. In the Colorado River below Glen Canyon Dam, production of P. antipodarum ranged from 11.7 g AFDM m−2 yr−1 to 14.7 g AFDM m−2 yr−1 (Cross et al. 2010). These estimates are equivalent to 13.0 g dry mass m−2 yr−1 and 16.3 g dry mass m−2 yr−1, respectively, using a dry mass to AFDM conversion factor of 0.9 (Benke et al. 1999). Hall et al. (2006) reported extremely high production estimates (~ 194 g AFDM m−2 yr−1 or ~ 215 g dry mass m−2 yr−1) in Polecat Creek, Wyoming. Why our estimates of P. antipodarum production in the Green River are relatively low compared to these other locations is unclear. The rivers studied by Hall et al. (2006) were warm, spring fed rivers with stable hydrographs and high rates of primary productivity (Hall et al. 2003). These factors likely helped sustain such high rates of P. antipodarum production in those rivers (Hall et al. 2006). In its native range, densities of P. antipodarum rarely exceed > 4000 individuals m−2 (Collier et al. 1998; Hall et al. 2003) and are often < 1000 individuals m−2 (Holomuzki and Biggs 1999). Furthermore, rates of P. antipodarum production in rivers of the western United States are generally higher than those from the New Zealand mud snail’s native range (~ 0.5 g AFDM m−2 yr−1; Huryn 1996). Hall et al. (2006) attributed the lower densities and secondary production to the relatively low temperatures P. antipodarum experiences in its native streams (~ 6 °C), which may limit P. antipodarum growth. Indeed, temperature appears to regulate biomass turnover rates of P. antipodarum (see Cross et al. 2010, Fig. 5). Our estimates of production:biomass ratios (P:B) for P. antipodarum (5.0–10.0 yr−1) are higher than those from its native range (~ 3.0 yr−1; Huryn 1996) and from the Colorado River (2.6–3.4 yr−1; Cross et al. 2010). Although the Colorado River below Glen Canyon Dam and the Green River below Flaming Gorge Dam have similar mean annual temperatures (9–10 °C), maximum daily temperatures are higher in the Green River (~ 18 °C) than in the Colorado (~ 16 °C). These higher maximum daily temperatures may explain the higher biomass turnover rates in our study, especially since population growth rates of P. antipodarum are highest ~ 18 °C (Dybdahl and Kane 2005). Regardless of the mechanisms for its success, it is clear that P. antipodarum is a successful invader outside of its native range. More estimates of P. antipodarum production from western rivers and other rivers throughout the world should help explain what factors drive P. antipodarum growth and production.
To date, New Zealand mud snails have been found in the Green River from Flaming Gorge dam downstream to the Colorado state line (M. Vinson, unpublished data). Although densities in the Green River remain relatively low compared to other locations in the western United States, the sudden increase in densities after 2015 at three sites below Flaming Gorge Dam is alarming. Furthermore, questions remain concerning the effects of New Zealand mud snails on communities downstream of Flaming Gorge Dam. Given the high degree of dominance in the community, the relatively high rates of secondary production, and the recent increase in densities of P. antipodarum at sites below Flaming Gorge Dam, we recommend that attention be brought to the potential effects of New Zealand mud snails on the Green River food web with particular emphasis on impacts to trout fitness and production.
Availability of data and material
The datasets generated during this study are available from the corresponding author upon reasonable request and from the Knowledge Network for Biocomplexity (KNB) repository—urn:uuid:f0ccd7d7-05e0-4393–9478-d43b0d984a00.
Code availability
Not applicable.
References
Alonso A, Castro-Díez P (2008) What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614:107–116
Benke AC (1979) A modification to the Hynes method for estimating secondary production with particular significance for multivoltine populations. Limnol Oceanogr 24:168–174
Benke AC, Huryn AD (2017) Secondary production and quantitative food webs. In: Lamberti GA, Hauer FR (eds) Methods in stream ecology (Third Edition). Academic Press, San Diego, pp 235–254
Benke AC, Huryn AD, Smock LA, Wallace JB (1999) Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc 18:308–343
Benson AJ, Kipp RM, Larson J, Fusaro A (2020) Potamopyrgus antipodarum (J.E. Gray, 1853): U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL, https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=1008, Revision Date: 1/6/2020, Access Date: 10/27/2020.
