Abstract
A confluence zone in the Upper Paraná River Floodplain was sampled to verify the influence of upstream reservoirs on the dispersion and colonisation of phytoplankton. We hypothesized that the dilutive effect and high discharge limit phytoplankton colonisation in the main stem and that the phytoplankton functional structure in floodplain lakes is influenced by upstream reservoirs. The recruitment of functional group (FG) M from Rosana Reservoir was evidenced with low establishment in the main stem and colonisation in the floodplain lake. The lower connectivity with the Paraná River during limnophase reflected in water column total mixing and dominance of the FGs C and P in floodplain lake and greater stability of the water column, with dominance of the FG H 1 in a secondary channel. In the potamophase, the Paraná River negatively influenced the phytoplankton biovolume in the associated environments. In this period, FG P was dominant in the secondary channel and FG X 2 in the floodplain lake. Our results emphasize that despite the contribution of inocula to the Parana River from the upstream inflow and from tributaries, the phytoplankton depletion was due to retention and seston sedimentation in the Porto Primavera Reservoir, high discharge and dilution in this confluence zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Species distributions depend on spatial and environmental processes. Spatial dynamics alter the local species diversity, which, in turn, alters the regional biota via dispersal and colonisation (Leibold et al., 2010).
The topology of fluvial systems, including the principal channel and associated environments, significantly affects dispersal, and it is fundamental in community structure (Brown & Swan, 2010). Most variation and heterogeneity of a river occur in confluence zones (Benda et al., 2004), and, according to the functional process zone perspective, a river is not continuous but is a series of patches. Primary production in these systems varies with the type of functional process zone and its environmental characteristics, particularly hydrological retention, connectivity, geomorphic complexity and inputs from the riparian zone and watershed (Thorp et al., 2008).
The relationship between hydrological connectivity and biodiversity depends on the exchange of organisms and gene flow through the processes of extinction and colonisation (Cloern, 2007). Maintaining the connectivity that results from the natural fluctuations in the water level is fundamental to the integrity, functioning and structure of aquatic communities in floodplain lakes (Leira & Cantonati, 2008). Alterations in these fluctuations, which are caused by climatic changes and flow regulation of the associated channels, may result in severe consequences for the water quality and biodiversity of these environments (Abrahams, 2008; Leira & Cantonati, 2008).
Reduced connectivity results in a reduced confluence effect from tributaries, in accordance with the ‘network dynamics hypothesis’ (NDH), which relates the key attributes of hydrographic networks with the heterogeneity of fluvial forms and processes (Benda et al., 2004). Moreover, with a limited dispersal of organisms, a decrease in connectivity can reduce the gene flow between local communities and lead to inbreeding and a loss of genetic diversity, as has been documented in fragmented environments (Coulon et al., 2004; Cloern, 2007).
Habitat fragmentation is considered to have a great impact on rivers worldwide (Nilsson et al., 2005), and fragmentation caused by dams, principally those located upstream of floodplain rivers, constitute one of the main anthropogenic alterations of these ecosystems (Souza Filho et al., 2004).
Studies have shown that reservoirs of the Upper Paraná River basin, particularly the Porto Primavera Reservoir (UHE Engenheiro Sérgio Motta), have caused significant alterations in the amplitude of variation of the hydrometric levels of this river in addition to reducing the discharge and connectivity between the river and the environments adjacent to the river’s floodplain (Souza Filho et al., 2004).
The present study aims to analyse the influence of the inputs from the Porto Primavera and Rosana Reservoirs on the dispersal and seasonal variation of biovolume and phytoplankton functional groups (FGs) in the Upper Paraná River, a secondary channel, and a floodplain lake. The following hypotheses were tested: (1) the segment of the Paraná River analysed receives phytoplankton biovolume from upstream inflows and from left and right bank tributaries, but the dilution effect and high discharge limit colonisation, which results in low phytoplankton biovolume in the main stem and (2) the biovolume of the taxonomic groups and phytoplankton FGs in floodplain lakes connected to the Paraná River is influenced by species dispersal originating from the inflows of the Porto Primavera and Rosana Reservoirs.
