Abstract
The toxicity of Roundup Original® (GLY), a glyphosate-based herbicide widely used in crops in Mato Grosso state, was determined in hybrid fish jundiara or pintado da Amazônia. The 96 h-LC50 of GLY was 13.57 mg L−1. Moreover, exposure to sublethal concentrations of GLY (0, 0.37, 0.75, 2.25, 4.5, 7.5 mg L−1) has not altered the survival rate (100% for all treatments). In fish liver, protein carbonyl (PC) levels as well as glutathione-S-transferase (GST) activity, reduced glutathione (GSH), and ascorbic acid (ASA) contents increased when compared to control group. Superoxide dismutase (SOD) activity was reduced and catalase (CAT) has not changed. PC content has grown in muscle and brain, and thiobarbituric acid-reactive species (TBARS) levels also increased in muscle, but in the brain, they remained unaltered. Acetylcholinesterase (AChE) activity reduced in muscle but increased in brain when compared to control group. Our results suggest that short-term exposure to GLY induced alterations in the oxidative stress biomarkers in fish and can be interfering with their survival in natural environment; besides, these findings may be considered of high ecotoxicological relevance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The agribusiness has been growing in the center-west region of Brazil (Figueiredo et al. 2005), and it is based on monocultures, intense mechanization, use of agrochemicals, and exports of grains (Camacho 2010; Belo et al. 2012). Soybeans are one of these outstanding monocultures with the state of Mato Grosso holding approximately 27% of the national production of this grain (MAPA 2017). Soybean production is accompanied by increasing use of herbicides, including glyphosate, which is widely used in genetically modified crops. This practice implies risks to the biodiversity (Moreira et al. 2012), including aquatic biota (Adams and Greeley 2000). Several studies point out to changes in water sources that surround cultivation areas, groundwater, and rainwater by pesticides (Marchesan et al. 2010; Belo et al. 2012), from mechanisms that include leaching, chemical-biological degradation, sorption, surface runoff, and volatilization of these substances (Rebelo and Caldas 2014).
Aquaculture has been used worldwide due to its potential to preserve natural biodiversity and to provide a continuous source of protein for increasing population consumption (Bostock et al. 2010; Nomura 2010). Gomes and Sato (2011) point out that the demand for this type of production in Brazil has increased, following the world scenario, which easily allows the correlation between the risks that the anthropogenic influence can cause to the aquatic environment. In this context, we propose to evaluate the so-called pintado da Amazônia or jundiara fish, a result of hybridization between the species Leiarius marmoratus and Pseudoplatystoma reticulatum, which is produced in the fish farms of Northern Mato Grosso state and it is of great commercial and economic importance. For the state, studies by Sinhorin et al. (2014a, b) with another hybrid species (surubim) showed evidence of metabolic, behavioral, and oxidative stress alterations after exposure to Roundup Original®, which is the most used glyphosate formulation in this region. In addition, other recent studies involving this class of herbicide have demonstrated its potentially negative effects on fish (Modesto and Martinez 2010a; Murussi et al. 2016; Moura et al. 2017a, b).
Therefore, the aim of this study was to evaluate the lethal concentration (LC50) of the glyphosate herbicide in 24, 48, 72, and 96 h of exposure in the jundiara fish, and from that, to assess parameters of oxidative stress in tests with sublethal concentrations.