Bruce RL, Moffitt CM, Dennis B (2009) Survival and passage of ingested New Zealand Mudsnails through the intestinal tract of Rainbow Trout. N Am J Aquacult 71:287–301
Bunn SE, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manage 30:492–507
Butkus R, Višinskienė G (2020) The aquatic invader Potamopyrgus antipodarum (Gray, 1843) is a poorer food item for benthivorous fishes than native snails. Aquat Invasions 15:473–481
Caton LW (1991) Improved subsampling methods for the EPA rapid bioassessment benthic protocols. Bull N Am Benthol Soc 8:317–319
Collado GA (2014) Out of New Zealand: molecular identification of the highly invasive freshwater mollusk Potamopyrgus antipodarum (Gray, 1843) in South America. Zool Stud 53:70
Collier KJ, Wilcock RJ, Meredith AS (1998) Influence of substrate type and physico-chemical conditions on macroinvertebrate faunas and biotic indices of some lowland, Waikato, New Zealand streams. New Zeal J Mar Fresh 32:1–19
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
Cross WF, Rosi-Marshall EJ, Behn KE, Kennedy TA, Hall RO Jr, Fuller AE, Baxter CV (2010) Invasion and production of New Zealand mud snails in the Colorado River, Glen Canyon. Biol Invasions 12:3033–3043
Dorgelo J (1987) Density fluctuations in populations (1982–1986) and biological observations of Potamopyrgus jenkinsi in two trophically differing lakes. Hydrol Bull 21:95–110
Dybdahl MF, Kane SL (2005) Adaptation vs. phenotypic plasticity in the success of a colonial invader. Ecology 86:1592–1601
Filbert RB, Hawkins CP (1995) Variation in condition of rainbow trout in relation to food, temperature, and individual length in the Green River, Utah. T Am Fish Soc 124:824–835
Gallardo B, Clavero M, Sánchez MI, Vilá M (2016) Global ecological impacts of invasive species in aquatic ecosystems. Glob Chang Biol 22:151–163
Greenwood DJ, Hall RO Jr, Tibbets TM, Krist AC (2020) A precipitous decline in an invasive snail population cannot be explained by a native predator. Biol Invasions 22:363–378
Gust M, Buronfosse T, André C, Mons R, Gagné F, Garric J (2011) Is exposure temperature a confounding factor for the assessment of reproductive parameters of New Zealand mudsnails Potamopyrgus antipodarum (Gray)? Aquat Toxicol 101:396–404
Hall RO Jr, Tank JL, Dybdahl MF (2003) Exotic snails dominate nitrogen and carbon cycling in a highly productive stream. Front Ecol Environ 1:407–411
Hall RO Jr, Dybdahl MF, VanderLoop MC (2006) Extremely high secondary production of introduced snails in rivers. Ecol Appl 16:1121–1131
Holomuzki JR, Biggs BJF (1999) Distributional response to flow disturbance by a stream-dwelling snail. Oikos 87:36–47
Holomuzki JR, Biggs BJF (2000) Taxon-specific responses to high-flow disturbances in streams: implications for population persistence. J N Am Benthol Soc 19:670–679
Holomuzki JR, Biggs BJF (2007) Physical microhabitat effects on 3-dimensioinal spatial variability of the hydrobiid snail, Potamopyrgus antipodarum. New Zeal J Mar Fresh 41:357–367
Huryn AD (1996) An appraisal of the Allen paradox in a New Zealand trout stream. Limnol Oceanogr 41:243–252
Kerans BL, Dybdhal MF, Gangloff MM, Jannot JE (2005) Potamopyrgus antipodarum distribution, abundance, and effects on native macroinvertebrates in the greater yellowstone ecosystem. J N Am Benthol Soc 24:123–138
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Loo SE, Mac Nally R, Lake PS (2007) Forecasting New Zealand Mudsnail invasion range: model comparisons using native and invaded ranges. Ecol Appl 17:181–189
