Abstract
Long-term phytoplankton dynamics in a floodplain lake, between periods of limnophase and potamophase was studied using the morphology-based functional classification (MBFG). The work was carried out to test the hypothesis that the temporal distribution of MBFGs is influenced by the hydrosedimentological regime of the Paraná River, and that these differences can be registered by analyzing the dominant MBFGs in the two periods. Samples were taken in an isolated floodplain lake on the Upper Paraná River floodplain, Brazil, from 2000 to 2012, and water level, water temperature, conductivity, pH, dissolved-oxygen, euphotic zone, maximum depth, and nutrients were used to explain the distribution of MBFGs. 478 taxa were identified and distributed in seven MBFGs (I–VII). MBFG V (flagellates algae) and VI (diatoms) showed 100% frequency of occurrence. MBFG II was associated exclusively with the limnophase; MBFG IV and VII were associated with limnophase periods with higher pH and dissolved-oxygen content; and MBFG I, III, V, and VI were associated with limnophase and potamophase, mainly associated with transparency, nutrients, and conductivity. The MBFG approach represented the trends of each group in terms of its occurrence and biovolume, according to the hydrosedimentological regime, providing broad-scale information on changes in the phytoplankton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton is a highly diverse ecological group, encompassing hundreds of species of algae and Cyanobacteria with different morpho-functional strategies. The co-occurrence of these species depends largely on differences in biological processes such as growth, acquiring resources (light and nutrients), ability to inhibit loss processes (predation and sedimentation), regulation of buoyancy, and vertical migration (Mihaljević et al., 2015). These organisms may show a highly variable morphology, according to the environmental variability and ecosystem characteristics (Naselli-Flores et al., 2007).
The need for understanding the relationships between structural and functional properties of ecosystems has led to the development of various classifications of the aquatic biota, based on structural, taxonomic and functional characteristics, to help explain the organization of ecological communities by simplifying the complexity of real ecosystems (Reynolds et al., 2002; Weithoff, 2003; Salmaso & Padisák, 2007; Padisák et al., 2009; Salmaso et al., 2015). Among the classifications that are currently applied in phytoplankton studies is the morphology-based functional classification (MBFG) proposed by Kruk et al. (2010), which considers the maximum linear dimension (MLD), volume (V), rate surface:volume (S/V), and the presence of flagella, siliceous structures, mucilage, and aerotopes.
In this classification system, the authors summarized the functional characteristics and the variability of the species in seven groups (MBFG I–VII), which reflect their different abilities to absorb light and nutrients, growth, and mechanisms to prevent sedimentation and predation (Pacheco et al., 2010). The formation of different groups facilitates predictions of community composition and relationships to particular environmental conditions (Kruk et al., 2011; Kruk & Segura, 2012).
The approach based on functional traits allows wider generalizations than the traditional phylogeny-based approaches, and has stimulated interest in classifying species into groups that are directly related to ecosystem functions (Litchman & Klausmeier, 2008; Brasil & Huszar, 2011) as well as many groups that comprise species with different structural and functional properties (Žutinić et al., 2014). Knowing of the factors that regulate the dynamics of phytoplankton is a prerequisite to understanding how the traits of the species influence their responses to the environment (Jamil et al., 2014).
In a floodplain system, the main hydrological and ecological features are dependent on the flood pulse. Maintaining connectivity resulting from natural fluctuations in water levels is critical to the integrity, functioning, and maintenance of the dynamic characteristics of the aquatic communities in floodplain lakes (Leira & Cantonati, 2008; Bovo-Scomparin et al., 2013) and the changes in connectivity may influence the distribution of morpho-functional strategies of planktonic algae in these environments. Although most rivers of South America are modified hydro-morphologically (Pelicice et al., 2014), there are long sections of rivers in the lowland systems that appear to be close to their natural state, such as the Upper Paraná River floodplain that is the last remaining undammed section of the Paraná River in Brazil, containing a variety of habitats, among which many lakes, supporting a high diversity of organisms (Agostinho et al., 2004). Thus, many floodplain lakes can significantly contribute to inoculation of the rivers’ phytoplankton by euplanktic species. In South America, the studies in floodplain lakes emphasize that the composition and dynamic phytoplankton are explained by external (hydrological) and internal (environmental filters) factors (De Emiliani, 1997; Huszar & Reynolds, 1997; Ibanez, 1998; Izaguirre et al., 2004; Bovo-Scomparin & Train, 2008; O’Farrell et al., 2011; Bortolini et al., 2014a; Granado & Henry, 2014; Pinto et al., 2014).
