Abstract
The use of ecological classification systems is becoming more and more widely used when studying phytoplankton. Grouping phytoplankton species into ecologically coherent groups allow to reduce redundancy and in this way, to handle a minor number of biological variables when investigating the ecological status of aquatic ecosystems. Three ecological classifications are mostly used when freshwater phytoplankton is studied: functional groups or coda, morpho-functional groups (MFGs) and morphology-based functional groups (MBFGs). In this study, these three ecological classifications were comparatively used along with two taxonomic classifications based on species and genera to analyse phytoplankton response to environmental variability in three sub-tropical Chinese reservoirs. Canonical correspondence analysis was performed to compare the five mentioned biological classifications. When ecological classifications were used, the percentage of variance explained in the biological groups–environmental variables was higher than that explained by the taxonomic classifications. Coda and MFGs showed a very high degree of overlapping, but since coda are associated to very detailed environmental templates, this method was more helpful in explaining phytoplankton variability in relation to environmental factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Functioning of aquatic ecosystems largely depends on phytoplankton morpho-functional diversity, as produced by the range of environmental pressures to which this group of organisms is adapted, which in turn allow them to cope with the environmental constraints. In particular, resource availability, water motion and temperature largely contribute to set the specific composition of the phytoplankton assemblages, the shape and size of the organisms involved and their seasonal succession (Naselli-Flores et al., 2007a; Naselli-Flores & Barone, 2011). For this reason, investigations on phytoplankton dynamics and structure are considered basic tools to understand the ecology of aquatic ecosystems. As an example, the main parameters used to describe the trophic state in most of the limnosystems are largely based on phytoplankton biomass or on its proxies [e.g. chlorophyll a (Chl a) concentrations, transparency and total phosphorus], and there are no studies on eutrophication, energy fluxes or primary production which may avoid phytoplankton analysis.
Moreover, the complexity of phytoplankton assemblages and their seasonal succession offer the opportunity to create models that can help the understanding of the vegetation processes in wider and less accessible, both on temporal and spatial scales (e.g. forests), ecosystems (Reynolds, 1997). However, phytoplankton is a phylogenetically heterogeneous group of organisms, and its assemblages are usually characterized by a quite high-species richness which reflects the high dynamism of aquatic ecosystems; this high number of species, on the other hand, complicates the development of ecological models and the interpretation of environmental patterns.
The identification of organisms at the species level is a time consuming activity which involves high costs in terms of time and expertise. Moreover, the development of molecular taxonomy is underlining more and more often how traditional taxonomy based on phenotypes is not always an adequate and sufficient tool to classify organisms.
The above-quoted reasons may have caused in the last years a general tendency aimed at the demise of traditional taxonomy. However, as shown by Boero (2010), a dismissal of taxonomy is in contradiction with all the efforts that have been put in the study of biodiversity since the establishment of the Rio Convention on Biological Diversity. Modern taxonomy needs to conjugate genotypic, phenotypic and chemical information as well as ecology to correctly identify organism. Colwell (1970) first pointed out this need and coined the term ‘polyphasic taxonomy’ to describe this combined approach (Cleenwerck & De Vos, 2008). The polyphasic approach in the taxonomy of phytoplankton is progressively leading to the clarification of real phylogenetic relationships among these organisms (e.g. Zapomělová et al., 2009; Moustaka-Gouni et al., 2010; Bock et al., 2011; Komárek & Mareš, 2012).
However, the polyphasic approach, although is a very promising tool to elucidate evolutionary relationships among organisms, does not solve the problem of a satisfactory taxonomic resolution when routine biomonitoring and ecological investigations are carried out.
To override these problems and to simplify the handling of long taxonomic lists, several attempts for an ecological classification of phytoplankton have been developed in the last years (Gallego et al., 2012). Pooling phytoplankton species with similar ecological characteristics into a fewer coherent categories is becoming a common approach when phytoplankton dynamics and structure are investigated, since it makes easier both the interpretation of ecological patterns and the comparison among different freshwater ecosystems.
The aim of this article is to verify the reliability of ecological classifications of phytoplankton compared to the traditional taxonomic ones. In addition, the article aims to compare the effectiveness of three ecological classifications in picking up the phytoplankton variability in relation to environmental variability. Two taxonomic (species, genera) and three non-taxonomic classifications of freshwater phytoplankton, which have been developed in the last years, were used in order to verify their usefulness in synthesizing the ecological responses of these organisms to environmental variability. Since flushing is well known as one of the main factors affecting environmental variability in reservoirs (Katsiapi et al., 2011) and it influences phytoplankton dynamics both directly (e.g. through dilution) and indirectly (e.g. by modulating resource availability), the study was carried out by analysing phytoplankton patterns in three selected reservoirs located in South China and subjected to a gradient of disturbance as represented by flushing.
