Abstract
Due to the wide use of glyphosate (GLY) in soybean cultivation, their residues in the environment may affect non-target organisms such as fish, developing toxic effects. Despite GLY being widely used in Brazil, there are few studies comparing the effects of commercial formulations in native freshwater fish species. Silver catfish (Rhamdia quelen) were exposed to three different commercial formulations of GLY 48 % (Orium®, Original® and Biocarb®) at 0.0, 2.5 and 5.0 mg/L for 96 h. The effects in thiobarbituric acid-reactive substances (TBARS), catalase (CAT), superoxide dismutase (SOD), glutathione-S-transferase (GST) and histological alterations were analysed in the liver, whereas alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were studied in the plasma. In the liver, TBARS levels increased and CAT decreased in all treatments and herbicides tested in comparison with the control group. The SOD increased at 2.5 mg/L of Orium®, Original® and 5.0 mg/L Orium® and Biocarb®, whereas GST increased at 2.5 mg/L Orium® and decreased at 2.5 mg/L Biocarb® when compared to the control group. The main histopathological alterations in hepatic tissue were vacuolisation, leucocyte infiltration, degeneration of cytoplasm and melanomacrophage in all GLY treatments. The ALT decreased after exposure to 2.5 mg/L of Biocarb® and AST increased at 2.5 mg/L of Orium®, Original® and 5.0 mg/L of Biocarb® in comparison with the control group. In summary, the oxidative damage generated by GLY may have caused the increased formation of free radicals that led to the histological alterations observed in hepatocytes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the production of genetically modified organisms, mainly soybean, the use of herbicides such as glyphosate (GLY) increased around the world, due the tolerance of this crop to pesticides (Giesy et al. 2000; Williams et al. 2000). Currently, several formulations of GLY based are registered in more than 100 countries, including Brazil, and are available under different brand names (Moreno et al. 2014). The GLY (N-phosphonomethyl glycine, C3H8NO5P) is a non-selective and post-emergence herbicide. Manufactured initially by Monsanto as Roundup®, it contains the isopropylamine salt of GLY as an active ingredient and polyoxyethylene amine (POEA) as a non-ionic surfactant, which increases the herbicide efficiency (Brausch and Smith 2007).
According to data of the World Health Organisation (WHO 1994), the toxicity of GLY is considered low. In addition, Williams et al. (2000) explained that to humans, others mammals and birds, when used in normal conditions, this herbicide does not represent risks to health; this study concluded that fish species in particular are more sensible than mammals. The extensive use of GLY may cause environmental problems and affect fish species. Several studies with different fish species have shown the toxic effects caused by GLY, such as Sinhorin et al. (2014) in Pseudoplatystoma sp., Langiano and Martinez (2008) in Prochilodus lineatus, Cattaneo et al. (2011) in Cyprinus carpio and Menezes et al. (2011) in Rhamdia quelen. Studies comparing the effects of commercial formulations in native fish species are scarce, and this is the major reason for this study.
The silver catfish (R. quelen) was chosen for this study due to the fact that it is an endemic fish species in South America that can support cold winters, grows fast during the summer and has excellent fillet quality for consumption. However, many sites in which the silver catfish live are located near agricultural areas that are continuously receiving pesticides from the agricultural activities. There are some research groups that chose this fish species to study (Barcellos et al. 2004; Baldisserotto 2009).
The herbicides that contain GLY could be responsible for inducing reactive oxygen species (ROS) formation, such as hydrogen peroxide (H2O2), superoxide anion (O2 −), hydroxyl radical (OH) or other nitrogen species, all of which may cause an imbalance between pro-oxidant and antioxidant defence mechanisms, resulting in oxidative damage (Modesto and Martinez 2010a; Glusczak et al. 2011). To neutralise the RS, the fish has an antioxidant defence system that is constituted of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and also a detoxificant enzyme glutathione-S-transferase (GST) (Modesto and Martinez 2010a; Menezes et al. 2011). As a result of oxidative damage, lipid peroxidation indicates alterations in the cellular membrane, cohesion, flow, permeability and metabolic function, leading to cellular instability with consequent damage and death of the cell (Langiano and Martinez 2008; Menezes et al. 2011).