McCarter NH (1986) Food and energy in the diet of brown and rainbow trout from Lake Benmore, New Zealand. New Zeal J Mar Fresh 11:357–373
McKenzie VJ, Hall WE, Guralnick RP (2013) New Zealand mudsnails (Potamopyrgus antipodarum) in Boulder Creek, Colorado: environmental factors associated with fecundity of a parthenogenic invader. Can J Zool 91:30–36
Moore JW, Herbst DB, Heady WN, Carlson SM (2012) Stream community and ecosystem responses to the boom and bust of an invading snail. Biol Invasions 14:2435–2446
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ribi G (1986) Within-lake dispersal of the prosobranch snails, Viviparus ater and Potamopyrgus jenkinsi. Oecologia 69:60–63
Riley LA, Dybdahl MF, Hall RO Jr (2008) Invasive species impact: asymmetric interactions between invasive and endemic freshwater snails. J North Am Benthol Soc 27:509–520
Ruhi A, Catford JA, Cross WF, Escoriza D, Olden JD (2019) Understanding the nexus between hydrological alteration and biological invasions. In: Sabater S, Elosegi A, Ludwig R (eds) Multiple stressors in river ecosystems. Elsevier, Cambridge, pp 45–64
Schisler GJ, Vieira NKM, Walker PG (2008) Application of household disinfectants to control New Zealand Mudsnails. N Am J Fish Manage 28:1172–1176
Schreiber ESG, Quinn GP, Lake PS (2003) Distribution of an alien aquatic snail in relation to flow variability, human activities, and water quality. Freshw Biol 48:951–961
Simberloff D, Gibbons L (2004) Now you see them, now you don’t! – population crashed of established introduced species. Biol Invasions 6:161–172
Strayer DL, D’Antonio CM, Essl F, Fowler MS, Geist J, Hilt S, Jöhnk IJK, Jones CG, Lambin X, Latzka AW, Pergl J, Pyšek RP, von Schmalensee M, Stefansson RA, Wright J, Jeschke JM (2017) Boom-bust dynamics in biological invasions: towards an improved application of the concept. Ecol Lett 20:1337–1350
Towers DJ, Henderson IM, Veltman CJ (1994) Predicting dry weight of New Zealand aquatic macroinvertebrates from linear dimensions. New Zeal J Mar Fresh 28:159–166
Vinson MR (2001) Long-term dynamics of an invertebrate assemblage downstream from a large dam. Ecol Appl 11:711–730
Vinson MR, Baker MA (2008) Poor growth of rainbow trout fed New Zealand mud snails, Potamopyrgus antipodarum. N Am J Fish Manage 28:701–709
Ward JV, Stanford JA (1983) The serial discontinuity concept of lotic ecosystems. In: Fontaine TD, Bartell SM (eds) Dynamics of lotic ecosystems. Ann Arbor Scientific Publishers, Ann Arbor, pp 29–42
Winterbourn M (1970) The New Zealand species of Potamopyrgus (Gastropoda: Hydrobiidae). Malacologia 10:283–321
Acknowledgements
We thank the taxonomists of the National Aquatic Monitoring Center and the numerous field technicians responsible for data collection. We also thank David Strayer and two anonymous reviewers for their comments and suggestions that greatly improved the manuscript.
Funding
Funding was provided by the Western Area Power Administration and Argonne National Laboratory.
Author information
Authors and Affiliations
Contributions
DN and TWA conceived the idea for the study. DN analyzed the data and led the writing of the manuscript. All authors contributed critically to drafts and gave approval for publication.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nelson, D., Armstrong, T.W. Invasion of the New Zealand mud snail (Potamopyrgus antipodarum) in the Green River below Flaming Gorge Dam. Biol Invasions 24, 775–785 (2022). https://doi.org/10.1007/s10530-021-02693-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02693-3