Abstract
Changes to the structure of the phytoplankton community and to the physical and chemical variables of the water were investigated in oxbow lakes with different levels of connection to a tropical river and subject to annual hydrological pulse variations. The selected lentic environments are located at the mouth region of the main tributary in a reservoir built for water storage and electric power generation. The temporal variation of phytoplankton in the studied lentic environments can be attributed mainly to the hydrological level of the river. A similar variation pattern of the ecological attributes was observed in the structure of the phytoplankton community in the connected lakes and Paranapanema River, evidencing the high degree of association that the lacustrine systems maintain with the river. The highest values of richness and diversity for connected environments were observed at the end of the emptying period and in the drought. However, considering the isolated lake, the highest values of these attributes were recorded during the flooding period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands, consisting of a river channel, islands, tributaries, oxbow lakes, and floodplains, form a single ecological unit, know as a “river–floodplain system” (Junk et al., 1989), which is functionally dependent on the horizontal flow of water (Junk, 1997; Neiff, 2003). Oxbow lakes are very important in the floodplain because they play a great role in the maintenance of the biodiversity of wetlands and present a high variety of ecological niches for aquatic organisms (Thomaz et al., 1997; Rodrigues et al., 2002; Taniguchi et al., 2004). These water bodies are considered rather complex environments, and the mechanisms that affect their metabolism are still much debated. However, the annual variation of the river level is a predominant controlling factor in these places (Domitrovic, 2003; Henry, 2005; Granado et al., 2009; Mihaljevíc et al., 2009; Henry et al., 2011). According to Thomaz et al. (2007), flooding joins water bodies with different hydrological characteristics within the landscape, and as a result, the ecological processes and biotic communities tend to be similar across the different environments during this phase due to the increase in the connectivity and in the exchange of water, sediment, nutrients, and organisms (Neiff, 2001; Rodrigues et al., 2002; Domitrovic, 2003).
Oxbow lakes linked to rivers are directly influenced by the patterns of variation of the physical and chemical factors of the water lateral inflow (Granado & Henry, 2008), affecting the structure of the biotic communities, especially with regard to biodiversity (Ward et al., 1999; Amoros & Bornette, 2002).
Variation in the water level is considered to be the main controlling factor of the changes in the structure of the phytoplankton community, such as in the tropical wetlands of the Pantanal of Mato Grosso (Oliveira & Calheiros, 2000; Loverde-Oliveira & Huszar, 2007), the Amazon (Huszar & Reynolds, 1997; Putz & Junk, 1997; Melo & Huszar, 2000), the Paraná River (Garcia De Emiliani, 1993, 1997; Domitrovic, 2003), the Araguia River (Nabout et al., 2006), the Mary River (Townsend, 2006), the Murray River (Butler et al., 2007) in Australia, and in the plains of rivers of temperate areas, such as the Daugava River in Letony (Paidere et al., 2007) and in the Danube in northeast Croatia (Mihaljevíc et al., 2009).
However, as ecosystems, wetlands are rarely found in their natural state today, as they have been modified or eliminated to meet the requirements of hydraulic constructions (dykes, canals, bed flattening, and construction of dams), particularly in highly industrialized countries in North America and Europe. Long stretches of the Rhine River channel were straightened in the nineteenth century (Junk, 1997). The mouth of the Danube River, the last floodable large plain of Europe, has also been extensively modified (Junk, 1980, 1997; Mihaljevíc et al., 2009). The alterations have led to a reduction in the investigation of the floodable areas in temperate regions. Ward et al. (1999) reported that artificial changes to the fluvial dynamic break the natural regime of disturbances that sustain the diversity of successional stages and high levels of connectivity in the fluvial region, resulting in less heterogeneity of habitats and, consequently, lower biodiversity.
In tropical regions, the floodplains have also been altered, mostly by the implementation of hydroelectric power plants (Junk, 1997). In Brazil, the main hydrographic basins have been altered with the construction of reservoirs for the generation of electric power (Junk, 1997). The dams constitute a fluvial discontinuity of anthropic origin which has ecological consequences, as damming results in the retention of organic matter, energy, and nutrients (Henry, 2003).
However, in the mouth areas of tributaries in reservoirs, floodable areas such as oxbow lakes can still be found, as, for example, those in the mouth zone of the Paranapanema River in the Jurumirim reservoir (São Paulo State, Brazil). In these locations, the inundation behavior can be distinct from that found in “true” floodplains, as the large volume of water accumulated in the reservoir acts as a system that dampens the hydrological pulses of the tributaries, altering their frequency, duration, and amplitude (Henry, 2005). Of the three oxbow lakes investigated in this region, two were permanently connected to the Paranapanema River, although with different levels of connectivity, determined by the modification of water levels.
The goal of the study was to examine the effects of water level on the modifications of phytoplankton community structure during a year in three marginal lakes, to understand the fluctuation patterns according to the connectivity level of lacustrine environments with a river. Two hypotheses were considered: (i) the temporal variation on community structure is determined predominantly by the hydrological level in the oxbow lakes; (ii) the phytoplankton composition pattern is influenced by their connection level with the river.