In general, biochemical and metabolic parameters measured in fish species are very sensitive to sublethal concentrations of pesticides. Fish exposure demonstrates a response relative to a stressor that may be measured although of several metabolic parameters related to energetic metabolism, enzyme varieties and histological alterations (Jiraungkoorskul et al. 2003; Pretto et al. 2011; Salbego et al. 2014). The importance of histology as sensitive and reliable indicators of health status in fish species has been studied. These studies showed histological alterations in the liver, such as fatty degeneration, multifocal necrotic processes and leucocyte infiltration (Szarek et al. 2000; Ayoola 2008; Ramírez-Duarte et al. 2008; Hued et al. 2012). Other alternatives for monitoring the exposure to pesticides in fish are through the analysis of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), because these enzymes indicate liver damage or hepatotoxicity (Ferreira et al. 2010). Thus, the main aim of this study was to investigate biochemical and histological alterations in the liver of silver catfish exposed to sublethal concentrations of GLY. Another point is to compare the responses obtained between the different commercial formulations to verify which presented more toxic effects to native fish species.
Materials and methods
GLY formulations
Three commercial formulations of GLY (N-phosphonomethyl) were used in this experiment with the trade name of Orium®, Roundup Original® and Biocarb® all with 48 % purity. These GLY formulations were manufactured by Dominus Chemistry, Monsanto and Bio carb Chemistry Industry, respectively.
Fish
The silver catfish (weight 12.0 ± 1.0 g; length 9.0 ± 1.0 cm) of both sexes were obtained from the fish farm at the Federal University of Santa Maria (UFSM). The fish were acclimated in dechlorinated tap water in boxes (250 L) for 10 days. They were maintained in continuously aerated water with a natural photoperiod (12-h light/12-h dark). Water quality parameters were measured every day and were: temperature 24.5 ± 2.0 °C, pH 6.8 ± 0.5 units, dissolved oxygen 8.0 ± 0.2 mg/L, non-ionised ammonia 0.56 ± 0.02 µg/L, nitrite 0.06 ± 0.01 mg/L and alkalinity 22.0 ± 2.0. During acclimation, the fish were fed once a day with commercial fish pellets (42 % crude protein, Supra®, Brazil). Faeces and pellet residues were removed by suction.
Experimental design
After an acclimatisation period, the fish were distributed randomly in 45-L boxes with dechlorinated tap water. The parameters of water quality during the experimental period were similar to the acclimatisation period. The experiment was composed of a control group (0.0 mg/L, without GLY) and two exposure groups (2.5 and 5.0 mg/L of GLY concentrations). Each experimental group contained eight fish in duplicate, so the final number was 16. During the experimental period, the fish were not feed. The exposure time of 96 h (acute toxicity) was according to Antón et al. (1994). We chose sublethal concentrations (2.5 and 5.0 mg/L) based on the value of LC50 for silver catfish which was determined to be 7.3 mg/L (Kreutz et al. 2008) and also because these concentrations may be environmentally realistic considering current application rates (Langiano and Martinez 2008; Topal et al. 2015). These were also considered as GLY is easily detected in rivers, agricultural and urban regions and causes damage to several fish species (Pesce et al. 2008; Tsui and Chu 2008).
To obtain the experimental concentrations (2.5 and 5.0 mg/L GLY), the herbicide was diluted in distilled water. The stock solution (300 mg/L GLY) was added with a micropipette at the beginning of the experiment to each of the boxes to obtain the desired concentrations, without herbicide replacement. The concentration of herbicide in the water was monitored at the beginning and end of the experimental period through liquid chromatography with previous derivatisation, as described by Hidalgo et al. (2004). After the experimental period, fish were anesthetised with benzocaine (0.01 %) (Antunes et al. 2008) and were sampled; the blood was collected from the caudal vein with heparinised syringe. After the fish were euthanised by spinal cord section, the liver was carefully removed, placed on ice and stored at −20 °C for posterior biochemical analyses. All protocols used in this study were approved by the Committee on Ethics and Animal Welfare of the Federal University of Santa Maria, protocol number 056/2013.
Determination of oxidative stress indicators
Thiobarbituric acid-reactive substances (TBARS) were determined according to method of Buege and Aust (1978). The antioxidant enzymes SOD and CAT activities were measured according to Misra and Fridovich (1972) and Nelson and Kiesow (1972), respectively. GST activity was measured according to Habig et al. (1974). All the methods were described in Menezes et al. (2011).
Protein determination
Protein was determined by the Coomassie blue method using bovine serum albumin as standard. Absorbance of samples was measured at 595 nm (Bradford 1976).
