Abstract
Central nervous system injuries and diseases, such as ischemic stroke, spinal cord injury, neurodegenerative diseases, glioblastoma, multiple sclerosis, and the resulting neuroinflammation often lead to death or long-term disability. MicroRNAs are small, non-coding, single-stranded RNAs that regulate posttranscriptional gene expression in both physiological and pathological cellular processes, including central nervous system injuries and disorders. Studies on miR-124, one of the most abundant microRNAs in the central nervous system, have shown that its dysregulation is related to the occurrence and development of pathology within the central nervous system. Herein, we review the molecular regulatory functions, underlying mechanisms, and effective delivery methods of miR-124 in the central nervous system, where it is involved in pathological conditions. The review also provides novel insights into the therapeutic target potential of miR-124 in the treatment of human central nervous system injuries or diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Central nervous system (CNS) injuries and diseases, such as stroke, spinal cord injury (SCI), neurodegenerative diseases (NDDs), glioblastoma (GBM), and multiple sclerosis (MS), as well as the excessive neuroinflammation they cause, are the major causes of morbidity and mortality (Collaborators 2019; Qureshi et al. 2009). As beneficial treatments for these complex pathologies are lacking and there are obstacles in the development of effective therapies, successful treatments for CNS injuries and disorders are needed to minimize both acute and chronic cellular damage, prevent secondary damage caused by treatment, and construct and maintain a beneficial microenvironment. The ability of microRNAs (miRNAs) to regulate molecular function, influence cellular behavior, and modulate inflammatory signaling is an area of ongoing research and has gained much attention in recent years (Akerblom and Jakobsson 2014; Bhalala et al. 2013; Juzwik et al. 2019).
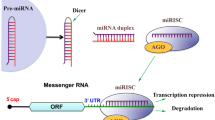
miRNAs are a new class of gene products, the discovery of which represents a novel insight into the regulatory network of gene expression during the development of plants and animals (Ambros 2004; Bartel 2004; Rodriguez et al. 2004). Mature miRNAs are small non-coding RNAs that are approximately 22 nucleotides in length. These small molecules are incorporated into the RNA-induced silencing complex (RISC) to silence gene expression through translational repression or mRNA degradation by binding to sites of antisense complementarity in the 3′ untranslated regions of target mRNAs (Lagos-Quintana et al. 2001). Generally, each miRNA can regulate hundreds of target genes, and the expression of more than one-third of all human genes is predicted to be mediated by miRNAs (Esquela-Kerscher and Slack 2006; Guarnieri and DiLeone 2008). In addition, miRNAs are implicated in various cellular processes, such as cell proliferation, cell differentiation, cellular metabolism, and immune responses, in both physiological and pathological conditions (Bi et al. 2009; Gauthier and Wollheim 2006; Hatfield and Ruohola-Baker 2008; Matsubara et al. 2007). Several miRNAs have been shown to play important roles in the regulation of CNS development (Saraiva et al. 2017), and the capacity of miRNAs to inhibit the action of hundreds of transcripts against CNS injuries and disorders makes them candidates for stabilizing the homeostatic state of the transcriptome (Manakov et al. 2012). The brain-specific miRNAs are predominantly miR-124, -101, -127, -128, -131, and -132 (Mishima et al. 2007). Additionally, miR-9, -21, -29b, -124, -137, 146a, and -155 have been shown to be involved in the modulation of CNS pathophysiology and are considered promising therapeutic targets (Bhalala et al. 2013; Su et al. 2016).
Among the highly expressed miRNAs, miR-124 accounts for 25–48% of all brain miRNAs (Lagos-Quintana et al. 2002). Moreover, miR-124 expression in the mouse CNS is more than 100 times higher than that in other organs. The expression ratios of miR-124 in the CNS are 60.7% for the cerebellum and 35.4% for the spinal cord (Mishima et al. 2007). Such abundant miR-124 expression in the CNS indicates that miR-124 may play an indispensable role in normal CNS functions, which has been attracting much attention for further research.