Materials and methods
Study area
The Paraná River is the second longest river in South America (4,695 km), the tenth largest river in the world in terms of water discharge, and the fourth largest in terms of drainage area (2.8 × 106 km2). The Upper Paraná River includes approximately the first third of the Paraná River basin, and it lies completely within Brazilian territory, except for one stretch along the Itaipu Reservoir that borders Paraguay. The Upper Paraná River has a drainage area of 891,000 km2, or 10.5% of the total area of Brazil (Souza Filho et al., 2004).
The rivers that form the Paraná River are similar to other plateau rivers, with an average slope of 0.8 m per km, decreasing in the middle portion to 0.3 m per km. Before the closure of the Porto Primavera Dam in 1998, a large floodplain existed from Três Lagoas (MS) to Guaíra (PR), extending for approximately 480 km. This floodplain currently includes a stretch of 230 km from the Porto Primavera Dam to the upper part of the Itaipu Reservoir (with a slope 0.18 m per km). This floodplain, especially on the western margin of the Paraná, can be up to 20 km wide and is composed of numerous secondary channels, lakes and rivers. Due to its importance as a representative of a local river–floodplain system, this floodplain was given status as an Environmental Protection Area (APA) to preserve the islands and ‘várzeas’ of the Paraná River (Souza Filho et al., 2004).
The reservoir of the Porto Primavera hydroelectric plant is located in the Paraná River, 28 km upstream of the river’s confluence with the Paranapanema River, the main tributary in the stretch studied. This reservoir is 2,250 km2 in area, 18,500 × 106 m3 in volume, and has an annual average discharge of 7,066 m³ s−1 and a theoretical water residence time of 33 days (Souza Filho et al., 2004).
The Paranapanema River is formed by various springs from the Serra do Paranapiacaba mountain range. The river stretches for 930 km to its mouth at the Paraná River. Rosana Reservoir occupies the last position in a cascade of eight reservoirs in the Paranapanema River (Fig. 1), forming part of the border between São Paulo and Paraná States, Brazil. The filling of this reservoir was completed in November 1986, and it has an inundated an area of 220 km2 and a volume of 1,920 km3 and has an average annual discharge of 1,203 m³ s−1 and a theoretical water residence time of 19 days (CESP, 1998).
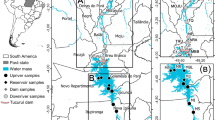
The study area is located downstream of the Porto Primavera Reservoir, in the middle section of the Upper Paraná, and comprises a stretch of the principal channel of the Upper Paraná River (stations: PR1—immediately downstream of the Porto Primavera Reservoir dam; PR2—downstream of the Paranapanema River mouth; PR3—in the main stem of the Paraná River, next to the right margin; and PR4–downstream of the channel that connects the secondary channel to the Paraná River), Baía River, one secondary channel (station SC), and one floodplain lake (station FL) (Fig. 1).
The secondary channel (Baía River) is sub-parallel to the Upper Paraná River along its right bank. The Baía River has a width:depth ratio of 18:1. During flood periods, the current velocities decrease, and the current may reverse and flow upstream due to the entry of water from the Paraná River. The construction of the Porto Primavera dam interrupted the Baía River system and diminished the watercourse volume (Souza Filho et al., 2004). The floodplain lake (Garças Lake) is located on the right bank of the principal channel of the Paraná River and is permanently connected to this channel. This lake is 2,128 m in length and 14.1 ha in area, and it has a perimeter of 4,328 m and an average depth of 2 m.
Field and laboratory methods
Phytoplankton samples were taken at the subsurface (depth 20 cm) in the studied environments. Samples were taken each month from May 2007 to April 2008. Phytoplankton samples were preserved with acidified Lugol’s solution.
Water temperature (WT; °C), pH, electrical conductivity (cond.; μS cm−1) and dissolved oxygen (O2; mg l−1) were measured with portable digital potentiometers. Water transparency (m) was measured with a Secchi disc and the turbidity was measured using a turbidimeter. Concentrations of total phosphorus, soluble reactive phosphorus (Golterman et al., 1978), total nitrogen (Mackereth et al., 1978), nitrate (Giné et al., 1980) and ammonium (Koroleff, 1978) were also determined. All of the abiotic variables were obtained in the same time than the biological ones.