Histological procedure
Each specimen had its liver removed and processed by routine histological techniques, embedded in paraffin and sectioned with a microtome of five to seven microns in thickness. The method of haematoxylin and eosin (HE) staining has been used for a general viewing of the affected tissues and organs (Michalany 1980). Histopathological alterations were classified on a scale from 0 to 3, where 0 = no alterations, 1 = slight alterations, 2 = moderate alterations and 3 = severe alterations (Hose et al. 1996). Definitions of slight, moderate and severe alterations were adapted from Poleksic and Mitrovic-Tutundzic (1994). The presence of histopathological alterations in each organ was semi-qualitatively evaluated by the degree of organ alteration (index alteration histopathological—IAH), which is based on the severity of the lesions (Table 1). In order, to calculate the IAH adapted from Poleksic and Mitrovic-Tutundzic (1994), the alterations of each organ were classified into progressive stages of tissue damage. IAH was calculated from each fish using the formula: IAH = (1 × SI) + (10 x SII) + (100 x SIII), where 1, 2 and 3 correspond to the numbers of alteration stages. In turn, S denoted the sum of alteration in a particular step.
IAH values between 0 and 10 indicate normal functioning of the organ; values between 11 and 20 indicate light organ damage; 21–50 denote moderate alterations; 50–100 reveal severe alterations; and above 100 means irreparable injuries to the organ (Poleksic and Mitrovic-Tutundzic 1994). Some cases were selected and photographed under an inverted light microscope fitted with a digital camera.
Transaminases analysis
The ALT and AST was measured in plasma with Bioclin test kit. These enzymes were expressed in U/L.
Statistical analysis
Data were tested for normality (Kolmogorov–Smirnov’s test) and for homogeneity of variances (Bartlett’s test). Statistical analyses were performed using a one-way analysis of variance (ANOVA) followed by Tukey’s test. Data exhibited homogeneous variance and were expressed as mean ± standard error (S.E.M.). The value of p ≤ 0.05 was considered statistically significant for all analyses. Analysis was performed using GraphPad Prism 6.01 (GraphPad Software, San Diego, USA).
Results
Herbicide monitoring
The measurements of GLY at the beginning and end of the experimental period showed that herbicide concentrations decreased by about 15–16 % of the initial level for all concentrations and formulations tested after 96 h (Table 2).
Oxidative stress indicators
The TBARS levels in the liver of silver catfish were high at 2.5 and 5.0 mg/L for all herbicides tested in relation to the control group. At the concentration of 2.5 mg/L, for the three products which differ significantly from each other, higher TBARS levels were observed in the Biocarb®. At the concentration of 5.0 mg/L, the Original® formulation was significantly different to Orium® and Biocarb®, which presented low TBARS levels (Fig. 1A). The CAT activity in the liver was lower at both concentrations and GLY formulations compared to the control fish. There was no significant difference in CAT activity between tested formulations (Fig. 1B). The SOD activity was significantly higher in the liver of fish exposed at both concentrations of Orium®, 2.5 mg/L Original® and 5.0 mg/L Biocarb® compared to the control group. Comparing between formulations at concentrations of 2.5 mg/L, the SOD activity was significantly lower in the liver of fish exposed to the Biocarb® in relation to Orium® and Original®. At 5.0 mg/L for the three herbicides, the SOD activity was significantly lower in the Original® compared to Orium® and no change was observed when compared to Biocarb® (Fig. 1C). In the liver, GST activity increased for Orium® (2.5 mg/L), but on the other hand, Biocarb® (2.5 mg/L) decreased in comparison with the control group. When comparing between 2.5 mg/L concentrations, all formulations presented different GST activity, with Orium® demonstrating a high elevation and Biocarb® a lower activity. At 5.0 mg/L concentrations, the Biocarb® formulation demonstrated an increase in GST activity in comparison with Original® (Fig. 1D), and none tested formulation at 5.0 mg/L had significant result in comparison with the control group.
TBARS levels (A), CAT (B), SOD (C) and GST (D) activities, in the liver of silver catfish exposed to three commercial formulations of GLY (2.5 and 5.0 mg/L) by 96 h. Asterisk indicates significant result compared to the control group. Lower-case letters indicate the difference between 2.5 mg/L concentrations and upper-case letters indicate the difference between 5.0 mg/L concentrations (p ≤ 0.05, n = 8)
Histological data
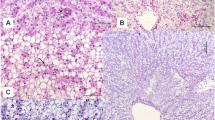
Histological analysis revealed that the liver of silver catfish had the same general pattern as other teleosts, with large hepatocytes and mild hyalinisation, a large and rounded nucleus, and a marked nucleolus (Fig. 2a). Hepatocytes form cords delimited by small blood vessels called sinusoids vessels, which radiate outward from the portal vein. Between the ridges, small bile ducts can be occasionally observed. These are formed by a simple cubic epithelium surrounded by connective tissue and were observed near the exocrine pancreatic tissue or isolated in the liver parenchyma (Fig. 2b).
