Abstract
In recent years, commercial glyphosate herbicide formulations have been widely used in agriculture to control aquatic weeds. These pesticides may result in disruption of ecological balance, causing damage to nontarget organisms including fish. Teleostean fish (Leporinus obtusidens) were exposed to commercial glyphosate herbicide formulation at 0 (control), 3, 6, 10 or 20 mg L−1 for 96 h. The effects of herbicide on plasmatic metabolic parameters, thiobarbituric acid reactive substances (TBARS), catalase activity, protein carbonyl, and mucus layer parameters were studied. Plasmatic glucose and lactate levels increased but protein levels showed reduction after herbicide exposure. TBARS levels in brain showed a reduction at all tested concentrations. However, liver demonstrated increased TBARS levels at all tested concentrations, whereas in white muscle TBARS production did not change after exposure to herbicide. Fish exposed to all concentrations of glyphosate showed increase in liver catalase activity and protein carbonyl. Herbicide exposure increased protein and carbohydrate levels of the mucus layer at all tested concentrations. The present results showed that, in 96 h, glyphosate changed toxicological parameters analyzed in piava. Parameters measured in this study may be useful in environmental biomonitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The increased use of herbicides in agriculture can affect aquatic ecosystems and cause ecological imbalance. In aquatic toxicology studies, fish are important indicators of the effects of toxic compounds. The commercial glyphosate formulation, which is the acid equivalent of the isopropylamine salt of glyphosate, contains in its formulation polyethoxylated tallow amine (POEA) as its predominant surfactant (Jiraungkoorskul et al. 2002), which is more acutely toxic to aquatic organisms than the active ingredient itself (Giesy et al. 2000). Glyphosate herbicide is nonselective and has been used for controlling aquatic weeds (Abdullah et al. 1995; Jiraungkoorskul et al. 2002). Glyphosate does not bioaccumulate in terrestrial or aquatic animals and presents low toxicity. The glyphosate formulation rapidly dissipates in surface waters, and soil microflora biodegrade it into aminomethylphosphonic acid (AMPA) and CO2. It is widely used worldwide due to its high efficiency and cost effectiveness (Giesy et al. 2000; Tomlin 2000).
There are various studies considering herbicide toxicity in fish, especially concerning metabolic, oxidative, and hematological parameters in response to Roundup® exposure (Fonseca et al. 2008; Cavalcante et al. 2008; Salbego et al. 2010). Modesto and Martinez (2010), studying the effects of Roundup® on biochemical biomarkers in the Neotropical fish Prochilodus lineatus, showed that the herbicide interferes with antioxidant defense, leading to occurrence of lipid peroxidation. Long-term exposure to Roundup® causes metabolic disruption affecting brain acetylcholinesterase activity as well as metabolic and hematologic parameters of Leporinus obtusidens (Salbego et al. 2010).
The response of some aquatic organisms to environmental contaminants has been studied through measurement of general physiological concentrations such as glucose, lactate, and protein (Fonseca et al. 2008; Gimeno et al. 1995; Sancho et al. 2000).
It is known that contaminants such as pesticides may induce formation of reactive oxygen species (ROS), resulting in imbalance between pro-oxidant and antioxidant defense mechanisms. Lipid peroxidation (LPO) induced by pollutants such as pesticides has been observed in fish species (Schlenk et al. 1997; Üner et al. 2005, 2006). Reactive oxygen species (ROS) produced in biological systems are detoxified by antioxidant defenses. Variations in the activities of antioxidant enzymes such as catalase have been proposed as indicators of pollutant-mediated oxidative stress (Ahmad et al. 2000; Sayeed et al. 2003; Üner et al. 2005). It has been established in mammals that protein damage or chemical modification of its amino acids during the oxidative stress process can produce high levels of protein carbonyls (Parvez and Raisuddin 2005). Many studies have suggested that protein carbonyl content may be used as a complementary biomarker of oxidative stress in humans and other vertebrates (Pey et al. 2003). However, few such studies have been reported in fish (Almroth et al. 2005; Parvez and Raisuddin 2005). The mucus layer that covers exposed surfaces of fish is important not only for its effective role as a protective barrier but also as a hydrodynamic lubricant, as well as an active antiparasitic and antibacterial agent (Sabóia-Moraes et al. 1996; Tromeur et al. 1992).