Although several studies have used the approach of MBFGs in different environments (Pacheco et al., 2010; Izaguirre et al., 2012; Hu et al., 2013; Segura et al., 2013; Mihaljević et al., 2013; Abonyi et al., 2014; Mihaljević et al., 2015), for Brazilian floodplains only the study of Bortolini et al. (2014b) has addressed the phytoplankton variability and the application of this classification, in a side channel of the Upper Paraná River.
Since planktonic algae have well defined traits that determine their niche, the study of phytoplankton communities would be an ideal approach to answer questions raised in trait-based ecology (Litchman & Klausmeier, 2008). Using this approach, we describe and compare the phytoplankton dynamics between periods of limnophase and potamophase in a subtropical floodplain lake, on a long-term scale, using the morpho-functional classification (MBFG). We hypothesized that the temporal distribution of MBFGs in the lake would be influenced by the hydrosedimentological regime of the Paraná River, and that the dominant MBFGs would differ between the two distinct periods.
Materials and methods
Study area
The Upper Paraná River includes the first third of the Paraná River basin, the second-longest river in South America, and the tenth largest in the world in terms of discharge. The Upper Paraná lies almost entirely within Brazil, except for a stretch in the Itaipu Reservoir on the border with Paraguay (Agostinho et al., 2008). The river width:depth ratio is 100:1 and the flow velocity is approximately 0.56 m s−1. The Upper Paraná River floodplain includes the segment between the Porto Primavera Hydroelectric Plant (Primavera, state of São Paulo) and the reservoir above the dam of the Itaipu Hydroelectric Plant (Guaíra, state of Paraná), forming a complex mosaic of wetlands. The floodplain is about 230 km long and 20 km wide (Souza Filho & Stevaux, 2004).
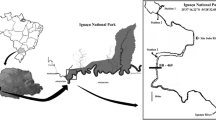
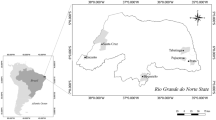
Osmar Lake (Fig. 1) is an isolated floodplain lake, located about 120 m from the Paraná River, in an area with dense riparian forest and sandy soil. During the limnophase period, the lake is approximately 60 m long and 15 m wide, on average; in potamophase periods the lake may cover an area of 0.15 ha, with a mean depth of 1.1 m. The connection between the lake and the main river channel forms only during the potamophase, when the river rises above the 3.5 m level (Souza Filho et al., 2004). In this lake, it was identified by Mormul et al. (2012) the possible occurrence of three water states, being the turbid state when the lake was at its shallowest phase and was characterized by high biomass of benthic fish and high values of total phosphorus (TP), turbidity and with high chlorophyll-a, and second state being macrophyte-dominated (Egeria densa Planc) occurred at the high water phase and exhibited the lowest values of TP, turbidity and chlorophyll-a. The third state would be a transient state that was observed during periods of intermediate water levels. The transient state was characterized by absence of submerged macrophytes, low benthic fish biomass, intermediate values of TP, turbidity and chlorophyll-a relative to the other two states, as well as high zooplankton densities, primarily small-sized filter-feeders.
Methods
The samples of phytoplankton were collected quarterly (except in 2000 and 2003 when only two samples were taken in each year and in 2001 when only one sample was taken) during the years 2000–2012. All sampling was conducted as part of the multidisciplinary research project “The Upper Paraná River floodplain”—Long Term Ecological Research Program (PELD), funded by MCT/CNPq. Phytoplankton samples were taken just below the water surface (depth 20 cm) in the pelagic zone of Osmar lake, being one sample collected at sampling date. The samples were collected directly in bottles and preserved with 1% Lugol solution. Counted fields were selected randomly, in an inverted microscope, according to Utermöhl (1958) and Lund et al. (1958). The phytoplankton biovolume was calculated by multiplying the density of each taxon by its respective volume. The cell volume was calculated from geometric models, according to the shape of the cells (Sun & Liu, 2003), being the encountered species identified and measured, on average thirty organisms by species.
The phytoplankton organisms were grouped in accordance with the seven MBFGs described by Kruk et al. (2010): The MBFG I includes small organisms with high s/v; MBFG II includes small flagellates organism with siliceous exoskeletal structures; MBFG III includes large filaments with aerotopes; MBFG IV includes organisms of medium size lacking specialized traits; MBFG V includes unicellular flagellates of medium to large size; MBFG VI includes non-flagellated organisms with siliceous exoskeletons; MBFG VII includes large mucilaginous colonies. The frequency of occurrence of the different groups was calculated according to Dajoz (2005), being constants groups those present in more than 50% of the samples, common groups those present between 25 and 50% of the samples and rare groups those present in less than 25% of the samples.