Histopathological alterations like hepatocyte vacuolisation (steatosis—hyalinisation), leucocyte infiltration, degeneration of cytoplasm areas, melanomacrophage, lateral position of the nucleus and lifting capsule were observed in the hepatic tissue in all GLY treatments. Vacuolisation of hepatocytes (hyalinisation) was the more frequent histopathological alteration observed and was seen in large areas of the liver, causing them to be classified as having moderate alterations.
The results obtained from analysing the intensity and frequency of alterations in silver catfish livers demonstrated that only slight alterations were observed in the control group and that the individuals undergoing treatments showed slight to severe histopathological alterations. We observed a higher frequency of moderate histopathological alterations in Orium® and severe alterations at 5.0 mg/L Biocarb® (Fig. 3). Using IAH analysis, it was observed that only Orium® treatments at both concentrations and Biocarb® at a concentration of 5.0 mg/L showed values above 10, indicating slight changes in the structure of the organ. The other treatments showed values lower than 10, indicating normal functioning of the body (Fig. 4).
Transaminases activities
The enzyme ALT in the plasma of silver catfish decreased only at 2.5 mg/L of Biocarb® in comparison with the control group. Comparing the results between the tested herbicides to a concentration of 2.5 mg/L, Biocarb® decreased significantly in relation at Orium® and Original® formulations. Comparisons between 5.0 mg/L concentrations did not show any significant results between the formulations tested (Fig. 5A). The enzyme AST in plasma increased with 2.5 mg/L of Orium® and Original® and 5.0 mg/L of Biocarb® when compared to the control group. Comparing between 2.5 mg/L concentrations, Orium® and Original® presented high values for AST activity in relation to Biocarb®. At 5.0 mg/L concentrations, no significant results were observed for any of the formulations tested (Fig. 5B).
ALT (A) and AST (B) activities in the plasma of silver catfish exposed to three commercial formulations of GLY (2.5 and 5.0 mg/L) by 96 h. Asterisk indicates significant result compared to the control group. Lower-case letters indicate the difference between 2.5 mg/L concentrations and upper-case letters indicate the difference between 5.0 mg/L concentrations (p ≤ 0.05, n = 8)
Discussion
As silver catfish have an important role both commercially and in aquaculture, this study is pertinent as monocultures of soybean are located close to fish farms that produce this species for consumption; therefore, the study presented some important results concerning glyphosate formulations and toxic effects on fish.
The results showed histological alterations in the liver, such as fatty degeneration, multifocal necrotic processes and leucocyte infiltration. Reductions in the initial concentration of GLY were recorded during the experimental period (15–16 %); these results are in agreement with other studies previously published by our research group (Salbego et al. 2010; Menezes et al. 2011; Sinhorin et al. 2014). This reduction in the concentration of GLY may be related to possible absorption by the fish, due to damage found in the liver of this species or by the transformation of GLY in metabolites, such as glyoxylate and aminomethyl-phosphonic acid. According to Aparicio et al. (2013), studies involving this herbicide in water are scarce, due to the fact that molecules of GLY are transitory in this situation; therefore, there is a tendency for these molecules in the environment to be located in the sediment.
Some authors consider glyphosate formulations to frequently induce oxidative damage, causing damage to unsaturated fatty acids, leading to lipid peroxidation (Modesto and Martinez 2010a; Cattaneo et al. 2011; Glusczak et al. 2011). In our study, the silver catfish exposed to GLY for 96 h showed an increase in TBARS levels in the liver for all concentrations and formulations tested. The lipid peroxides formation is the general response of fish species to glyphosate exposure. This increase in TBARS levels in the liver was also observed by Sinhorin et al. (2014), when Pseudoplatystoma sp. were exposed to 7.5 mg/L of GLY (Roundup Original®) for 96 h, and by Glusczak et al. (2011), when Leporinus obtusidens was exposed to GLY (Roundup®) for 96 h. The liver is the main xenobiotics detoxifying organ and is particularly susceptible to oxidative damage. These results suggest that the fish develop responses to each commercial formulation of the GLY herbicide in an attempt to adapt to or eliminate the xenobiotic.
