Abstract
Phytoplankton functional classification based on simple morphological traits may simplify our understanding of variation in this community as a function of environmental filters. We tested the effectiveness of the morphology-based functional group (MBFG) approach as a model of phytoplankton temporal dynamics in a Brazilian subtropical river. The São João River has an area of approximately 79.10 km2, with 28.09 km2 located within the Iguaçu National Park, in Foz do Iguaçu, Paraná, Brazil. We collected phytoplankton samples and measured environmental variables in the intermediate river section on a monthly basis between August 2008 and July 2009. We tested for differences between the environmental variables, phytoplankton biovolume and sampled months and identified the environmental variables with the greatest influence on MBFGs. Our results revealed clear temporal variability of environmental conditions in this river. We recorded the presence of seven MBFGs (I, II, III, IV, V, VI and VII) in the lotic environment, with MBFG IV (chlorococcal chlorophyceans and desmids), V (flagellates) and VI (diatoms) being the most frequent and most important groups for phytoplankton biomass. Significant temporal differences were found for MBFGs I, II, IV, V and VI, with a clear seasonal succession, especially among MBFGs V and VI. Temperature, pH, electrical conductivity, transparency and nutrients were the main predictors of MBFGs in the São João River. Approaches based on traits have been increasingly applied in community ecology, and we believe that the MBFG approach can increase our understanding of environmental dynamics as well as improve the assessment of general ecological issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to predict the dynamics of biological communities, it is essential to understand the mechanisms determining community structure and function, since they vary greatly in space and time (Heino et al. 2015). In rivers, the phytoplankton community consists of species that originated in this environment and have the ability to grow and reproduce in the main channel (Reynolds 2006). In many cases, the potamoplankton can receive a large contribution of ticoplankton taxa, which come from tributaries and dead zones, hence contributing to the development of populations in the main channel (Descy et al. 2017). According to the river continuum concept (RCC), the potamoplankton can form dominant populations, especially along intermediate sections (Vannote et al. 1980).
Phytoplankton plays a central role in the structure and function of river ecosystems as it determines their primary productivity (El-otify and Iskaros 2015). However, in freshwater ecosystems, the phytoplankton in rivers is less well studied than that in lake ecosystems; a gradual increase in the amount of research has been occurring only over the last 50 years (Dokulil 2014). In South America, some relevant studies on river phytoplankton have been conducted in the last decades and have contributed to our understanding of the ecology of this community in the lotic environment (O’Farreal et al. 1996; Train and Rodrigues 1998; Zalocar De Domitrovic 2002; Devercelli 2006; Zalocar De Domitrovic et al. 2007; Rodrigues et al. 2009; Bortolini and Bueno 2013).
In rivers, the phytoplankton is subject to unidirectional flow, which has an effect on the physical and geographical characteristics of the drainage basin, and results in a community composition that is strongly influenced by seasonal hydrological patterns (Abonyi et al. 2014). Phytoplankton species exhibit widely different responses to environmental filters, including their method of resource acquisition, such as light and nutrients, tolerance of high levels of turbulence, ability to inhibit loss processes, sedimentation and advective downstream transport (Reynolds 1994; Reynolds and Descy 1996; Naselli-Flores and Padisák 2016). The impacts of these differences are observed in biomass proportion, community composition and morphological traits (Chen et al. 2015). A combination of these features can describe the species habitat template using particular species traits (Reynolds et al. 2002; Padisák et al. 2009; Kruk et al. 2010). Thus, it is now common in ecological studies to use functional classifications based on various criteria such as morphological, physiological or behavioral characteristics, along with environmental gradients (Török et al. 2016).
In this context, trait-based approaches, which are relatively easy to implement, have been increasingly applied to explain and predict the response of phytoplankton species to environmental conditions in different aquatic systems (Kruk and Segura 2012), using phytoplankton morphological traits that are associated with their ecological performance (Litchman and Klausmeier 2008). The morphology-based functional group (MBFG) approach proposed by Kruk et al. (2010) is among the classifications currently applied in phytoplankton studies. Originally proposed for lakes, its application to lotic systems has been suggested by authors such as Bortolini et al. (2014), Chen et al. (2015) and Mihaljević et al. (2013, 2015). Forming different groups facilitates predictions of community composition and its relationship with particular environmental conditions. The advantages of this approach are that it does not require specific knowledge of physiological traits or taxonomy and that it is simple to apply in many scenarios (Kruk et al. 2011; Kruk and Segura 2012; Salmaso et al. 2015).