The data for water temperature (WT, °C), pH, electrical conductivity (Cond, μS cm−1), and dissolved oxygen (DO, mg L−1) were obtained in situ using Digimed portable digital potentiometers. Water transparency (m) was obtained using a Secchi disk, and the euphotic zone (Z eu, m) was calculated as 2.7 times the depth of the Secchi disk (Cole, 1994). The maximum depth (Z max) was obtained in each habitat. The light availability in the lake was obtained from the Z eu/Z max ratio. Levels of soluble reactive phosphorus (SRP, µg L−1; Golterman et al., 1978), nitrate (NO3−, µg L−1; Giné et al., 1980), nitrite (NO2−, µg L−1; Giné et al., 1980) and ammonium (NH4+, µg L−1; Koroleff, 1978) were determined. The dissolved inorganic nitrogen (DIN) content was determined from the NO3−, NO2−, and NH4+ concentrations.
Daily water level (WL) data for the Paraná River were obtained from Itaipu Binational (Itaipu Binacional), the National Water Agency (Agência Nacional das Águas—ANA) and the Ichthyology, Limnology and Aquaculture Research Center (Núcleo de Pesquisas em Ictiologia, Limnologia e Aquicultura—Nupélia). Potamophase was considered when the water level of the Paraná River was above 3.5 m, indicating the start of the flood period; while limnophase was considered when the water level was less than 3.5 m (Souza Filho et al., 2004). The amplitude of the limnophase (number of days when the water level was below the 3.5 m reference level), amplitude of the potamophase (number of days when the water level was above the reference level), and the connectivity index (ratio of the number of days of potamophase to days of limnophase) were calculated using the PULSE program (Neiff & Neiff, 2003).
A principal component analysis (PCA) was performed to reduce the number of predictor variables. Analysis of variance (two-way ANOVA) was performed to test significant differences between the scores of the first two PCA axes, between periods (limnophase and potamophase) and among years (2000–2012). Analysis of variance was also used to identify significant differences in mean values of biovolume of each MBFG between periods and among years. To investigate the relationship of MBFGs to environmental variability, we performed a redundancy analysis (RDA) using the biovolume of MBFGs as the response variable and environmental variables as the explanatory variables. The existence of multicollinearity between the explanatory variables was examined by the variance inflation factor (VIF). PCA was performed using the program PC-Ord 6.0 (McCune & Mefford, 1999), Anova by the Statistica program (StatSoft Inc., 2005), and RDA through the R program (R Development Core Team, 2014).
Results
Environmental variability
Different flood patterns, defined according to the water levels of the Paraná River, were observed on the annual scale (CV = 28.7%; p = 0.0001). The largest number of days of limnophase (355 and 362 days, respectively) occurred in the years 2000 and 2001, when the lake had no connectivity with the main channel during almost the entire period. From 2005 onward, the number of days of limnophase decreased, and the connectivity between the lake and the main channel increased and the largest number of days of potamophase and highest connectivity indexes were observed in 2009, 2010, and 2011 (Table 1).
Nutrient concentrations showed high temporal variability (DIN: 141–74% and SRP: 141–38%). Z eu and Z max showed the highest variability in years with a potamophase. According to the Z eu/Z max ratio, the water column had light continually available in the majority of sampling periods. The mean water temperature was highest in 2001, during a period of extreme drought in the region. Mean dissolved-oxygen concentrations in the subsurface water were lowest in 2012. The mean pH was around 6. The highest mean value of electrical conductivity was recorded in 2010. Means and variation coefficients of abiotic parameters in the lake are shown in Table 2.
The PCA explained 50% of the environmental variability, distinguishing the limnophase and potamophase periods. On axis 1, Z max (0.92), Z eu (0.88), and WL (0.80) positively correlated, while the Z eu/Z max ratio (−0.22) negatively. On axis 2, WT (0.54) and SRP (0.48) positively correlated, while DO (−0.73) and DIN (−0.56) negatively (Fig. 2). The ANOVA identified significant differences for the scores of PCA axis 1 among years (F = 4.51; p = 0.002) and between periods versus years (F = 3.96; p = 0.005).
Dispersion of month-year scores along the first two axes of the principal components analysis, using the abiotic variables in Osmar Lake during 2000–2012 (WL, water level; Z eu, euphotic zone; Z max, maximum depth; Z eu/Z max, Z eu/Z max ratio; DO, dissolved oxygen; DIN, dissolved inorganic nitrogen; WT, water temperature; SRP, soluble reactive phosphorus)
Phytoplankton community and morphologically based functional groups (MBFG)
Of the 478 taxa identified, Chlorophyceae (146), Euglenophyceae (87), and Zygnematophyceae (71) were the most species-rich groups. Seven MBFGs were represented. MBFG V, represented by unicellular flagellates—Volvocales, euglenophyceans, cryptophyceans, dinophyceans, and raphidophyceans, and MBFG VI, represented by diatoms, showed 100% frequency of occurrence. MBFG I, represented by small organisms with high s/v—small cyanobacteria (71%), MBFG II, represented by small flagellated organisms with siliceous exoeskeletal structures—chrysophyceans (87%), MBFG IV, represented by organisms of medium size lacking specialized traits—chlorophyceans, zygnematophyceans, Oscillatoriales, and xanthophyceans (95%), and VII, represented by large mucilaginous colonies—cyanobacteria e chlorophyceans with large dimensions (84%) were also considered constant. Only MBFG III, represented by large filaments with aerotopes—heterocytic cyanobacteria (48%), was found to be common. MBFGs II, IV, V, VI, and VII were important for phytoplankton biovolume mainly in the limnophase period. MBFGs III, IV, V, and VI also made important contributions to the biovolume during potamophase periods (Table 3).