Total alkalinity (Alc.) was measured according to Mackereth et al. (1978), the euphotic zone (Zeu) was calculated as being 2.7-fold the depth of the Secchi disc (Cole, 1994) and the concentrations of total suspended matter (TSM) were determined according to Wetzel & Likens (2000).
The period between September 2007 and April 2008 was under the influence of La Niña (C.P.C., 2012). Rainfall and hydrometric levels (H. Level) of the Paraná River were provided by the Brazilian National Water Agency (ANA). Periods of potamophase were considered when the hydrometric level of the Paraná River was greater than 3.5 m, which signals the beginning of the flooding process of the lentic environments associated with the Upper Paraná River (Souza Filho et al., 2004). Periods of limnophase were considered when the hydrometric level of the Paraná River was lower than 3.5 m.
Phytoplankton density was estimated according to Utermöhl (1958) and Lund et al. (1958). The phytoplankton biovolume was estimated by multiplying the density of different taxa by their respective volumes. The volume of each cell was calculated from geometric models approximate the form of individuals (Sun & Liu, 2003).
Species contributing more than 5% to the total phytoplankton biovolume according to the methodology proposed by Kruk et al. (2002) and Huszar et al. (2003) were grouped in FGs according to Reynolds et al. (2002), Borics et al. (2007) and Padisák et al. (2009).
The relationships between the abiotic data and the biovolume of the phytoplankton FGs were analysed through canonical correspondence analysis (CCA; Ter Braak, 1986). The FGs biovolume values were log-transformed to minimise data variability. The statistical significance for the first two axes was tested by permutation test (Legendre et al., 2011). To identify which variables explained the greatest variability of phytoplankton FGs, forward selection of explanatory variables (Blanchet et al., 2008) were made. The CCA analysis was performed in the R-language environment (R Development Core Team, 2012).
Results
The pluviometric index values were less than 30 mm during most of the study period, with values greater than 40 mm recorded only in July and November 2007. The hydrometric levels were below 3.5 m between May 18, 2007 and March 11, 2008 (Fig. 2a). This period was characterised as limnophase. The highest discharge values were documented between March 18 and April 30, 2008 (Fig. 2b), being this period characterised as potamophase.
The water temperature was between 18 and 30°C with values less than 22°C (>18°C) only in the months of June, July and August. The pH remained close to neutral. The Paraná River had high Z eu values, which were very close to the Z max values. The electrical conductivity and alkalinity did not display clear seasonality, and the greatest variability was observed in the secondary channel (CV = 23 and 44%, respectively) (Table 1).
TSM and turbidity values showed high variation (CV >40%) in all of the sampling stations, with a trend of decreasing values in the period between May and October 2007. Higher values were verified between February and April 2008 to sampling stations located in the main stem and between November 2007 and April 2008 to floodplain lake and secondary channel (Table 1).
The greatest N-NO3 − concentrations values were registered in the Paraná River. In the secondary channel, the greatest TN concentrations values were found. SRP and TP average concentrations were between 7.1–9.4 and 9.4–13.9, respectively in the Paraná River, and the highest average values were observed in the floodplain lake and the secondary channel (Table 1).
We documented 168 taxa, which were distributed among the following groups: Chlorophyceae (35%), Bacillariophyceae (23%), Cyanobacteria (15%), Euglenophyceae (10%), Xanthophyceae (5%), Zygnemaphyceae (4%), Cryptophyceae (4%), Chrysophyceae (3%) and Dinophyceae (1%).
We recorded 73 taxa in the Paraná River (28 taxa at station PR1, 33 at station PR2, 28 at station PR3, and 46 at station PR4), 74 taxa in the secondary channel, and 129 taxa in the floodplain lake. Biovolume values were high in the floodplain lake and secondary channel and low in the stations located in the Paraná River, with the exception of station PR4 in November and PR2 in November and December, when there were Dolichospermum planctonicum (Brunnth.) Wacklin, Hoffm. et Komárek (basionym: Anabaena planctonica Brunnthler) and Radiocystis fernandoi Komárek & Komárk.-Legn. blooms, respectively. The greatest values occurred during limnophase, with the maximum value found in the secondary channel in November. An accentuated reduction of biovolume values was documented during the month of April, during potamophase, particularly in the floodplain lake and secondary channel (Fig. 3).