Considering that there is no information available about changes in plasmatic metabolism, oxidative stress, and mucus layer parameters in piava exposed to herbicides, the present study aimed to investigate the effects of commercial glyphosate formulation on oxidative stress parameters in piava (Leporinus obtusidens) as a complementary study concerning the toxicity of this herbicide.
Materials and Methods
Fish
The piava (Leporinus obtusidens) species was chosen for this study because it is a native freshwater fish of Southern Brazil with good acceptance in the consumer market (Andrian et al. 1994; Baldisserotto and Gomes 2005). Piava of both sexes were obtained from the Santa Maria Federal University (UFSM) fish farm (Rio Grande do Sul, Brazil). Fish (weight, 12.0 ± 1.0 g; length, 8.0 ± 1.0 cm) were acclimated to laboratory conditions for 10 days. They were kept in continuously aerated tanks (250 L) with a static system and a natural photoperiod (12 h light/12 h dark). Throughout the experimental period, water quality was as follows: temperature 23 ± 0.5°C, pH 7.5 ± 0.05, and dissolved oxygen 7.1 ± 0.2 mg L−1. Fish were fed once a day with commercial fish pellets (42% crude protein; Supra, Brazil), and feces and pellet residues were removed daily by suction only in the acclimation period.
Experimental Design
Acute toxicity assays were carried out in static fashion for 96 h, according to Antón et al. (1994). Previous experiments carried out in our laboratory were not able to obtain a lethal concentration (LC50) of glyphosate at 96 h, because all fish survived even at the highest concentration tested (100 mg L−1) and showed normal swimming and feeding behavior. Therefore, experimental glyphosate concentrations were chosen considering nominal sublethal concentrations. This study was approved by the Ethics and Animal Welfare Committee of the Federal University of Santa Maria (no. 23081.016049/2005-40). After acclimation, groups of eight fish were placed in 45-L continuously aerated glass tanks and exposed for 96 h to 0 (control), 3, 6, 10 or 20 mg L−1 commercial glyphosate formulation (480 g L−1 acid equivalent, 692 g L−1 inert ingredients; Monsanto Company, St. Louis, MO, USA). Before experiments, stock solution (500 mg L−1) was prepared by diluting commercial glyphosate formulation (480,000 mg L−1), then diluted to the required concentrations. All tests were carried out in triplicate. Herbicide was added to the water only at the beginning of the experiment. Water quality did not change throughout the experimental period, and water was not replaced.
Analytical Procedures
At the end of the exposure period (96 h), mucus was carefully scraped from dorsal body surface (total area 6 cm2) using a cotton-tipped swab. After scraping, the cotton was immersed in 2 mL distilled water, and the sample was used to determine soluble sugar (Duboie et al. 1956) and protein (Lowry et al. 1951). All fish were sampled, and blood was collected from the caudal vein with a 1-mL heparinized syringe. One blood aliquot was centrifuged at 1,500×g for 10 min, and plasma was separated. Plasma glucose was measured by the glucose oxidase method with Bioclin test kit. Plasma was dissolved in 10% trichloroacetic acid (1:20 dilution), and lactate was estimated according to Harrower and Brown (1972). Plasma total protein levels were measured according to Lowry et al. (1951) using bovine serum albumin (Sigma) as standard. Brain, white muscle, and liver samples were quickly removed, washed with 150 mM saline solution, packed in Teflon tubes, and kept at −20°C for posterior analyses.
Lipid peroxides produced from oxidative stress were quantified by TBARS assay, performed using the malondialdehyde (MDA) reaction with 2-thiobarbituric acid (TBA), measured optically. Tissues samples (brain, white muscle, and liver) were homogenized in a Potter–Elvejhem glass/Teflon homogenizer with 20 mM potassium phosphate buffer, pH 7.4 (with 0.1% Triton X100 and 150 mM NaCl) (1:20 dilution), centrifuged at 10,000×g for 10 min at 4°C. Brain, white muscle, and liver homogenates (100–400 μL) were added to 8.1% sodium dodecyl sulfate (SDS), 2.5 M acetic acid (pH 3.4), 0.8% thiobarbituric acid were added to adjust to final volume of 2.0 mL. The reaction mixture was placed in a microcentrifuge tube and incubated for 90 min at 95°C. After cooling, it was centrifuged at 5,000×g for 10 min, and the optical density at 532 nm was determined. TBARS levels are expressed in units of nmol MDA mg protein−1 according to Ohkawa et al. (1979).