The highest mean values of phytoplankton biovolume were recorded in 2007 (Fig. 3a). Significant temporal differences between the values of biovolume of the MBFGs (Fig. 3b–h) were observed only for MBFG V (Period—F = 23.52; p = 0.0000 and Period versus Year—F = 2.79; p = 0.0283).
MBFG VII, represented mainly by Sphaerocystis planctonica (Korshikov) Bourrelly, was most dominant during the limnophase period, in December 2007, when it contributed over 90% of the phytoplankton biovolume. However, from 2008 onward, this group declined in biovolume. MBFG VI, represented by Aulacoseira herzogii (Lemmermann) Simonsen, contributed more than 50% of the total biovolume in March 2005 and June 2007, and was represented by Ulnaria ulna (Nitzsch) P. Compère in June 2010. MBFG V contributed significantly to the biovolume in both the limnophase and potamophase. MBFG IV had its greatest contribution in December 2008, with the dominance of Scenedesmus indicus Philipose, which at that time comprised 82% of the total biovolume. MBFG III, represented by Dolichospermum planctonicum (Brunnthaler) Wacklin, L. Hoffmann & Komárek, contributed significantly to the biovolume in February 2000, June 2006, and December 2009. MBFG II, represented by chrysophyceans, showed a significant contribution to biovolume only in limnophase periods, especially in June 2004, September 2005, December 2006, September 2007, June 2009, and September 2011. MBFG I, represented by Synechococcus sp., contributed significantly to the relative biovolume only in September 2004, during limnophase. Overall, MBFGs V and VI were the main contributors in terms of biovolume (Fig. 4).
The RDA showed the variability of the biovolume of MBFGs during the 12 years of the study (axis 1 = 0.05; axis 2 = 0.01, p < 0.05) and explained 25% of the data variance. MBFG I, with its only significant contribution in September 2004, was directly correlated with DIN. MBFG II, was correlated only with the limnophase period, distributed in the upper-left portion of the diagram, due to the high contribution of this group in the driest months. MBFG III and MBFG V, associated with both the limnophase and potamophase, were related mainly to the distribution of light and nutrients in the lake, especially SRP. MBFGs I, III, and V were also associated with the water level. MBFGs IV, VI, and VII recorded in both hydrological periods were correlated in the RDA with pH and dissolved oxygen (Fig. 5).
Diagram of the first and second RDA axes based on the biomass of MBFGs, including all samples from 2000 through 2012, and environmental variables (WL, water level; Z eu, euphotic zone; Z max, maximum depth; Z eu/Z max, Z eu/Z max ratio; DO, dissolved oxygen; DIN, dissolved inorganic nitrogen; WT, water temperature; SRP, soluble reactive phosphorus; Cond, electrical conductivity; I, II, III, IV, V, VI, VII, MBFGs I–VII; triangles indicate environmental variables; stars indicate MBFGs; full circles indicate potamophase; empty circles indicate limnophase)
Discussion
Knowledge of the morphological traits of phytoplankton can assist in predicting the niche of a particular species and comparing its behavior along an environmental gradient (Kruk & Segura, 2012). In Osmar Lake, the MBFGs were defined according to the environmental variability resulting from conditions imposed by the hydrosedimentological regime of Paraná River, which influenced the environmental conditions in the lake and consequently the distribution of these groups throughout the study period.
The low level of connectivity, the shallow depth of the lake, and the light availability (high Z eu/Z max ratio), as well as the availability of nutrients, influenced the occurrence of all MBFGs. However, the constancy of MBFGs V and VI is probably due to their ecophysiological characteristics as well as to the lake dynamics. Shallow lakes alternate constantly between clear and turbid states (Scheffer, 1998), as recorded by Mormul et al. (2012) for Osmar Lake. This alternation may have favored the alternating dominance between the MBFGs.