Materials and methods
Experimental design
Juvenile jundiara fish (45.3 ± 8.0 g and 16.0 ± 1.0 cm) were obtained from a fish farm in Sinop, Mato Grosso, Brazil. Before the experimentation for LC50 determination, the fishes were acclimated to laboratory conditions for 10 days in 300-L fiberglass tanks containing aerated and dechlorinated water, under natural photoperiod conditions (12 h light/12 h dark). During this period, the fish were fed once a day with commercial fish pellets containing 42% crude protein and the water parameters were monitored every day. Those parameters were as follows: temperature, 26 ± 1 °C; dissolved oxygen, 6.4 ± 0.5 mg L−1; pH values, 6.8 ± 0.3; non-ionized ammonia, 0.08 ± 0.01 μg L−1; and total hardness, 18 ± 0.2 mg L−1 CaCO3. Feces and pellet residue were removed every day by suction. Firstly, after the acclimation period, fish were distributed in exposure groups (0, 0.37, 0.75, 2.25, 4.5, 7.5, 11.25, 15, 22.5, 30 mg L−1; nominal concentration of glyphosate) of Roundup Original® GLY). The herbicide used in this study presents in its formulation 480 g L−1 containing isopropylamine salt of glyphosate, 360 g L−1 acid equivalent, N-(phosphonomethyl) glycine (glyphosate), and 684 g L−1 of inert ingredients, MAPA SOB No. 00898793 (Monsanto, St. Louis, MO, USA). Fish were placed in 50-L tanks, which were continuously aerated and contained 4 fish per tank. All tests were carried out in triplicate (n = 12), and the fish were not fed during this period. Dead fish were removed after 24, 48, 72, and 96 h of exposure. The determined value of LC50 at 13.57 mg L−1 of GLY was estimated according to Hamilton et al. (1977) by the trimmed Spearman-Karber method.
Afterwards, based on the previous studies performed by Sinhorin et al. (2014a, b), other fish were exposed to sublethal concentrations of 0, 0.37, 0.75, 2.25, 4.5, and 7.5 mg L−1 of Roundup Original® for 96 h. The water quality has not changed throughout the experimental period, and fish were maintained in a static system. Following exposure, the fish were removed from the tanks, immediately anesthetized with eugenol (50 mg L−1) (Kreutz et al. 2011) and killed by medular section. Samples of liver, brain, and muscle were removed by dissection. The samples were frozen at − 80 °C until biochemical assays, and all these tests were carried out in triplicate. The study was approved by the Committee Guidelines (Ethics in Animal Research of the Federal University of Mato Grosso - UFMT), Reference number 23108.780290/11-5.
Biochemical assays
The protein carbonyl (PC) content in liver, muscle, and brain was determined by spectrophotometry after DNPH derivation according to Yan et al. (1995), with some modifications. The total carbonyl content was assessed using a molar extinction coefficient of 22.000 M−1 cm−1 and expressed as nmol carbonyl mg protein−1.
The lipid peroxidation (LPO) in muscle and brain was estimated by determining the levels of thiobarbituric acid reactive substances (TBARS) (Konn and Liversedge 1944). The lipid peroxides produced from oxidative stress were quantified by reaction between the malondialdehyde (MDA) with 2-thiobarbituric acid (TBA), measured optically at 532 nm, and results were expressed in nmol MDA mg protein−1 following the calibration curve for MDA.
Superoxide dismutase (SOD) activity was assessed in the liver by inhibition of adrenaline oxidation, measured spectrophotometrically at 480 nm, using the UV-VIS spectrophotometer according to Misra and Fridovich (1972) and expressed as UI SOD mg protein−1. Catalase (CAT) activity in liver was assessed by ultraviolet spectrophotometry according to Nelson and Kiesow (1972). The principle is based on decomposition of H2O2 and measured spectrophotometrically at 240 nm, and results are expressed in μmol H2O2 min−1 mg protein−1. Glutathione-S-transferase (GST) activity was determined in liver according to Habig et al. (1974), and enzyme activity was measured according to the formation of GS-DNB adduct. The result was expressed in μmol GS-DNB min−1 mg protein−1. In addition, non-protein thiols (GSH, reduced glutathione) levels were measured by the colorimetric method consisting in a reaction of sulfhydryl groups developed by Sedlack and Lindsay (1968) in the liver and quantified at 412 nm. The result was expressed in μmol GSH mg protein−1 and compared to a standard GSH curve. Ascorbic acid (ASA) levels in the liver were determined according to Roe (1954), by colorimetric method and read at absorbance of 520 nm. The result was expressed in μmol ASA g tissue−1 and compared to a standard curve of ascorbic acid.