Cyanobacteria contributed the most to the phytoplankton biovolume at all of the sampling stations except for the floodplain lake, where Bacillariophyceae was dominant (Fig. 3). We documented nine FGs (A, C, E, J, K, M, P, W 1 and X 2 ) in the floodplain lake, five (C, H 1 , M, P and X 2 ) in the secondary channel, four (C, M, P and X 2 ) at station PR1, four (C, M, P and X 2 ) at station PR2, four (H 1 , M, P and X 2 ) at station PR4 and three (A, M and X 2 ) at station PR3 (Fig. 4; Table 2).
The two first axes of the CCA analysis were significant (eigenvalue = 0.63 and 0.41, respectively, P < 0.05) and explained 31% of the total variance. Secondary channel was discriminated in the first axis of the CCA and represented by FGs H 1 (Dolichospermum planctonicum) and P (Aulacoseira granulata Ehrenberg (Simonsen) var. granulata), which were correlated with higher N-NH4 + (−0.38) and SRP (−0.42) concentrations (Fig. 5).
The first CCA axis also evidenced a temporal gradient for the stations PR1, PR3 and PR4 and particularly discriminated February, May, July and November from the others months, as seen in the right of the diagram, influenced by higher values of pH (0.76) with lower N-NH4 + and SRP concentrations (Fig. 5).
Station PR2 was discriminated in the second axis of the CCA and represented by FG M (R. fernandoi), which was correlated with higher N-NO3 − concentrations (0.67) and Z eu (0.52). Floodplain lake was also discriminated in the second axis of the CCA and represented by FGs A, C, E, J, K, W 1 and X 2 , which were correlated with lower SRP concentrations and Paraná River hydrometric levels (Fig. 5). However, the results of forward selection of explanatory variables showed that only the variables N-NO3 − (P = 0.01) and pH (P = 0.02) were significant.
Discussion
The increase in transparency and the decrease in total phosphorus concentrations of the Upper Paraná River as compared with data obtained in sample series from 1987 to 1988 (Thomaz et al., 1992) and 2000 to 2008 (Roberto et al., 2009) may be attributed to the retention and sedimentation processes of seston in the cascade of reservoirs located upstream. These processes have shown an increase after the construction of the dam of the Porto Primavera Reservoir. The high concentrations of TN observed in the Upper Paraná River, which were similar to those recorded before the construction of the reservoir (Thomaz et al., 1992), proved to have the smallest influence of this reservoir on nitrogen concentrations.
Because light and nitrogen were not limiting to the phytoplankton development in the Upper Paraná River, the low biovolume values may be attributed to low phosphorous concentrations and high discharge. Despite the Upper Paraná River discharge has suffered reduction after construction of the Porto Primavera (Souza Filho et al., 2004), it has strongly influenced the phytoplankton and abiotic variables of this river.
Fluctuations in the hydrometric levels of fluvial systems play a fundamental role in the structure, function and integrity of the adjacent aquatic environments (Leira & Cantonati, 2008). Impacts caused by climatic changes on these systems can provoke alterations in adjacent environments, communities and species (Abrahams, 2008). In addition to the influence of operational procedures of the upstream dams in the Upper Paraná River, the hydrological cycle was under the influence of La Niña (C.P.C., 2012). This influence was reflected in the low hydrometric levels of the Paraná River and characterised most of the study period as occurring during limnophase, with low connectivity between the river and the secondary channel and floodplain lake.
A sharp reduction in the hydrometric level of shallow lakes promotes the total mixing of the water column by the action of winds (Hofmann et al., 2008). The lower connectivity between the Paraná River and the floodplain lake, the low Z max values and the high nutrient concentrations observed during limnophase, period under the influence of La Niña, may have contributed to the dominance of the FGs C and P in the floodplain lake. However, in the secondary channel, in this period, the lower connectivity and the greater stability of the water column favoured FG H 1 (D. planctonicum).