Study area
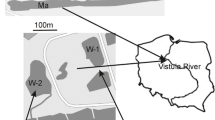
The present study was conducted on the Paranapanema River and three oxbow lakes Camargo Lake, Coqueiral Lake, and Cavalos Lake, which have different levels of connection with the lotic system in the mouth zone at the Jurumirim Reservoir, São Paulo, Brazil (Fig. 1).
The Paranapanema River rises in the Paranapiacaba mountain region (to the east of São Paulo State, Brazil) and discharges into the River Paraná. It is one of the main affluents, and as it flows from the coast (Atlantic Plateau) to the countryside is classified as endorheic (Henry & Nogueira, 1999).
The study area is located upstream of the Jurumirim Reservoir (the first of a series of reservoirs for electric power generation), in the south east region of São Paulo State. The transition region between the Paranapanema River and the Jurumirim Reservoir is characterized by a pronounced reduction in the water velocity (Casanova & Henry, 2004) and a great rate of sedimentation of allochthonous material transported by the River (Henry & Maricato, 1996). The local landscape is made up of numerous oxbow lakes, three of which—Camargo Lake, Coqueiral Lake, and Cavalos Lake—have been intensively studied (Moschini-Carlos et al., 1998; Henry, 2003, 2005). The lakes have different morphometric characteristics and degrees of connection with the Paranapanema River (Henry, 2005; Panarelli et al., 2008). Coqueiral Lake has the greatest surface area (641,000 m2), low mean depth (1.8 m), and great connection with the river (around 40 m width), while Camargos Lake has a small surface area (224,500 m2), greater mean depth (3.2 m), and little connection with the river (around 5 m width). The third lake (Cavalos) is isolated from the river channel and has the smallest surface area (8,600 m2) and smallest mean depth (1.4 m). A dominant floating graminea (Echinochloa polystachya (H.B.K.) Hitchcock) is observed at the margins of both oxbow lakes, at connection sites with Paranapanema River, and also at the shoreline of the isolated lake.
Materials and methods
Water samples were collected on a monthly basis for 1 year (from July 2004 to July 2005) in the central region of the Cavalos Lake and at two sites of the Paranapanema River and lakes Camargo and Coqueiral.
Phytoplankton community was quantified at 400× magnification with an inverted microscope (Utermohl, 1958). Counting was performed for 100 individuals of the most frequent species or by the stabilization curve of the number of species. Richness was evaluated as a function of the number of species found during the study period. The density of phytoplankton organisms was calculated according to APHA (1995). The biovolume (mm3 l−1) was expressed by multiplying the density of each species by the mean volume of cells (Wetzel & Likens, 1991; Hillebrand et al., 1999). The predominant species were those which contributed with at least 1% of the total value of the community totaling around 80% of the biovolume of each environment (Sommer et al., 1993). The Shannon index was used to calculate species diversity (H′) (Shannon & Weaver, 1963).
Correlate physical and chemical variables of the water were also analyzed: temperature (Thermistor Toho Dentan ET-3), water level, electric conductivity (Hatch conductivimeter with values corrected for 25°C, following Golterman et al., 1978), stream velocity (ELE stream flowmeter), water transparency (Secchi disk), suspended solids (Teixeira & Kutner, 1962), alkalinity (Mackeret et al., 1978), pH (pH meter, Micronal B380), dissolved oxygen (Winkler method, described by Golterman et al., 1978), total nitrogen and total phosphorus (Valderrama, 1981), and reactive silicate (Golterman et al., 1978).
The rainfall data were obtained from the pluviometric station E5-017 (Water and Electric Power Department, DAAE), located in the city of Angatuba, 30 km away from the study site. Water level values were provided by the Jurumirim Reservoir operating department of the Duke Energy Company. A previous study showed that the water levels at the dam of Jurumirim and at the site of the inflowing river in the reservoir present a similar pattern of variation (Pompêo et al., 1999). A detailed description of the results for the physical and chemical variables of the water was presented in Granado and Henry (2008).
Data analysis
A factorial variance analysis (ANOVA) was applied using the GML procedure of the SAS system (version 9.12) to compare the abiotic data on spatial (environments and stations, a total of seven sampling sites) and temporal scales (the 13 months of samplings). When the analysis indicated a significant difference (P < 0.05), the Tukey test was applied to determine the origin of the differences.
Principal component analysis (PCA) was also performed with the abiotic data (alkalinity, conductivity, dissolved oxygen, suspended solids, temperature, pH, transparency, total nitrogen, total phosphorus, silicate and water level), and canonical correspondence analysis (CCA) with the phytoplankton community data (biomass of the predominant species). These analyses were performed based on the covariance matrices with the abiotic data fitted by the ranging amplitude of variation ([(xx min)/(x max − x min)]) and the biological data (biovolume of the describing species) by [log (x + 1)]. To test the significance level of the first two canonical axes, Monte Carlo test (99 permutations; P < 0.05) was used. Pearson correlations (r) were computed from ordination values (position of the sampling unities on the axes) and individual biotic and abiotic variables used in the ordination shape (McCune & Mefford, 1997). Data fitting was performed with the software FITOPAC (Shepherd, 1996), and the PCA and the CCA with the software PCORD, version 3.1, for Windows (McCune & Mefford, 1997).