Acetylcholinesterase (AChE) activity in muscle and brain was measured as described by Ellman et al. (1961). Tissues were homogenized in 50 mM sodium phosphate buffer pH 7.2, containing 1% Triton X-100, and after centrifugation, the supernatant was removed for analysis. The supernatant was incubated at 25 °C for 2 min with 100 mM phosphate buffer pH 7.5, and 10 mM DTNB was used as chromogen. The substrate, 1 mM acetylthiocholine (AcSCh), was added after 2 min to promote the reaction. Absorbance was measured at 412 nm for duration of 2 min, and the activity was expressed as μmol AcSCh min−1 mg protein−1.
Protein levels for oxidative stress, with exception of ASA and AChE, were estimated spectrophotometrically by the method of Bradford (1976), using bovine serum albumin as standard.
Statistical analysis
Data are given as mean ± standard deviation (S.D.) (n = 6–7), which were analyzed by one-way (ANOVA) followed by the Dunnett’s test. The Bartlett’s test was performed to compare the homogeneity of variances among the groups. The results were considered statistically significant at P < 0.05.
Results
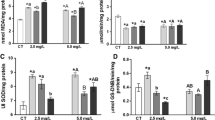
We determined the LC50 in jundiara fish (13.57 mg L−1). The exposure to sublethal concentrations of GLY has not altered the survival rate (100% for all treatments) of jundiara. The water quality parameters measured in all experimental tanks have not varied as a function of the treatment (control and agrichemical exposure). The liver of fish exposed to GLY increased PC content about 21% at 0.37 and 4.5 mg L−1 concentration when compared to control group (Table 1). In the same way, PC content increased in muscle and brain (34.6% at 2.25 mg L−1 and 29% at 4.5 mg L−1) when compared to the control fish (Table 2). TBARS levels also increased in muscle (31.7% at 2.25 mg L−1), but in the brain, they remained unaltered (Table 2). For enzymatic antioxidants, there was a different pattern of results. For example, SOD activity in the liver of fish exposed to GLY was significantly reduced (22.6% at 0.75 mg L−1) when compared to the control group and CAT has not changed (Table 1). On the other hand, GST activity in the liver of fish exposed to GLY was remarkably increased (66.3% at 0.37 mg L−1) as well as the non-enzymatic antioxidants, GSH (51.5 and 47.8% at 0.37 and 0.75 mg L−1, respectively) and ASA (17.6% at 4.5 mg L−1) when compared to the control values (Table 1). AChE activity reduced in muscle (21 and 26.7% at 2.25 and 4.5 mg L−1, respectively), and it was increased about 42% in the brain (2.25 mg L−1) as compared to the control group (Fig. 1a, b, respectively).
Acetylcholinesterase (AChE) activity in muscle (a) and brain (b) of jundiara exposed to different concentrations of GLY or only water (control) for 96 h. Data represent the mean ± standard deviation (S.D.) (n = 6). Asterisks indicate a difference between groups and control values (ANOVA followed by Dunnett’s test) P < 0.001
Discussion
This study focused on the oxidative stress parameter changes of pintado da Amazônia, hybrid fish species, concerning to understand the possible mechanisms of GLY toxicity. Differently from Jiraungkoorskul et al. (2002) which observed a variation in the LC50 = 2–55 mg L−1 in different fish species, life stage, and test conditions after Roundup exposure, we observed a LC50 of 13.57 mg L−1 for jundiara after 96 h of exposure; afterwards, the fish were exposed to sublethal concentrations of GLY in order to find out how the animals would react to the toxic substance. It is very important to clarify that the concentrations chosen for our experiment are supported by Langiano and Martinez (2008), who pointed that concentrations of GLY under 10 mg L−1 might be considered environmentally relevant and realistic, inasmuch as at the current application rates of this substance, a water body with no intercepting vegetation can have a maximum concentration of 3.7 mg L−1 of glyphosate as a contaminant, which means 9 mg L−1 of GLY that can be in direct contact with fish and other non-target organisms (Langiano and Martinez 2008).