Catalase (EC 1.11.1.6) activity was assayed by ultraviolet spectrophotometry (Nelson and Kiesov 1972). Liver samples were prepared as reported for TBARS assay. Briefly, the assay mixture consisted of 2.0 mL potassium phosphate buffer (50 mM, pH 7.0), 0.05 mL H2O2 (0.3 M), and 0.05 mL homogenate. Catalase (CAT) activity was determined by following the H2O2 decrease using absorbance at 240 nm. Enzyme activity was expressed as micromoles of H2O2 reduced per milligram of protein per minute (μmol mg protein−1 min−1).
Liver tissue was homogenized in 10 volumes (w/v) of 10 mM Tris–HCl buffer, pH 7.4 using a glass homogenizer. Protein carbonyl content was determined by the method described by Yan et al. (1995), with some modifications. Briefly, homogenates were diluted to 0.7–0.8 mg mL−1 of protein in each sample, and 1-mL aliquots were mixed with 0.2 mL 10 mM 2,4-dinitrophenylhidrazine (DNPH) or 0.2 mL 2 M HCl. After incubation at room temperature for 1 h in dark, 0.5 mL denaturing buffer (150 mM sodium phosphate buffer, pH 6.8, containing SDS 3%), 2.0 mL heptane (99.5%), and 2.0 mL ethanol (99.8%) were added sequentially, followed by vortexed agitation for 40 s and centrifugation for 15 min. Next, the protein isolated from the interface was washed two times with 1 mL ethyl acetate:ethanol 1:1 (v/v) and suspended in 1 mL denaturing buffer. Each DNPH sample was read at 370 nm using a Femto Scan spectrophotometer against the corresponding sample (blank), and total carbonylation was calculated using a molar extinction coefficient of 22,000 M−1 cm−1.
Protein levels for oxidative stress parameters were estimated spectrophotometrically by the method of Bradford (1976), using bovine serum albumin as standard.
Statistical Procedures
One-way analysis of variance (ANOVA) and Duncan’s multiple-range tests were used. Data (n = 3), representing the mean of each triplicate (8), were expressed as mean ± standard deviation (SD), and mean differences were considered significant at P < 0.05.
Results and Discussion
Plasma glucose and lactate levels increased after exposure to glyphosate (Table 2). The hyperglycemia present in fish exposed to the herbicide may partially be a consequence of brain cholinesterase inhibition shown in this fish species (Glusczak et al. 2006). Blockage of the neuroeffector in the adrenal medulla favors glycogen breakdown and glucose export due to hypersecretion of adrenaline (Aguiar et al. 2004; Crestani et al. 2006). In addition, Roundup® causes cholinesterase inhibition in brain and muscle of Prochilodus lineatus (Modesto and Martinez 2010), and brain of Leporinus obtusidens after 90 days exposure (Salbego et al. 2010). The changes in lactate levels also indicate metabolic disorders. Lactate has been widely used as a measure of anaerobic metabolism, and this increase has been demonstrated to be a rapid and clear response to energy depletion caused by lack of oxygen (Gimeno et al. 1995). Lactate levels also increase in liver and muscle of the same fish Leporinus obtusidens. However, increase in lactate levels was recorded after 90 days of exposure (Salbego et al. 2010). The results observed after long-term exposure are in agreement with those obtained in the present study, where plasma lactate increase indicates anaerobic preference. The decreased plasma protein in L. obtusidens at all tested concentrations may indicate use of plasmatic protein to supply energy metabolism disrupting of any tissue. In addition, these results may indicate physiological adaptability of fish to compensate for oxidative damage caused by this herbicide. Leporinus obtusidens exposed to environmentally relevant concentrations of Roundup® showed similar results with plasma protein reduction after exposure to 1 or 5 mg L−1 for 90 days (Salbego et al. 2010). The results presented herein, when compared with those obtained for long-term exposure, could indicate a compensatory mechanism due to increased demand for energy expenditure for herbicide biotransformation. Hypoproteinemia is usually associated with fish exposure to pesticide and has been correlated with disturbance in osmoregulation (Begum 2004; Salbego et al. 2010; Sancho et al. 2000).