Results
The annual fluctuation of the water level, a consequence of the hydrological variation and of the operation of the downstream reservoir, enabled the distinction of five phases during the year: emptying in 2004 (July, August, and September of 2004), drought (October, November, and December 2004), filling (January and February 2005), high water (March and April 2005), and emptying in 2005 (May, June, and July 2005) (Fig. 2). During the year of study, the water level of the Jurumirim Reservoir was always higher than 563.6 m (frontier between connection/disconnection of Camargo Lake and Coqueiral Lake with the Paranapanema River). In the study period, the lakes remained thus connected to the water course.
No variations on abiotic factors (Tables 1, 2) and on the structure of phytoplankton community (Table 3) between sampling stations in two of the oxbow lakes (Coqueiral and Camargo) and in Paranapanema River were found.
The variation pattern of the connected lakes (Camargo and Coqueiral) is similar to that of the Paranapanema River in relation to the main physical and chemical variables of the water (Tables 1, 2). The average values of the main abiotic variables used in the PCA and in the CCA are presented in Table 2, together with the Tukey test results. With the exception of dissolved oxygen, none of the other abiotic variables showed significant differences between the Paranapanema River and the connected lakes, according to the ANOVA and Tukey test (Tables 1, 2) and the PCA (Fig. 3; Table 4). In Cavalos Lake, high alkalinity, conductivity, total nitrogen, and total phosphorus were significantly different from those of the other two connected lakes and the Paranapanema River (Table 2).
Monthly ordination by PCA (axes 1 and 2) of samples from the Paranapanema River and Lakes Camargo, Coqueiral, and Cavalos from July 2004 to July 2005. Letters together with sample units indicate the beginning of the sampling months (jl/4 July 2004, ag August, s September, o October, n November, d December, ja January, f February, mr March, ap April, my May, jn June, jl/5 July 2005). Other legends: Alk alkalinity, Cond conductivity, DO dissolved oxygen, PT phosphorus, NT nitrogen, SS suspended solids, Si silicate, Level hydrometric level, Transp water transparency
The PCA enabled the distinguishing of three clusters for the sample units from the Paranapanema River and the Camargo Lake and Coqueiral Lake: (1) the emptying phase of 2004, characterized by high transparency and depth values and low quantities of suspended solids; (2) the end of the emptying phase of 2005, with high dissolved oxygen concentrations; and (3) the end of the filling and high-water phases and the beginning of the emptying phase of 2005, for which the distribution of the sampling units, close to the axis intersection, demonstrated correlation with the low values of all of the variables, probably due to the effects of dilution. The PCA also showed that the Cavalos Lake behaves differently from the other environments, principally toward the end of the filling and high-water phases, until the middle of the emptying phase of 2005, which was characterized by sampling units with high alkalinity and conductivity values and low oxygen concentrations.
With regard to the phytoplankton community, 180 species were identified in the Paranapanema River and the oxbow lakes during the study phase. The most representative class was Chlorophyceae with 40% of the total richness with 73 species, followed by Bacillariophyceae (30) and Cyanobacteria (27), Euglenophyceae (18), Zygnemaphyceae (17), and Cryptophyceae (7). Chrysophyceae (4), Dinophyceae (3), and Xanthophyceae (1) also contributed to the richness.
In Camargo Lake, 117 and in Cavalos Lake, 97 species were identified. The greatest richness values were recorded in the Paranapanema River (135 taxa) and in Coqueiral Lake (138 taxa), both of which had similar richness values, with higher values in the drought season, a decrease in the filling phase, an increase in the high-water phase, and another reduction in the emptying phase of 2005 (Fig. 4). In Camargo Lake, low richness was observed in the filling phase (January and February 2005). However, the drought phase and high-water phase had a high number of species. In the Cavalos Lake, the values were lower in the emptying phase, increased during the filling phase, and reached the greatest richness value during the high-water phase (Fig. 4).
In the Paranapanema River and in Coqueiral Lake, a similar pattern of variation in diversity was found. It was lower at the end of the filling phase and during the high-water phase, and higher in the drought phase, when the greatest values were recorded in the river and the lake. In Camargo Lake, the diversity was greater in the beginning of the emptying phase of 2004 and during the drought phase, decreased at the end of the drought phase and during the filling phase, and increased during the high-water phase. In Cavalos Lake, high values were found at the end of the filling phase, which remained high during the high-water phase (Fig. 4).
With regard to the phytoplankton density, the River and the connected lakes (Camargo and Coqueiral) showed a similar pattern of variation. Lower values were recorded in the emptying phase, with an increase in the drought phase. A reduction was recorded in the filling phase and a new increase occurred in the high-water phase, when the attained density was maximum (Fig. 5). In Cavalos Lake, the greatest density of phytoplankton was found in the end of the filling phase (February 2005) (6,552 org ml−1), with the highest value of the other two oxbow lakes and the river. In the emptying (July–September 2004) and drought (October–December 2004) phases, low densities were found (Fig. 5).