The D. planctonicum blooms in the secondary channel may have resulted from recruitment from the lentic environments associated with the channel, in which blooms of various species of Dolichospermum are common (Train & Rodrigues, 2004). The lentic characteristics of the Baía River certainly favoured D. planctonicum blooms, supporting the findings of Train & Rodrigues (1998). However, the higher values of biovolume of this species recorded in the secondary channel in the present study may be related to the decrease in the river’s discharge after construction of the Porto Primavera Reservoir.
As reported in a study in the Baía River during the hydrological cycle of 1993–1994, Dolichospermum circinalis, Dolichospermum solitarium and Dolichospermum spiroides were dominant in the period of low water and water column stability and were substituted by Aulacoseira granulata var. granulata in conditions of total mixing and higher N-NO3 − concentrations (Train & Rodrigues, 1998). The absence of FG H 1 and the dominance of FG P in this secondary channel in April, in the present study, in conditions of water column instability, indicate a likely interruption in the successional sequence and a reversion to a previous stage of phytoplankton succession.
The higher concentrations of N-NO3 − and N-NH4 + documented during the dominance of FG P and the low concentrations of these nutrients when FG H 1 was dominant might also contribute to the successional dynamics of the phytoplankton community in the secondary channel.
The low contribution of Aulacoseira granulata (FG P) to Upper Paraná River phytoplankton biovolume values, which was dominant in previous periods in the same stretch (Rodrigues et al., 2009) and in the Middle Paraná River (Devercelli, 2010) is probably due to seston sedimentation and retention in the Porto Primavera Reservoir and reduction in discharge and phosphorus concentrations in the Upper Paraná River, after construction of the dam reservoir (Souza Filho et al., 2004; Roberto et al., 2009).
The higher water level in the Paraná River in April led to a greater connectivity with the floodplain lake, dilution of the phytoplankton community in this environment and changes in dominance of FGs. The high contribution of FG X 2 in the floodplain lake, which was the principal dominant in all stations of Upper Paraná River, confirms the greater influence of the river on the lake during this month.
The dominance of FG X 2 in Upper Paraná River and in others aquatic systems in the Paraná Basin (Garcia de Emiliani, 1997; O’Farrell et al., 2003; Devercelli, 2010) may be attributed to opportunistic characteristics of cryptomonads, which are adapted to a turbulent water column, a high surface:volume ratio and the rapid absorption of nutrients (Reynolds et al., 2002). According to Reynolds (2000), phytoplankton species recruitment upstream in rivers is intensified when the species have a small cell size, high growth rate and photosynthetic efficiency, and low rate of sedimentation.
Dispersion acts as a homogenising agent, independent of the local environmental conditions (Leibold et al., 2010). In addition to the low environmental variability among the sampling sites in the main channel, the dominance of FGs M and X 2 in all the stations may be attributed to species dispersion.
The high biovolume values of R. fernandoi (FG M) recorded at station PR2 in November and December were the result of inflows of inocula into the Paranapanema River, likely from the Rosana Reservoir. Although potamoplankton is represented by forms that are able to survive selective forces that act on rivers (Reynolds, 2000), some populations are maintained by inocula from ‘dead zones’ (Reynolds, 2000; Thorp et al., 2008).
Radiocystis fernandoi showed high values of biovolume in the Rosana Reservoir in 2001 and 2002 (Rodrigues et al., 2005; Train et al., 2005, cited as Microcystis aeruginosa) and in tributaries of the left bank of this reservoir in 2003 (Borges et al., 2008) and 2006 (Fonseca et al., 2009; Borges et al., 2010). Although this species was registered at stations PR1, PR3 and PR4, colonisation of the principal channel of the Paraná River was likely limited by the high discharge and low concentrations of phosphorus.