In general, miR-124 matures from three different precursor variants, called primary (pri) miRNA, located on human chromosomal positions 8p23.1 (miR-124-1), 8q12.3 (miR-124-2), and 20q13.33 (miR-124-3) (Gebauer et al. 2013; Lagos-Quintana et al. 2002; Mishima et al. 2007). Although the loci they are transcribed from are different, generally, it is accepted that most pre-miRNAs all cleave to the same mature miRNAs and thus possess the same characteristics once loaded into RISC. In addition, a study on miR-124 monitoring by a fluorescence tracer system revealed that it was significantly upregulated during the direct neuronal conversion of mouse embryonic fibroblasts reprogrammed by the ectopic expression of Ascl1, Brn2, and Myt1L (Sano et al. 2017). Moreover, miR-124a knockout mice exhibited CNS abnormalities, including small brain size and axonal mis-sprouting of dentate gyrus granule cells (Sanuki et al. 2011). Similarly, some miR-124 knockdown planarians displayed a reduction in overall brain size during their brain regeneration (Sasidharan et al. 2017). These studies suggest that miR-124 plays a significant role in neuronal wiring and neuronal maturation.
In this review, we provide an overview of miR-124, its roles in CNS development, and summarize recent data of its role in neuroinflammation, ischemic stroke, SCI, NDDs, GBM, MS, and the methods for the delivery of miR-124. The mechanisms underlying miR-124 actions are also discussed. Finally, the potential of miR-124 as a therapeutic target to ameliorate CNS injuries and diseases is highlighted.
MiR-124 in Neuroinflammation
Neuroinflammation acts as a central component of CNS injuries and diseases and appears to be responsible for their progression. Upon CNS damage, resident glial cells, including microglia and astrocytes, become activated and induce proinflammatory cytokine, chemokine, and matrix metalloproteinase production, contributing to blood–brain barrier (BBB) disruption, followed by the infiltration of macrophages from the peripheral blood into the damaged core, and the above processes have been extensively reviewed elsewhere (Burda and Sofroniew 2014). In the recovery outcome, the immune system plays a dual beneficial and detrimental role, which has also been well documented (Jacobs et al. 2012; Medzhitov 2008; Varley et al. 2015). An excessive inflammatory response may be more harmful than the original insult, leading to neurological dysfunction (Jayaraj et al. 2019). miR-124 acts as one of the key miRNAs regulating neuroinflammation in various CNS pathologies, which may shed light on novel therapies targeting miR-124 for treating CNS insults, and we will review that in this section.
Neuroinflammation is a process that involves omnidirectional communication of multiple cells and molecular interactions to mount an inflammatory response and disease pathogenesis or to maintain CNS functions by modifying inflammation-related neuroprotection. Therefore, to target neuroinflammation, reconstructing an appropriate immune microenvironment should be beneficial to enhance therapeutic effects, rather than the broad immune suppression that is typically perceived.
Among the cell types involved in neuroinflammation, microglia are critical tissue-specific immune cells acting as resident macrophages that influence brain development, maintain the neuronal environment, as well as respond to and induce CNS diseases (Orihuela et al. 2016; Prinz et al. 2019). In response to the different stages of CNS injuries, microglia and macrophages are activated to subsequently increase the release of inflammatory cytokines; however, miR-124 was demonstrated to maintain microglia in a quiescent state. After splenectomy, the secretion of proinflammatory cytokines, such as TNF-α and IL-6, predisposes individuals to mental or cognitive disorders (Wan et al. 2007). However, miR-124 was shown to reduce microglial activation-mediated neuroinflammation by targeting vesicle-associated membrane protein 3 (Chen et al. 2019). In addition, miR-124 inhibited TNF-α and IL-6 production by lipopolysaccharide (LPS)-stimulated macrophages by targeting signal transducer and activator of transcription 3 (STAT3) and TNF-α converting enzyme (TACE) (Sun et al. 2013). In a model of cocaine-induced neuroinflammation, intranasally delivered miR-124-loaded engineered extracellular vesicles (EVs) were detected in the CNS where they significantly reduced the expression of inflammatory markers, such as TLR4, MYD88, STAT3, NF-κB p65, and the microglial activation marker IBA1(Chivero et al. 2020). These data indicate that engineered EVs can deliver miR-124 into the CNS to alleviate cocaine-mediated microglial activation.
