Abstract
Sugarcane is one of the world’s most important commodities. In order to control weeds in the plantations and increase productivity, the mixing of different herbicides during spraying operations is commonplace. This practice is unregulated, and the impact on water quality and nontarget tropical species is poorly understood. In the present work, exposure and recovery assays were used to evaluate antioxidant enzyme activity and histopathological alterations in the liver of tilapia (Oreochromis niloticus) following exposure to mixtures of the herbicides widely used in sugarcane crops: ametryn (AMT), tebuthiuron (TBUT), diuron (DIU), and hexazinone (HZN). The greatest biochemical changes were observed for the mixture (DIU+HZN)+AMT+TBUT at the highest concentration tested (1/10 96hLC50). This mixture caused a significant increase (p < 0.01) of approximately 82% in GST activity after 14 days of exposure. For three of the mixtures evaluated, GST and CAT could be considered potential biochemical biomarkers of exposure to the herbicide mixtures due to the frequency, intensity, and statistical significance of alterations in the assimilation phase. Although morphological changes were evident in the hepatic tissue, severe damage was only noted in a few samples, and there were no statistically significant differences, relative to the control. The results of hepatic lesion recovery assays suggested that the most sensitive individuals affected by the xenobiotics were unable to achieve full recovery. It is anticipated that the data obtained may assist in the selection of biomarkers for monitoring purposes, as well as in reinforcing standards of conduct in the use of agrochemical mixtures in agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sugarcane is one of the world’s most important commodities, with an estimated harvested area of more than 26 million hectares. In 2014, Brazil contributed the highest harvested area (10.42 million ha), but other countries such as India and China also produce significant quantities of this commodity (FAO 2014). The herbicides widely used in sugarcane plantations to increase crop productivity include mixtures of ametryn (AMT), tebuthiuron (TBUT), hexazinone (HZN), and diuron (DIU). These chemicals are used in the following proportions: AMT+TBUT = 2.0 + 1.2 kg active ingredient (a.i.) ha−1; (DIU+HZN)+TBUT = 2.0 + 1.2 kg a.i. ha−1; (DIU+HZN)+TBUT+AMT = 2.0 + 1.2 + 1.5 kg a.i. ha−1 (Moura and Jonsson 2016).
Mixing of pesticides in spray tanks is a common practice among rural producers. However, despite some advantages for agriculturalists, the use of mixtures can amplify the toxic effect of each component in nontarget organisms (Laetz et al. 2009; Prestes et al. 2011; Silva et al. 2015).
In recent analyses, ametryn and tebuthiuron were detected in São Paulo State waters at levels of 0.29 and 0.01 μg L−1, respectively (Monteiro et al. 2014). Other herbicides have also been detected in Brazilian water bodies (Santos et al. 2013), some at concentrations exceeding current maximum permissible levels. For example, atrazine was detected at a concentration of 9.3 μg L−1 in river water from Campo Verde in Mato Grosso State (Moreira et al. 2012), while the maximum permissible level is 2 μg L−1 (Brasil 2005). Pinheiro et al. (2010) detected 2,4-D at concentrations of 34.8 and 74.5 μg L−1 in water bodies of the Itajaí river basin. According to Brazilian legislation (Brasil 2005), the maximum permissible concentrations of 2,4-D are 4 μg L−1 (class 1 freshwater) and 30 μg L−1 (class 3 freshwater).
The presence of glyphosate was detected at a concentration of 7 μg L−1 in water near rice fields (Mattos et al. 2002). This was lower than the maximum permissible value for surface waters (Brasil 2005) but exceeded the maximum limit (0.5 μg L−1) established by the European Union for water intended for human consumption (Prammer 1998; Pinheiro et al. 2010).
Brazilian legislation (Brasil 2005) only sets limit values applicable to the aquatic environment for some of the many products routinely used in agriculture. The herbicides described above for use in sugarcane plantations are not mentioned in such legislation. However, Pathiratne and Kroon (2016) reported that the following maximum concentrations would protect 95% of the species exposed: ametryn 0.038 μg L−1, hexazinone 2.0 μg L−1, diuron 1.3 μg L−1, and tebuthiuron 11.0 μg L−1. These were considered threshold values for the protection of tropical freshwater ecosystems in Australia.
In addition to the limited legislation, the presence of some compounds, such as atrazine, at concentrations below the limit values can cause structural, biochemical, and physiological alterations in fish (Mela et al. 2013). This constitutes another source of concern about the possible impacts of agrochemicals in the aquatic environment.
Consideration of possible adverse effects of a given contaminant at sublethal concentrations is extremely important in environmental risk assessment, since there can be cascade effects leading to consequences at the levels of individuals, communities, and ecosystems. The use of biomarkers in risk assessments offers the advantage of enabling the detection of potentially toxic exposure, well before real adverse effects occur (Walker et al. 1995).
Even at concentrations of 1/100 of the median lethal concentration (LC50), some pesticides and other toxic substances can cause adverse effects in aquatic organisms (Sancho et al. 2009; Saka et al. 2013). According to Das and Gupta (2012) and Hassaan et al. (2014), the exposure of fish to 1/100 LC50 is sufficient for the detection of morphological alterations induced by sublethal doses of pesticides.
Under natural conditions, organisms are constantly exposed to a variety of toxic agents, which can act in combination in biological systems (Tallarida 2001). Although the effects of chemical interactions have been known for many years, there are relatively few studies devoted to observing the outcomes of simultaneous exposure to two or more substances (Brodeur et al. 2014; Tang and Escher 2014).
Toxicity data for tropical and neotropical species are often lacking for ecological risk assessment. Consequently, tropical and subtropical countries use water quality criteria derived from temperate regions (such as the USA, Canada, or Europe) to assess ecological risks in aquatic systems (Kwok et al. 2007). For many herbicide formulations widely used in Brazilian commercial crops, the doses that can lead to the decline of aquatic communities are more restrictive than the values adopted in temperate countries, which are normally used as references (Moura et al. 2008).
The likelihood of the simultaneous presence of these compounds in water bodies, resulting in synergistic toxic effects, makes it important to study their impacts using suitable biochemical biomarkers able to provide an indication of alterations in nontarget organisms after brief periods of exposure (Modesto and Martinez 2010).
Reactive oxygen species (ROS) are produced by biological systems as a result of normal cellular metabolism and environmental factors, such as pollutants. Cell components including carbohydrates, nucleic acids, lipids, and proteins can be damaged by the high reactivity of ROS. The condition of “oxidative stress” is associated with a shift in the balance between oxidants and antioxidants, in favor of oxidants. Organisms possess integrated antioxidant systems, which include enzymatic antioxidants that are usually effective in blocking the harmful action of ROS (Birben et al. 2012). Antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GST) have been studied as biomarkers of oxidative stress (Nava-Álvarez et al. 2014) caused by herbicides in fish (Toni et al. 2010; Stara et al. 2012) and other organisms present in the aquatic environment (Mofeed and Mosleh 2013).