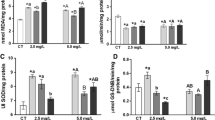
Measurement of lipid peroxidation through TBARS quantification has been used as an indicator of oxidative stress in fish. In this work, TBARS levels were altered after exposure of fish to glyphosate. TBARS levels in brain tissue showed a reduction at all concentrations tested when compared with the control group (P < 0.05). Similar results were observed in our laboratory concerning brain TBARS levels, where L. obtusidens exposed to clomazone (0.5 mg L−1) or propanil herbicides (3.6 mg L−1) showed a decrease in TBARS levels in this tissue (Moraes et al. 2009). Hepatic tissues showed an increase in TBARS levels at all tested concentrations. In the liver, elevation in TBARS suggests participation of free-radical-induced oxidative cell injury caused by glyphosate toxicity. In muscle tissues, TBARS production did not change at any tested concentration (Fig. 1). Apparently, glyphosate caused lipid peroxidation only in liver tissues, and changes in TBARS varied depending on the tissue considered. Recently, Roundup® exposure was reported to cause lipid peroxidation and also to impair oxidative defenses of Prochilodus lineatus (Modesto and Martinez 2010).
As in this study, L. obtusidens exposed to clomazone (0.5 mg L−1) or propanil herbicide (3.6 mg L−1) exhibited a TBARS increase in the liver (Moraes et al. 2007). The level of lipid peroxidation may differ among the fish species and tissues considered; for example, TBARS levels in L. obtusidens poisoned with herbicides at rice field conditions showed reduced TBARS levels in white muscle after exposure to quinclorac (0.375 mg L−1), propanil (3.6 mg L−1), and metsulfuron methyl herbicide (0.002 mg L−1). However, another fish species, Rhamdia quelen, exposed to clomazone (0.5 or 1.0 mg L−1) showed an increase in TBARS levels, particularly in the liver, after 12, 24, 48, 96 or 192 h exposure (Crestani et al. 2007). Li et al. (2003) also observed elevated TBARS levels in the liver of Carassius auratus after exposure to 3,4-dichloroaniline. The differences in peroxide levels have also been attributed to the variation in antioxidant mechanisms of fish species (Radi et al. 1985; Ahmad et al. 2000).
The antioxidant enzyme catalase showed an increase in activity in the liver with increasing herbicide concentration (Fig. 2). This enzyme seems to be important as an antioxidant defense against possible lipid damage generated by glyphosate. Catalase activity showed an increase in hepatic tissue after exposure to herbicide (Fig. 2). According to previous experiments (Moraes et al. 2007), elevation in catalase activity was observed in the liver of Leporinus obtusidens exposed to commercial formulations of clomazone (0.5 mg L−1) or propanil herbicide (3.6 mg L−1). However, Crestani et al. (2006) showed a reduction in catalase activity in hepatic tissue of silver catfish exposed to clomazone (0.5 or 1.0 mg L−1) after 12, 24, and 96 h. Sayeed et al. (2003) also observed a 45% decrease in hepatic catalase activity as well as high levels of TBARS in freshwater fish (Channa punctatus) exposed to the insecticide deltamethrin. Thus, oxidative stress generated by water containing glyphosate-based herbicides may suppress the antioxidant defense represented by catalase, leading to a loss of this compensatory mechanism.
Fish exposed to glyphosate concentrations showed an increase in protein carbonyls in liver tissue (Fig. 3). Parvez and Raisuddin (2005) also observed an increase in protein carbonyls after 48 h of exposure to deltamethrin, endosulfan or paraquat. Few studies have been carried out using protein carbonyl formation in teleost fish. The presence of carbonyl groups in protein has been used as a marker of ROS-mediated protein oxidation (Madhusudhanan et al. 2004; Parvez and Raisuddin 2005). The reduction of TBARS formation shown in our study may affect protein oxidation. In addition, the increase in protein carbonyl levels would indicate that normal protein metabolism is disrupted, resulting in accumulation of damaged molecules. The relationship between TBARS, catalase activity, and protein carbonylation in this study may indicate a response of the fish to survive after herbicide toxicity.