Activated microglia will be polarized toward different activation states, M1 and M2, which represent the two extremes. Generally, polarized M1 microglia and macrophages release destructive proinflammatory mediators, such as TNF-α, IL-6, and IL-1β, causing further damage to host tissue. Polarized M2 microglia and macrophages clear cellular debris and release neuroprotective trophic factors and anti-inflammatory mediators, such as IL-10, TGF-β, and IL-4 (Mosser and Edwards 2008; Orihuela et al. 2016). Modulating the beneficial polarization of M1/M2 is important for nerve recovery. The contribution of miR-124 in the polarization of macrophages to the M2 phenotype has been described in a recent review, and it has been well studied in various CNS diseases (Ponomarev et al. 2013). Ponomarev et al. found that miR-124 was expressed in microglia but not in peripheral monocytes or macrophages. They demonstrated that miR-124 induced microglial quiescence and suppressed experimental autoimmune encephalomyelitis (EAE) by deactivating macrophages through direct inhibition of C/EBP-α, one of the M1 polarization transcription factors (Ponomarev et al. 2011). When performing more detailed gene expression profiling in miR-124-transfected macrophages, they found that miR-124 not only downregulated the M1-associated markers, such as IL-6, TNF-α, and inducible nitric oxide synthase but also upregulated the expression of M2-associated markers, including TGF-β1, ARG1, and FIZZ1 (Ponomarev et al. 2011). Bone marrow mesenchymal stem cell-derived exosomal miR-124 attenuated neurological damage in a model of spinal cord ischemia–reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization (Li et al. 2020). Intracerebral miR-124 administration after stroke resulted in a significant increase in the M2 phenotype, marked by ARG1 upregulation, paralleled by a decrease in the M1 phenotype. This substantial decrease in the M1/M2 ratio improved neurological deficits after stroke and correlated strongly with functional outcomes (Hamzei Taj et al. 2016a, b).
In neurons, miR-124 overexpression was shown to improve the adverse effects of oxygen–glucose deprivation (OGD) and reperfusion-induced PC12 cellular injuries, and significantly repress NF-κB signaling activation and proinflammatory cytokine production by regulating NOX2 (Wu et al. 2020). In addition, microglial exosomal miR-124 treatment in traumatic brain injury suppressed neuronal inflammation in scratch-injured neurons by suppressing the activity of mTOR signaling, mediated via PDE4B; this effect contributed to neurite outgrowth when microglial exosomal miR-124 was transported into neurons (Huang et al. 2018). Consistently, a recent study detected that M2-exosomes could improve the outcome of middle cerebral artery occlusion (MCAO). The associated mechanism in neurons might be partly related to exosomal miR-124 and its downstream target USP14 (Song et al. 2019b). These results suggest that M2 microglia and macrophages not only modulate the microenvironment via anti-inflammatory or neurotrophic factors but also deliver exosomal miR-124 to neurons and other cell types to achieve a more beneficial crosstalk.
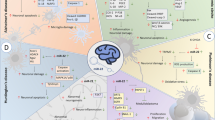
Pro/anti-inflammatory mediators act not only on microglia and neurons but also on astrocytes, which presents another opportunity for therapeutic miR-124-mediated modulation of the neuroinflammatory microenvironment. In response to a wide variety of CNS insults of varying nature and severity, astrocytes undergo reactive astrogliosis and become reactive astrocytes (Mori et al. 2008; Sofroniew 2009). Similar to the M1/M2 microglia and macrophages, there are different subtypes of reactive astrocytes, termed A1 and A2. Transcriptome analysis of reactive astrocytes reveals that LPS-induced A1 reactive astrocytes upregulate many genes that have been previously shown to be destructive to the synapses. In contrast, ischemia-induced A2 reactive astrocytes were shown to upregulate many neurotrophic factors, which promoted synapse repair (Zamanian et al. 2012). Indeed, A1 astrocytes are toxic and accumulate in many CNS diseases, such as amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and MS (Liddelow and Barres 2017; Liddelow et al. 2017). Therefore, preventing the formation of A1 astrocytes, promoting