Materials and methods
Description of sites
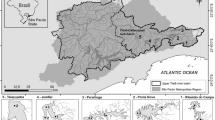
Three small reservoirs located in the offshore plain of Zhuhai city (South China) were selected for this study. They form a connected water system to supply drinking water to the cities of Zhuhai and Macao (Fig. 1) by storing the water pumped from the downstream of the Pearl River and re-distributing it to water plants through underwater pipes when needed. The water from the river is characterized by high-nutrient loads which promote eutrophication processes in these reservoirs as underlined by the recurrent algal blooms. The main difference among the reservoirs is represented by their flushing rates which reflect the pumping operations. Precipitation in the area is mostly concentrated in the period May–September (summer monsoon) with mean annual values of 1,991 mm. Table 1 summarizes the main characteristics of the studied reservoirs.
NanPing (NP) reservoir (N 22°12′09″ E 113°28′59″) has a watershed area of 2.36 km2 and a volume of 5.01 × 106 m3. It receives inflow water from two upstream pumping stations and has a water retention time (WRT) of 20–30 days.
ZhuXianDong (ZXD) reservoir (N 22°12′21.3″ E 113°30′57.6″) has a watershed area of 2.81 km2 and a volume of 1.96 × 106 m3. It is the terminal reservoir in the chain and supply water to Gongbei water plant and Macao. ZXD reservoir receives inflow water from both NP reservoir and a downstream pumping station. About 2.2 × 105 m3 (11.5 % of total volume) is pumped to Macao from ZXD reservoir every day, so the water residence time in the reservoir is the shortest (<10 days).
SheDiKeng (SDK) reservoir (N 22°11′13.9″ E 113°28′45.5″) is the smallest reservoir with a total volume of 1.28 × 106 m3. Its watershed covers an area of 2.31 km2. This reservoir only receives water from the pumping station when the waters from Pearl River are clear. It is used as an emergency reservoir to supply drinking water when severe saltwater intrusions in the Pearl River make its waters unsuitable for drinking. The pumping occurs only in January and March, so the reservoir shows the highest average water residence time (295 days) among the studied water bodies.
Sampling and measurements
Phytoplankton was collected every 2 months, from April to December 2006, in the three studied reservoirs at discrete depths (0.5, 5 and 10 m). The three samples collected at different depths were mixed, and subsamples were used for phytoplankton, Chl a and chemical analyses.
Water transparency was measured using a Secchi disk, and euphotic zone (z eu) was estimated by multiplying water transparency by 2.7 (Cole, 1994). Water temperature, electrical conductivity and pH were measured along vertical profiles at 1 m intervals with a SONDE (Yellow Spring Instruments, Ohio). Mixing depth (z mix) was estimated from thermal profiles as the upper water layer where temperature decrease was less than 1°C per m. WRT was calculated as the ratio of reservoir volume divided by the average daily outflow measured in the 15 days before sampling.
Chlorophyll a was measured spectrophotometrically by filtering 500 ml of water through a 0.45-μm cellulose acetate membranes (Lin et al., 2005).
Nutrients (SRP, N-NO3), total nitrogen (TN) and total phosphorus (TP) analyses were carried out according to APHA (1989). All the analyses were completed within 24 h from sampling.
For phytoplankton counting, 1 l of water preserved with 5 % formalin and 1 % Lugol’s iodine solution was allowed to settle in a graduated flask. After 2 weeks, the supernatant was siphoned off with a 2-mm diameter hose, and the residual (35 ml) was collected for microscopic counting. At least 400 algal units (≥2 μm) were counted in each sample using a Sedgewick-Rafter chamber under an Olympus microscope at 400× magnification (APHA, 1989). Three replicates for each sample were counted, and phytoplankton cells or colonies were measured to estimate biovolume. The biomass (mg l−1) was calculated from biovolume estimates according to Hillebrand et al. (1999) and assuming a specific gravity of 1 mg mm−3.
Phytoplankton was identified at the lowest taxonomical rank possible, and species were grouped into coda (FGs) according to Padisák et al. (2009); species not listed in this article were sorted into coda according to the methodology described in Reynolds et al. (2002). Phytoplankton species were also pooled in morpho-functional groups (MFG) following Salmaso & Padisák (2007) and in morpho-functional based groups (MFBG) as described in Kruk et al. (2010).
Data analysis
Ordination was performed using CANOCO 4.5. Canonical correspondence analysis (CCA) was used to analyse the distribution of taxonomic and ecological groups along environmental gradients. This unimodal ordination method was preferred because the longest gradient length was higher than 4 (see Lepš & Šmilauer, 2003 for more details). Moreover, it was selected because it is a constrained method, i.e. it forces the biological variables to fit a pre-arranged environmental template, thus allowing a comparison among the ordinations obtained using the different grouping methods. Five biological matrices, built on relative biomass values as resulting from the five adopted ways of grouping phytoplankton, were used in the CCA analyses. One matrix contained the biomass values of single species, whereas in the second one the species were grouped into genera. In the other three matrices, initial phytoplankton species were grouped using the three ecological classifications above quoted. Only the groups which were present with a relative biomass greater than 5 % were included in the analyses. Environmental data were normalized using the formula y i = log(x i + 1). The significance with which environmental variables explain the variance of species data was tested using Monte Carlo simulations with 99 unrestricted permutations. Variables were considered to be significant when P < 0.05.
For each reservoir, eight independent limnological variables were taken into consideration: three chemical (SRP, NO3-N and pH) and five hydrological and physical (epilimnetic temperature, mixing depth–euphotic depth ratio, conductivity, WRT and inflow).