In this study, L. obtusidens exposed to glyphosate showed an increase in protein and carbohydrate levels of the mucus layer when compared with controls (Table 1). Glycoproteins represent the major component of the mucus coating of fish skin. The changes in carbohydrate and protein content in the fish surface observed in L. obtusidens could be a mechanism to protect against external agents and microbial development. The suggested functional significance of the fish epidermal mucus includes osmoregulation, protection from abrasions, entanglement of particulate materials, defense against pathogens and parasites, reduction of swimming drag or friction, and protection against environmental contaminants (Tromeur et al. 1992; Hinton et al. 2001). In this context, our results can represent a protective mechanism against glyphosate toxicity.
In summary, the present work demonstrates that the concentrations of glyphosate-based herbicide used in agricultural fields cause changes in oxidative stress parameters in piava (L. obtusidens). It is evident that, from an ecophysiological point of view, use of this herbicide formulation in agriculture and aquaculture must be carefully evaluated. We conclude that the health of this fish species may be affected by the presence of glyphosate in water. However, more studies are necessary to discriminate which compound of the commercial formulation could be responsible for the oxidative liver damage found in the present study. In this context, TBARS, protein carbonyl, CAT, and mucous layer analyses can be used as biomarkers of fish poisoning by glyphosate. The observed alterations indicate also the potential use of native fish species as toxicity bioindicators in biomonitoring studies considering the environment risk of contamination by pesticides of rivers near areas of rice or soy cultivation in Southern Brazil.
References
Abdullah PM, Daud J, Hong SK, Yew HC (1995) Improved method for the determination of glyphosate in water. J Chromatogr A 697:363–369
Aguiar LH, Moraes G, Avilez IM, Altran AE, Corrêa CF (2004) Metabolical effects of folidol 600 on the neotropical freshwater fish matrinxã, Brycon cephalus. Environ Res 95:224–230
Ahmad I, Hamid T, Fatima M, Chand HS, Jain SK, Athar M, Raisuddin S (2000) Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim Biophys Acta 1523:37–48
Almroth CB, Sturve J, Berglund A, Förlin L (2005) Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat Toxicol 73:171–180
Andrian IF, Dória CC, Torrente G, Ferreti CML (1994) Espectro alimentar e similaridade na composição de quatro espécies de Leporinus (Characiformes, Anostomidae) do Rio Paraná, Brasil. Revista Unimar 16(3):97–106
Antón FA, Laborda E, Ariz M (1994) Acute toxicity of the herbicide glyphosate to fish. Chemosphere 28:745–753
Baldisserotto B, Gomes CL (2005) Espécies nativas para piscicultura no Brasil. In: Tataje RD, Filho ZE (eds) Cultivo do gênero Leporinus. Editora UFSM, Santa Maria, pp 81–103
Begum G (2004) Carbofuran insecticide induced biochemical alterations in liver and muscle tissues of the fish Clarias batrachus (Linn) and recovery response. Aquat Toxicol 66:83–92
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cavalcante DGSM, Martinez CBR, Sofia SH (2008) Genotoxic effects of Roundup (R) on the fish Prochilodus lineatus. Mutat Res-Genet Toxicol Environ 655:41–46
Crestani M, Menezes C, Glusczak L, Miron dos SD, Lazzari R, Duarte FM, Morsch MV, Pippi LA, Vieira PV (2006) Effects of clomazone herbicide on hematological and some parameters of protein and carbohydrate metabolism of silver catfish Rhamdia quelen. Ecotoxicol Environ Saf 65:48–55
Crestani M, Menezes C, Glusczak L, Miron dos SD, Spanevello R, Silveira A, Gonçalves FF, Zanella R, Loro LV (2007) Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 67:2305–2311
Duboie MG, Gilles KA, Hamilton JK, Roberts PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–358
Fonseca MB, Glusczak L, Moraes BS, Menezes CC, Pretto A, Tierno MA, Zanella R, Gonçalves FF, Loro VL (2008) The 2,4-D herbicide effects on acetylcholinesterase activity and metabolic parameters of piava freshwater fish (Leporinus obtusidens). Ecotoxicol Environ Saf 69:416–420
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup herbicide. Rev Environ Contam Toxicol 167:35–120