The SOD–CAT system is an important line of defence against oxygen toxicity, due to the inhibitory effects on the formation of oxyradicals. Taken together, these enzymes are frequently used as biomarkers, indicating the production of ROS (van der Oost et al. 2003; Langiano and Martinez 2008). The CAT activity was inhibited at all concentrations of the herbicides tested. The reduction in CAT activity may have occurred due to superoxide ions, which were probably not neutralised efficiently by SOD, as observed in this study; this hypothesis may also be reinforced by the study of Modesto and Martinez (2010a), where P. lineatus was exposed to GLY for 96 h. Our results were similar to those reported by Ferreira et al. (2010), who demonstrated the inhibition of CAT activity to exposure silver catfish to GLY (Roundup®) by 96 h. In our research, the protection of the organism against ROS by the SOD-CAT in the liver was not efficient in any concentrations and formulations tested. This result may be ascribed to the increased TBARS levels registered in this organ, showing oxidative damage.
GST is an important enzyme involved in catalysing the conjugation of a wide variety of electrophilic substrates to reduce glutathione (van der Oost et al. 2003; Langiano and Martinez 2008). Furthermore, it protects the cell against the effects of xenobiotics. In the present study, GST activity decreased in the liver at a concentration of 2.5 mg/L of Biocarb® in relation to the control group. This decrease in GST activity could suggest a failure of detoxification and the occurrence of oxidative damage as evidence in the same concentration in the TBARS levels. Toni et al. (2013) mentioned the activity reduction in this enzyme might be related to cytochrome P450 enzymes biotransformation steps and to the production of different metabolites that may compete with GST substrates for the active sites of the enzyme. Similar results were also reported by Menezes et al. (2011) when silver catfish were exposed to GLY (Roundup®) for 8 days.
Thus, the presence of oxidants may lead to the activation or inhibition of enzymatic activity. The induction of GST in fish organs is considered beneficial for handling stressful conditions, as observed at 2.5 mg/L Orium®. According to Modesto and Martinez (2010b) exposing P. lineatus to GLY (Roundup®) for several experimental periods, the activation of this enzyme may have occurred due to metabolisation of the produced lipoperoxides. However, in the present study, the activation of GST was not sufficient to eliminate GLY. These results show that formulations of GLY herbicides can have different effects on the same species, being effective or not, still no effect was observed in high concentrations in comparison with the control group. This response may indicate the exposed fish tried to use others pathways in the attempt of the detoxification.
The oxidative damage situation induced in the liver of silver catfish by GLY may also be observed in several histological alterations shown in this organ. In the present study, vacuolisation was the main alteration observed in all treatments. The study by Ayoola (2008), in which Oreochromis niloticus were exposed to GLY at different concentrations for 96 h, also demonstrated vacuolisation. Others herbicides such as 2,4-D and clomazone (Cattaneo et al. 2008; Crestani et al. 2007) and insecticide-class pesticides (carbosulfan) and fungicides (benomyl and propineb) are able to produce similar results such as vacuolisation and focal necrosis as found in the present study (Capkin et al. 2010). According to Jiraungkoorskul et al. (2003), vacuolisation might be an imbalance between the rate of synthesis of lipid substances in the parenchymal cells and the rate of release in the systemic circulation. Nevertheless, lipid changes in hepatocytes may indicate that fish are concentrating lipophilic substances (such as surfactant POEA) in the hepatocytes with the end result being the reduced availability of the xenobiotic (Sarkar et al. 2005; Ramírez-Duarte et al. 2008).
In our research, all GLY treatments demonstrated moderate alterations indicating degeneration of the cytoplasm and severe alterations (focal necrosis) at 5.0 mg/L Biocarb®. These results allow us to hypothesise that an increase in the detoxifying function of glyphosate by the liver may have occurred, and the results demonstrated that it was not possible to regenerate new hepatocytes in response to this exposure. Similar results were described by Langiano and Martinez (2008) after GLY (Roundup®) exposure in P. lineatus considering different experimental periods. Kumar et al. (2014) exposing Oreochromis mossambicus to endosulfan reported results of focal necrosis as observed in our investigation. According to Mela et al. (2013), focal necrosis represents irreversible functional damage on homoeostasis of the organism. Nevertheless, in the present study, treatments of GLY demonstrated infiltration of leucocytes and melanomacrophages. These results are considered a biomarker of immune toxicity and are related to degenerative necrotic conditions. These cells acted to remove debris (cell death) by increasing the phagocytic activity (Hued et al. 2012; Mela et al. 2013). By observing this set of results, it is possible to deduce that GLY might cause an inflammatory process in the liver of silver catfish.
According to the IAH analysis, the formulations Orium® at both concentrations and Biocarb® at 5.0 mg/L were able to cause slight changes to organs that were still reversible. The histological alterations observed in this study may be dependent on the time of exposure and concentrations used; this hypothesis was reinforced by Topal et al. (2015) when Oncorhynchus mykiss was exposed to GLY for different experimental periods.