We analyzed the structure of the phytoplankton community in an intermediate section of a Brazilian subtropical river in different seasons, with respect to environmental variability. We applied the morphology-based functional classification proposed by Kruk et al. (2010) to this community. We predicted that despite the unidirectional transport in the river, temporal variability in MBFGs would occur depending on environmental filters, such as temperature, nutrients and light. Moreover, we sought to investigate whether the morphological approach is useful in explaining the phytoplankton structural alterations in this lotic system on a temporal scale.
Materials and methods
Study area
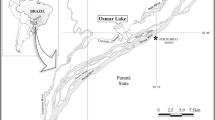
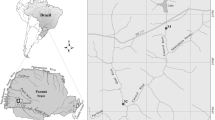
– We carried out this study in the São João River (25°35.52′S and 54°24.17′W); a subtropical Brazilian river (Fig. 1) with a total area of 79.10 km2. Of these, 28.09 km2 is located in the Iguaçu National Park (INP), in Foz do Iguaçu, Paraná, Brazil. The INP covers the largest fragment of Atlantic Forest within a Brazilian protected area and is considered one of the last remnants of this type of vegetation in the southern region of the country (Ibama 2008). The São João River is a third-order river with waters flowing north–south and an average flow of 0.88 m3 s−1, bordering the INP in the west. Its mouth can be found in the Iguaçu River (Salamuni et al. 2002), which is in turn an important affluent of the Paraná River Basin—the second largest hydrological system in South America and the fifth largest in the world (Devercelli 2006). The sampling stations are located in the middle section of the river within the INP region, with dense surrounding vegetation and a some aquatic macrophytes, including Cyperaceae, Poaceae, Pontederiaceae and Utriculariaceae.
Sampling and sample analysis
– The samples of phytoplankton were collected monthly between August 2008 and July 2009, from three sampling stations in longitudinal sections in the pelagic region of the São João River. Samples were obtained directly with bottles 20 cm below the surface of the water and fixed with 1% acetic Lugol solution (Bicudo and Menezes 2006). Counts were performed randomly by field using an inverted microscope, following the method of Lund et al. (1958) and Utermöhl (1958). The phytoplankton biomass was estimated from the biovolume, which was calculated by multiplying the density of each taxon by its respective volume. The cell volume was calculated from geometric models, according to the shape of the cells (Sun and Liu 2003). The phytoplankton was grouped in accordance with the seven MBFGs described by Kruk et al. (2010). The frequency of occurrence of the different groups (constancy = C) was calculated according to Dajoz (2005), where they were classified as constants (C ≥ 70), common (30 ≥ C ≤ 70), sporadic (10 ≥ C ≤ 30) or rare (C ≤ 10). All the samples were deposited at the UNOP-ALGAE herbarium, Unioeste/Cascavel, Paraná, Brazil.
Measurements of water temperature (°C), dissolved oxygen (mg L−1), pH and electrical conductivity (µS cm−1) were obtained with portable digital potentiometers. Water transparency (m) was obtained using a Secchi disk. The ammonium concentration (NH4 +), nitrite (NO2 −), nitrate (NO3 −) and total phosphorus (TP) were determined according to APHA (1995). Meteorological data regarding precipitation were supplied by the Meteorological Institute of Paraná (SIMEPAR/CURITIBA).
Data analyses
– To test for significant differences between each environmental variable and sampled month, a Kruskal–Wallis nonparametric test was performed, since the assumptions of normality and homoscedasticity of the variances were not met. A Kruskal–Wallis test was also used to identify significant differences in the values of total biovolume and of each MBFG among the sampled months. The similarity between biovolume values of MBFGs on the temporal scale was assessed using Nonmetric Multidimensional Scaling (NMDS), where the distances were calculated as the Bray–Curtis similarity (Clarke 1993). The distortion of the resolution in two dimensions was expressed by the S value (stress), wherein the closer the value to zero stress, the better the fit between the original distance of objects and the configuration obtained by the analysis (Legendre and Legendre 1998). Multiple regression analyses were conducted to identify the environmental variables that influenced the MBFGs in the São João River, where the response variable was the biovolume of MBFGs and the explanatory variable was the environmental variables. Residuals were analyzed to verify the validity of the multiple regression assumptions (normal distribution and homogeneity of variances) (Manly 1994). The Kruskal–Wallis and multiple regression tests were performed using the Statistica program (StatSoft Inc. 2005), and NMDS was performed using the R program (R Development Core Team 2016).