Results
Hydraulic patterns
All the reservoirs showed their minimum volumes in April, at the end of the dry seasons (Fig. 2a). The inflow increased in the flood season (June to October) in all the reservoirs (Fig. 2b) and then declined in December when pumping decreased to avoid saltwater intrusions. SDK reservoir was characterized by flushes much lower than NP and ZXD. Stored volume increased in all the reservoirs from April to December because of both the water pumping from Pearl River and the direct effect of precipitation (Fig. 2c). The outflow varied greatly among reservoirs. However, SheDiKeng reservoir had quite smaller outflows compared to the other two reservoirs (Fig. 2d), and no water was spilled out in June and August.
Physical and chemical scenarios
The reservoirs were characterized by high-water temperatures and relatively low-transparency values throughout the studied period. The surface water temperature exceeded 25°C from June to October in all the reservoirs (Fig. 3). Even in April and December, it showed values above 20°C throughout the whole water columns. In ZXD reservoir, thermal stratification never occurred in the studied period, whereas NP and SDK reservoirs showed a weak stratification in spring (April) and early summer (June).
The lowest mean transparency (SD) was measured in ZXD reservoir that had the largest water inflow, and the highest mean SD was in SDK which was characterized by the lower inflows. In ZXD and NP reservoirs, SD was lower in June and August when more water was pumped into the reservoir (Fig. 4a). The values of pH were generally higher than 8.0, varying from 7.5 to 9.7. Values above 9 were associated to high values of phytoplankton biomass. Conductivity values decreased from April to June in all the reservoirs and then tended to increase again from August to December (Fig. 4b). ZXD and NP reservoirs showed values ranging between 200 and 400 μS cm−1, whereas SDK reservoir was characterized by much lower values, ranging between 60 and 110 μS cm−1.
Light penetration in the reservoirs was low. All the reservoirs showed z mix/z eu > 1 but SDK reservoir in June. The latter water body was generally characterized by lower values of the z mix/z eu and thus by a higher light availability in its mixing layer (Fig. 4c).
The concentrations of both TP and TN in the reservoirs (Fig. 4d, e) were high at the end of the dry season (April) and showed lower values in the middle of the flood season (August) when precipitation reaches its peak. ZXD reservoir, which received the highest amount of water from Pearl River, had the highest concentration of nutrients, whereas SDK reservoir showed the lowest value for the opposite reason (Fig. 4g, f).
Phytoplankton biomass, species composition and dominant groups
In NP reservoir, biomass values were above 20 mg l−1 from April to August, with a maximum in June (26 mg l−1). October was characterized by lower values (5.2 mg l−1), which further declined in December (3.0 mg l−1). In April, total phytoplankton biomass was 24.8 mg l−1, and the assemblage was dominated by the green flagellated Carteria sp. (21.5 mg l−1) accompanied by the dinoflagellate Peridinium pusillum (Pénard) Lemmermann (2.0 mg l−1). From June to October, these species were replaced by diatoms: Cyclotella meneghiniana Kützing, which formed about 65 % of total biomass in June and then declined, and Synedra sp., which showed its highest relative contribution (49 %) in August. In these months, filamentous Cyanobacteria, mainly represented by Pseudanabaena sp., were sub-dominant. The green algae Botryococcus braunii Kützing and Pandorina sp. dominated the phytoplankton assemblage in December.
The highest biomass value (>80 mg l−1) in ZXD reservoir was recorded in April, mainly due to the green alga Carteria sp. (≈60 mg l−1). Conversely, in June, total biomass declined to values <7 mg l−1. In this month, the filamentous green alga Planctonema sp. and diatoms [mainly represented by Aulacoseira ambigua (Grunow) Simonsen] were the most abundant taxa. These species also dominated the August and October assemblages, when total biomass was below 2 mg l−1. In December, Planktothrix sp. (Cyanoprokaryota) and Ochromonas sp. (Chrysophyta) were the dominant taxa although quite low-total biomass values (≈1 mg l−1) were recorded.
SDK reservoir also showed the highest phytoplankton biomass values (37.5 mg l−1) in April. In this month, the nostocalean Dolichospermum circinalis (Rabenhorst ex Bornet et Flahault) Wacklin, Hoffman & Komárek formed more than 90 % of total phytoplankton biomass. Large colonies of Botryococcus braunii were dominant in June when total biomass was 23.1 mg l−1. In August, the structure of the assemblage changed again, and the dinoflagellate Ceratium hirundinella (O.F.Müller) Dujardin and the desmid Staurastrum natator West formed, respectively, the 21 and 26 % of total biomass (15.5 mg l−1). Phytoplankton biomass declined in October (2.5 mg l−1) and December (2.0 mg l−1). In this last month, the centric diatoms Cyclotella meneghiniana (21 %) and Aulacoseira ambigua (67 %) dominated the phytoplankton assemblage.
Table 2 summarizes the main dominant and subdominant species identified in the three studied reservoirs.