Members of MBFG V, including most unicellular flagellated species, which had intermediate values for most attributes and low maximum growth rates, have wide physiological plasticity, motility, and tolerance to light limitation and disturbance (Kruk & Segura, 2012). The presence of flagella helps algae to remain in the water column, independently of the hydrological period, being this group already registered in a shallow lake with alternative steady states (Izaguirre et al., 2012). MBFG VI, represented by diatoms, non-flagellated organisms with siliceous exoskeletons, typically shows high sinking rates, and often maintains a high biomass. The high proportions of diatoms may be related to the extensive interaction between the pelagic and littoral region of the lake, due to its small dimension, especially in limnophase, which favored the occurrence of both meroplanktonic and periphytic diatoms. However, this fact may hinder the evaluation of the results obtained for MBFG VI. The MBFG VI has been registered in turbid shallow lakes (Izaguirre et al., 2012) and associated with high concentration of nitrates (Mihaljević et al., 2015).
Morpho-functional traits related to loss from sedimentation, such as the presence of mucilage, large dimensions and a low surface:volume ratio probably favored the dominance of MBFG VII in most limnophase periods. In particular, the dominance of S. planctonica in December 2007 was related mainly to high availability of light and nutrients, especially SRP. However, according to Pacheco et al. (2010), MBFG VII includes species that are typical of environments both rich and poor nutritionally, and therefore the potential usefulness of this MBFG to classify lake conditions is limited and requires further evaluation. This group can show relatively low sensitivity to detect certain phytoplankton functional aspects that may be relevant.
The dominance of MBFG II, represented in this study mainly by Dinobryon spp. and Mallomonas spp. exclusively in the limnophase, in conditions of high transparency, lower concentrations of SRP and DIN, and temperatures around 22°C, may be associated with their advantage in oligotrophic to mesotrophic conditions, in transparent, neutral to slightly alkaline water (Kruk & Segura, 2012). Dinobryon species, which can form large colonies, are tolerant to low temperatures and can resort to mixotrophy to obtain nutrients from bacteria in oligotrophic conditions, which suggests that it is favored by decreases in nutrients (Kamjunke et al., 2007). Mallomonas spp. are single-celled flagellates, covered externally with a highly organized layer of flat, siliceous structures (Jo et al., 2011). They are common in freshwaters, particularly in oligotrophic waters with low alkalinity (Siver et al., 2013). The same pattern of occurrence of MBFG II was registered by Pacheco et al. (2010) in oligotrophic lakes Clotilde and García in the southern coast of Uruguay, besides that Mihaljević et al. (2015) recorded high contribution (>70%) of MBFG II (represented by Chrysococcus and Dinobryon spp.) to the total biomass in Lake Sakadas.
The dominance of MBFG III, represented by k-strategist heterocytic cyanobacteria, in 2000, 2006, and 2009, years with low connectivity, occurred in eutrophic conditions with a more-stable water column as recorded by Izaguirre et al. (2012). The absence of a potamophase in 2000 was attributed to a synergism between the La Niña climate phenomenon (McPhaden et al., 2006; CPTEC, 2012) and the formation of the Porto Primavera reservoir, the last reservoir in the cascade upstream of the Upper Paraná River floodplain (Rodrigues et al., 2009; 2015; Bovo-Scomparin et al., 2013). In 2006 and 2009, there was a low-amplitude potamophase and therefore the Paraná River had less influence on the lake. The presence of traits, such as larger MLD, high mean biomass, low maximum growth rate, low sinking velocities, aerotopes, as well as tolerance to light limitation (Kruk & Segura, 2012) were probably decisive for the success of MBFG III under these conditions.
MBFG IV, which includes medium-sized to large single-celled organisms, colonial and filamentous taxa without aerotopes, flagella, heterocysts, mucilage and siliceous exoskeletal structures (Kruk et al., 2010), was represented mainly by chlorophyceans and zygnematophyceans. MBFG IV is widely distributed and recorded in lakes with different environmental conditions (Izaguirre et al., 2012). This fact is probably due to the grouping of phytoplankton groups, such as chlorophyceans and zygnematophyceans, which exhibit high number of species. The colonial Chlorococcales S. indicus was the dominant taxon in December 2008, during the limnophase period, with mesotrophic conditions, slightly acid pH, and high water transparency.
The high relative contribution of MBFG I (mainly Synechococcus sp.) in September 2004, during the limnophase period, occurred during periods of high nutrient concentrations, particularly DIN (>140 μg L−1). According to Kruk & Segura (2012), these r-selected organisms have effective resource acquisition and high specific growth rates in saturated and limited-resource environments. The small body size of members of this group may have contributed to their low representation in biomass in most sampling periods, distinguishing this group from others, as verified by Mihaljević et al. (2015) along a river–floodplain gradient.