In the three oxbow lakes and in the river, high values of biomass occurred during the high-water phase, with the exception of the Cavalos Lake, which had the highest biovolume (4.1 mm3 l−1), twofold that of the other two lentic and lotic ecosystems at the end of the filling phase (February 2005) (Fig. 5). The predominant groups of phytoplankton in the Paranapanema river were Chlorophyceae and Bacillariophyceae, except in February 2005. In the Camargo Lake, Cryptophyceae were predominant in the filling period. Cyanobacteria were predominant in the final phase of high water and in the emptying. In Coqueiral Lake, Chlorophyceae were predominant in the same two last mentioned phases. In the Cavalos Lake, the predominant groups were Cyanobacteria in the drought phase and the initial filling period and the Euglenophyceae in the final of the filling and the high water. Cryptophyceae presented high contribution in the emptying phase in 2004 and 2005 (Fig. 6).
The predominant species in the Paranapanema River were Closteriopsis acicularis (Chodat) J.H. Belcher & Swale during the whole of the high-water phase (March and April 2005) and Cryptomonas brasiliensis A. Castro, C. Bicudo, and D. Bicudo in July 2005. In Camargo Lake, C. brasiliensis predominated in the end of the drought phase and the beginning of the filling phase and the emptying phase of 2005. This species also predominated in Coqueiral Lake in part of the drought phase (November), the filling phase (January), the high-water phase (April), and in the emptying phase of 2005. Cavalos Lake had a pattern different from those of the other two oxbow lakes and the river; C. brasiliensis predominated in the emptying phase of 2004 and Chroococus minutus (Kützing) Nägeli predominated in the same phase in 2005.
Some predominant species were common to the River and the connected lakes, such as Aulacoseira granulata var. granulata (Ehrenberg) Simonsen, Cyclotella meneghiniana Kützing, Botryococcus braunii Kützing, Eutetramorus fottii (Hindak) Komárek, Radiococcus planctonicus Lund, and Dinobryon bavaricum Imhof; Eudorina elegans Ehrenberg and C. brasiliensis predominated in the oxbow lakes and in the river. The latter stood out in nearly the entire assemblage, especially in the most unstable periods, as in the end of the drought season and during the filling phase (Table 5).
CCA analysis (Fig. 7; Table 6) of the biomass data for the 16 predominant species in all of the environments (the three lakes and the river) and of the 11 environmental variables revealed a spatial gradient along axis 1, with sampling units allocated from left to right, that is, from Paranapanema River to Cavalos Lake.
Monthly ordination by CCA (axes 1 and 2) of the sampling units as a function of abiotic and biological (biomass) variables of samples from the Paranapanema River and Camargo, Coqueiral, and Cavalos Lake from July 2004 to July 2005. Letters together with sample units indicate the beginning of the sampling months (jl/4 July 2004, ag August, s September, o October, n November, d December, ja January, f February, mr March, ab April, mi May, jn June, jl/5 July 2005). Other legends: Alc alkalinity, Cond conductivity, OD dissolved oxygen, PT phosphorus, NT nitrogen, Adeli Aphanocapsa delicatissima, Aminu Aphanotece minutíssima, Agrg Aulacoseira granulata var. granulata, Cmen Cyclotella meneghiniana, Cstel C. stelligera, Cacic Closteriopsis acicularis, Cbras Cryptomonas brasiliensis, Dbav Dinobryon bavaricum, Eleg Eudorina elegans, Efott Eutetramorus fottii, Rplanc Radiococcus planctonicus, Psp.2 Peridinium sp.2, Sachr Sphaerocystis achroeterii, Eacu Euglena acus, Esp. Euglena sp., Tvolv T. volvocina
A majority of sampling units from the Paranapanema River were distributed in the upper left part of the graph. They were associated with high concentrations of dissolved oxygen and correlated with the species A. granulata var. granulata, D. bavaricum, C. acicularis, and Discotella stelligera (Cleve & Grunow) Houk & Klee. The sampling units from the Camargo and Coqueiral Lakes were observed in the same position occupied by the river samples. Some are closer to the center of the axes, in association with the high densities of the species C. brasiliensis, E. elegans, Aphanotece minutíssima (W. West) J., and R. planctonicus. The sampling units from Cavalos Lake were distributed to the right of the graph, along axis 2 (Fig. 7).
Discussion
In the present study, the investigated two greater lakes (Camargo Lake and Coqueiral Lake) were permanently connected to the Paranapanema River, the main tributary of Jurumirim Reservoir. The water mass stored in the reservoir reduces significantly the natural flood pulses in the mouth zone of the main tributary, differently to unimodal or plurimodal flood pulses during the year in floodplains (Hamilton et al., 1998; Krusche & Mozeto, 1999). According to Granado & Henry (2008), a similar variation pattern was found for most abiotic factors in connected lakes (Camargo and Coqueiral) and the Paranapanema River demonstrating the high degree of association that the lake systems maintain with the River. An isolation pattern for Cavalos Lake was identified, mainly in the end of the filling and in the high-water phases up to the middle of the emptying phase of 2005, when high alkalinity, conductivity, and low dissolved oxygen concentrations were found. The increase in the concentrations of CO2 and a decrease in the concentrations of oxygen during the filling phase are probably related to macrophytes’ decomposition at the margins of the isolated lake, when submerged after the increase of water level. Pithart et al. (2007) also observed chemical alterations of the water, with the reduction of the amount of dissolved oxygen in 29 shallow lakes in the floodplains of the Luznice River in the Czech Republic, as a function of the decomposition of the surrounding vegetation, which is flooded during the flood pulse.