According to phytoplankton biomass trends, Chl a concentrations showed a wide range of values (4 < Chl a < 120 μg l−1) in the studied reservoirs. A general decreasing trend occurred in all the studied reservoirs proceeding from April onward. Very high concentrations of Chl a (>100 μg l−1) were measured in April in SDK and ZXD reservoirs when a cyanobacterial bloom caused by Dolichospermum circinalis (SDK) and one caused by the volvocalean Carteria sp. (ZXD) were observed (Fig. 4h).
A total of 96 species belonging to 72 genera were identified in the three reservoirs (Appendix 1—Supplementary Material). The highest species richness (65) was recorded in SDK reservoirs. The other two water bodies, in spite of their connection, showed a different number of species: 61 were found in ZXD and 45 in NP. Only 31 species belonging to 24 genera showed a relative biomass value >5 % in at least one of the three studied reservoirs. Allocation of phytoplankton species in ecological groups allowed to identify 23 coda (FGs), 25 MFGs and all the 7 morphology-based functional groups (MBFGs) described. The composition of ecological groups is reported in Appendix 2—Supplementary Material. Among these groups, 19 FGs, 19 MFGs and 7 MBFGs showed a relative biomass >5 %. A much higher number of species contributed to these ecological groups: 90 species were considered in the 19 FGs, 88 in the 19 MFGs and all the 96 identified species in the 7 MBFGs.
As regard FGs, 13 were common to all the reservoirs; codon T was exclusively found in ZXD reservoir and codon L M in SDK. These two reservoirs also shared W1 and H1 coda, whereas the codon Y was recorded in NP and ZXD and the codon A in NP and SDK.
Among the 19 identified MFGs, 9 were recorded in NP and 11 in both ZXD and SDK. However, only 3 MFGs (7a, 5a and 2b) were common to all the reservoirs. MFGs 10a, 2d, 2a and 1c were exclusively found in ZXD; MFGs 8a, 6a, 5e, 5b and 1b were present only in SDK, and MFG 4 was detected only in NP (Table 3).
All the seven described MFBGs were identified in both ZXD and SDK reservoirs, whereas in NP Group 2 was not recorded.
Data ordination
Canonical correspondence analysis was performed initially on the five selected taxonomic/ecological groups dataset constrained to the eight independent environmental variables. However, results generally improved when forward selection was applied excluding nutrients and pH, and including only five hydrological and physical variables (water residence time, inflow, z mix/z eu, temperature and conductivity). In particular, the eigenvalues of CCA axes 1 and 2 constrained to the five variables slightly decreased compared to the initial analyses as well as the biological groups–environmental variables correlations of CCA axes 1 and 2. However, the percentage of variance explained by these two axes in the biological group–environment relationships increased (Table 4). Thus, only these results are described below.
When using species, the eigenvalues of CCA axis 1 (0.762) and axis 2 (0.659) accounted for 22.9 % of the cumulative variance. The species–environment correlations of CCA axes 1 and 2 were high (0.966 and 0.991, respectively), and the first two axes accounted for 55.7 % of the variance in the species–environment relationships.
When species were grouped into genera, the eigenvalues of CCA axis 1 (0.743) and axis 2 (0.638) accounted for 25.4 % of the cumulative variance. The genera–environment correlations of CCA axes 1 and 2 are also high (0.958 and 0.987, respectively), and the first two axes accounted for 59.2 % of the variance in the genera–environment relationships.
When using MFGs, the eigenvalues of CCA axis 1 (0.677) and axis 2 (0.605) accounted for 42.2 % of the cumulative variance. The MFGs–environment correlations of CCA axes 1 and 2 were slightly lower than those obtained using taxonomic groups (0.935 and 0.947, respectively), but the first two axes accounted for 66.3 % of the variance in the MFGs–environment relationships.
Ecological classification performed using Reynolds coda (FGs) showed comparable results. The eigenvalues for axis 1 (0.665) and axis 2 (0.650) accounted for 41.6 % of the cumulative variance. Also in this case, the FGs–environment correlations of CCA axes 1 and 2 were high (0.922 and 0.962, respectively), and the first two axes accounted for 63.6 % of the variance in the coda–environment relationships.
The results of CCA analyses obtained using species (Fig. 5a) and genera (not shown) show a cloud of overlapping points with an unclear trend.
Conversely, the biplots obtained using the phytoplankton ecological classifications into FGs and MFGs show clearer trends as illustrated in Fig. 5b and c. The two ordinations are quite similar and indicate a good correspondence between the FGs and MFGs identified in the studied reservoirs. In particular, two main gradients appear in both the biplots, one driven by the water residence time and the z mix/z eu and the second one by inflow. Phytoplankton ecological groups are distributed along these gradients. Contrasting results were obtained when using the MBFGs. The eigenvalues of CCA axis 1 and axis 2 are much lower (0.402 and 0.258, respectively) but they explain a higher cumulative percentage of variance (56.5 %). The MBFGs–environment correlations of CCA axes 1 and 2 are lower than those obtained when using FGs and MFGs (0.907 and 0.801, respectively), and the first two axes accounted for 35.1 % of the variance in the MFBGs–environment relationships. The biplot obtained using this ecological classification offers a less clear interpretation, and MBFGs seem to be distributed along a temperature-driven gradient but G3 which contains only Dolichospermum circinalis and thus exhibits a negative correlation to inflow as codon H1 and MFG 5e (Fig. 5d).