Results
Environmental variables in the São João River
– High temporal variability was observed for nutrient concentrations and precipitation in the São João River (CV > 50%); lower mean values of NH4 +, NO2 −, NO3 − and TP were found during the study period. The highest precipitation occurred in January and May 2009 (254 and 243 mm, respectively), and the lowest in November 2008 (0 mm), reflecting the seasonality. Lower temporal variability was observed for dissolved oxygen, pH and transparency of the water column. Electrical conductivity showed a variation of >40%. Significant temporal differences were found for dissolved oxygen, pH, electrical conductivity, NH4 +, NO2 −, NO3 − and TP (Table 1).
Biological responses and morphology-based functional groups (MBFG)
– Among the 127 taxa identified, Bacillariophyceae (39), Chlorophyceae (30), Euglenophyceae (26) and Zygnematophyceae (10) had the highest species richness. For the full list of taxa, see Bortolini and Bueno (2013). Seven MBFGs were present. Three groups had 100% occurrence frequency: MBFG IV, represented by chlorococcal chlorophyceans, desmids and xanthophyceans; MBFG V, represented by euglenophyceans, dinophyceans and cryptophyceans; MBFG VI, represented by diatoms. Three groups were considered common: MBFG I, represented by unicellular cyanobacteria (58% occurrence); MBGF III, represented by heterocytic cyanobacteria (36% occurrence); MBFG VII, represented by colonial chlorophyceans and cyanobacteria (44% occurrence). Only MBFG II, which was represented by chrysophyceans, was found to be sporadic (28% occurrence). MBFGs I, II, IV, V and VI presented significant differences in biovolume according to the sampling months (Table 2).
We observed high temporal variability in the total phytoplankton biovolume (P = 0.0007) as well as in MBFGs during the study period in the São João River (Fig. 2). The highest values of total phytoplankton biovolume occurred in March and April 2009. MBFG VI indicated a greater contribution to biovolume in August and September 2008 and July 2009. MBFG V had a greater contribution to phytoplankton biovolume in October 2008 and June 2009. MBFG II had a significant contribution to biovolume in March and April 2009 (Fig. 3). The remaining groups were less representative of the phytoplankton biovolume.
The similarity analysis (NMDS) indicated a stress value of around 0.10, implying that the results of the temporal MBFG biovolume distribution can be interpreted reliably (Fig. 4). Values of MBFG biovolume varied among the sampling months. MBFG I was associated with periods of lower precipitation, such as October, November and December 2008, when this MBFG had the highest biovolume values recorded. MBFG II had the highest biovolume in March 2009. MBFGs III, IV and VI were associated with August and September 2008 and July 2009. MBFG V and MBFG VII were associated with February, March, April and May 2009.
A multiple regression analysis (Table 3) revealed a significant relationship between the environmental variables and total phytoplankton biovolume (R 2 = 0.58), MBFG I (R 2 = 0.32), MBFG II (R 2 = 0.14), MBFG V (R 2 = 0.50) and MBFG VII (R 2 = 0.49). The total phytoplankton biovolume was negatively influenced by pH and positively influenced by water temperature and conductivity.
MBFG I had a negative association with NH4 + and a positive association with the TP in the São João River. MBFG II had a positive association with water temperature, while MBFG V had a positive correlation with water temperature and conductivity, but a negative correlation with pH. Finally, MBFG VI had a positive correlation with water transparency. The residuals of the models were analyzed, and no outliers, trends or serial correlation were verified, corroborating the assumptions of the analysis (Table 3).
Discussion
Our results indicate that the functional groups based on morphological criteria responded to the temporal variability in environmental filters and the seasonality during the study period in the São João River. The correlations between physiological traits and morphological aspects, along with the fact that the MBFGs have different functional traits, suggest that morphology is a reasonable proxy for function (Kruk et al. 2010; Kruk and Segura 2012). The use of certain morphological traits as indicators of the functional properties of communities can be also applied to river phytoplankton (Chen et al. 2015) and can reflect the habitat template of the groups, as verified in our study.