A set of biomarkers is necessary for understanding the response of the organism when exposed to pesticides. Thus, biomarkers that indicate oxidative damage, antioxidant enzymes, histology and plasma enzymes were integrated into the present study. These parameters were chosen for being usually changed by the exposed organisms. Therefore, these biomarkers may predict the toxicity of the compound and be useful in aquaculture and environmental risk assessment. ALT, the enzyme that is present preferentially in the cytoplasm showed decreased activity at 2.5 mg/L Biocarb®. This result may be due to a deficiency of available amino acids, leading to a decrease in alpha keto acids and a consequential decrease in the activity of ALT. The enzyme AST may be found in the cytoplasm and mitochondria; in our research, an increase in this enzyme was observed at 2.5 mg/L Orium® and Original® and at 5.0 mg/L Biocarb®. These observed results of increased AST activity in Orium® and Biocarb® may be related to the damage frequency, which in these groups was moderate and severe, being indicative of damage to the hepatocytes and the excessive production of said enzyme, or as a result of necrosis where disruption of the membranes occur, releasing them into the blood. Still, it is possible to hypothesise that GLY is causing an initial damage in the hepatocytes of silver catfish after acute exposure. Similar results were observed by Crestani et al. (2006) after exposing silver catfish to clomazone (0.1 and 5.0 mg/L) for several experimental periods. Considering that AST is found in the mitochondria, it is possible to imply that GLY may cause more toxic effects in mitochondria of the hepatocytes compared to other organelles, leading to a deficit in cellular respiration.
Conclusion
The knowledge of sublethal effects of GLY formulations considering this set of analyses used at present study is important to better understanding the different pathway of detoxification utilised by organisms exposed in attempt of eliminate the same active ingredient. The values obtained for TBARS, SOD and GST showed that 2.5 mg/L of Biocarb® was the more extreme treatment compared with others formulations tested. The difference observed for the formulation Biocarb® may be related to the components of the formula, such as the surfactant used in the manufacturing of this product, which were not described by the manufacturer. However, in histological analyses, more damage was observed at 5.0 mg/L Biocarb®; although this damage is reversible according to IAH, the damage in the environment if this poisoning is prolonged might lead to irreversible damage, mainly to the liver, impairing homoeostasis and detoxification processes. It is important to emphasise the severe damage in the hepatocytes may have occurred due to the increase in the free radicals, which induce the oxidative damage and the alterations for AST might have caused injury to the mitochondria. Thus, the present study allowed to identify the responses of native organism and predict potential damages this organism may have in the environmental situations. This condition may occur in near regions to soybean cultivation where GLY is widely used on genetically modified organisms and other situations where GLY herbicide is used.
References
Antón FA, Laborda E, Ariz M (1994) Acute toxicity of the herbicide glyphosate to fish. Chemosphere 28:745–753. doi:10.1016/0045-6535(94)90228-3
Antunes MIPP, Spurio RS, Godoi DA (2008) Cloridrato de benzocaína na anestesia de carpas (Cyprinus carpio). Semina Cienc Agrár 29:151–156. doi:10.5433/1679-0359.2008v29n1p151
Aparicio VC, Gerónimo ED, Marino D et al (2013) Environmental fate of glyphosate and aminimethyl-phosphonic acid in surface waters and soil of agricultural basins. Chemosphere 93:1866–1873. doi:10.1016/j.chemosphere.2013.06.041
Ayoola SO (2008) Toxicity of glyphosate herbicide on Nile tilapia (Oreochromis niloticus) juvenile. Afr J Agric Res 3:825–834 (Article Number: 6E4299538453)
Baldisserotto B (2009) Piscicultura continental no Rio Grande do Sul: situação atual, problemas e perspectivas para o futuro. Cienc Rural 39:291–299. doi:10.1590/S0103-84782008005000046
Barcellos LJG, Kreutz LC, Quevedo RM et al (2004) Nursey rearing of jundiá, Rhamdia quelen (Quoy and Gaimard) in cages: cage type, stocking density and stress response to confinement. Aquaculture 232:383–394. doi:10.1016/S0044-8486(03)00545-3