Fatima et al. (2007) evaluated the combined effects of herbicides, at concentrations present in water bodies in Belgium, on the activities of CAT and SOD extracted from the liver, kidney, and spleen of goldfish exposed to the compounds. In the liver, the combined effects of the herbicides atrazine (42 μg L−1), simazine (41 μg L−1), diuron (41 μg L−1), and isoproturon (47 μg L−1) significantly increased SOD activity, while CAT activity decreased.
In selection of biomarkers in fish, ecotoxicological evaluations that combine different methodological tools have been shown to provide the best strategy for understanding toxic effects. The combined use of histological biomarkers (which enable identification of the specific location of cellular dysfunction) and biochemical analyses has been widely reported to give satisfactory results (Poleksic and Karan 1999; Oropesa et al. 2009; Husak et al. 2014), including in the evaluation of xenobiotic mixtures (Strmac and Braunbeck 2002; Gonçalves et al. 2014). Assessments of the effects of herbicide mixtures are quite scarce in the literature, especially in the case of long-term assays of the biochemical and histological profiles of fish (Rossi et al. 2011; Babut et al. 2013).
The aim of the present work was to evaluate the effects in fish of prolonged exposure to a set of herbicide mixtures, employing biochemical and histopathological biomarkers. This study provides new data for herbicide risk assessment in tropical environments, contributing to sustainable agricultural development.
2 Material and Methods
2.1 Herbicide Exposure Assays
The assays were performed using mixtures of herbicide formulations based on tebuthiuron (TBUT—Combine 500 SC®; concentrated suspension; 500 g L−1), ametryn (AMT—Gesapax 500®; concentrated suspension; 500 g L−1), and a commercial mixture of diuron (DIU) and hexazinone (HZN) (Velpar K®, which consists of dispersible granules containing DIU at 468 g kg−1 and HZN at 132 g kg−1). All the formulations were obtained from local suppliers. Analytical standards (> 98% purity) were purchased from Dr. Ehrenstorfer (Augsburg, Germany).
2.2 Toxicity Assays
Tilapia (Oreochromis niloticus) was selected as the test organism because it is representative of tropical aquatic ecosystems and is widely used in ecotoxicological testing (Jordaan et al. 2013). Tilapia is economically important, since it is the second largest group of farmed fish (after carp), and is the most widely cultivated (FAO 2016).
Fish with average weight of 33.48 ± 6.15 g and average length of 9.98 ± 0.56 cm were obtained from a commercial fishery in São Paulo State (Brazil) and were transferred to polyethylene tanks (115 L usable volume). The experimental systems were installed in a climate-controlled room, with a photoperiod of 16:8 h (light/dark), temperature of 26 ± 2 °C, and constant aeration. The water used had the following characteristics: pH = 7.7, dissolved oxygen = 6.2 mg L−1, electrical conductivity = 3.8 mS cm−1, and total hardness = 53.6 mg L−1 CaCO3. The fish were exposed in duplicate (n = 15 per tank) to the control treatment (clean water) and to two sublethal concentrations of each mixture, corresponding to 1/100 and 1/10 of the median lethal concentrations for 96 h exposure (96hLC50). The 96hLC50 values determined for each mixture in previous tests (Moura and Jonsson 2016) were as follows: AMT+TBUT 10.76 mg L−1, (DIU+HZN)+TBUT 43.09 mg L−1, and (DIU+HZN)+TBUT+AMT 11.90 mg L−1. These values represent the total amounts of herbicides in each mixture. The amounts of the active ingredients in the mixtures tested were proportional to the quantities used in sugarcane plantations, as described above. Therefore, at the highest exposure level (1/10 96hLC50), the nominal concentrations (mg L−1) in the mixtures were 0.67 + 0.40 (AMT+TBUT), 2.70 + 1.60 ((DIU+HZN)+TBUT), and 0.51 + 0.30 + 0.38 ((DIU+HZN)+TBUT+AMT). At the lowest exposure level (1/100 96hLC50), the concentrations were 1/10 of these values.
The animals were fed twice daily and the media in the tanks were totally renewed every 2 days. Fish were collected (n = 6 per treatment) after 7 and 14 days of exposure (assimilation phase). The fish were then kept in clean water for 14 days, and individuals (n = 6 per treatment) were collected after 7 and 14 days (recovery phase).
At the end of the experiment, the animals were anesthetized with benzocaine diluted in water and were euthanized by spinal medulla section. The livers were removed and stored at −80 °C until they were processed for biochemical analysis. Hepatic samples for histopathological analysis were also collected from the animals exposed to the mixtures for up to 14 days and from those allowed to recover for up to 14 days. The samples were fixed in 10% formalin solution for 24 h at ambient temperature.
The procedures used in this study were authorized by the Animal Experimentation Ethics Committee of Embrapa Environment (Registration No. 002/2012).
2.3 Analysis of Herbicides in the Test Solutions
Samples of water were collected from the tanks after preparation of the test solutions and before renewal. The samples were stored in a freezer (at −18 °C) prior to the chemical analyses.
An aliquot of approximately 0.5 mL of the sample was filtered through a 0.22-μm membrane and transferred to a vial, followed by dilution with 0.5 mL of the chromatography 80% mobile phase (20% (v/v) aqueous 0.1% formic acid mixed with 80% (v/v) acetonitrile). Aliquots were injected into an ultra-performance liquid chromatograph coupled to a triple quadrupole mass spectrometer equipped with electrospray ionization (UPLC-ESI-MS/MS) (model Quattro Premier XE, Waters).
The chromatographic separation employed an ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm ID, 50 mm) maintained at 40 °C. Gradient elution was performed using an aqueous solution containing 0.1% formic acid (phase A) and acetonitrile (phase B).
The mass spectrometer was operated in MRM mode, with positive ion electrospray ionization. The capillary voltage used was 3.0 kV. Nitrogen was used as the desolvation gas, at 400 °C and a flow rate of 500 L h−1, and argon was used as the collision gas, at a flow rate of 20 mL min−1. The limit of detection (LOD) was 0.010 mg L−1, the recoveries were between 107.7 and 127.3%, and the coefficient of variation was < 3%.
2.4 Biochemical Analyses
The hepatic samples were homogenized in 0.5 mol L−1 cold phosphate buffer at pH 7 (1:4, w/v), followed by centrifugation (10,000×g) for 20 min at 4 °C. Aliquots of the supernatants were stored in Eppendorf tubes, at −80 °C, for subsequent biochemical analyses. Measurements were made of the specific activities of catalase (CAT) (Aebi 1984), superoxide dismutase (SOD) (Ukeda et al. 1997), glutathione S-transferase (GST) (Keen et al. 1976), and glutathione peroxidase (GPx) (Catalgol et al. 2007). The data were normalized to the sample protein concentration (Bradford 1976).
2.5 Histopathological Evaluation
After fixation, the hepatic samples were embedded in paraffin (Paraplast Plus P3683, Sigma-Aldrich, St. Louis, MO). Sections (6-μm thick) of each fragment were stained using the hematoxylin-eosin technique.