Discussion
Although the present study was carried out on a relatively low number of phytoplankton samples, the considered season is strongly subjected to the monsoon climate. In this period, the reservoirs are filling, stored water is contemporarily used and the hydrological constraints are quite strong. Thus, in the considered period, sharp environmental gradients occurred as underlined by the large number of recorded ecological groups (23 FGs, 25 MFGs and 7 MBFGs) which made possible to perform the comparative analysis.
Among the three studied reservoirs, SDK was the one less subjected to operational procedures. Its volume did not increase as much as the other two reservoirs, and its thermal structure was less disturbed by water inflow from Pearl River. This, as already shown by Wang et al. (2012) in a closely located reservoir, allowed SDK to exhibit a more stable stratification although it is shallower than NP and ZXD. The minor depth of the mixing zone and the minor amount of turbid waters coming from the river contributed to the lower z mix/z eu values observed in SDK. At the same time, phytoplankton biomass in this reservoir was less diluted by the water pumped from the river.
The dominant species in the phytoplankton assemblages of the three studied reservoirs reflect the different hydrological constraints to which these water bodies are subjected. Just before the beginning of the pumping season, phytoplankton assemblages of both NP and ZXD reservoir were dominated by Carteria sp. This genus is typically found in nutrient-rich stagnating water columns and storage reservoirs (Padisák et al., 2009). When inflow and outflow increase, these reservoirs, which exhibit a weaker thermal stability, are dominated by species sensitive to stratification (Cyclotella meneghiniana and/or Aulacoseira ambigua) or by species which can tolerate flushing and turbid waters (Synedra spp.). Conversely, the clearer and more thermally stable SDK reservoir shows species typical of clear epilimnia (e.g. Botryococcus braunii) or adapted to mixed layers 2–3 m thick (Staurastrum spp.). Moreover, the latter reservoir showed a higher number of coexisting phytoplankton species with a similar relative contribution to total biomass, ranging between 5 and 10 %, both in August and October. This likely occurred because of the lower flushes, mainly due to precipitation, which may act within the limit of an intermediate disturbance preventing the establishment of competitive exclusion (Naselli-Flores et al., 2003). Conversely, in the other reservoirs, subjected to very high flushes from Pearl River, only a narrower set of stress-adapted species prevailed.
The high number of species recorded in SDK is not surprising; phytoplankton assemblages can be formed by dozens of coexisting species; in addition, each population includes a certain degree of phenotypic variability (Naselli-Flores & Barone, 2003). This richness, coupled to low-generation time, allow phytoplankton to respond very fast to environmental variability through the selection of the phenotype best fitting the new environmental scenario (Naselli-Flores & Barone, 2000, 2007; Naselli-Flores et al., 2007a) or, if the variation exceed the possibilities given by species-specific phenotypic plasticity, by allowing one of the rare species to ‘immediately’ fill a newly formed niche (Padisák et al., 2010a and literature therein).
Moreover, reservoirs generally represent very dynamic environments where resource availability, and thus phytoplankton composition, can change more quickly than in natural lakes (Naselli-Flores, 2003). When studying and comparing reservoirs, the use of phytoplankton species can generate a confusing account of their ecological status. Conversely, the identification of coherent ecological groups, by reducing the inherent species redundancy shown by ecosystems (Walker, 1992), can allow a better explanation of environmental patterns and represents a more appropriate tool to understand the assemblage evolution under a changing environment.
The CCA performed using species and genera showed that the first two axes accounted for percentage of the variance in the biological groups–environmental variables relationships lower than that obtained when using ecological classifications. Moreover, pooling species in ecological groups allowed to consider a higher number of species in the analyses. Actually, ordination analyses were carried out considering those items (species or groups) contributing more than 5 % to total biomass. Species accounting less than 5 % to total biomass were not considered but when belonging to the same ecological group they were summed and together gave a contribution higher than the limit set and thus they could be considered in the analysis carried out using the ecological classifications.
Two taxonomic classifications based on species and genera, respectively, as well as three ecological classifications of phytoplankton have been comparatively tested in the present study.
The Reynolds’ functional classification of freshwater phytoplankton (Reynolds et al., 2002) is probably the most used non-taxonomic approach to group phytoplankton and it has been successfully applied to investigate phytoplankton assemblages in South China reservoirs (Xiao et al., 2011; Hu & Xiao, 2012). Originally formed by 31 groups called ‘coda’ (FGs), it has been successively enlarged to 39 coda by Padisák et al. (2009). Two main ideas lie beneath the functional groups theory: (1) a functionally well-adapted species is likely to tolerate the constraining conditions of factor deficiency more successfully than individuals of a less well-adapted species; (2) a habitat shown typically to be constrained by light, nutrients or whatever, is more likely to be populated by species with the appropriate adaptations to be able to function there (this of course does not imply that those species will be there). A well-defined habitat template is associated to each codon as well as its tolerance and sensitivity to one or more environmental factor. This classification is largely based on the combination of three morphological descriptors as surface area (s), volume (v) and maximum linear dimension (m) which are powerful predictors of optimum dynamic performance (Reynolds & Irish, 1997).