Bradford MMA (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Brausch MJ, Smith NP (2007) Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch Environ Contam Toxicol 52:217–221. doi:10.1007/s00244-006-0151-y
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–309. doi:10.1016/S0076-6879(78)52032-6
Capkin E, Terzi E, Boran H et al (2010) Effects of some pesticides on the vital organs of juvenile rainbow trout (Oncorhynchus mykiss). Tissue Cell 42:376–382. doi:10.1016/j.tice.2010.10.001
Cattaneo R, Loro VL, Spanevello R et al (2008) Metabolic and histological parameters of silver catfish (Rhamdia quelen) exposed to commercial formulation of 2,4-D (dichlorophenoxiacetic acid) herbicide. Pestic Biochem Physiol 92:133–137. doi:10.1016/j.pestbp.2008.07.004
Cattaneo R, Clasen B, Loro VL et al (2011) Toxicological responses of Cyprinus carpio exposed to a commercial formulation containing glyphosate. Bull Environ Contam Toxicol 87:597–602. doi:10.1007/s00128-011-0396-7
Crestani M, Menezes C, Glusczak L et al (2006) Effects of clomazone herbicide on hematological and some parameters of protein and carbohydrate metabolism of silver catfish Rhamdia quelen. Ecotoxicol Environ Toxicol 65:48–55. doi:10.1016/j.ecoenv.2005.06.008
Crestani M, Menezes C, Glusczak L et al (2007) Effects of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 67:2305–2311. doi:10.1016/j.chemosphere.2006.090.70
Ferreira D, Motta AC, Kreutz LC et al (2010) Assessment of oxidative stress in Rhamdia quelen exposed to agrochemicals. Chemosphere 79:914–921. doi:10.1016/j.chemosphere.2010.03.024
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup® herbicide. Rev Environ Contam Toxicol 16:35–120. doi:10.1007/978-1-4612-1156-3_2
Glusczak L, Loro VL, Pretto A et al (2011) Acute exposure to glyphosate herbicide affects oxidative parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 61:624–630. doi:10.1007/s00244-011-9652-4
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hidalgo C, Rios C, Hidalgo M et al (2004) Improved coupled-column liquid chromatographic method for the determination of glyphosate and aminomethylphosphonic acid residues in environmental waters. J Chromatogr A 1035:153–157. doi:10.1016/j.chroma.2004.02.044
Hose JE, Mcgurk MD, Marty GD et al (1996) Sublethal effects of the Exxon Valdez oil spill on herring embryos and larvae: morphological, cytogenetic, and histopathological assessment 1989–1991. Can J Fish Aquat Sci 53:2355–2365
Hued AC, Oberhofer S, Bistoni MA (2012) Exposure to a commercial glyphosate formulation (Roundup®) alters normal gill and liver histology and affects male sexual activity of Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Arch Environ Contam Toxicol 62:107–117. doi:10.1007/s00244-011-9686-7
Jiraungkoorskul W, Upatham ES, Kruatrachue M et al (2003) Biochemical and histophatological effects of glyphostate herbicide on nile tilapia (Oreochromis niloticus). Environ Toxicol 18:260–267. doi:10.1002/tox.10123
Kreutz LC, Barcellos LJG, Silva TO et al (2008) Acute toxicity test for agricultural pesticides on silver catfish (Rhamdia quelen) fingerlings. Cienc Rural 38:1050–1055. doi:10.1590/S0103-84782008000400022
Kumar N, Sharma R, Tripathi G et al (2014) Cellular metabolic, stress and histological response on exposure to acute toxicity of endosulfan in tilapia (Oreochomis mossambicus). Environ Toxicol. doi:10.1002/tox.22026
Langiano VC, Martinez CBR (2008) Toxicity and effects of a glyphosate-based herbicide on the neotropical fish Prochilodus lineatus. Comp Biochem Physiol 147:222–231. doi:10.1016/j.cbpc.2007.09.009
Mela M, Guiloski IC, Doria HB et al (2013) Effects of the herbicide atrazine in neotropical catfish (Rhamdia quelen). Ecotoxicol Environ Saf 93:13–21. doi:10.1016/j.ecoenv.2013.03.026
Menezes CC, Fonseca MB, Loro VL et al (2011) Roundup effects on oxidative stress parameters and recovery pattern of Rhamdia quelen. Arch Environ Contam Toxicol 60:665–671. doi:10.1007/s00244-010-9574-6
Michalany J (1980) Técnica histológica em Anatomia Patológica, com instruções para o cirurgião, enfermeira e citotécnico. EPU, São Paulo, p 277p
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Modesto KA, Martinez CBR (2010a) Effects of Roundup Transorb® on fish: hematology, antioxidant defenses and acetylcholineserase activity. Chemosphere 81:781–787. doi:10.1016/j.chemosphere.2010.07.005