According to PCA, Cavalos Lake presents a similar behavior to the other two lentic environments in the dry season, a period characterized by high concentrations of suspended solids and nutrients and low values for water transparency and depth, a consequence of the concentration effect resulting from the reduction in the water volume of the lakes.
Phytoplankton structure in oxbow lakes and connection level
The variation patterns of the phytoplankton community structure of the Paranapanema River and the connected lakes were similar as also observed for the abiotic variables (Granado & Henry, 2008, 2012). In contrast, in the isolated lake, the community attributes and the variation pattern were distinct, confirming the hypothesis proposed that the high level of connectivity would lead to similar species composition and variation patterns of richness, diversity, and biomass (biovolume).
High values of richness and diversity were found in the Paranapanema River and the connected lakes (Camargo and Coqueiral) in the drought phase, just as recorded by Descy (1993) for the Moselle River (France); by Garcia De Emiliani (1997) for El Tigre Lake and the Correntoso River in the floodplain of the Upper Paraná River; by Ibañez (1998) in Camaleão Lake in the Amazon plain; by Nabout et al. (2006) in oxbow lakes of the Araguaia River; and by Taniguchi et al. (2005) in Diogo Lake on the Mogi-Guaçu River. The high richness and diversity values were associated to greater environmental stability, a consequence of the absence of hydrometric variability, which favored the development of various algal groups over exclusively opportunistic species (Taniguchi et al., 2005). Flooding led to a reduction in the richness and diversity of the phytoplankton in the environments in the Paranapanema-Jurumirim Reservoir transition zone, which even increased in the high-water phase; however, both the richness and diversity values were lower than the values found during the drought phase.
According to Henry et al. (2006a), the attributes of the flood pulse in the mouth zones of rivers in lakes are greatly modified by the connection of the waters of the lotic and lentic (reservoir) systems. As the connection with the river is permanent, the hydrological pulses are attenuated, producing only lateral input into the lakes from the river, due to the variability of the water level (Henry, 2003). Thus, in the study period, after the flooding, the values of diversity of the phytoplankton species increased, but they did not reach maximum diversity, at least not during the sampling period.
Relationships between water level and phytoplankton composition
The constant presence of C. brasiliensis and its dominance in some phases of the study may be related to its opportunistic survival strategy. According to Klaveness (1988), the Cryptophyceae are found in various systems throughout the year. Peaks in the development of species are observed after perturbations, such as water mixing, by the wind, and rainfall periods. Flooding appears to favor C. brasiliensis, the only predominant organism in the connected lentic environments during the filling phase. In the other months, the peaks in development of the species may have been related to the wind and rainfall phases that occurred in the days before collection.
High concentrations of nitrogen and of phosphorus were also observed in the water during the filling phase, as well as low luminosity, as demonstrated by the reduced depth of Secchi disk and by high coefficients of light attenuation. According to Isaksson (1998), Reynolds (1984), and Reynolds et al. (2002), cryptophytes can grow under low light intensities and develop mixotrophy. The possibility of vertical migration also contributes to the success of these phytoflagellates (Reynolds et al., 2002) common to shallow eutrophic lakes in different regions of the planet.
Henry et al. (2006b) also reported the constant presence of C. brasiliensis in the oxbow lakes of the Paranapanema River during an extreme drought in the region (October 1999–December 2000), as well as a high abundance of Chroomonas spp. High densities were attributed to the adverse conditions (extreme drought) of the lakes, which made it impossible for other algae to develop.
In Cavalos Lake, the pattern for the physical and chemical variables of the water and the attributes of the phytoplankton community were observed to be different from those of the other two oxbow lakes and the Paranapanema River. In the CCA, these sampling units clustered separately from the other three environments (Fig. 7). Greater richness and diversity in the isolated lake (Cavalos Lake) were achieved at the end of the filling phase when the oxbow lakes were recorded to be at their deepest. The hydrometric level varied because of water input from underground flow (Carmo, 2007) and rainfall. The significant increase in the richness and diversity of species of algae, as well as their density and biomass (biovolume) during the filling phase, can be attributed to the following: (a) the increase in nutrients, in particular the concentrations of total phosphorus, which was higher in this phase, originating from adjacent flooded areas and the decomposition of submersed macrophytes (indicated by the low concentrations of dissolved oxygen in the water); (b) the inocula of the algal species in the sediment or in aquatic macrophytes that developed due to the beneficial nutritional conditions, or also by periphyton species, dragged by the mass of filling water.
In the Paranapanema River and the Camargo and Coqueiral Lakes, densities and biomass (biovolumes) were high in the high-water phase and low in the emptying and filling phases. However, in many studies of floodplains (Garcia de Emiliani, 1997; Huszar & Reynolds, 1997; Train & Rodrigues, 1998; Domitrovic, 2003; Nabout et al., 2006), the high-water phase is characterized by low phytoplankton densities and biomass related to the dilution effect of flooding. Higher values were observed during the drought phase, when the lakes were usually isolated from the main river channel.