The intensities of the different histopathological alterations in the liver were evaluated using semi-quantitative analysis, as described by Bernet et al. (1999), where importance factors and weights were allocated according to the importance represented by the lesion in terms of hepatic functionality. The attribution of these weights was achieved by classifying the observed alterations according to the criteria described by Bernet et al. (1999) and Costa et al. (2011). The alterations and their corresponding importance factors (weights) were as follows: (1) hemorrhage/congestion and hepatocyte cytoplasmic vacuolization; (2) vacuolated nuclei, pyknotic nuclei, leukocytes infiltration, and intracellular eosinophilic bodies; and (3) hepatocyte necrosis.
For each animal, the degree of hepatic damage was calculated using the method of Poleksic and Mitrovic-Tutundzic (1994), with modifications. An alteration severity index (I), considering the degree of importance (1, 2, or 3) given to each alteration as described above, was constructed as follows:
Once the index value had been obtained, the severity of impairment of the organ was described according to the value of I as follows: 0–10 = normal functioning, 11–20 = light to moderate damage, 21–50 = moderate to severe alterations, 51–100 = severe alterations, > 100 = irreparable damage (Poleksic and Mitrovic-Tutundzic 1994).
2.6 Statistical Analysis
In the case of the biochemical analyses, the effects of the treatments on the response variables were investigated using a general linear model (GLM). For those variables that showed evidence of heterogeneous variance, the GLM was adjusted using the weighted least squares method, considering the variances of the treatments as weightings. These analyses were performed using Statgraphics Plus v.5.1 software (Manugistics 2001). Statistical evaluation of differences between the groups that served as controls and the groups exposed to the herbicides employed a significance level of p < 0.05.
In the case of the semi-quantitative histopathological analyses, after obtaining the scale of severity of the liver injury for each animal, Fisher’s exact test (Mehta and Patel 1983; Valz and Thompson 1994) was used for comparative analysis among the three concentrations tested for each mixture evaluated and between the exposure and recovery assays.
3 Results and Discussion
During the experimental period, there was no mortality of animals in the control group or in the groups exposed to the herbicide mixtures. There was also no evidence of any behavioral changes in the fish.
3.1 Concentrations of Active Agents in the Water
The real concentrations of the active agents during the assimilation phase (days 7, 11, and 14) were considered as the average concentrations before and after renewal of the test solutions (Table 1). For some of the active agents, the real concentrations were substantially lower than the nominal values, with recoveries of approximately 50%. The recovery percentages showed almost no differences between the water renewal periods, suggesting that the low recoveries were not due to degradation of the compounds.
Instead, they could probably be explained by rapid adsorption of the herbicides onto the walls of the containers. Wheelock et al. (2005) evaluated the influence of the containers in tests of the toxicity of cyhalotrin towards aquatic invertebrates. It was noticed that plastic tanks showed greater adsorption of the insecticide, compared to glass or Teflon containers. Topp and Smith (1992) also reported that pesticides could be adsorbed onto plastic materials. Furthermore, the adjuvants present in pesticide formulations include adherence agents that act to increase adhesion of the active agents to target surfaces in order to enhance their effectiveness (Herzfeld and Sargent 2011).
In the present work, the assay was not invalidated by the decreased concentrations of the compounds relative to the nominal concentrations because the levels of the xenobiotics remaining in the water were measured and were reported as real concentrations.
3.2 Enzymatic Activity
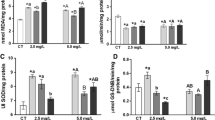
The results obtained for exposure of the fish to the herbicide mixtures at the two sublethal concentrations evaluated are presented in Tables 2, 3, 4, and 5. The animals exposed to AMT+TBUT C1 showed a significant decrease of 44.6% in CAT activity after 14 days of the assimilation phase (Table 2). After the first week of the recovery phase, the animals showed a significant decrease in GPx activity of 49.3% (Table 5).
The exposure to the highest concentration of DIU+HZN+TBUT resulted in a 46.4% decrease in GPx activity after the first week of the recovery phase (Table 5). This mixture also induced substantial alterations of the enzymatic activities of CAT (Table 2; 0.063 units) and GST (Table 4; 0.051 units) during the assimilation phase. Although these changes were not statistically significant at the 5% level, the significances obtained were very close to this level (p < 0.07 and 0.06, respectively).
Exposure to the highest concentration of DIU+HZN+AMT+TBUT resulted in an increase of around 33.3% (p < 0.05) in SOD activity after 7 days (Table 3) and an increase of 82.8% in GST activity after 14 days (Table 4). For the other experimental conditions, there were no significant differences relative to the control groups.
Statistically significant effects, relative to the controls, mainly occurred during the exposure phase, consistent with the progressive accumulation of the compounds in the tissues of the fish, with alterations becoming more apparent after longer exposure times. Based on the liver biochemical data and the real concentrations measured in the water, the estimated NOEC values (mg L−1) for the compounds of the (DIU+HZN)+AMT+TBUT mixture were 0.038 (AMT), 0.025 (DIU), 0.036 (HZN), and 0.023 (TBUT). For the DIU+HZN+TBUT mixture, the NOEC values were 0.110 (DIU), 0.045 (HZN), and 0.116 (TBUT). As use of the AMT+TBUT combination resulted in biochemical changes at the lowest concentration tested, it is not possible to establish NOEC values. The estimated NOEC values were higher than the proposed threshold values for tropical freshwater in Australia that would protect 95% of the species exposed (Pathiratne and Kroon 2016).
The enzymes SOD, CAT, and GPx are considered antioxidant enzymes. SOD has the capacity to neutralize the potential adverse effects of superoxide radicals, with the final product of the reaction being hydrogen peroxide, which is degraded by the CAT and GPx enzymes (Birben et al. 2012). The change in SOD activity after exposure for 7 days to the highest concentration of the (DIU+HZN)+AMT+TBUT mixture was in agreement with this mechanism. Hence, this significant increase in activity (Table 3) was probably due to increased enzyme expression in order to counteract the undesirable effects of reactive oxygen species.
Moraes et al. (2007) found that exposure of the fish Leporinus obtusidens to mixtures of the herbicides clomazone and propanil increased the activities of oxidative enzymes such as CAT in the liver, which was suggested to assist in detoxification of the herbicides. Although expression of the enzymes involved in neutralization of the effects caused by oxidative stress may increase in an attempt to minimize damage, the inhibition of their activity can also occur (Crestani et al. 2007), as observed for CAT and GPx in this work.
This has been reported elsewhere for CAT (Ortiz-Ordoñez et al. 2011) and GPx (Monteiro et al. 2006), when fish were exposed to the action of agrochemicals. Therefore, oxidative stress generated by herbicides may act to suppress the activities of antioxidant defense enzymes, due to their oxidative damage and a loss of compensatory mechanisms (Moraes et al. 2007).