The biovolume values of Chroococcales at station PR3 were higher than those measured in the period from March 1993 to February 1994 (Train & Rodrigues, 2004; Rodrigues et al., 2009). Although R. fernandoi had been recorded at station PR1, which is situated immediately downstream of the Porto Primavera Reservoir, the low values recorded show the low contribution of inocula in this reservoir to the downstream stretch of the Paraná River.
There are three stages in phytoplankton species recruitment: the dispersion of pre-existing populations, establishment of low population density, and posterior colonisation with an increase in spatial occupation. The downstream flow in rivers permits the dispersion of viable inocula, which can colonise downstream regions (Talling & Prowse, 2010). Considering the ‘network dynamics hypothesis’ and the effect of confluence in floodplain rivers (Benda et al., 2004), it is possible that the colonisation of R. fernandoi observed in the floodplain lake resulted from the inflow of inocula from the Paranapanema River.
The high biovolume values of R. fernandoi documented in the floodplain lake confirm the hypothesis that the biovolume of taxonomic and functional phytoplankton groups is influenced by the dispersion of species from reservoirs located upstream of the stretch of the Upper Paraná River, particularly from the Rosana Reservoir.
Other floodplain environments of the Upper Paraná River might also act in the dispersion of R. fernandoi to the floodplain lake, as blooms of this species have been documented there (Train et al., 2004 (cited as Microcystis aeruginosa); Bovo-Scomparin & Train, 2008).
Radiocystis fernandoi was placed in FG M due to its dominance in the floodplain lake and in other tropical environments (Bovo-Scomparin & Train, 2008; Borges et al., 2008, 2010) under eutrophic conditions and water column mixing, in addition to the morphological and functional similarities that this species shares with Microcystis aeruginosa. These results contrast with the proposal by Padisák et al. (2009) to place this species in FG L O , which is characteristic of mesotrophic environments and is represented by species that are sensitive to conditions of deep or prolonged mixing (Reynolds et al., 2002).
The recruitment of phytoplankton in the Upper Paraná River was evidenced mainly to FG M, with dispersion of R. fernandoi populations from Rosana Reservoir, establishment of low population biovolume in the main stem of Upper Paraná River and posterior colonisation in the floodplain lake.
The low values of phytoplankton biovolume documented in the main stem of the Upper Paraná River indicate that the stretch studied here does not offer favourable conditions for phytoplankton development. These results confirm the hypothesis that although this river, in this confluence zone, receives phytoplankton biovolume from upstream inflow and from left and right bank tributaries, the depletion of phytoplankton is due to retention and seston sedimentation in the Porto Primavera Reservoir in addition to the high discharge and dilution in this section of the Upper Paraná River.
References
Abrahams, C., 2008. Climate change and lakeshore conservation: a model and review of management techniques. Hydrobiologia 613: 33–43.
Benda, L., K. Andras, D. Miller & P. Bigelow, 2004. Confluence effects in rivers: interactions of basin scale, network geometry, and disturbance regimes. Water Resources Research 40: W05402.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632.
Borges, P. A. F., S. Train & L. C. Rodrigues, 2008. Estrutura do fitoplâncton, em curto período de tempo, em um braço do reservatório de Rosana (ribeirão do Corvo, Paraná, Brasil). Acta Scientiarum Biological Sciences 30(1): 57–65.
Borges, P. A. F., S. Train, J. D. Dias & C. C. Bonecker, 2010. Effects of fish farming on plankton structure in a Brazilian tropical reservoir. Hydrobiologia 649: 279–291.
Borics, G., G. Várbíró, I. Grigorszky, E. Krasznai, S. Szabó & K. T. Kiss, 2007. A new evaluation technique of potamo-plankton for the assessment of the ecological status of rivers. Archives fur Hydrobiologie Supplement 161(3–4): 465–486.
Bovo-Scomparin, V. M. & S. Train, 2008. Long-term variability of the phytoplankton community in an isolated floodplain lake of the Ivinhema River State Park, Brazil. Hydrobiologia 610: 331–344.
Brown, B. L. & C. M. Swan, 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580.
CESP, 1998. Conservação e manejo nos reservatórios: limnologia, ictiologia e pesca. Série Divulgação e Informaçãom, São Paulo: 220.