A1/A2 astrocyte shift, or blocking the A1 neurotoxin hold great potential for treating CNS diseases (Yun et al. 2018). Activated microglia induce A1 astrocytes by secreting IL-1α, TNF-α, and C1q, and A1 astrocytes lose the ability to promote neuronal survival, outgrowth, synaptogenesis, and phagocytosis and induce the death of neurons and oligodendrocytes (Liddelow et al. 2017). In vivo inhibition of microglia-mediated formation of A1 astrocytes prevented the death of axotomized CNS neurons (Liddelow et al. 2017; Yun et al. 2018). Neuron-derived exosomes enriched with miR-124-3p promoted function recovery by suppressing myosin heavy chain 9 activity, which led to the downregulation of M1 microglia and A1 astrocytes in vitro and in vivo (Jiang et al. 2020). In addition, it has been reported that the Notch-STAT3 axis and the NF-κB/C3/C3aR pathway are involved in A1 astrocyte activation (Lian et al. 2015; Qian et al. 2019). Coincidently, Notch and NF-κB are extensively studied targets of miR-124, although additional experimental data are required to fill this knowledge gap (Gan et al. 2019; Jiang et al. 2016). The roles and potential mechanisms of miR-124 in neuroinflammation are summarized in Fig. 1. Emerging evidence indicates that during neuroinflammation, there must be crosstalk among neurons, microglia, and astrocytes through the release of diverse signaling molecules that determine the fates of astrocytes and microglia (Jha et al. 2019). Thus, an indirect approach for modulating the neuroinflammatory microenvironment could involve miR-124-mediated downregulation of proinflammatory factors, such as TNF-α and IL-1, that typically induce M1 microglia polarization and subsequent microglia-induced A1 astrocyte formation (Fig. 2).
Proposed communication of multicellular model regulated by miR-124 in neuroinflammation after CNS injuries/diseases. Under pathological condition of the CNS, miR-124 maintains microglia in a quiescent state or inhibits M1 microglia polarization. Polarized M1 microglia and macrophages release destructive proinflammatory mediators to further damage host tissues. Additionally, miR-124 promotes M2 microglia/macrophage polarization and induces neuroprotective trophic factors and the release of anti-inflammatory mediators. Activated microglia further induce A1 astrocytes by secreting Il-1α, TNF, and C1q. Exosomes loaded with miR-124 secreted by M2 microglia can promote the reparation of damaged neurons. Analogously, exosomes enriched with miR-124 promote function recovery by suppressing the activation of M1 microglia and A1 astrocytes. (Created with BioRender.com.)
Neuroinflammation is now recognized as the hallmark of virtually all neurological disorders (Glass et al. 2010). It is a complex and well-orchestrated process by various groups of neurons and glial cells in nervous systems. In addition, it is not considered synonymous with poor CNS outcomes anymore. As discussed above, there are now multiple examples of the significant benefits of inflammatory responses to the injured CNS for neuroprotection and for regenerative responses. A major strategy is to employ existing medications that polarize immune subsets into those beneficial phenotypes (Yong et al. 2019). According to the studies reviewed above, miR-124 has a comprehensive anti-inflammatory and neuroprotective effect when it acts on groups of cells within the CNS in the process of the neuroinflammatory response; thus, it is promising for the treatment of CNS injuries and diseases.
miR-124 in Cerebral Stroke and Brain Injury
Cerebral stroke is a major cause of mortality and morbidity (Feigin et al. 2017). Cerebral ischemia (ischemic stroke) accounts for about 80–90% of all strokes, and intracerebral hemorrhage (ICH) accounts for approximately 10–20% of strokes (Feigin et al. 2009; Sacco et al. 2009). Cerebral stroke involves a complex pathology, which triggers a cascade of cellular responses, and ultimately results in focal neurological deficits as well as long-term cognitive and motor impairment. miRNAs are involved in regulating many aspects of the pathogenic mechanisms following a stroke, thereby providing insights into new therapeutic avenues. Continuing research on the brain-specific miR-124 has accelerated efforts to explore whether it is an effective target for stroke treatment.