The temporal variations in the availability of resources resulted in changes in the temporal distribution and alternating dominance of different MBFGs, representing changes in the composition and phytoplankton community structure in this floodplain lake, which is subject to disturbances such as flooding. Competitive abilities were likely important for the distribution of the different MBFGs, as also observed by Pacheco et al. (2010).
The functional classification based on morphology is useful to define and understand the dynamics of groups of phytoplankton species in relation to environmental variation. As Kruk et al. (2010) noted, clearly, many more adaptive traits could also be important to ecological success than those tested in their study. Nonetheless, studies using this classification suggest that MBFGs are indeed suitable and might represent ecological functioning in a broader sense (Salmaso et al., 2015). The community characteristics are best understood if grouped species have similar traits or behave similarly (Salmaso & Padisák, 2007).
In the classification based on morphology, the presence of similar structures, such as sizes and shapes in phylogenetically distantly related species, can be interpreted as a set of common analogous traits under strong natural selection. Although morphology and structure have implicitly functional roles, physiological complexities are not taken into account and characters such as pigment composition and photosynthetic efficiency which are vital characteristics cannot be predicted and modeled by size and shape (Salmaso et al., 2015). However, according Izaguirre et al. (2012) the classification based on purely morphological traits, can offer advantages, among them, its objectivity and the relative ease of its application to the majority of species for which physiological traits are unknown and are not readily determined. The identification of common traits under a range of environmental conditions can improve our ability to generalize results, finding common patterns useful also in predicting shifts in the phytoplankton.
The classification based not only on the functional characteristics of individual species, but also on the range of conditions within which the species occur together proposed by Reynolds et al. (2002) and revised by Padisák et al. (2009) are now often used in ecological studies of phytoplankton. However, new categorizations should be tested so that we can evaluate their applicability to the study of the phytoplankton community. Nevertheless, functional groups are not meant to be a substitute for the whole extent of information that can be gathered from species. The knowledge of which species dominate a functional group is of primary importance and is essential in addressing particular ecological questions or environmental issues (Padisák et al., 2009; Salmaso et al., 2015).
Our study is one of the first descriptions of phytoplankton variability in floodplain lakes in Brazil that uses the functional classification based on morphology. Although simple, the MBFG approach well represented the biovolume trends of each group and its occurrence, as well as the functioning trends in this shallow lake, according to environmental variability. The environmental factors were influenced by the hydrosedimentological regime of the Paraná River, influencing the distribution of MBFGs. In this case, the model provided useful information on the phytoplankton variability on a large time scale.
References
Abonyi, A., M. Leitão, I. Stankovi, G. Borics, G. Várbíró & J. Padisák, 2014. A large river (River Loire, France) survey to compare phytoplankton functional approaches: do they display river zones in similar ways? Ecological Indicators 46: 11–22.
Agostinho, A. A., L. Rodrigues, L. C. Gomes, S. M. Thomaz & L. E. Miranda, 2004. Structure and Functioning of the Paraná River and its Floodplain. Eduem, Maringá.
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68(4, Suppl.): 1119–1132.
Bortolini, J. C., V. M. Bovo-Scomparin, A. C. M. de Paula, G. A. Moresco, L. M. Reis, S. Jati & L. C. Rodrigues, 2014a. Composition and species richness phytoplankton in a subtropical floodplain lake: a long-term study. Acta Limnologica Brasiliensia 26(3): 296–305.
Bortolini, J. C., L. C. Rodrigues & S. Train, 2014b. Phytoplankton functional and morphological groups as indicators of environmental variability in a lateral channel of the Upper Paraná River floodplain. Acta Limnologica Brasiliensia 26(1): 98–108.
Bovo-Scomparin, V. M. & S. Train, 2008. Long-Term variability of the phytoplankton community in an isolated floodplain lake of the Ivinhema River State Park, Brazil. Hydrobiologia 610: 331–344.
Bovo-Scomparin, V. M., S. Train & L. C. Rodrigues, 2013. Influence of reservoirs to dispersion and seasonal variation of the phytoplankton community in the Upper Paraná River, Brazil. Hydrobiologia 702: 115–127.
Brasil, J. & V. L. M. Huszar, 2011. O papel dos traços funcionais na ecologia do fitoplâncton continental. Oecologia Australis 15(4): 799–834.
CPTEC, 2012. Centro de Previsão do tempo e estudos climáticos. Available at: http://www.cptec.inpe.br/.
Dajoz, R., 2005. Princípios de Ecologia. Artmed, Porto Alegre.
De Emiliani, M. O. G., 1997. Effects of water level fluctuations on phytoplankton in a river-floodplain lake system (Paraná River, Argentina). Hydrobiologia 357: 1–15.
Cole, G. A., 1994. Textbook of Limnology. Waveland Press Inc, Long Grove.