Cloern, J. E., 2007. Habitat connectivity and ecosystem productivity: implications from a simple model. American Naturalist 169(1): 21–33.
Cole, G. A., 1994. Textbook of Limnology. Waveland Press Inc., Illinois.
Coulon, A., J. F. Cosson, J. M. Angibault, B. Cargnelutti, M. Galan, N. Morellet, E. Petit, S. Aulagnier & J. M. Hewison, 2004. Landscape connectivity influences gene flow in a roe deer population inhabiting a fragmented landscape: an individual-based approach. Molecular Ecology 13: 2841–4850.
C.P.C., 2012. Climate Prediction Center. National Weather Service. Available at: http://www.cpc.noaa.gov.
Devercelli, M., 2010. Changes in phytoplankton morpho-functional groups induced by extreme hydroclimatic events in the Middle Paraná River (Argentina). Hydrobiologia 639: 5–19.
Fonseca, I. A., N. S. Siqueira & L. Rodrigues, 2009. Algas Perifíticas a montante e jusante do local de instalação de tanques-rede em tributários do reservatório de Rosana – Paraná, Brasil. Acta Scientiarum 31(2): 135–141.
Garcia de Emiliani, M. O., 1997. Effects of water level fluctuations on phytoplankton in a river-floodplain lake system (Paraná River, Argentina). Hydrobiologia 357: 1–15.
Giné, M. F., H. Bergamin, E. A. G. Zagatto & B. F. Reis, 1980. Simultaneus determination of nitrite and nitrate by flow injection analysis. Analytica Chimica Acta 114: 191–197.
Golterman, H. L., R. S. Clymo & M. A. M. Ohstad, 1978. Methods for Physical and Chemical Analysis of Freshwater. Blackwell Scientific Publication, Oxford.
Hofmann, H., A. Lorke & F. Peeters, 2008. Temporal scales of water-level fluctuations in lakes and their ecological implications. Hydrobiologia 613: 85–96.
Huszar, V. L. M., C. Kruk & N. Caraco, 2003. Steady-state assemblages of phytoplankton in four temperate lakes (NE U.S.A.). Hydrobiologia 502: 97–109.
Koroleff, K., 1978. Determination of ammonia. In Grasshoff, K. & E. Kremling (eds), Methods of Seawater Analysis. Verlag Chemie, Winhein.
Kruk, C., N. Mazzeo, G. Lacerot & C. S. Reynolds, 2002. Classification schemes for phytoplankton: a local validation of functional approach to the analysis of species temporal replacement. Journal of Plankton Research 24: 901–912.
Legendre, P., J. Oksanen & C. J. F. Ter Braak, 2011. Testing the significance of canonical axes in redundancy analysis. Methods in Ecology and Evolution 2: 269–277.
Leibold, M. A., E. P. Economo & P. Peres-Neto, 2010. Metacommunity phylogenetics: separating the roles of environmental filters and historical biogeography. Ecology Letters 13: 1290–1299.
Leira, M. & M. Cantonati, 2008. Effects of water-level fluctuations on lakes: an annotated bibliography. Hydrobiologia 613: 171–184.
Lund, J. W. G., C. Kipling & E. D. Lecren, 1958. The inverted microscope method of estimating algal number and the statistical basis of estimating by counting. Hydrobiologia 11: 980–985.
Mackereth, F. Y. H., J. R. Heron & J. F. Tailing, 1978. Water analysis: some revised methods for limnologists. Freshwater Biological Association (Scientific Publication No. 36).
Nilsson, C., C. A. Reidy, M. Dynesius & C. Revenca, 2005. Fragmentation and flow regulation of the world’s large river systems. Science 308: 405–408.
O’Farrell, I., R. Sinistro, I. Izaguirre & F. Unrein, 2003. Do steady state assemblages occur in shallow lentic environments from wetlands? Hydrobiologia 502: 197–209.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
R Development Core Team, 2012. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
Reynolds, C. S., 2000. Hydroecology of river plankton: the role of variability in channel flow. Hydrological Processes 14: 3119–3132.