In addition to being considered an antioxidant enzyme, GST is responsible for the detoxification and elimination of electrophilic compounds, including various substances used in agriculture. Stimulation of this enzyme involves conjugation reactions in the presence of glutathione (GSH) (Jonsson et al. 2002). In the present work, the increase in GST activity could have been related to induction of the synthesis of this enzyme during the assimilation phase, as observed after 14 days of exposure to DIU+HZN+AMT+TBUT. Similar findings were reported by Koutnik et al. (2015) in fishes and by Güngördü (2013) in amphibians exposed to the herbicides atrazine, metribuzine, or glyphosate. Vieira et al. (2014) observed the induction of hepatic and gill GST in fish contained in cages at various points of a watershed close to areas of intensive agriculture. Increased activity of gill GST was observed by Paulino et al. (2012) in the fish Prochilodus lineatus exposed subchronically to the herbicide atrazine.
It has been reported previously that in terms of enzymatic activity, there is no consistent concentration-response relationship, given that low concentrations of a xenobiotic might inhibit an antioxidant enzyme, while higher concentrations might have no effect (Otitoju and Onwurah 2005). Reduced activity of GPx may be caused by negative feedback due to an excess of substrate (Monteiro et al. 2006) or damage resulting from oxidative alterations (Tabatabaie and Floyd 1994). Decreased GPx activity can also indicate that its antioxidant capacity is exceeded by the quantity of hydroperoxides generated from lipid peroxidation (Remacle et al. 1992). These effects could have contributed to the decrease in GPx activity observed in the present work after 7 days of the recovery phase. There is no obvious explanation for the detection of this effect in the recovery phase, since complex biochemical pathways and regulatory mechanisms may be involved. There was a delay in the decrease of GPx activity in the presence of the xenobiotics, which only became manifested in the recovery phase. Similar to the results of the present study, Branco et al. (2011) observed inhibition of the activity of an antioxidant enzyme (thioredoxin reductase) in fish during the phase of clearance of a toxic agent.
According to the data obtained, the enzymes studied did not appear to be strong biomarkers of exposure to any of the herbicide mixtures evaluated under the conditions employed. Nonetheless, exposure to some of the mixtures resulted in changes in the activities of the CAT, SOD, and GST enzymes during the exposure phase.
However, considering the intensities and the statistical significances of the alterations in the assimilation phase, the GST and CAT enzymes appeared to be the most promising biochemical biomarkers of exposure to the mixtures of herbicides evaluated, with high frequencies of alterations for both enzymes. The GPx enzyme presented highly significant inhibition of activity for two of the mixtures evaluated, but this effect was only detected after the exposure period, during the recovery phase.
Environmental pollutants such as insecticides can cause effects in the cellular structures of invertebrates at concentrations of 1/100 of their median lethal concentrations (Oliveira et al. 2014). In agreement with the present work, concentrations of herbicides at levels up to 1/100 of the 96hLC50 have previously been found to induce alterations in aquatic organisms (Sancho et al. 2009; Saka et al. 2013).
3.3 Histopathological Alterations
The tilapia used in the present work presented the typical hepatic morphology described for this teleost (Figueiredo-Fernandes et al. 2006). The hepatocytes were large, and some were binucleate. In control samples, as well as in samples from the groups exposed to the herbicide mixtures, the hepatocytes were vacuolated and were only slightly colored by eosin, which is typical of teleost fingerlings and probably indicates a good stock of cytoplasmic glycogen, which is not stained by the method employed here (Fig. 1a).
Light micrographs of sections through livers of tilapia (Oreochromis niloticus) from the control group (a, b) and groups exposed to herbicides mixture (c–e). In a, tubules of hepatocytes are observed (T). The surface of these cells is facing the bile duct or pre-ductules; in this case it is possible to observe the core of preductulares cells (arrows). Capillaries (s) surrounding the tubules flow into the hepatic vein (V). Finally, the bile duct and the hepatic artery branch may be noted (asterisk). Bar, 50 μm. In b is noted peri-venous infiltration of plasma cells (arrows), Bar, 100 μm. In c is the liver of tilapia exposed to a mixture of (DIU+HZN)+TBUT, where scattered hepatocytes were observed with nuclear inclusion (arrow). Bar, 50 μm. In d is a tilapia liver exposed to the mixture AMT+TBUT, where eosinophilic inclusions, with varying size, in the cytoplasm of hepatocytes can be noted (arrows), as well as areas of cellular degeneration where the cell limit and the tubular arrangement of hepatocytes were lost (asterisk). Bar, 50 μm. In e is the area of necrosis above the venous vessel in tilapia liver exposed to a mixture of AMT+TBUT. Bar, 100 μm. Used in a–e was hematoxylin-eosin stain
Sparse collagenous stroma was observed, with the largest blood vessels showing collagen fibers in the adventitia. No stromal alterations were detected in the case of fish exposed to the herbicide mixtures. In samples from both the control and the exposed groups, the adventitia of the blood vessels showed leukocyte infiltration, mainly involving mast cells, also known as eosinophilic granulocyte cells (Fig. 1b). This feature was therefore not related to the effects of the different herbicide treatments.
Given that the tilapia were obtained from fish farms, the occurrence of inflammatory infiltrate can be considered normal. In these environments, the fish are subjected to the action of parasites, bacteria, and other organisms, all of which can stimulate an inflammatory response in the host (Baccari et al. 2011). However, with the techniques employed in this work, none of these agents was observed. In addition to these pathogenic organisms, other factors can favor the accumulation of mast cells in fish tissues, such as the stress of confinement (Holland and Rowley 1998).
The hepatic changes found only in the groups exposed to the herbicide mixtures included nuclear inclusions, with sparse distribution through the parenchyma (Fig. 1c), and eosinophilic cytoplasmic inclusions (EIs) in the hepatocytes (Fig. 1d). In areas where hepatocytes with EIs were observed, there were also regions of cellular degeneration, with loss of the cell boundary and disarray of the hepatocyte tubules (Fig. 1d). Only a few samples presented focal hepatic necrosis (Fig. 1e). The distributions of these alterations in the experimental population are provided in Table 6. The frequencies of different degrees of liver damage in the experimental groups are provided in Table 7.
The comparative analysis revealed no significant differences among the groups in terms of the severity index values. However, it is worth noting that the group treated with the AMT+TBUT mixture presented the greatest number of animals with eosinophilic inclusions, as well as focal necrosis in both assay phases (exposure and recovery), including in fish exposed to the lowest concentration of the mixture. The group treated with AMT+TBUT+(DIU+HZN) also presented liver cell degeneration and focal necrosis in the recovery phase.