A similar approach was used by Salmaso & Padisák (2007) who used 31 MFGs to investigate phytoplankton structure and dynamics in two deep oligotrophic lakes. This method is also based on the assumption that morphological characteristics (s, v and m) define the ecological niche of phytoplankton by influencing their efficiency in resource exploitation (maximum nutrient uptake rate, half-saturation constant for uptake, subsistence quotas and uptake affinity) as well as their sinking properties, light harvesting efficiency and susceptibility to grazing (Padisák et al., 2003; Naselli-Flores et al., 2007b; Zohary et al., 2010; Naselli-Flores & Barone, 2011; Edwards et al., 2012). These features, along with buoyancy regulation, requirement for specific resources (e.g. silica), mixotrophy and nitrogen fixation, are considered effective factors which select the best competitors under different environmental constraints (Weithoff, 2003).
A third grouping method has been recently proposed by Kruk et al. (2010). These authors developed a MBFG approach which further simplify the MFGs approach described above by clustering phytoplankton organisms in seven groups discriminated by their morphological traits (e.g. volume, presence of flagella, mucilage, siliceous exoskeleton). Significant differences in growth rate, sinking rates, population structure and competitive ability have been shown for these groups. Moreover, their potential ecological performance in terms of resources acquisition and avoidance of loss processes (consumption and sinking) have been derived, and their environmental preferences have been established allowing the definition of an habitat template for each group (Kruk & Segura, 2012 and literature therein).
As already shown in literature, the results obtained in the present study confirm that physical constraints in reservoirs have effects in shaping the structure of phytoplankton assemblages stronger than those exerted by nutrients availability (e.g. Naselli-Flores, 2000; Naselli-Flores & Barone, 2005; Hoyer et al., 2009; Padisák et al., 2010b; Rigosi & Rueda, 2012). In particular, the pumping of more turbid water from the saltier Pearl River in the reservoirs decreases their water residence time and increases their z mix/z eu and their conductivity values. In addition, these strong inflows interfere with the stratification patterns and cause a dilution effect on phytoplankton assemblages.
The results achieved from the constrained ordination rather obviously show a progressive improvement as the number of biological items involved in the analyses decreases, from species to MBFGs. However, although the best results from the statistical analyses were obtained using the MBFGs approach, the chances to clearly interpret how the environmental variables influence phytoplankton are diminished by the wideness of each group which collect a variety of organisms with different ecological requirements. In particular, all the diatoms are contained in one group in the MBFGs classification, whereas they are divided in four groups in the MFGs approach and in five groups in the FGs classification. In particular, the latter species distribution into ecological groups allow to separate oligotrophic (codon A) from mesotrophic (codon B), eutrophic (codon C) or even hypertrophic (codon P) lakes according to resource (both nutrients and light) availability, thus facilitating a fine tuning in the interpretation of environmental conditions. Analogously, flagellates are grouped in two MBFGs, in seven MFGs and in a dozen of FGs.
In the present study, we compared a functional approach with two morphological approaches, one based on 31 descriptors and another one on 7 descriptors. Our results showed that functional classification and morphological classification can overlap when the number of morphological descriptors involved is detailed enough to depict a large number of adaptive strategies, which at the end constitute the ecological response of phytoplankton to environmental variability. As a consequence, according to the results achieved, MBFGs are less sensitive in summarizing phytoplankton response to environmental variability than FGs and MFGs because of the low number of groups considered.
The definition of MFGs, based on easily recognizable morphological features, allows to reduce a high number of species into a more easy to handle number of coherent groups. However, an immediate reconstruction of the ecological environment is quite hard and an accurate knowledge about phytoplankton morphological adaptations to environmental gradients is required to interpret environmental patterns. Our results show a good coincidence among MFGs and coda; this make possible to extend the habitat template defined for coda to the correspondent MFG (see Appendix 2—Supplementary Material) and facilitate the attribution of an environmental template to each MFG.
Functional group classification does not require skills to interpret the relationships between the biota and its environment, since it already provides a description of the habitat template associated to each codon. Moreover, it can offer support for the correct taxonomic identification of species. This is probably the reason why FGs classification is widely used in literature as indicated by the high number of citations collected in the Web of Science. It actually offers a better support in the assessment of ecological state both when different environments are compared (Stanković et al., 2012) or when inter-annual variability of phytoplankton is investigated (Naselli-Flores & Barone, 2012). In the present study, two ecological gradients were identified: one is driven by decreasing values of the water residence time. This gradient is underlined by the sequence of coda D → J → T → G. According to Padisák et al. (2009), these coda progressively correspond to rivers → low-gradient rivers → persistently mixed layers → stagnating environment. As water residence time increases, z mix/z eu decreases due to the lower amounts of turbid and saltier waters pumped in from Pearl River. This tendency indicating an improvement of underwater light condition is analogously underlined by the sequence L M → F → L O → A → N. The second gradient is mainly driven by flushing as shown by the sequence of coda H1 → C → D → J → S1.