Protein carbonilation is a phenomenon already observed after sublethal exposure of fish to different toxic substances (Glusczak et al. 2011; Moura et al. 2017a). The increased levels of protein carbonyl suggest an important alteration of the normal protein metabolism (Glusczak et al. 2011), what provokes an accumulation of damaged molecules in the organism, process that corroborates to the oxidative stress. In our study, we could observe that different concentrations of the herbicide, for all the tested tissues (liver, brain and muscle), were able to provoke the carbonilation condition. Alterations of PC levels in liver of fish were also observed in the study of Loro et al. (2015) after exposing R. quelen and L. obtusidens to 0.2 or 0.4 mg L−1 of Roundup for 96 h.
At the same time, the induction of TBARS levels in the muscle is noticed in several studies that indicated GLY or other related herbicides as causes of this biomarker to increase in different fish tissues and also while studying different fish species (Modesto and Martinez 2010a; Menezes et al. 2011; Moura et al. 2017a). The study conducted by Sinhorin et al. (2014a) is also among them, where the authors detected a raise in TBARS levels for the muscle of surubim after exposing fish to Roundup Original® in very similar concentrations to the ones we used. The lipid peroxidation that occurs in this situation affects various aspects of the metabolism, and products of this reaction may be harmful to other cell components, just as Vieira et al. (2016) observed genetic disruption in animals with high LPO.
The action of the herbicide on the jundiara generated a rise of the reactive oxygen species (ROS), which had a negative influence on the activity of SOD and stimulated the increase of GST. It is important to consider that the antioxidant enzymes are endowed with a complex pathway of regulation (Modesto and Martinez 2010a). SOD is responsible for the conversion of the superoxide anion into H2O2, which is converted into water and oxygen by CAT. Both lipid peroxidation and protein carbonilation may cause loss of enzymatic activity and disturb the function of the proteins. Due to this circumstance, the enzymes may be inactivated by the excess of oxidant substances, even if the oxidants are their own substrates as discussed by Modesto and Martinez (2010a). Based on that, we can consider that the reduction of SOD levels, presented in our results, is probably a reflex of such incident.
In the same way, Sinhorin et al. (2014a) observed similar results in surubim fish using Roundup Original®, in which protein carbonyl was increased causing a reflection on SOD activity. The raise in SOD or CAT activity is a positive response against the production of ROS; however, high levels of oxidant substances may lead to an impairment of this compensatory mechanism.
GST is recognized as an enzyme involved in the reduction of glutathione in order to prevent harm to the cells caused by the presence of xenobiotics according to Braz-Mota et al. (2015). The induction of its activity is a favorable response against intoxication since it helps the animal handle the stress condition. Vieira et al. (2016) and Moura et al. (2017a) perceived the same behavior of this enzyme in their researches as well as Vieira et al. (2014) who also mentioned high levels of GST and GSH in the liver of fish collected from different sites in the Água das Araras stream.
With respect to non-enzymatic antioxidants, the levels of GSH and ASA were found raised in the liver of jundiara. Sinhorin et al. (2014a) commented that when the normal enzymatic defenses are stressed or not working properly, the non-enzymatic defenses may rise, contributing to a scenario of an organism’s response against the damage preventing the auto-oxidation chain. Moura et al. (2017a) observed an analogous condition after exposing jundiara to a concentration of 1.357 mg L−1 of Roundup Original®, close to the lowest concentrations employed in our experiments.
Several xenobiotics can induce the production of ROS, and increased TBARS and GSH levels as well as reduced SOD activity, events that we have seen in jundiara, are symbols of that, as mentioned by Zhang et al. (2012) who studied the effects of textile effluent on zebrafish. Ferreira et al. (2010) detailed consonant results, since Rhamdia quelen exposed to 1.21 mg L−1 of Roundup showed increased levels of GST, GSH, and ASA.