After the reduction in algal biomass caused by the filling phase, the Chlorophyceae class, represented principally by B. braunii and E. elegans species, predominated in terms of biovolume. Inocula of the latter are very common in the sediment (Happey-Wood, 1988) and may have been resuspended by strong currents in the flooding phase. The author also highlighted the relationship of the genus Eudorina with the availability of nitrogen. Organisms of this genus were found in high concentrations in the Paranapanema River in the high-water phase. In Camargo Lake, the increase in biomass in the high-water phase can be attributed to Cyanobacteria A. minutíssima and the Chlorococcales, which have high reproductive potential.
In the emptying and the drought phase of 2004, Bacillariophyceae predominated, principally in terms of biomass, with individuals of the Centrales order standing out. The species Aulacoseira granulata contributed more than 50% of the total biomass in the Paranapanema River in these two phases. Oliveira and Calheiros (2000) also recorded the predominance of this group on the Pantanal floodplain, especially A. granulata and Cyanobacteria, in the emptying phase. In Camargo and Coqueiral Lakes, Bacillariophyceae (C. meneguiniana and D. stelligera) predominated in the end of the emptying phase of 2004 and on the surface of Coqueiral Lake during the drought phase. Although wind velocity was not measured, it is known that August and September (emptying phase) are marked by strong winds, which may have contributed to the resuspension of these algae, as they require the moving of the mass of water in order to stay in the euphotic zone. Possible wind action on the column of water can be demonstrated by the presence of isotherms in the lakes, particularly in August.
Although the Paranapanema River—Jurumirim Reservoir transition region—behaves differently from the majority of the floodplains, variations of the depths of the lakes were observed through the annual cycle as a consequence of lateral water input from the River. The variations in water level imply changes in the physical and chemical structure of the environments and in the dynamic of the phytoplankton community, which were comparable to those found in wetlands.
In conclusion, temporal variation on phytoplankton in the studied lentic environments can be attributed mainly to hydrological level of the river (first hypothesis). A similar variation pattern of the ecological attributes was observed in the structure of the phytoplankton community in the connected lakes and Paranapanema River, evidencing the high degree of association that the lacustrine systems maintain with the river.
References
American Public Health Association, 1995. Standart Methods for the Examination of Water and Wastewater, 19th ed. Byrd Prepess Spingfield, Washington, DC.
Amoros, C. & G. Bornette, 2002. Connectivity and biocomplexity in water bodies of riverine floodplains. Freshwater Biology 47: 761–776.
Butler, J., R. Croome & G. N. Rees, 2007. The composition and importance of the phytoneuston in two floodplain lakes in south-eastern Australia. Hydrobiologia 579: 135–145.
Carmo, C. F., 2007. Influência do aqüífero freático na dinâmica de nutrientes (nitrogênio e fósforo) em lagoas com diferentes características hidrodinâmicas. PhD Thesis. Escola de Engenharia de São Carlos, Universidade de São Paulo: 257 pp.
Casanova, S. M. C. & R. Henry, 2004. Longitudinal distribution of Copepoda populations in the transition zone of Paranapanema River and Jurumirim Reservoir (São Paulo) and interchange with two lateral lakes. Brazilian Journal of Biology 64: 11–26.
Descy, J. P., 1993. Ecology of the phytoplankton of the river Moselle: effects of disturbances on community structure and diversity. Hydrobiologia 249: 111–116.
Domitrovic, Y. Z., 2003. Effect of fluctuation in water level of phytoplankton development in three lakes of the Paraná River floodplain (Argentina). Hydrobiologia 510: 175–193.
Garcia de Emiliani, M. O., 1993. Seasonal succession of phytoplankton in a lake of the Paraná River floodplain, Argentina. Hydrobiologia 264: 101–114.
Garcia de Emiliani, M. O., 1997. Effects of water level fluctuations on phytoplankton in a river–floodplain lake system (Paraná River, Argentina). Hydrobiologia 357: 1–15.
Golterman, H. L., R. S. Clymo & M. A. M. Ohnstad, 1978. Methods for Physical and Chemical Analysis of Freshwater, 2nd ed. Blackwell Scientific, Oxford: 214 pp.
Granado, D. C. & R. Henry, 2008. The influence of the hydrologic pulse on the water physical and a chemical variables of lateral lakes with different connection levels to Paranapanema River in the mouth zone at Jurumirim Reservoir. Acta Limnologica Brasiliensia 20: 265–275.
Granado, D. C. & R. Henry, 2012. Changes on water quality of Paranapanema River and three lateral lakes in its mouth zone into Jurumirim Reservoir (São Paulo, Brazil). Latin America Journal of Aquatic Research 40: 79–89.
Granado, D. C., R. Henry & A. Tucci, 2009. Influência da variação hidrométrica na comunidade fitoplanctônica do rio Paranapanema e de uma lagoa marginal na zona de desembocadura na represa de Jurumirim (SP). Hoehnea 36: 113–129.
Hamilton, S. K., O. C. Souza & M. E. Coutinho, 1998. Dynamics of floodplain inundation in the alluvial fan of the Taquari River (Pantanal, Brazil). Verhandlung Internationale Vereinging Limnologie 26: 912–926.
Happey-Wood, C. M., 1988. Ecology of freshwater planktonic green algae. In Sangren, C. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 175–226.
Henry, R. (ed.), 2003. Ecótonos nas interfaces dos ecossistemas aquáticos. Rima, São Carlos: 349 pp.