Hepatocyte EIs, also known as hyaline degeneration or eosinophilic bodies, were recently included among histopathological biomarkers of hepatic alterations in fish and were used to obtain degeneration index values for the liver. Histochemical analysis of the inclusions confirmed the presence of proteins and lipoproteins (Costa et al. 2011). In mammals, this type of hepatocellular inclusion is associated with the presence of enlarged lysosomes (Koller 1973), autophagy (Hanada et al. 2012), and the accumulation of poorly formed proteins due to compromised cellular function, caused by factors such as cellular stress (Hanada et al. 2007). Although there have been several recorded instances of the occurrence of eosinophilic inclusions in the hepatocytes of teleosts exposed to xenobiotics (Costa et al. 2009), the precise significance of this hepatic alteration has not yet been fully elucidated.
In this work, the presence of EIs occurred in association with areas of cellular degeneration and/or focal necrosis. However, in another work, no correlation was observed between the presence of these inclusions and the occurrence of more severe alterations (Costa et al. 2009).
As no significant differences were observed between the control and the treated groups, the results described above indicated that it was probably only very sensitive animals that showed hepatic areas with degenerative lesions such as focal necrosis. In another work, common carp exposed to copper did not show histopathological changes in the kidney, but the biochemical profiles revealed disturbances in osmoregulation in this organ (Sevcikova et al. 2016). Stara et al. (2014) did not find any histopathological differences in the hepatopancreas of Procambarus clarkia exposed chronically to a triazine herbicide, although changes were observed in the activities of antioxidant enzymes.
The period of recovery employed here was insufficient to achieve full recovery following the occurrence of these lesions. Similar findings have been reported previously in studies using herbicide concentrations compatible with those employed in agriculture, with persistent biochemical and histopathological alterations, even after long recovery periods. After a recovery period of 20 days, Xing et al. (2012) observed histopathological lesions in the brain and kidney of carp exposed to sublethal chronic concentrations of atrazine and chlorpyrifos, used alone or in combination, over a period of 40 days. Unfortunately, the literature contains few reports of studies investigating liver recovery in fish subjected to long-term exposure to sublethal concentrations of herbicides. Using acute exposure assays, Crestani et al. (2007) observed vacuolation in hepatocytes of Rhamdia quelem after exposure to clomazone for 96 and 192 h, and the alteration was maintained in samples from animals allowed to recover for similar periods.
4 Conclusions
Exposure to the 1/10 96hLC50 DIU+HZN mixture caused alterations in biochemical biomarkers. Surprisingly, biochemical alterations in animals exposed to AMT+TBUT were only observed for the group exposed to the lowest concentration (1/100 96hLC50).
Biochemical alterations are biomarkers of sublethal exposure. Changes at this level can be observed before the appearance of histopathological and physiological alterations. In this study, no significant effects on the histopathology of the fish were observed, but there were alterations in biomarkers, which were mainly associated with oxidative stress. However, for the parameters considered, there were no concentration-response relationships. This could have been due to the inconstant concentrations of the herbicides, due to their adsorption onto the walls of the plastic tanks used to house the fish. In addition, the observed alterations were not persistent and disappeared after leaving the fish in clean water for 14 days. It is also possible that the biochemical alterations could have been related to homeostasis mechanisms.
This work suggests NOEC values related to fish physiology for some herbicides present in mixtures commonly used in sugarcane crops. These values, derived from our observed alterations of normal biological function, could assist in establishing concentration limits for pesticides in Brazilian water bodies. However, the estimated NOEC values are higher than the threshold concentrations proposed elsewhere in order to protect 95% of the species inhabiting tropical freshwater compartments.
The data obtained here should contribute to the implementation of more effective measures designed to provide guidance to agriculturalists and to monitor the use of herbicides and other agrochemicals. The findings should also assist in the selection of biomarkers suitable for use in the biomonitoring and recovery of tropical aquatic systems’ resources impacted by agrochemicals.
References
Aebi, H. (1984). Catalase in vitro. Academic Press, 105, 121–126. doi:10.1016/S0076-6879(84)05016-3.
Babut, M., Arts, G. H., Caracciolo, A. B., Carluer, N., Domange, N., Friberg, N., et al. (2013). Pesticide risk assessment and management in a globally changing world—report from a European interdisciplinary workshop. Environmental Science and Pollution Research, 20(11), 8298–8312. doi:10.1007/s11356-013-2004-3.
Baccari, G. C., Pinelli, C., Santillo, A., Minucci, S., & Rastogi, R. K. (2011). Mast cells in nonmammalian vertebrates: an overview. In K. W. Jeon (Ed.), International review of cell and molecular biology (Vol. 290, pp. 1–50). Amsterdam: Elsevier Inc..
Bernet, D., Schmidt, H., Meier, W., Burkhardt-Holm, P., & Wahli, T. (1999). Histopathology in fish: proposal for a protocol to assess aquatic pollution. Journal of Fish Diseases, 22(1), 25–34. doi:10.1046/j.1365-2761.1999.00134.x.
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organization (WAO) Journal, 5, 9–19 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3488923/pdf/waoj-5-9.pdf. Accessed 22 Feb 2017.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. doi:10.1016/0003-2697(76)90527-3.
Branco, V., Canário, J., Holmgren, A., & Carvalho, C. (2011). Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicology and Applied Pharmacology, 251(2), 95–103 http://dx.doi.org/10.1016/j.taap.2010.12.005.
Brasil, Ministry of the Environment, National Environmental Council – CONAMA. (2005). CONAMA Framework Resolution 357/2005. Official Gazette, Brasília, 18th march 2005. 58–63 http://www.mma.gov.br/port/conama/res/res05/res35705.pdf. (In Portuguese). Accessed 22 Feb 2017.
Brodeur, J. C., Poliserpi, M. B., D'Andrea, M. F., & Sánchez, M. (2014). Synergy between glyphosate- and cypermethrin-based pesticides during acute exposures in tadpoles of the common South American toad Rhinella arenarum . Chemosphere. doi:10.1016/j.chemosphere.2014.02.065.
Catalgol, B. K., Ozden, S., & Alpertunga, B. (2007). Effects of trichlorfon on malondialdehyde and antioxidant system in human erythrocytes. Toxicology in Vitro, 21, 1538–1544. doi:10.1016/j.tiv.2007.06.002.
Costa, P. M., Diniz, M. S., Caeiro, S., Lobo, J., Martins, M., Ferreira, A. M., et al. (2009). Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquatic Toxicology, 92, 202–212. doi:10.1016/j.aquatox.2008.12.009.
Costa, P. M., Caeiro, S., Lobo, J., Martins, M., Ferreira, A. M., Caetano, M., et al. (2011). Estuarine ecological risk based on hepatic histopathological indices from laboratory and in situ tested fish. Marine Pollution Bulletin, 62(1), 55–65. doi:10.1016/j.marpolbul.2010.09.009.
Crestani, M., Menezes, C., Glusczak, L., Miron, D. S., Spanevello, R., Silveira, A., et al. (2007). Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere, 67(11), 2305–2311. doi:10.1016/j.chemosphere.2006.09.070.
Das, S., & Gupta, A. (2012). Effect of malathion (EC) on gill morphology of Indian flying barb, Esomus danricus (Hamilton-Buchanan). World Journal of Fish and Marine Sciences, 4(6), 626–628. doi:10.5829/idosi.wjfms.2012.04.06.6490.