Gimeno L, Ferrando MD, Sanchez S, Gimeno LO, Andreu E (1995) Pesticide effects on eel metabolism. Ecotoxicol Environ Saf 31:153–157
Glusczak L, Miron SD, Crestani M, Fonseca BM, Pedron AF, Duarte FM, Vieira PLV (2006) Effect of glyphosate herbicide on acetylcholinesterase activity, metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf 65:237–241
Harrower JR, Brown CH (1972) Blood lactic acid. A micromethod adapted to field collection of microliter samples. J Appl Physiol 32:709–711
Hinton DE, Segner H, Braunbeck T (2001) Toxic responses of the liver. In: Schlenk D, Benson WH (eds) Target organ toxicity in marine and freshwater teleosts. Taylor & Francis, London
Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P (2002) Histopathological effects of Roundup, a glyphosate herbicide, on Nile tilapia (Oreochromis niloticus). Sci Asia 28:121–127
Li W, Yin D, Zhou Y, Hu S, Wang L (2003) 3,4-Dichloroaniline-induced oxidative stress in liver of crucian carp (Carassius auratus). Ecotoxicol Environ Saf 56:251–255
Lowry OH, Rosbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Madhusudhanan N, KavithaLakshi SN, Radha Shanmugasundaram K (2004) Oxidative damage to lipids and proteins induced by aflatoxin B1 in fish (Labeo rohita)—protective role of Amrita Bindu. Environ Toxicol Pharmacol 17:73–77
Modesto KA, Martinez CBR (2010) Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Moraes BS, Loro VL, Glusczak L, Pretto A, Menezes C, Marchezan E, Machado SO (2007) Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere 68:1597–1601
Moraes BS, Loro VL, Pretto A, Fonseca MB, Menezes C, Marchesan E, Reimche GB, Avila LA (2009) Toxicological and metabolic parameters of the teleost fish (Leporinus obtusidens) in response to commercial herbicides containing clomazone and propanil. Pest Biochem Physiol 95:57–62
Nelson DP, Kiesov LA (1972) Enthalphy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solution in the UV). Anal Biochem 49:474–478
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Pharmacol 20:112–117
Pey A, Saborido A, Blázquez I, Delgado J, Megías A (2003) Effects of prolonged stanozolol treatment on antioxidant enzyme activities, oxidative stress markers, and heat shock protein HSP72 levels in rat liver. J Steroid Biochem Mol Biol 87:269–277
Radi AAR, Hai DQ, Matkovics B, Gabrielak T (1985) Comparative antioxidant enzyme study in freshwater fish with different types of feeding behavior. Comp Biochem Physiol 81C:395–399
Sabóia-Moraes SMT, Hernandez-Blazquez FJ, Mota DL, Bittencourt AM (1996) Mucous cell types in the branchial epithelium of the euryhaline fish Poecilia vivipara. J Fish Biol 49:545–548
Salbego J, Pretto A, Gioda C, de Menezes C, Lazzari R, Radünz Neto J, Baldisserotto B, Loro V (2010) Herbicide formulation with glyphosate affects growth, acetylcholinesterase activity, and metabolic and hematological parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 58:740–745
Sancho E, Fernandez-Véga C, Sanchez M, Ferrando MD, Andreu-Moliner E (2000) Alterations on AChE activity of the fish Anguilla anguilla as response to herbicide-Contaminated Water. Ecotoxicol Environ Saf 46:57–63
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Rizwanul H, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Schlenk D, Wolford L, Chelius M, Steevens J, Chan KM (1997) Effect of arsenite, arsenate, and the herbicide monosodium methyl arsonate (MSMA) on hepatic metallothionein expression and lipid peroxidation in channel catfish. Comp Biochem Physiol C 118(2):177–183
Tomlin CDS (2000) The pesticide manual. A world compendium, 12th edn. BCPC, Surrey
Tromeur F, Guerard F, Le Gal Y (1992) Mucous glycoproteins from the ray Raja batis. Comp Biochem Physiol 102B:773–778
Üner N, Oruç E, Sevgiler Y (2005) Oxidative stress-related and ATPase effects of etoxazole in different tissues of Oreochromis niloticus. Environ Toxicol Pharmacol 20:99–106
Üner N, Oruç E, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245
Yan LJ, Traber MG, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glusczak, L., Loro, V.L., Pretto, A. et al. Acute Exposure to Glyphosate Herbicide Affects Oxidative Parameters in Piava (Leporinus obtusidens). Arch Environ Contam Toxicol 61, 624–630 (2011). https://doi.org/10.1007/s00244-011-9652-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9652-4