Henry, R., 2005. The connectivity of the Paranapanema River with two lateral lakes in its mouth zone into the Jurumirim Reservoir. Acta Limnologica Brasiliensia 17: 57–69.
Henry, R. & F. E. Maricato, 1996. Sedimentation rates of tripton in Jurumirim Reservoir (São Paulo, Brazil). Limnologica 26: 15–25.
Henry, R. & M. G. Nogueira, 1999. A represa de Jurumirim (São Paulo): Primeira síntese sobre o conhecimento limnológico. In Henry, R. (ed.). Ecologia de Reservatórios: Estrutura, função e aspectos sociais. FUNDIBIO-FAPESP, Botucatu: 651–686.
Henry, R., E. A. Panarelli, S. M. C. Casanova, M. R. Suiberto & A. A. O. Afonso, 2006a. Interações hidrológicas entre lagoas marginais e o rio Paranapanema na zona de sua desembocadura na Represa de Jurumirim. In Nogueira, M. G., A. Jorcin & R. Henry (eds), Ecologia de reservatórios: impactos potenciais, ações de manejo e sistemas em cascata, 2a ed. Rima, São Carlos: 57–82.
Henry, R., E. Ushinohama & R. M. R. Ferreira, 2006b. Fitoplâncton em três lagoas marginais ao Rio Paranapanema em sua desembocadura no Reservatório de Jurumirim (São Paulo, Brasil) durante um período prolongado de seca. Revista Brasileira de Botânica 29: 399–414.
Henry, R., E. A. Panarelli, S. M. C. Casanova, D. C. Granado, R. M. Mortari & J. Abra, 2011. Plankton richness and abundance in several different hydrological situations in lakes lateral to a river: a case study in mouth zone of a tributary into a tropical river. Oecologia Australis 15: 537–558.
Hillebrand, H., C. D. Dürselen, D. Kirschiel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Huszar, V. L. M. & C. S. Reynolds, 1997. Phytoplancton periodicity and sequences of dominance in a Amazonian floodplain lake (Lago Batata, Pará, Brazil): responses to gradual environmental change. Hydrobiologia 346: 169–181.
Ibañez, M. S. R., 1998. Phytoplankton composition and abundance of a central Amazonian floodplain lake. Hydrobiologia 362: 79–83.
Isaksson, A., 1998. Phagotrophic phytopflagellates in lakes: a literature review. Archives of Hydrobiology, Special Issues on Advanced Limnology 51: 63–90.
Junk, W. J., 1980. Áreas inundáveis – Um desafio para a Limnologia. Acta Amazônica 10: 775–795.
Junk, W. J., 1997. The Central Amazon Floodplain: Ecology of a Pulsing System. Springer Verlag, Berlin: 525 pp.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river floodplain systems. Canadian Special Publication of the Fisheries and Aquatic Sciences 106: 110–127.
Klaveness, D., 1988. Ecology of the Cryptomonadida: a first review. In Sangren, C. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 105–133.
Krusche, A. V. & A. A. Mozeto, 1999. Seasonal variation in water quality of an oxbow lake in response to multiple short-time pulse of flooding (Jataí Ecological Station, Mogi-Guaçu River, Luiz Antonio, SP, Brazil). Anais da Academia Brasileira de Ciências 71: 777–790.
Loverde-Oliveira, S. M. & V. L. M. Huszar, 2007. Phytoplankton ecological responses to the flood pulse in a Pantanal lake, Central Brazil. Acta Limnologica Brasiliensia 19: 117–130.
Mackeret, F. I. H., J. Heron & J. F. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association, London: 121 pp.
McCune, B. & J. J. Mefford, 1997. PC-ord. Multivariate analysis of ecological data, version 3.0. Oregon MjM Software Design: 47 pp.
Melo, S. & V. L. Huszar, 2000. Phytoplankton in an Amazonian flood-plain lake (Lagoa Batata, Brasil): diel variation and species strategies. Journal of Plankton Research 22: 63–76.
Mihaljevíc, M., F. Stevic, J. Horvatic & B. H. Kutuzovic, 2009. Dual impact of the flood pulses on the phytoplankton in a Danubian floodplain lake (Kopacki Rit nature Park, Croatia). Hydrobiologia 618: 77–88.
Moschini-Carlos, V., M. L. M. Pompêo & R. Henry, 1998. Caracterização limnológica de uma baía marginal do Rio Paranapanema (zona de desembocadura da Represa de Jurumirim, SP). Acta Limnologica Brasiliensia 10: 1–19.
Neiff, J. J., 2001. Diversity in some tropical wetland systems of South America. In Gopal, B., W. J. Junk & J. A. Davis (eds), Biodiversity in Wetlands: Assessment, Function and Conservation, Vol. 2. Backhuys Publishers, Leiden: 157–186.
Neiff, J. J., 2003. Planícies de inundação são ecótonos? In Henry, R. (ed.), 2003. Ecótonos nas interfaces dos ecossistemas aquáticos, Rima, São Carlos: 29–45.
Nabout, J. C., I. S. Nogueira & L. G. Oliveira, 2006. Phytoplankton community of floodplain lakes of the Araguaia River, Brazil, in the rain and dry seasons. Journal of Plankton Research 28: 181–193.