FAO—Food and Agriculture Organization of the United Nations. (2014). FAO STAT, food and agriculture data: Crops—sugarcane. http://www.fao.org/faostat/en/#data/QC. Accessed 03 Mar 2017.
FAO—Food and Agriculture Organization of the United Nations. (2016). Cultured aquatic species information programme: Oreochromis niloticus. Text by Rakocy, J. E. In: FAO Fisheries and Aquaculture Department [online]. Rome. Updated 18 February 2005. http://www.fao.org/fishery/culturedspecies/Oreochromis_niloticus/en. Accessed 24 Feb 2017.
Fatima, M., Mandiki, S. N., Douxfils, J., Silvestre, F., Coppe, P., & Kestemont, P. (2007). Combined effects of herbicides on biomarkers reflecting immune-endocrine interactions in goldfish. Immune and antioxidant effects. Aquatic Toxicology, 81(2), 159–1,67. doi:10.1016/j.aquatox.2006.11.013.
Figueiredo-Fernandes, A., Fontaínhas-Fernandes, A., Monteiro, R., Reis-Henriques, M. A., & Rocha, E. (2006). Effects of the fungicide mancozeb on liver structure of Nile tilapia, Oreochromis niloticus: assessment and quantification of induced cytological changes using qualitative histopathology and the stereological point-sampled intercept method. Bulletin of Environmental Contamination and Toxicology, 76(2), 249–255. doi:10.1007/s00128-006-0914-1.
Gonçalves, C., Martins, M., Diniz, M. S., Costa, M. H., Caeiro, S., & Costa, P. M. (2014). May sediment contamination be xenoestrogenic to benthic fish? A case study with Solea senegalensis. Marine Environmental Research, 99, 170–178 http://dx.doi.org/10.1016/j.marenvres.2014.04.012.
Güngördü, A. (2013). Comparative toxicity of methidathion and glyphosate on early life stages of three amphibian species: Pelophylax ridibundus, Pseudepidalea viridis, and Xenopus laevis. Aquatic Toxicology, 140–141, 220–228. doi:10.1016/j.aquatox.2013.06.012.
Hanada, S., Harada, M., Kumemura, H., Omary, M. B., Koga, H., Kawaguchi, T., et al. (2007). Oxidative stress induces the endoplasmic reticulum stress and facilitates inclusion formation in cultured cells. Journal of Hepatology, 47(1), 93–102. doi:10.1016/j.jhep.2007.01.039.
Hanada, S., Harada, M., Abe, M., Akiba, J., Sakata, M., Kwan, R., et al. (2012). Aging modulates susceptibility to mouse liver Mallory-Denk body formation. Journal of Histochemistry & Cytochemistry, 60(6), 475–483. doi:10.1369/0022155412441478.
Hassaan, M. S., Goda, A. M. A. S., Mahmoud, S. A., & Tayel, S. (2014). Protective effect of dietary vitamin E against fungicide copperoxychloride stress on Nile tilapia, Oreochromis niloticus (L.), fingerlings. International Aquatic Research, 6, 58. doi:10.1007/s40071-014-0058-6.
Herzfeld, D., & Sargent, K. (2011). Pesticide formulations. In D. Herzfeld & K. Sargent (Eds.), Private pesticide applicator training manual. 19 th edition. Pesticide safety and environmental education program (pp. 85–107). Minnesota: University of Minnesota Extension http://www.extension.umn.edu/agriculture/pesticide-safety/ppat_manual/Chapter%204.pdf. Accessed 22 Feb 2017.
Holland, J. W., & Rowley, A. F. (1998). Studies on the eosinophilic granule cells in the gills of the rainbow trout, Oncorhynchus mykiss. Comparative Biochemistry and Physiology. Part C, Pharmacology, Toxicology & Endocrinology, 120(2), 321–328. doi:10.1016/S0742-8413(98)10016-6.
Husak, V. V., Mosiichuk, N. M., Maksymiv, I. V., Sluchyk, I. Y., Storey, J. M., Storey, K. B., & Lushchak, V. I. (2014). Histopathological and biochemical changes in goldfish kidney due to exposure to the herbicide Sencor may be related to induction of oxidative stress. Aquatic Toxicology, 155, 181–189. doi:10.1016/j.aquatox.2014.06.020.
Jonsson, C. M., Ferracini, V. L., Paraiba, L. C., Rangel, M., & Aguiar, S. R. (2002). Biochemical changes and accumulation in “pacu” fish (Metynnis argenteus) exposed to paclobutrazol. Scientia Agricola, 59(3), 441–446. doi:10.1590/S0103-90162002000300005 (In Portuguese).
Jordaan, M. S., Reinecke, S. A., & Reinecke, A. J. (2013). Biomarker responses and morphological effects in juvenile tilapia Oreochromis mossambicus following sequential exposure to the organophosphate azinphos-methyl. Aquatic Toxicology, 144-145, 133–140. doi:10.1016/j.aquatox.2013.10.007.
Keen, J. H., Habig, W. H., & Jakoby, W. B. (1976). Mechanism for several activities of the glutathione S-transferase. The Journal of Biological Chemistry, 251, 6183–6188. http://www.jbc.org/content/251/20/6183.full.pdf. Accessed 12 Aug 2017.
Koller, L. D. (1973). A note on eosinophilic cytoplasmatic bodies in the liver of a rabbit. Veterinary Pathology, 10(4), 295–298 http://journals.sagepub.com/doi/pdf/10.1177/030098587301000402. Accessed 22 Feb 2017.
Koutnik, D., Stara, A., & Velisek, J. (2015). The effect of selected triazines on fish: a review. Slovenian Veterinary Research, 52(3), 107–131.
Kwok, K. W. H., Leung, K. M. Y., Lui, G. S. G., Chu, V. K. H., Lam, P. K. S., Morritt, D., et al. (2007). Comparison of tropical and temperate freshwater animal species’ acute sensitivities to chemicals: implications for deriving safe extrapolation factors. Integrated Environmental Assessment and Management, 3(1), 1551–3793 http://dx.doi.org/10.1002/ieam.5630030105.
Laetz, C. A., Baldwin, D. H., Collier, T. K., Hebert, V., Stark, J. D., & Scholz, N. L. (2009). The synergistic toxicity of pesticide mixtures: implications for risk assessment and the conservation of endangered Pacific salmon. Environmental Health Perspectives, 117(3), 348–353 https://ehp.niehs.nih.gov/wp-content/uploads/117/3/ehp.0800096.pdf. Accessed 22 Feb 2017.
Manugistics. (2001). Statgraphics plus: Version 5.1 for Windows. Rockville.