The difficulties in applying FGs classification are mainly due to assigning species to coda; this process requires knowledge of the autoecology of single species or species groups and a minimal expertise on phytoplankton taxonomy, even though placing phytoplankton in the proper codon is largely facilitated by an accurate analysis of environmental conditions. Conversely, MFG and MBFG approaches are based on easily recognizable features and do not require particular taxonomic skills. Thus, they can result more suitable in monitoring programs carried out by environmental agencies. In particular, the results of the present study show that MFG approach offers the possibility to infer the ecological features of the studied environment with the same accuracy offered by FGs, once the ecological relationships between morphology and environmental constraints are clarified. Moreover, the present study can offer the possibility to extrapolate the environmental template of MFGs by comparing them with the correspondent coda as reported in Appendix 1—Supplementary Material. MBFG classification showed a higher sensitivity to temperature variation and it can thus be useful for screening the seasonal patterns followed by phytoplankton thus offering a complementary aid when the analysis of phytoplankton is used in monitoring programs.
References
APHA, 1989. Standard Methods for the Examination of Water and Wastewater. American Water Works Association and Water Pollution Control Federation, Washington, DC.
Bock, C., T. Proeschold & L. Krienitz, 2011. Updating the genus Dictyosphaerium and description of Mucidosphaerium gen. nov. (Trebouxiophyceae) based on morphological and molecular data. Journal of Phycology 47: 638–652.
Boero, F., 2010. The study of species in the era of biodiversity: a tale of stupidity. Diversity 2: 115–126.
Cleenwerck, I. & P. De Vos, 2008. Polyphasic taxonomy of acetic acid bacteria: an overview of the currently applied methodology. International Journal of Food Microbiology 125: 2–14.
Cole, G. A., 1994. Textbook of Limnology, 4th ed. Waveland Press Inc., Prospect Heights, IL.
Colwell, R. R., 1970. Polyphasic taxonomy of the genus Vibrio: numerical taxonomy of Vibrio cholerae, Vibrio parahaemolyticus and related Vibrio species. Journal of Bacteriology 104: 410–433.
Edwards, K. F., M. K. Thomas, C. A. Klausmeier & E. Litchman, 2012. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnology and Oceanography 57: 554–566.
Gallego, I., T. A. Davidson, E. Jeppesen, C. Pérez-Martínez, P. Sánchez-Castillo, M. Juan, F. Fuentes-Rodríguez, D. León, P. Peñalver, J. Toja & J. J. Casas, 2012. Taxonomic or ecological approaches? Searching for phytoplankton surrogates in the determination of richness and assemblage composition in ponds. Ecological Indicators 18: 575–585.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hoyer, A. B., E. Moreno-Ostos, J. Vidal, J. M. Blanco, R. L. Palomino-Torres, A. Basanta, C. Escot & F. J. Rueda, 2009. The influence of external perturbations on the functional composition of phytoplankton in a Mediterranean reservoir. Hydrobiologia 636: 49–64.
Hu, R. & L. J. Xiao, 2012. Functional classification of phytoplankton assemblages in reservoirs of Guangdong Province, South China. In B.-P. Han & Z. Liu (eds), Tropical and Sub-tropical Reservoir Limnology in China. Monographiae Biologicae, 91. Springer, Dordrecht: 59–70.
Katsiapi, M., M. Moustaka-Gouni, E. Michaloudi & K. Kormas, 2011. Phytoplankton and water quality in a Mediterranean drinking-water reservoir (Marathonas Reservoir, Greece). Environmental Monitoring and Assessment 181: 563–575.
Komárek, J. & J. Mareš, 2012. An update to modern taxonomy (2011) of freshwater planktic heterocytous cyanobacteria. Hydrobiologia. doi:10.1007/s10750-012-1027-y.
Kruk, C. & A. Segura, 2012. The habitat template of phytoplankton morphology-based functional groups. Hydrobiologia. doi:10.1007/s10750-012-1072-6.
Kruk, C., V. L. M. Huszar, E. T. H. M. Peeters, S. Bonilla, L. Costa, M. Lürling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Lepš, J. & P. Šmilauer, 2003. Multivariate Analysis of Ecological Data using CANOCO. Cambridge University Press, New York, NY.
Lin, Sh. J., L. J. He, P. Sh. Huang & B. P. Han, 2005. Comparison and improvement on the extraction method for chlorophyll a in phytoplankton. Chinese Journal of Ecological Science 24: 9–11.
Moustaka-Gouni, M., K. A. Kormas, P. Polykarpou, S. Gkelis, D. C. Bobori & E. Vardaka, 2010. Polyphasic evaluation of Aphanizomenon issatschenkoi and Raphidiopsis mediterranea in a Mediterranean lake. Journal of Plankton Research 32: 927–936.
Naselli-Flores, L., 2000. Phytoplankton assemblage in twenty-one Sicilian reservoirs: relationships between species composition and environmental factors. Hydrobiologia 424: 1–11.
Naselli-Flores, L., 2003. Man-made lakes in Mediterranean semi-arid climate: the strange case of Dr Deep Lake and Mr Shallow Lake. Hydrobiologia 506(509): 13–21.
Naselli-Flores, L. & R. Barone, 2000. Phytoplankton dynamics and structure: a comparative analysis in natural and man-made water bodies of different trophic state. Hydrobiologia 438: 65–74.