Giné, M. F., H. Bergamim, E. A. G. Zagatto & B. F. Reis, 1980. Simultaneus determination of nitrite and nitrate by flow injection analysis. Analytica Chimica Acta 114: 191–197.
Golterman, H. L., R. S. Clymo & M. A. Ohstad, 1978. Methods for Physical and Chemical Analysis of Freshwater. Blackwell Scientific Publication, Oxford.
Granado, D. C. & R. Henry, 2014. Phytoplankton community response to hydrological variations in oxbow lakes with different levels of connection to a tropical river. Hydrobiologia 721: 223–238.
Hu, R., B. Han & L. Naselli-Flores, 2013. Comparing biological classifications of freshwater phytoplankton: a case study from South China. Hydrobiologia 701: 219–233.
Huszar, V. L. M. & C. S. Reynolds, 1997. Phytoplankton periodicity and sequences of dominance in an Amazonian flood-plain lake (Lago Batata, Pará, Brazil): responses to gradual environmental change. Hydrobiologia 346: 169–181.
Ibanez, M. S. R., 1998. Phytoplankton composition and abundance of a central Amazonian floodplain lake. Hydrobiologia 362: 79–83.
Izaguirre, I., I. O´Farrell, F. Unrein, R. Sinistro, M. S. Afonso & G. Tell, 2004. Algal assemblages across a wetland, from a shallow lake to relictual oxbow lakes (Lower Paraná River, South America). Hydrobiologia 511: 25–36.
Izaguirre, I., L. Allende, R. Escaray, J. Bustingorry, G. Pérez & G. Tell, 2012. Comparison of morpho-functional phytoplankton classifications in human-impacted shallow lakes with different stable states. Hydrobiologia 698: 203–216.
Koroleff, K., 1978. Determination of ammonia. In Grasshoff, K. & K. Kremling (eds), Methods of Seawater Analysis. Verlag Chemie, Winhein.
Jamil, T., C. Kruk & C. J. F. ter Braak, 2014. A unimodal species response model relating traits to environment with application to phytoplankton communities. PLOS One 9(5): 1–14.
Jo, B. Y., W. Shin & S. M. Boo, 2011. Studies on ultrastructure and three-gene phylogeny of the genus Mallomonas (Synurophyceae). Journal of Phycology 47: 415–425.
Kamjunke, N., T. Henrichs & U. Gaedke, 2007. Phosphorus gain by bacterivory promotes the mixotrophic flagellate Dinobryon spp. during re-oligotrophication. Journal of Plankton Research 29(1): 39–46.
Kruk, C. & A. M. Segura, 2012. The habitat template of phytoplankton morphology-based functional groups. Hydrobiologia 698: 191–202.
Kruk, C., V. L. M. Huszar, E. H. M. Peeters, S. Bonilla, L. Costa, M. Lurling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Kruk, C., E. H. M. Peeters, E. H. Van Nes, V. L. M. Huszar, L. S. Costa & M. Scheffer, 2011. Phytoplankton community composition can be predicted best in terms of morphological groups. Limnology and Oceanography 56(1): 110–118.
Leira, M. & M. Cantonati, 2008. Effects of water-level fluctuations on lakes: an annotated bibliography. Hydrobiologia 613: 171–184.
Litchman, E. & C. A. Klausmeier, 2008. Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution and Systematics 39: 615–639.
Lund, J. W. G., C. Kipling & E. D. Lecren, 1958. The inverted microscope method of estimating algal number and the statistical basis of estimating by couting. Hydrobiologia 11: 980–985.
McCune, B. & M. J. Mefford, 1999. PC-ORD. Multivariate analysis of ecological data, version 4.0. MjM Software Design, Gleneden Blach, Oregon. Hydrobiologia 11: 980–985.
McPhaden, M. J., S. E. Zebiak & M. H. Glantz, 2006. ENSO as an Integrating concept in earth science. Science 314: 1740–1745.
Mihaljević, M., D. Špoljarić, F. Stević & T. Pfeiffer, 2013. Assessment of flood-induced changes of phytoplankton along a river–floodplain system using the morpho-functional approach. Environmental Monitoring and Assessment 185: 8601–8619.
Mihaljević, M., F. Stević, D. Špoljarić & T. Žuna Pfeiffer, 2015. Spatial pattern of phytoplankton based on the Morphology-Based Functional approach along a river–floodplain gradient. River Research and Applications 31: 228–238.
Mormul, R. P., S. M. Thomaz, A. A. Agostinho, C. C. Bonecker & N. Mazzeo, 2012. Migratory benthic fishes may induce regime shifts in a tropical floodplain pond. Freshwater Biology 57: 1592–1602.