Mattos, M. L. T., Peralba, M. C. R., Dias, S. L. P., Prata, F., & Camargo, L. (2002). Environmental monitoring of glyphosate and its metabolite (aminomethylphosphonic acid) in tillage water of irrigable rice. Pesticidas: Revista de Ecotoxicologia e Meio Ambiente, 2(4), 145–154. doi:10.5380/pes.v12i0.3156 (In Portuguese).
Mehta, C. R., & Patel, N. R. (1983). A network algorithm for performing Fisher’s exact test in r x c contingency tables. Journal of the American Statistical Association, 78, 427–434 http://www.cytel.com/hubfs/0-library-0/pubs/a-network-algorithm-for-performing-fishers-exact-test-in-r--c-contingency-tables.pdf?t=1485372895302. Accessed 24 Feb 2017.
Mela, M., Guiloski, I. C., Doria, H. B., Randi, M. A., Oliveira Ribeiro, C. A., Pereira, L., et al. (2013). Effects of the herbicide atrazine in neotropical catfish (Rhamdia quelen). Ecotoxicology and Environmental Safety, 93, 13–21. doi:10.1016/j.ecoenv.2013.03.026.
Modesto, K. A., & Martinez, C. B. R. (2010). Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere, 78(3), 294–299. doi:10.1016/j.chemosphere.2009.10.047.
Mofeed, J., & Mosleh, Y. Y. (2013). Toxic responses and antioxidative enzymes activity of Scenedesmus obliquus exposed to fenhexamid and atrazine, alone and in mixture. Ecotoxicology and Environmental Safety, 95(1), 234–240. doi:10.1016/j.ecoenv.2013.05.023.
Monteiro, D. A., Almeida, J. A., Rantin, F. T., & Kalinin, A. L. (2006). Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 143(2), 141–149. doi:10.1016/j.cbpc.2006.01.004.
Monteiro, R. T. R., Silva, G. H., Messias, T. G., Queiroz, S. C. N., Assalin, M. R., Cassoli, D. R., et al. (2014). Chemical and ecotoxicological assessments of water samples before and after being processed by a water treatment plant. Revista Ambiente & Água, 9(1), 6–18. doi:10.4136/ambi-agua.1292.
Moraes, B. S., Loro, V. L., Glusczak, L., Pretto, A., Menezes, C., Marchezan, E., & Machado, S. O. (2007). Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere, 68(8), 1597–1601. doi:10.1016/j.chemosphere.2007.03.006.
Moreira, J. C., Peres, F., Simões, A. C., Pignati, W. A., Dores, E. C., Vieira, S. N., Strüssmann, C., & Mott, T. (2012). Groundwater and rainwater contamination by pesticides in an agricultural region of Mato Grosso state in central Brazil. Ciência & Saúde Coletiva, 17(6), 1557–1568. doi:10.1590/S1413-81232012000600019 (In Portuguese).
Moura, M. A. M., & Jonsson, C. M. (2016). Acute toxicity of mixture of sugarcane herbicides to tilapia fingerlings. Ecotoxicology and Environmental Contamination, 11(1), 15–20. doi:10.5132/eec.2016.01.03.
Moura, M. A. M., Franco, D. A. S. F., & Matallo, M. B. (2008). Herbicides impact on water resources. Revista Tecnologia & Inovação Agropecuária, 1, 142–151 http://www.dge.apta.sp.gov.br/publicacoes/T&IA/T&IAv1n1/Revista_Apta_Artigo_117.pdf (In Portuguese). Accessed 02 Mar 2017.
Nava-Álvarez, R., Razo-Estrada, A. C., García-Medina, S., Gómez-Olivan, L. M., & Galar-Martínez, M. (2014). Oxidative stress induced by mixture of diclofenac and acetaminophen on common carp (Cyprinus carpio). Water, Air, & Soil Pollution, 225, 1873. doi:10.1007/s11270-014-1873-5.
Oliveira, R. A., Roat, T. C., Carvalho, S. M., & Malaspina, O. (2014). Side-effects of thiamethoxam on the brain and midgut of the Africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environmental Toxicology, 29(10), 1122–1133. doi:10.1002/tox.21842.
Oropesa, A. L., García-Cambero, J. P., Gómez, L., Roncero, V., & Soler, F. (2009). Effect of long-term exposure to simazine on histopathology, hematological, and biochemical parameters in Cyprinus carpio. Environmental Toxicology, 24(2), 87–99. doi:10.1002/tox.20412.
Ortiz-Ordoñez, E., Uria-Galicia, E., Ruiz-Picos, R. A., Duran, A. G. S., Trejo, Y. H., Sedeno-Diaz, J. E., & Lopez-Lopez, E. (2011). Effect of Yerbimat herbicide on lipid peroxidation, catalase activity, and histological damage in gills and liver of the freshwater fish Goodea atripinnis. Archives of Environmental Contamination and Toxicology, 61(3), 443–452. doi:10.1007/s00244-011-9648-0.
Otitoju, O., & Onwurah, I. N. E. (2005). Superoxide dismutase (SOD) activity and serum calcium level in rats exposed to a locally produced insecticide “Rambo insect powder”. Animal Research International, 2(1), 261–266. doi:10.4314/ari.v2i1.40850.
Pathiratne, A., & Kroon, F. J. (2016). Using species sensitivity distribution approach to assess the risks of commonly detected agricultural pesticides to Australia’s tropical freshwater ecosystems. Environmental Toxicology and Chemistry, 35(2), 419–428. doi:10.1002/etc.3199.
Paulino, M. G., Souza, N. E. S., & Fernandes, M. N. (2012). Subchronic exposure to atrazine induces biochemical and histopathological changes in the gills of a neotropical freshwater fish, Prochilodus lineatus. Ecotoxicology and Environmental Safety, 80(1), 6–13. doi:10.1016/j.ecoenv.2012.02.001.
Pinheiro, A., Silva, M. R., & Kraisch, R. (2010). Presence of pesticides in surface water and groundwater in the basin of Itajaí, SC. REGA—Revista de Gestão de Água da América Latina, 7(2), 17–26 http://www.abrh.org.br/SGCv3/UserFiles/Sumarios/5600b5161b6d9eabf8b99a621bd33c16_eb0bba188410253222b079565d16fd2e.pdf (In Portuguese) Accessed 02 Mar 2017.
Poleksic, V., & Karan, V. (1999). Effects of trifluralin on carp: biochemical and histological evaluation. Ecotoxicology and Environmental Safety, 43, 213–221.
Poleksic, V., & Mitrovic-Tutundzic, V. (1994). Fish gills as a monitor of sublethal and chronic effects of pollution. In R. Müller & R. Lloyd (Eds.), Sublethal and chronic effects of pollutants on freshwater fish (Vol. 30, pp. 339–352). Oxford: Fishing News Books.
Prammer, B. (1998). Directiva 98/83/CE do Conselho de 3 de novembro de 1998 relativa à qualidade da água destinada ao consumo humano. Jornal Oficial das Comunidades Européias, 1, 1–23.