In fish muscle, besides increasing the oxidant substances, GLY also decreased AChE activity. Comparable situation was observed by Moura et al. (2017a). Increase of TBARS in muscle is likely to have influenced the reduced activity of AChE, an important enzyme responsible for the cholinergic impulses when hydrolyzing acetylcholine into choline and acetic acid (Vieira et al. 2014).
This reduction could be related to the generation of ROS in the fish during exposure to GLY, which can negatively impact the activity of AChE, a mechanism already observed in toxic events (Cattaneo et al. 2011). According to Gholami-Seyedkolaei et al. (2013), cholinergic hyperactivity induced by an AChE inhibitor promotes accumulation of ROS leading to lipid peroxidation, reinforcing this decrease. On the other hand, the non-production of TBARS in the brain has allowed the appearance of a different result, which was an increase of its activity for the 2.25 mg L−1 concentration of GLY. Depending on the fish species, the concentration, and also the tissue tested, this enzyme sensitivity varies in accordance with Modesto and Martinez (2010a), who investigated diverse other studies demonstrating equivalent reactions.
Since AChE is frequently used to evaluate the impact of a toxic event in organisms (Pessoa et al. 2011; Moura et al. 2017a) and its activity has been altered in muscle and in brain, our results suggest that the fish has tried to adapt its physiological conditions to survive during exposure to the herbicide.
The contrasts among the various responses to the different concentrations tested in our study are a common situation when one is working with a concentration curve of a toxic substance. Not necessarily the highest concentrations will produce the worst effects, and it all depends on many individual characteristics, including the tissue tested, the concentration used, and the time of exposure, what we can see by the results of Modesto and Martinez (2010b) who identified different behaviors for SOD and CAT after exposing Prochilodus lineatus to the concentrations of 1 and 5 mg L−1 Roundup Transorb for 6, 24, or 96 h.
Thusly, this work evaluated the effect of GLY on jundiara hybrid fish, as well as determined the LC50 for this substance. In addition, it was shown that fish exposure to sublethal concentrations promoted alterations in biomarkers of oxidative stress in liver, muscle, and brain of these animals, also interfering in AChE activity. The extensive application of pesticides easily introduces them accidentally in fresh and marine surface waters (Fiorino et al. 2018), and because of that, it is truly important to evaluate and comprehend the impacts of such practices to start discouraging them to avoid the total impairment of the aquatic biota.
Conclusion
This investigation demonstrated that the pintado da Amazônia, a hybrid fish, is sensitive to glyphosate in sublethal concentrations. PC levels in liver, muscle, and brain and TBARS contents in muscle were elevated in this research, which indicates cellular damage. Additionally, the herbicide induced an oxidative stress and it causes changes in both enzymatic and non-enzymatic antioxidants, as well as in AChE activity in muscle and brain. The alterations observed in this study could be used as bioindicators of exposure to glyphosate, and our findings suggest that exposure to this herbicide, in these concentrations, may be impairing the fish health. Moreover, further studies related to the stress response and oxidative stress in non-target organisms exposed to GLY are necessary to understand the mechanisms of toxicity of this herbicide, mainly for farmed fish and hybrid animals.