Naselli-Flores, L. & R. Barone, 2003. Steady-state assemblages in a Mediterranean hypertrophic reservoir. The role of Microcystis ecomorphological variability in maintaining an apparent equilibrium. Hydrobiologia 502: 133–143.
Naselli-Flores, L. & R. Barone, 2005. Water-level fluctuations in Mediterranean reservoirs: setting a dewatering threshold as a management tool to improve water quality. Hydrobiologia 548: 85–99.
Naselli-Flores, L. & R. Barone, 2007. Pluriannual morphological variability of phytoplankton in a highly productive Mediterranean reservoir (Lake Arancio, Southwestern Sicily). Hydrobiologia 578: 87–95.
Naselli-Flores, L. & R. Barone, 2011. Fight on plankton! Or, phytoplankton shape and size as adaptive tools to get ahead in the struggle for life. Cryptogamie Algologie 32: 157–204.
Naselli-Flores, L. & R. Barone, 2012. Phytoplankton dynamics in permanent and temporary Mediterranean waters: is the game hard to play because of hydrological disturbance? Hydrobiologia. doi:10.1007/s10750-012-1059-3.
Naselli-Flores, L., J. Padisák, M. T. Dokulil & I. Chorus, 2003. Equilibrium/steady state concept in phytoplankton ecology. Hydrobiologia 502: 395–403.
Naselli-Flores, L., J. Padisák & M. Albay, 2007a. Shape and size in phytoplankton ecology: do they matter? Hydrobiologia 578: 157–161.
Naselli-Flores, L., R. Barone, I. Chorus & R. Kurmayer, 2007b. Toxic cyanobacterial bloom in reservoirs under a semi-arid Mediterranean climate: the magnification of a problem. Environmental Toxicology 22: 399–404.
Padisák, J., É. Soróczki-Pintér & Z. Rezner, 2003. Sinking properties of some phytoplankton shapes and the relation of form resistance to morphological diversity of plankton – an experimental study. Hydrobiologia 500: 243–257.
Padisák, J., L. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Padisák, J., É. Hajnal, L. Krienitz, J. Lakner & V. Üveges, 2010a. Rarity, ecological memory, rate of floral change in phytoplankton – and the mystery of the Red Cock. Hydrobiologia 653: 45–64.
Padisák, J., É. Hajnal, L. Naselli-Flores, M. T. Dokulil, P. Nõges & T. Zohary, 2010b. Convergence and divergence in organization of phytoplankton communities under various regime of physical and biological control. Hydrobiologia 639: 205–220.
Reynolds, C. S., 1997. Vegetation processes in the pelagic: a model for ecosystem theory. Ecology Institute, Oldendorf/Luhe: 371.
Reynolds, C. S. & A. E. Irish, 1997. Modelling phytoplankton dynamics in lakes and reservoirs: the problem of in situ growth rates. Hydrobiologia 349: 5–17.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rigosi, A. & F. J. Rueda, 2012. Hydraulic control of short-term successional changes in the phytoplankton assemblage in stratified reservoirs. Ecological Engineering 44: 216–226.
Salmaso, N. & J. Padisák, 2007. Morpho-Functional Groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112.
Stanković, I., T. Vlahović, M. Gligora Udovič, G. Várbíró & G. Borics, 2012. Phytoplankton functional and morpho-functional approach in large floodplain rivers. Hydrobiologia. doi:10.1007/s10750-012-1148-3.
Walker, B. H., 1992. Biodiversity and ecological redundancy. Conservation Biology 6: 18–23.
Wang, S., X. Qian, B. P. Han, L. C. Luo & D. Hamilton, 2012. Effects of local climate and hydrological conditions on the thermal regime of a reservoir at Tropic of Cancer, in southern China. Water Research 46: 2591–2604.
Weithoff, G., 2003. The concepts of ‘‘plant functional types’’ and ‘‘functional diversity’’ in lake phytoplankton - a new understanding of phytoplankton ecology? Freshwater Biology 48: 1669–1675.
Xiao, L. J., T. Wang, R. Hu, B.-P. Han, S. Wang, X. Qian & J. Padisák, 2011. Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large canyon-shaped reservoir. Water Research 45: 5099–5109.
Zapomělová, E., K. Řeháková, J. Jezberová & J. Komárková, 2009. Polyphasic characterization of eight planktonic Anabaena strains (Cyanobacteria) with reference to the variability of 61 Anabaena populations observed in the field. Hydrobiologia 639: 99–113.
Zohary, T., J. Padisák & L. Naselli-Flores, 2010. Phytoplankton in the physical environment: beyond nutrients, at the end, there is some light. Hydrobiologia 639: 261–269.
Acknowledgments
This study was supported by a Science and Technology Planning Project of Guangdong Province (No. 2009B050200001). A grant provided by the Water Resource Department of Guangdong Province to develop a warning and control plan of cyanobacterial blooms in small reservoirs is highly appreciated. The managers of the studied reservoirs are also thanked for kindly providing the hydrological data.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling editor: Judit Padisak
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, R., Han, B. & Naselli-Flores, L. Comparing biological classifications of freshwater phytoplankton: a case study from South China. Hydrobiologia 701, 219–233 (2013). https://doi.org/10.1007/s10750-012-1277-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1277-8