Naselli-Flores, L., J. Padisák & M. Albay, 2007. Shape and size in phytoplankton ecology: do they matter? Hydrobiologia 578: 157–161.
Neiff, J. J. & M. Neiff, 2003. PULSO: software para análisis de fenómenos recurrentes. Available at: http://www.neiff.com.ar.
O’Farrell, I., I. Izaguirre, G. Chaparro, F. Unrein, R. Sinistro, H. Pizarro, P. Rodríguez, P. T. Pinto, R. Lombardo & G. Tell, 2011. Water level as the main driver of the alternation between a free-floating plant and a phytoplankton dominated state: a long-term study in a floodplain lake. Aquatic Science 73: 275–287.
Pacheco, J. P., C. Iglesias, M. Meerhoff, C. Fosalba, G. Goyenola, F. Teixeira de Mello, S. García, M. Gelós & F. García-Rodrígues, 2010. Phytoplankton community structure in five subtropical shallow lakes with different trophic status (Uruguay): a morphology-based approach. Hydrobiologia 646: 187–197.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and missue in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Pelicice, F. M., P. S. Pompeu & A. A. Agostinho, 2014. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries. doi:10.1111/faf.12089.
Pinto, P. T., R. Lombardo, I. O´Farrell & I. Izaguire, 2014. Drivers shaping phytoplankton diversity and composition in a humid Pampean floodplain lake (natural reserve). Hydrobiologia. doi:10.1007/s10750-014-2008-0.
R Development Core Team, 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
Reynolds, C. S., V. L. M. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rodrigues, L. C., S. Train, V. M. Bovo-Scomparin, S. Jati, C. C. J. Borsalli & E. Marengoni, 2009. Interannual variability of phytoplankton in the main rivers of the Upper Paraná River floodplain, Brazil: influence of upstream reservoirs. Brazilian Journal of Biology 69: 501–516.
Rodrigues, L. C., N. R. Simões, V. M. Bovo-Scomparin, S. Jati, N. F. Santana, M. C. Roberto & S. Train, 2015. Phytoplankton alpha diversity as an indicator of environmental changes in a neotropical floodplain. Ecological Indicators 48: 334–341.
Salmaso, N. & J. Padisák, 2007. Morpho-Functional Groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112.
Salmaso, N., L. Naselli-Flores & J. Padisák, 2015. Functional classifications and their application in phytoplankton ecology. Freshwater Biology 60: 603–619.
Scheffer, M., 1998. Ecology of Shallow Lakes, 1st ed. Chapman and Hall, London.
Segura, A. M., C. Kruk, D. Calliari & H. Fort, 2013. Use of a morphology-based functional approach to model phytoplankton community succession in a shallow subtropical lake. Freshwater Biology 58: 504–512.
Siver, P. A., A. P. Wolfe, F. J. Rohlf, W. Shin & B. Y. Jo, 2013. Combining geometric morphometrics, molecular phylogeny, and micropaleontology to assess evolutionary patterns in Mallomonas (Synurophyceae: Heterokontophyta). Geobiology 11: 127–138.
Souza Filho, E. E. & J. C. Stevaux, 2004. Geology and geomorphology of the Baía-Curutuba-Ivinheima river complex. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 1–30.
Souza Filho, E. E., P. C. Rocha, E. Comunello & J. C. Stevaux, 2004. Effects of the Porto Primavera Dam on physical environment of the downstream floodplain. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 55–74.
Statisoft., 2005. Inc. Statistica (data analysis software system) version 7.1. Available in: www.statisoft.inc.
Sun, J. & D. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen phytoplankton-methodic. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 9: 1–39.
Weithoff, G., 2003. The concepts of ‘plant functional types’ and ‘functional diversity’ in lake phytoplankton – a new understanding of phytoplankton ecology? Freshwater Biology 48: 1669–1675.
Žutinić, P., G. U. Marija, K. B. Koraljka, P. Andelka & J. Padisák, 2014. Morpho-functional classifications of phytoplankton assemblages of two deep karstic lakes. Hydrobiologia 740: 147–166.
Acknowledgments
We thank to the Long Term Ecological Research Program (Programa de Pesquisa Ecológica de Longa Duração, PELD/CNPq), the Center of Research in Limnology, Ichthyology, and Aquaculture of State University of Maringá (Nupélia/UEM) for logistical and financial support and CAPES and CNPq for post-graduate and post-doctoral scholarships. We would like to thank the Limnology Basic Laboratory/Nupelia for abiotic data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Bortolini, J.C., Moresco, G.A., de Paula, A.C.M. et al. Functional approach based on morphology as a model of phytoplankton variability in a subtropical floodplain lake: a long-term study. Hydrobiologia 767, 151–163 (2016). https://doi.org/10.1007/s10750-015-2490-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2490-z