References
Adams SM, Greeley MS (2000) Ecotoxicological indicators of water quality: using multi-response indicators to assess the health of aquatic ecosystems. Water Air Soil Pollut 123:103–115
Belo MSS, Pignati W, Dores EFGC, Moreira JC, Peres F (2012) Pesticide use in soybean production in Mato Grosso state, Brazil: a preliminary occupational and environmental risk characterization. Rev Bras Saúde Ocup 125:78–88
Bostock J, McAndrew B, Richards R, Jauncey K, Telfer T, Lorenzen K, Little D, Ross L, Handisyde N, Gatward I, Corner R (2010) Aquaculture: global status and trends. Philos Trans R Soc Lond Ser B Biol Sci 365:2897–2912
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Braz-Mota S, Sadauskas-Henrique H, Duarte RM, Val AL, Almeida-Val VMF (2015) Roundup® exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma macropomum. Chemosphere 135:53–60
Camacho SR (2010) A barbárie moderna do agronegócio latifundiário exportador e suas implicações sócio-ambientais. Agrária 13:169–195
Cattaneo R, Clasen B, Loro VL, de Menezes CC, Pretto A, Baldisserotto B, Santi A, de Avila LA (2011) Toxicological responses of Cyprinus carpio exposed to a commercial formulation containing glyphosate. Bull Environ Contam Toxicol 87:597–602
Ellman GL, Courtney KD, Andres V (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ferreira D, Motta AC, Kreutz LC, Toni C, Loro VL, Barcellos LJ (2010) Assessment of oxidative stress in Rhamdia quelen exposed to agrichemicals. Chemosphere 79(9):914–921
Figueiredo MG, Barros ALM, Guilhoto JJM (2005) Relação econômica dos setores agrícolas do estado do Mato Grosso com os demais setores pertencentes tanto ao estado quanto ao restante do Brasil. Rev Econ Sociol Rural 43(3):557–575
Fiorino E, Sehonova P, Plhalova L, Blahova J, Svobodova Z, Faggio C (2018) Effects of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pollut Res Int 25(9):8542–8549
Gholami-Seyedkolaei S, Mirvaghefi A, Farahmand H, Kosari AA (2013) Effect of a glyphosate-based herbicide in Cyprinus carpio: assessment of acetylcholinesterase activity, hematological responses and serum biochemical parameters. Ecotoxicol Environ Saf 98:135–141
Glusczak L, Loro VL, Pretto A, Moraes BS, Raabe A, Duarte MF, da Fonseca MB, de Menezes CC, de Sousa Valladão DM (2011) Acute exposure to glyphosate herbicide affects oxidative parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 61:624–630
Gomes MVT, Sato Y (2011) Avaliação da contaminação por metais pesados em peixes do Rio São Francisco à jusante da represa de Três Marias, Minas Gerais, Brasil. Saúde & Amb Rev 6:24–30
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grama S, Pokethitiyook P (2002) Histopathological effects of Roundup, a glyphosate herbicide, on Nile tilapia (Oreochromis niloticus). Sci Asia 28:121–127
Konn HI, Liversedge M (1944) On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine and menadione. J Pharmacol Exp Ther 82:292–300
Kreutz LC, Barcellos LJG, Valle SF et al (2011) Altered hematological parameters in silver catfish (Rhamdia quelen) following short term exposure to subtlethal concentration of glyphosate. Fish ShellfishImmunol 30:51–57
Langiano VC, Martinez CB (2008) Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus. Comp Biochem Physiol C 147:222–231
Loro VL, Glusczak L, Moraes BS, Leal CAM, Menezes C, Murussi CR, Leitemperger J, Schetinger MRC, Morsch VM (2015) Glyphosate-based herbicide affects biochemical parameters in Rhamdia quelen (Quoy & Gaimard, 1824 and) Leporinus obtusidens (Valenciennes, 1837). Neotrop Ichthyol 13(1):229–236
MAPA (2017) Ministério da Agricultura, Pecuária e Abastecimento. In: Projeções do Agronegócio, Brasil 2014/2015 a 2024/2025. Brasília. http://www.agricultura.gov.br/assuntos/politica-agricola/todas-publicacoes-de-politica-agricola/projecoes-do-agronegocio/projecoes-do-agronegocio-brasil-2014-2015-a-2024-2025.pdf/view/. Cited 14 May 2017
Marchesan E, Sartori GMS, Avila LAD et al (2010) Residues of pesticides in the water of the depression central rivers in the state of Rio Grande do Sul, Brazil. Cienc Rural 40(5):1053–1059