For most of the months, we recorded low phytoplankton biovolume values in the São João River (<2 mm3 L−1), which generally has a lower biomass than the lentic environment (Devercelli and O’Farell 2013; Bortolini et al. 2014). In addition, in this type of environment, the phytoplankton has to tolerate high levels of turbulence, limited light and continuous transport downstream, which have direct impacts on their biomass. Nutrients can also be a limiting factor for potamoplankton in certain situations (Reynolds and Descy 1996), as illustrated by the low concentrations recorded in this river. These conditions favor hardy species such as diatoms (MBFG VI), chlorococcal green algae (MBFG IV) and flagellated algae with mixotrophic potential (MBFG V), which remained constant throughout the study period.
MBFG VI was represented by nonflagellated organisms with siliceous exoskeletons. Silica is one of the main traits of diatoms and potentially increases sink rate and sedimentation (Kruk and Segura 2012), making these algae dependent on hydrodynamic conditions for their re-suspension in the water column. This may explain why this MBFG was favored in lotic environments. In addition, their cell wall is made of silica, promoting moderate vulnerability to consumption (Kruk et al. 2010). The biovolume of MBFG VI varied significantly according to the sampling month, presenting the highest contributions in months with lower temperatures, such as August and September 2008 as well as June and July 2009, which had an average temperature of 20 °C. Even though in our study we did not find a correlation between MBFG VI and temperature, we believe that this factor may be, even if indirectly, important for the development of this MBFG, since many diatoms are generally better adapted to lower temperatures than other species (Lürling et al. 2013). The positive relationship between this group and water transparency is probably due to the low variability of this parameter during the study period, which is tolerated by MBFG VI. According to Abonyi et al. (2014), to study different functional approaches regarding river phytoplankton, including the MBFG approach, a finer resolution for diatoms, especially pennate forms, is needed for ecological and river status assessment, since these taxa can respond in different ways to environmental conditions. In addition, many pennate diatoms found in the plankton are carried from dead zones to the main channel, thus contributing ticoplanktonic taxa to the phytoplankton.
The high occurrence of MBFG V, represented by flagellated algae with mixotrophic and phagotrophic ability, such as euglenophyceans, dinophyceans and cryptophyceans, may be related to their greater tolerance of limited nutrient availability and higher concentrations of organic matter in the river. Considering the evolution of photosynthesis in eukaryotes, mixotrophy is suggested as a nutritional strategy that is easier to emerge, since mixotrophic protists have a wide spectrum of nutritional strategies (Jones 2000). Flagellated algae representatives of MBFG have a high S/V ratio, which can reduce predation pressure by zooplankton (Kruk and Segura 2012). In addition, the presence of flagella can be an important functional trait to beat the current in lotic systems and remain in the water column. Many taxa of this MBFG can also be associated with dead zones of the river and therefore contribute to the phytoplankton community of the lotic environment.
The success of mixotrophs over autotrophs may vary according to environmental conditions (Saad et al. 2016), as evidenced in our study, where MBFG V exhibited a positive relationship with temperature and electrical conductivity, and a negative relationship with pH. The major contribution of organic matter derived from the banks in lotic environments results in higher concentrations of organic matter suspended in the water column. This can have a direct impact on pH values and electrical conductivity, which favors MBFG V. In our study, we found a succession between MBFG VI and MBFG V, where there was a greater diatom contribution in months of lower temperatures and more flagellated algae at higher temperatures (25 °C on average), corroborating the positive relationship between MBFG V and temperature.
The green algae of MBFG IV, mainly represented by chlorococcal chlorophyceans and desmids, were associated with a low concentration of nutrients and had a mild tolerance to limited resources (Kruk et al. 2010). The absence of specialized morphological traits to minimize losses from sedimentation emphasizes that members of this group depend on water-column mixing to remain in the photic layer (Reynolds et al. 2002; Padisák et al. 2009; Rodrigues et al. 2009; Kruk et al. 2010). They may therefore be favored in environments with more turbulence, such as rivers. Although this group had 100% frequency of occurrence during the study, the proportion of contribution to the biovolume phytoplankton was lower than that of MBFGs V and VI, because of the smaller cell size of these algae, especially chlorococcal chlorophyceans, in comparison with flagellated algae and diatoms.