Prestes, E. B., Jonsson, C. M., & Castro, V. L. S. (2011). Toxicity of formulations based on piraclostrobin, epoxiconazole and its combination on algae Pseudokirchneriella subcapitata. Pesticidas: Revista de Ecotoxicologia e Meio Ambiente, 21, 39–46. doi:10.5380/pes.v21i0.25943 (In Portuguese).
Remacle, J., Lambert, D., Raes, M., Pigeolet, E., & Michiels, C. (1992). Importance of various antioxidant enzymes for cell stability: confrontation between theoretical and experimental data. Biochemical Journal, 286, 41–46 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1133015/pdf/biochemj00129-0048.pdf. Accessed 02 Mar 2017.
Rossi, S. C., Silva, M. D., Piancini, L. D. S., Ribeiro, C. A. O., Cestari, M. M., & Assi, H. C. S. (2011). Sublethal effects of waterborne herbicides in tropical freshwater fish. Bulletin of Environmental Contamination and Toxicology, 87(6), 603–607. doi:10.1007/s00128-011-0397-6.
Saka, M., Tada, N., & Kamata, Y. (2013). Application of an amphibian (Silurana tropicalis) metamorphosis assay to the testing of the chronic toxicity of three rice paddy herbicides: simetryn, mefenacet, and thiobencar. Ecotoxicology and Environmental Safety, 92(1), 135–143. doi:10.1016/j.ecoenv.2013.03.023.
Sancho, E., Fernández-Vega, C. E., Villarroel, M. J., Andreu-Moliner, E., & Ferrando, M. D. (2009). Physiological effects of tricyclazole on zebrafish (Danio rerio) and post-exposure recovery. Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology, 150(1), 25–32. doi:10.1016/j.cbpc.2009.02.004.
Santos, E. A., Correia, N. M., & Botelho, R. G. (2013). Herbicides residues in water bodies—a review. Revista Brasileira de Herbicidas, 12(2), 188–201. doi:10.7824/rbh.v12i2.245 (In Portuguese). Accessed 03 Mar 2017.
Sevcikova, M., Modra, H., Blahova, J., Dobsikova, R., Plhalova, R. L., Zitka, O., et al. (2016). Biochemical, haematological and oxidative stress responses of common carp (Cyprinus carpio L.) after sub-chronic exposure to copper. Veterinární Medicína, 61(1), 35–50 http://vri.cz/docs/vetmed/61-1-35.pdf. Accessed 03 Mar 2017.
Silva, E., Daam, M. A., & Cerejeira, M. J. (2015). Predicting the aquatic risk of realistic pesticide mixtures to species assemblages in Portuguese river basins. Journal of Environmental Sciences, 31(1), 12–20. doi:10.1016/j.jes.2014.11.006.
Stara, A., Machova, J., & Velisek, J. (2012). Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.) Environmental Toxicology and Pharmacology, 33(2), 334–343. doi:10.1016/j.etap.2011.12.019.
Stara, A., Kouba, A., & Velisek, J. (2014). Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in red swamp crayfish (Procambarus clarkii). BioMed Research International, Article ID 680131, 6 pages. doi:10.1155/2014/680131.
Strmac, M., & Braunbeck, T. (2002). Cytological and biochemical effects of a mixture of 20 pollutants on isolated rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxicology and Environmental Safety, 53(2), 293–304. doi:10.1006/eesa.2002.2221.
Tabatabaie, T., & Floyd, R. A. (1994). Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Archives of Biochemistry and Biophysics, 314(1), 112–119. doi:10.1006/abbi.1994.1418.
Tallarida, R. J. (2001). Drug synergism: its detection and applications. Journal of Pharmacology and Experimental Therapeutics, 298(3), 865–872 http://jpet.aspetjournals.org/content/jpet/298/3/865.full.pdf. Accessed 03 Mar 2017.
Tang, J. Y., & Escher, B. I. (2014). Realistic environmental mixtures of micropollutants in surface, drinking, and recycled water: herbicides dominate the mixture toxicity toward algae. Environmental Toxicology and Chemistry, 33(6), 1427–1436. doi:10.1002/etc.2580.
Toni, C., Menezes, C. C., Loro, V. L., Clasen, B. E., Cattaneo, R., Santi, A., et al. (2010). Oxidative stress biomarkers in Cyprinus carpio exposed to commercial herbicide bispyribac-sodium. Journal of Applied Toxicology, 30(6), 590–595. doi:10.1002/jat.1530.
Topp, E., & Smith, W. (1992). Sorption of the herbicides atrazine and metolachlor to selected plastics and silicone-rubber. Journal of Environmental Quality, 21(3), 316–317. doi:10.2134/jeq1992.00472425002100030002x.
Ukeda, H., Maeda, S., Ishii, T., Sawamura, M. (1997). Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-1-[(phenylamino)-carbonyl]-3, 4-tetrazolium-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Analytical Biochemistry, 251, 206–209. doi:10.1006/abio.1997.2273.
Valz, P. D., & Thompson, M. E. (1994). Exact inference for Kendall’s S and Spearman’s Rho with extensions to Fisher’s exact test in r x c contingency tables. Journal of Computational and Graphical Statistics, 3(4), 459–472. doi:10.1080/10618600.1994.10474658.
Vieira, C. E. D., Almeida, M. S., Galindo, B. A., Pereira, L., & Martinez, C. B. R. (2014). Integrated biomarker response index using a Neotropical fish to assess the water quality in agricultural areas. Neotropical Ichthyology, 12(1), 153–164. doi:10.1590/S1679-62252014000100017.
Walker, C.H. (1995). Biochemical biomarkers in ecotoxicology - some recent developments. The Science of the Total Environment, 171(1-3), 189–195. doi:10.1016/0048-9697(95)04720-6.
Wheelock, C. E., Miller, J. L., Miller, M. G., Shan, G., Geem, S. J., & Hammock, B. D. (2005). Influence of container adsorption upon observed pyrethroid toxicity to Ceriodaphnia dubia and Hyalella azteca. Aquatic Toxicology, 74(1), 47–52. doi:10.1016/j.aquatox.2005.04.007.
Xing, H., Li, S., Wang, Z., Gao, X., Xu, S., & Wang, X. (2012). Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere, 88(4), 377–383. doi:10.1016/j.chemosphere.2012.02.049.
Acknowledgements
Financial support was provided by São Paulo Research Foundation (FAPESP) (grant nos. 2010/06294-8 and 2011/09579-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Animals
The procedures described in the present paper were authorized by the Animal Experimentation Ethics Committee of Embrapa Environment (Registration No. 002/2012) and respect national and international safety regulations and ethical principles for animal welfare and the 3Rs principle.
Rights and permissions
About this article
Cite this article
Jonsson, C.M., Arana, S., Ferracini, V.L. et al. Herbicide Mixtures from Usual Practice in Sugarcane Crop: Evaluation of Oxidative Stress and Histopathological Effects in the Tropical Fish Oreochromis niloticus . Water Air Soil Pollut 228, 332 (2017). https://doi.org/10.1007/s11270-017-3506-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3506-2