Menezes CC, Fonseca MB, Loro VL, Santi A, Cattaneo R, Clasen B, Pretto A, Morsch VM (2011) Roundup effects on oxidative stress parameters and recovery pattern of Rhamdia quelen. Arch Environ Contam Toxicol 60:665–671
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation o epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Modesto KA, Martinez CBR (2010a) Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Modesto KA, Martinez CBR (2010b) Effects of Roundup Transorb on fish: hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere 81:781–787
Moreira JC, Peres F, Simões AN et al (2012) Groundwater and rainwater contamination by pesticides in an agricultural region of Mato Grosso state in Central Brazil. Cien Saude Colet 18:1557–1568
Moura FR, Brentegani KR, Gemelli A, Sinhorin AP, Sinhorin VDG (2017a) Oxidative stress in the hybrid fish jundiara (Leiarius marmoratus × Pseudoplatystoma reticulatum) exposed to Roundup Original®. Chemosphere 185:445–451
Moura FR, Lima RRS, Cunha APS et al (2017b) Effects of glyphosate-based herbicide on pintado da Amazônia: hematology, histological aspects, metabolic parameters and genotoxic potential. Environ Toxicol Pharmacol 56:241–248
Murussi CR, Costa MD, Leitemperger JW, Guerra L, Rodrigues CCR, Menezes CC, Severo ES, Flores-Lopes F, Salbego J, Loro VL (2016) Exposure to different glyphosate formulations on the oxidative and histological status of Rhamdia quelen. Fish Physiol Biochem 42(2):445–455
Nelson DP, Kiesow LA (1972) Enthalphy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solution in the UV). Anal Biochem 49:474–478
Nomura I (2010) O futuro da pesca e da Aquicultura marinha no mundo. Cienc Cult 62(3):28–32
Pessoa PC, Luchmann KH, Ribeiro AB, Veras MM, Correa JRMB, Nogueira AJ, Bainy ACD, Carvalho PSM (2011) Cholinesterase inhibition and behavioral toxicity of carbofuran on Oreochromis niloticus early life stages. Aquat Toxicol 105:312–320
Rebelo RM, Caldas ED (2014) Environmental risk assessment of aquatic systems affected by pesticide use. Quim Nova 37(7):1199–1208
Roe JH (1954) In: Glick D (ed) Chemical determination of ascorbic, dehydroascorbic and diketogulonic acids. Methods Biochem Anal Interscience Publishers Inc., New York, pp 115–139
Sedlack J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sinhorin VDG, Sinhorin AP, Teixeira JMS et al (2014a) Effects of the acute exposition to glyphosate-based herbicide on oxidative stress parameters and antioxidant responses in a hybrid Amazon fish surubim (Pseudoplatystoma sp). Ecotoxicol Environ Saf 106:181–187
Sinhorin VDG, Sinhorin AP, Teixeira JMS, Miléski KML, Hansen PC, Moeller PR, Moreira PSA, Baviera AM, Loro VL (2014b) Metabolic and behavior changes in surubim acutely exposed to a glyphosate-based herbicide. Arch Environ Contam Toxicol 67(4):659–667
Vieira CED, Almeida MS, Galindo BA, Pereira L, Martinez CBR (2014) Integrated biomarker response index using a Neotropical fish to assess the water quality in agricultural areas. Neotrop Ichthyol 12(1):153–164
Vieira CED, Costa PG, Lunardelli B, de Oliveira LF, da Costa Cabrera L, Risso WE, Primel EG, Meletti PC, Fillmann G, Bueno dos Reis Martinez C (2016) Multiple biomarker responses in Prochilodus lineatus subjected to short-term in situ exposure to streams from agricultural areas in southern Brazil. Sci Total Environ 542:44–56
Yan L J, Traber M G, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351
Zhang W, Wei L, Zhang J et al (2012) Characterisation of acute toxicity, genotoxicity and oxidative stress posed by textile effluent on zebrafish. J Environ Sci 24(11):2019–2027
Acknowledgements
We are grateful to Universidade Federal de Mato Grosso (UFMT) for the support and facilities, as well as to colleagues, who contributed with the development of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
dos Santos Teixeira, J.M., da Silva Lima, V., de Moura, F.R. et al. Acute toxicity and effects of Roundup Original® on pintado da Amazônia. Environ Sci Pollut Res 25, 25383–25389 (2018). https://doi.org/10.1007/s11356-018-2630-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2630-x