Modesto KA, Martinez CBR (2010b) Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the Prochilodus lineatus. Chemosphere 78:294–299. doi:10.1016/j.chemosphere.2009.10.047
Moreno NC, Sofia SH, Martinez CBR (2014) Genotoxic effects of the herbicide Roundup Transorb® and its active ingredient glyphosate on the fish Prochilodus lineatus. Environ Toxicol Pharmacol 37:448–454. doi:10.1016/j.etap.2013.12.012
Nelson DP, Kiesow LA (1972) Enthalpy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solutions in the UV). Anal Biochem 49:474–478. doi:10.1016/0003-2697(72)90451-4
Pesce S, Fajon C, Bardot C et al (2008) Longitudinal changes in microbial planktonic communities of a French river in relation to pesticide and nutrients inputs. Aquat Toxicol 86:352–360. doi:10.1016/j.aquatox.2007.11.016
Poleksic V, Mitrovic-Tutundzic V (1994) Fish gills as a monitor of sublethal and chronic effects of pollution. In: Müller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Cambridge Univ. Press, Cambridge, pp 339–352
Pretto A, Loro VL, Menezes C et al (2011) Commercial formulation containing quinclorac and metsulfuron-methyl herbicides inhibit acetylcholinesterase and induce biochemical alterations in tissues of Leporinus obtusidens. Ecotoxicol Environ Saf 74:336–341. doi:10.1016/j.ecoenv.2010.10.003
Ramírez-Duarte WF, Rondón-Barrangán IS, Eslava-Mocha PR (2008) Acute toxicity and histopathological alterations of Roundup® herbicide on “cachama blanca” (Piaractus brachypomus). Pesqui Vet Bras 28:547–554. doi:10.1590/S0100-736X2008001100002
Salbego J, Pretto A, Gioda CR et al (2010) Herbicide formulation with glyphosate affects growth, acetycholinesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 58:740–745. doi:10.1007/s00244-009-9464-y
Salbego J, Pretto A, Silva VMM et al (2014) Glyphosate on digestive enzymes activity in piava (Leporinus obtusidens). Cienc Rural 44:1603–1607. doi:10.1590/0103-8478cr20131399
Sarkar B, Chatterjee A, Adhikari S et al (2005) Carbofuran and cypermethrin-induced histophatological alterations in liver of Labeo rohita (Hamilton) and its recovery. J App Ichthyol 21:131–135. doi:10.1111/j.1439-0426.2004.00590.x
Sinhorin VDG, Sinhorin AP, Teixeira JMS et al (2014) Effects of the acute exposition to glyphosate-based herbicide on oxidative stress parameters and antioxidant responses in a hybrid Amazon fish surubim (Pseudoplatystoma sp). Ecotoxicol Environ Saf 106:181–187. doi:10.1016/j.ecoenv.2014.04.040
Szarek A, Siwicki A, Andrzejewska E et al (2000) Effects of the herbicide Roundup® on the ultraestructural pattern of hepatocytes in carp (Cyprinus carpio). Mar Environ Res 50:253–266. doi:10.1016/S0141-1136(00)00088-X
Toni C, Menezes C, Clasen B et al (2013) Oxidative stress in carp exposed to quinclorac herbicide under rice field condition. Ecotoxicol Environ Saf 92:27–31. doi:10.1016/j.ecoenv.2013.01.028
Topal A, Atamanalp M, Uçar A et al (2015) Effects of glyphosate on juvenile rainbow trout (Oncorhynchus mykiss): transcriptional and enzymatic analyses of antioxidant defence system, histopathological liver damage and swimming performance. Ecotoxicol Environ Saf 111:206–214. doi:10.1016/j.ecoenv.2014.09.027
Tsui MTK, Chu LM (2008) Environmental fate and non-target impact of glyphosate-based herbicide (Roundup®) in a subtropical wetland. Chemosphere 71:439–446. doi:10.1016/j.chemosphere.2007.10.059
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. doi:10.1016/S1382-6689(02)00126-6
WHO—World Health Organization (1994) Environmental health criteria: glyphosate. Geneva
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharm 31:117–165. doi:10.1006/rtph.1999.1371
Acknowledgments
We would like to thank the Federal University of Santa Maria for the support and facilities and the financial support and fellowships from the Brazilian agency CAPES (Coordination for the Improvement of Higher Education Personnel).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murussi, C.R., Costa, M.D., Leitemperger, J.W. et al. Exposure to different glyphosate formulations on the oxidative and histological status of Rhamdia quelen . Fish Physiol Biochem 42, 445–455 (2016). https://doi.org/10.1007/s10695-015-0150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0150-x