MBFG I was found to be common in the São João River. This group consists of r-selected species and has a wide trophic spectrum, so is favored in a broad ecological niche (Kruk et al. 2010). In our study, this MBFG had a positive correlation with NH4 + and TP. Chen et al. (2015) subdivided the MBFG I into two groups and found that the group RIa, represented by algae without flagella, which are the representatives of this MBFG in our study, had a strong association with nutrients.
MBFG II, represented by chrysophyceans, was sporadic, with significant contributions in just 2 months of the study. This MBFG has a direct association with environments with lower nutrient concentrations (Reynolds et al. 2002; Kruk and Segura 2012), such as the São João River. According to Kruk et al. (2010) and Kruk and Segura (2012), the representatives of this MBFG may be vulnerable to consumption, in addition to presenting low to moderate sinking losses. Moreover, Dinobryon, one of the main representatives of this MBFG in the São João River, can form resistance propagules and can be facultative mixotrophic in unfavorable conditions (Saad et al. 2016). The highest biomass of MBFG II occurred in March 2009, when the temperature was around 30 °C, which reinforces the positive relationship between this group and temperature that was found in the regression analysis. According to Heinze et al. (2013), the factors proposed to affect the seasonal distribution of Dinobryon, such as light and temperature, are mutually exclusive, but an overall importance of temperature is suggested.
MBFG III, represented by heterocytic cyanobacteria, was associated with August and September 2008 as well as July 2009. Due to their morphology, the species of this MBFG may be considered k-selected due to their high S/V ratio and low losses from consumption and sinking; therefore, the success of these organisms relies on low-light, high-trophic status environments (Train and Rodrigues 1998; Kruk et al. 2010). Thus, in our study this MBFG was not favored by the hydrodynamics of this habitat or by the low nutrient concentrations. Likewise, MBFG VII, represented by colonial chlorophyceans and cyanobacteria, had low biovolume contributions, despite being considered common in our study. This is probably a result of the low nutrient concentration and hydrodynamics of the environment, since the taxa of this MBFG were large in size and volume, but low in surface area/volume ratio, suggesting that it tends to make species sensitive to low resource supply (Kruk et al. 2010).
Different phytoplankton groups are known to occupy different niches in the water column according to their size. This difference in phytoplankton behavior has been attributed to several factors, such as sinking rate, photosynthetic efficiency, the uptake of nutrients, advection, grazing and coagulation (Serra et al. 2003). This can be seen in the São João River, since predominated algae with larger cell size, such as MBFG VI (diatoms) and MBFG V (flagellates), were favored in this river. The export of larger phytoplankton from the surface to the bottom is crucial in explaining the transport of carbon through the water column, where environmental turbulence is a decisive factor in maintaining different taxa in the water column according to their morpho-functional traits (Reynolds and Descy 1996).
The morpho-functional approach can offer advantages over other functional approaches that require a more accurate knowledge of the species, because it is purely based on morphological traits of organisms so it is not necessary to know the species’ taxonomy and autoecology (Kruk et al. 2010; Izaguirre et al. 2012; Hu et al. 2013; Salmaso et al. 2015). In comparison with other concepts, the MBFG approach has been reported to be easier to summarize phytoplankton responses to environmental variability; in addition, the use of morphological traits and the easier group classification (Salmaso et al. 2015) means that predictions can be made and applied to simplify studies of complex patterns.
Grouping species with similar traits or behavior favors our understanding of community characteristics (Salmaso and Padisák 2007). However, functional groups are not meant to be a substitute for the entirety of information that can be gathered from species (Bortolini et al. 2016), but rather a tool to summarize this information. Thus, investigating the phytoplankton functional composition using a simple morphological approach, in accordance with environmental filters, can be a useful instrument to understand community temporal variability, support management decisions and provide a better understanding of environmental change and the assessment of general ecological issues in lotic systems.