Oliveira, M. D. & D. F. Calheiros, 2000. Flood pulse influence on phytoplankton communities of the south Pantanal floodplain, Brazil. Hydrobiologia 427: 101–112.
Paidere, J., D. Gruberts & A. Skute, 2007. Impact of two different flood pulses on planktonic communities of the largest floodplain lakes of the Daugava River (Latvia). Hydrobiologia 592: 303–314.
Panarelli, E. A., S. M. C. Casanova & R. Henry, 2008. The role of resting eggs in the recovery of zooplankton community in a marginal lake of the Paranapanema River (São Paulo, Brazil), after a long drought period. Acta Limnologica Brasiliensia 20: 73–88.
Pithart, D., R. Pichlová, M. Bílý, J. Hrbáček, K. Novotná & L. Pechar, 2007. Spatial and temporal diversity of small shallow waters in river Lužnice floodplain. Hydrobiologia 584: 265–275.
Pompêo, M. L. M., R. Henry & V. Moschini-Carlos, 1999. Ecologia de Echinochloa polystachya do Rio Paranapanema – SP, Brasil. In Henry, R. (ed.), Ecologia de reservatórios: estrutura, função e aspectos sociais. FAPESP/FUNDIBIO, Botucatu: 737–767.
Putz, R. & W. J. Junk, 1997. Phytoplankton and periphyton. In Junk, W. J. (ed.), The Central Amazon Floodplain: Ecology of a Pulsing System. Springer Verlag, Berlim: 525 pp.
Reynolds, C. S., 1984. The Ecology of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 384 pp.
Reynolds, C. S., V. L. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rodrigues, L. C., S. Train, M. C. Roberto & T. A. Pagioro, 2002. Seasonal fluctuation of some limnological variables on a floodplain lake (Patos lagoon) of the Upper Paraná River, Mato Grosso do Sul State, Brazil. Brazilian Archives of Biology and Technology 45: 499–513.
Shannon, C. E. & W. Weaver, 1963. A Mathematical Theory of Communication. University of Ilinois Press, Urbana: 117 pp.
Shepherd, G. J., 1996. Fitopac 1: manual de usuário. Departamento de Botânica. Unicamp, Campinas: 95 pp.
Sommer, U., J. Padisák, C. S. Reynolds & P. Juhász-Nagy, 1993. Hutchinson’s heritage: the diversity-disturbance relationship in phytoplankton. Hydrobiologia 249: 1–7.
Taniguchi, G. M., D. C. Bicudo & P. A. C. Senna, 2004. Abiotic variables in littoral – limnetic of an oxbow lake of Mogi-Guaçu River Floodplain, Southeastern, Brazil. Brazilian Archives of Biology and Technology 47: 961–971.
Taniguchi, G. M., D. C. Bicudo & P. A. C. Senna, 2005. Gradiente litorâneo-limnético do fitoplâncton e ficoperifíton em uma lagoa da planície de inundação do Rio Mogi-Guaçu. Revista Brasileira de Botânica 28: 137–147.
Teixeira, C. & M. B. Kutner, 1962. Plankton studies in a mangrove environment. I – first assessment of standing stock and ecological factors. Boletim do Instituto Oceanográfico 12: 101–124.
Thomaz, S. M., M. C. Roberto & L. M. Bini, 1997. Caracterização limnológica dos ambientes aquáticos e influência dos níveis fluviométricos. In Vazzoler, A. E. M., A. A. Agostinho & N. S. Hahn (eds), A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Eduem, Maringá: 73–102.
Thomaz, S. M., l. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river–floodplain systems. Hydrobiologia 579: 1–13.
Townsend, S. A., 2006. Hydraulic phases, persistent stratification and phytoplankton in a tropical floodplain lake (Mary River, northern Australia). Hydrobiologia 556: 163–179.
Train, S. & L. C. Rodrigues, 1998. Temporal fluctuations of the phytoplankton community of the Baía River, in the upper Paraná River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 361: 125–134.
Utermohl, H., 1958. Zur Vervollkommung der quantitativen Phytoplankton-Methodic. Verhandlung Internationale Vereinging Limnologie 9: 1–38.
Valderrama, J. C., 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Marine Chemistry 10: 109–122.
Ward, J. V., K. Tockner & F. Schiemer, 1999. Biodiversity of floodplain river ecosystems: ecotones and connectivity. Regulated Rivers: Research and Management 15: 125–139.
Wetzel, R. G. & G. E. Likens, 1991. Limnological Analysis. 2nd ed. Springer Verlag, New York: 391 pp.
Acknowledgments
The authors are grateful to FAPESP—São Paulo Research Foundation (Proc. 03/12473-9)—for the scholarship granted to D.C. Granado, to Hamilton A. Rodrigues and Lúcio Miguel de Oliveira for helping with fieldwork, to the two anonymous reviewers for comments and suggestions, to Dr. Antonio C. S. Pião for statistical analysis (ANOVA and Tukey test), and to Laerte José da Silva, from the American Translators Association, for the English language revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Rights and permissions
About this article
Cite this article
Granado, D.C., Henry, R. Phytoplankton community response to hydrological variations in oxbow lakes with different levels of connection to a tropical river. Hydrobiologia 721, 223–238 (2014). https://doi.org/10.1007/s10750-013-1664-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1664-9