Reynolds, C. S., V. L. M. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Roberto, M. C., N. F. Santana & S. M. Thomaz, 2009. Limnology in the Upper Paraná River floodplain: large-scale spatial ad temporal patterns, and the influence of reservoirs. Brazilian Journal of Biology 69(2 Suppl): 717–725.
Rodrigues, L. C., S. Train, B. M. Pivato, V. M. Bovo-Scomparin, P. A. F Borges & S. Jati, 2005. Assembléias fitoplanctônicas de 30 Reservatórios do estado do Paraná. In Rodrigues, L., S. M. Thomaz, A. A. Agostinho & L. C. Gomes (eds), Biocenoses em reservatórios: padrões espaciais e temporais. RIMA, São Carlos: 57–72.
Rodrigues, L. C., S. Train, V. M. Bovo-Scomparin, S. Jati, C. C. J. Borsalli & E. Marengoni, 2009. Interannual variability of phytoplankton in the main rivers of the Upper Paraná River floodplain, Brazil: influence of upstream reservoirs. Brazilian Journal of Biology 69(2 Suppl): 501–516.
Souza Filho, E. E., P. C. Rocha, E. Comunello & J. C. Stevaux, 2004. Effects of the Porto Primavera Dam on physical environment of the downstream floodplain. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and Its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 55–74.
Sun, J. & D. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25(2): 1331–1346.
Talling, J. F. & G. A. Prowse, 2010. Selective recruitment and resurgence of tropical river phytoplankton: evidence from the Nile system of lakes, rivers, reservoirs and ponds. Hydrobiologia 637: 187–195.
Ter Braak, C. J. F., 1986. Canonical correspondence analysis: a new eigenvector -technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
Thomaz, S. M., M. C. Roberto, F. A. Lansac Toha, A. F. Lima & F. A. Esteves, 1992. Características limnológicas de uma estação de amostragem do alto rio Paraná e outra do baixo rio Ivinhema – (PR, MS-Brasil). Acta Limnologica Brasiliensia 4: 32–51.
Thorp, J. H., M. C. Thoms & M. D. Delong, 2008. The Riverine Ecosystem Synthesis: Toward Conceptual Cohesiveness in River Science. Elsevier, London.
Train, S. & L. C. Rodrigues, 1998. Temporal fluctuations of the phytoplankton community of the Baía River, in the upper Paraná River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 361: 125–134.
Train, S. & L. C. Rodrigues, 2004. Phytoplankton assemblages. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation. Backhuys, Leiden: 103–124.
Train, S., L. C. Rodrigues, V. M. Bovo, P. A. F. Borges & B. M. Pivato, 2004. Phytoplankton composition and biovolume in environments of the Upper Paraná river. In Agostinho, A. A., L. Rodrigues, L. C. Gomes, S. M. Thomaz & L. E. Miranda (eds), Structure and Functioning of the Paraná River and Its Floodplain. EDUEM, Maringá: 63–74.
Train, S., S. Jati, L. C. Rodrigues & B. M. Pivato, 2005. Distribuição espacial e temporal do fitoplâncton em três reservatórios da Bacia do Rio Paraná. In Rodrigues, L., S. M. Thomaz, A. A. Agostinho & L. C. Gomes (eds), Biocenoses em reservatórios: padrões espaciais e temporais. RIMA, São Carlos: 73–85.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen phytoplankton-methodic. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Wetzel, R. G. & G. E. Likens, 2000. Limnological Analysis. Springer, New York.
Acknowledgments
The authors are grateful to the Núcleo de Pesquisas em Limnologia, Ictiologia e Aqüicultura (Nupélia) at Universidade Estadual de Maringá for logistic support; CNPq/PELD for financial support and CAPES for a scholarship granted to the first author. Special thanks are due to the two anonymous reviewers whose suggestions helped to improve the original submission.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Bovo-Scomparin, V.M., Train, S. & Rodrigues, L.C. Influence of reservoirs on phytoplankton dispersion and functional traits: a case study in the Upper Paraná River, Brazil. Hydrobiologia 702, 115–127 (2013). https://doi.org/10.1007/s10750-012-1313-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1313-8