References
Abonyi A, Leitão M, Stankovi I, Borics G, Várbíró G, Padisák J (2014) A large river (River Loire, France) survey to compare phytoplankton functional approaches: Do they display river zones in similar ways? Ecol Ind 46:11–22
American Public Health Association (1995) Standard methods for the examination of water and wastewater, 19th edn. APHA, Washington DC
Bicudo CEM, Menezes M (2006) Gêneros de Algas de Aguas Continentais do Brasil: Chave para identificação e descrições. RiMa, Sao Carlos
Bortolini JC, Bueno NC (2013) Seasonal variation of the phytoplankton community structure in the São João River, Iguaçu National Park, Brazil. Braz J Biol 73:1–14
Bortolini JC, Rodrigues LC, Train S (2014) Phytoplankton functional and morphological groups as indicators of environmental variability in a lateral channel of the Upper Paraná River floodplain. Acta Limnol Bras 26:98–108
Bortolini JC, Moresco GA, Paula ACM, Jati S, Rodrigues LC (2016) Functional approach based on morphology as a model of phytoplankton variability in a subtropical floodplain lake: a long-term study. Hydrobiologia 767:151–163
Chen N, Liu L, Li Y, Qiao D, Li Y, Zhang Y, Lv Y (2015) Morphology-based classification of functional groups for potamoplankton. J Limnol 74:559–571
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust Ecol 18:117–143
Dajoz R (2005) Princípios de Ecologia. Artmed, Porto Alegre
Descy JP, Darchambeau F, Lambert T, Stoyneva-Gaertner MP, Bouillon S, Borges AV (2017) Phytoplankton dynamics in the Congo River. Freshw Biol 62:87–101
Devercelli M (2006) Phytoplankton of the Middle Paraná River during an anomalous hydrological period: a morphological and functional approach. Hydrobiologia 563:465–478
Devercelli M, O’Farrell I (2013) Factors affecting the structure and maintenance of phytoplankton functional groups in a nutrient rich lowland river. Limnologica 43:67–78
Dokulil MT (2014) Potamoplankton and primary productivity in the River Danube. Hydrobiologia 729:209–227
El-otify AM, Iskaros IA (2015) Water quality and potamoplankton evaluation of the Nile River in Upper Egypt Qualidade da água e avaliação do potamoplâncton do rio Nilo no Alto Egito. Acta Limnol Bras 27:171–190
Heino J, Melo AS, Bini LM (2015) Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw Biol 60:223–235
Heinze AW, Truesdale CL, Devaul SB, Swinden J, Sanders R (2013) Role of temperature in growth, feeding, and vertical distribution of the mixotrophic chrysophyte Dinobryon. Aquat Microb Ecol 71:155–163
Hu R, Han B, Naselli-Flores L (2013) Comparing biological classifications of freshwater phytoplankton: a case study from South China. Hydrobiologia 701:219–233
IBAMA (2008) Plano de manejo. Ministério do meio ambiente, Brasil. http://www.ibama.gov.br/siucweb/mostraUc.php?seqUc=17
Izaguirre I, Allende L, Escaray R, Bustingorry J, Pérez G, Tell G (2012) Comparison of morpho-functional phytoplankton classifications in human-impacted shallow lakes with different stable states. Hydrobiologia 698:203–216
Jones RI (2000) Mixotrophy in planktonic protists: an overview. Freshw Biol 45:219–226
Kruk C, Segura AM (2012) The habitat template of phytoplankton morphology-based functional groups. Hydrobiologia 698:191–202
Kruk C, Huszar VLM, Peeters EHM, Bonilla S, Costa L, Lurling M, Reynolds CS, Scheffer M (2010) A morphological classification capturing functional variation in phytoplankton. Freshw Biol 55:614–627
Kruk C, Peeters EHM, Van Nes EH, Huszar VLM, Costa LS, Scheffer M (2011) Phytoplankton community composition can be predicted best in terms of morphological groups. Limnol Oceanogr 56:110–118
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Litchman E, Klausmeier CA (2008) Trait-based community ecology of phytoplankton. Annu Rev Ecol Evol Syst 39:615–639
Lund JWG, Kipling C, Lecren ED (1958) The inverted microscope method of estimating algal number and the statistical basis of estimating by counting. Hydrobiologia 11:980–985
Lürling M, Eshetu F, Faassen EJ, Kosten S, Huszar VLM (2013) Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw Biol 58:552–559
Manly BFG (1994) A primer of multivariate statistics. Chapmam & Hall, London
Mihaljević M, Špoljarić D, Stević F, Pfeiffer T (2013) Assessment of flood-induced changes of phytoplankton along a river–floodplain system using the morpho-functional approach. Environ Monit Assess 185:8601–8619
Mihaljević M, Stević F, Špoljarić D, Žuna T, Pfeiffer T (2015) Spatial pattern of phytoplankton based on the morphology-based functional approach along a river–floodplain gradient. River Res Appl 31:228–238
Naselli-Flores L, Padisák J (2016) Blowing in the wind: how many roads can a phytoplanktont walk down? A synthesis on phytoplankton biogeography and spatial processes. Hydrobiologia 764:303–313
O’Farreal IO, Izaguirre I, Vinocur A (1996) Phytoplankton ecology of the Lower Paraná River (Argentina). Arch Hidrobiol 1:75–89
Padisák J, Crossetti LO, Naselli-Flores L (2009) Use and m issue in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621:1–19
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Reynolds CS (1994) The long, the short and the stalled: on the attributes of phytoplankton selected by physical mixing in lakes and rivers. Hydrobiologia 289:9–14
Reynolds CS (2006) Ecology of phytoplankton. Cambridge University Press, New York
Reynolds CS, Descy JP (1996) The production, biomass and structure of phytoplankton in large rivers. Archiv für Hydrobiologie 113:161–167
Reynolds CS, Huszar VLM, Kruk C, Naselli-Flores L, Melo S (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:417–428
Rodrigues L, Train S, Bovo-Scomparin VM, Jati S, Borsalli C, Marengoni E (2009) Interannual variability of phytoplankton in the main rivers of the Upper Paraná River floodplain, Brazil: influence of upstream reservoirs. Braz J Biol 69:501–516
Saad JF, Unrein F, Tribelli PM, López N, Izaguirre I (2016) Influence of lake trophic conditions on the dominant mixotrophic algal assemblages. J Plankton Res. doi:10.1093/plankt/fbw029
Salamuni R, Salamuni E, Rocha LA, Rocha AL (2002) Parque Nacional do Iguaçu, PR: Cataratas de fama mundial. In: Schobbenhaus C, Campos DA, Queiroz ET, Winge M, Berbert-Born MLC (eds) Sítios Geológicos e Paleontológicos do Brasil. Comissão Brasileira de Sítios Geológicos e Paleobiológicos (SIGEP), Brasília, pp 313–321
Salmaso N, Padisák J (2007) Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578:97–112
Salmaso N, Naselli-Flores L, Padisák J (2015) Functional classifications and their application in phytoplankton ecology. Freshw Biol 60:603–619
Serra T, Granata T, Colomer J, Stips A, Møhlenberg F, Casamitjana X (2003) The role of advection and turbulent mixing in the vertical distribution of phytoplankton. Estuar Coast Shelf Sci 56:53–62
Statisoft Inc. (2005) Statistica (data analysis software system) version 7.1. www.statisoft.inc
Sun J, Liu D (2003) Geometric models for calculating cell biovolume and surface area for phytoplankton. J Plankton Res 25:1331–1346
Török P, T-Krasznai E, B-Beres V, Bácsi I, Borics G, Tóthmérész B (2016) Functional diversity supports the biomass–diversity humped-back relationship in phytoplankton assemblages. Funct Ecol. doi:10.1111/1365-2435.12631
Train S, Rodrigues LC (1998) Temporal fluctuations of the phytoplankton community of the Baía River, in the upper Paraná River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 361:125–134
Utermöhl H (1958) Zur Vervollkommnung der quantitativen phytoplankton-methodic. Verhandlungen der Internationalen Vereinigung fu¨r Theoretische und Angewandte Limnologie 9:1–39
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushin CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Zalocar De Domitrovic Y (2002) Structure and variation of the Paraguay River phytoplankton in two periods of its hydrological cycle. Hydrobiologia 472:177–196
Zalocar De Domitrovic Y, Poi De Neiff ASG, Casco SL (2007) Abundance and diversity of phytoplankton in the Paraná River (Argentina) 220 km downstream of the Yacyretá reservoir. Braz J Biol 67:53–63
Acknowledgements
The authors thank the Brazilian Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio, Chico Mendes Institute for the Conservation of Biodiversity) for providing the necessary research permits, as well as the staff of Iguaçu National Park, for the use of the facilities to complete these studies and Sisbio for the permission to carry out the research in the protected area (13134-2). NCB is grateful to the CNPq of Brazil for a Research Productivity Grant (process 307196/2013-5). JCB is grateful to the CNPq for providing post-doctoral scholarship (process 165796/2015-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bortolini, J.C., Bueno, N.C. Temporal dynamics of phytoplankton using the morphology-based functional approach in a subtropical river. Braz. J. Bot 40, 741–748 (2017). https://doi.org/10.1007/s40415-017-0385-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-017-0385-0