Ischemic Stroke
Multiple reports have shown that miR-124 levels increased after an acute ischemic stroke in both experimental MCAO models and in patients. Jeyaseelan et al. (2008) first reported an increased level of miR-124 in brain samples from a rat model following MCAO and 24-h reperfusion. Consistently, miR-124 concentration increased in rat plasma after MCAO induction (Laterza et al. 2009; Weng et al. 2011). miR-124 expression levels were significantly high in the plasma, serum, and cerebrospinal fluid of stroke patients as well as in the brain samples of men who died of brain stroke (Ji et al. 2016; Leung et al. 2014; Sessa et al. 2019; Sorensen et al. 2017). In addition, studies suggest that a higher concentration of miR-124 was related to a larger infarct volume after stroke and more unfavorable outcomes after stroke treatment (He et al. 2019; Sorensen et al. 2017). The mechanisms that result in changes in circulating miR-124 levels after stroke are still unclear. Possibly, it could be the result of increased miR-124 released from damaged brain tissue into circulation, which may be explained by the significant correlation between plasma miR-124 and lesion size (Rainer et al. 2016). In summary, miR-124 is not only a promising candidate biomarker after stroke but also an early prediction and risk stratification indicator for stroke treatment.
Under ischemic stroke conditions, the availability of oxygen and glucose to tissues is delayed, which elicits multifaceted responses and triggers primary and secondary insults. It involves a complex pathology, including multiple types of cellular responses, such as astrogliosis, microgliosis, and peripheral source of macrophage activation, as well as excitotoxicity, oxidative stress, inflammation, neuronal apoptosis, neurodegeneration, and BBB destruction. Ultimately, ischemic stroke leads to the death of brain tissue and causes focal neurological deficits (Burda and Sofroniew 2014; Tobin et al. 2014). The mechanisms of miR-124 as therapeutic targets for ischemic stroke should be based on its actions against stroke pathology; some of these mechanisms are discussed here.
Neurogenesis after stroke occurs even in adult rodents and humans (Arvidsson et al. 2002; Jin et al. 2006). However, the self-repair of injured brain through neuronal replacement from the differentiation of neuronal precursors is insufficient (Adamczak et al. 2017; Shimada et al. 2010). For efficient repair, a promising avenue is the optimization of this neurogenesis process to promote the self-repair mechanism. miR-124 has been shown to play an important role in promoting neurogenesis under physiological and pathological conditions. Some evidence has also demonstrated that miR-124 promoted the survival and neuronal differentiation of neuronal stem cells (NSCs), partly via direct suppression of paired box 3 expression or enhanced proliferation (Wei et al. 2018). It also induced the differentiation of NSCs into neurons by activation of the Wnt/β-catenin pathway through direct targeting of the disheveled binding antagonist of β-catenin 1 (Jiao et al. 2018). In addition, miR-124 also contributed to the regulation of neurite outgrowth during neuronal differentiation. Functional enrichment analysis revealed that miR-124 influenced axon regeneration mainly by regulating cellular component organization, axonogenesis, and cell morphogenesis (Su et al. 2018). Suppression of miR-124 led to reciprocal increases in mRNA levels of target genes that inhibited axonal and dendritic projections (Morris et al. 2015). Studies have also shown that miR-124 directly targeted and downregulated the endogenous expression of oxysterol-binding protein to promote neurite outgrowth and elongation during the development of the C57BL/6 mouse cortex (Gu et al. 2016). In addition, miR-124 directly targeted CBX2, a negative regulator of neuronal differentiation, to stimulate neurite development (Gu et al. 2018). According to its multiple functions in promoting neuronal differentiation and neurite outgrowth, miR-124 is a potential therapeutic target after ischemic stroke. Under ischemic conditions, miR-124 was reported to inhibit neuronal progenitor cell proliferation and promote neuronal differentiation by targeting JAG1 (Liu et al. 2011). Modified exosomes loaded with miR-124 crossed the BBB and were efficiently delivered to the infarct site, where they induced robust cortical neurogenesis to protect against ischemic injury (Yang et al. 2017). Despite the evidence supporting the neurogenesis-promoting function of miR-124, its in vivo application is less straight forward; although it has been shown that miR-124-loaded nanoparticles increased survival and neuronal differentiation of NSCs in vitro, it did not contribute to stroke outcomes in vivo (Saraiva et al. 2018). However, before the substantial use of miR-124 in the therapeutic promotion of neurogenesis, the dosage, mode of administration, time frame, and therapeutic window need to be clarified.
OGD, as well as injury-induced cell death, including apoptosis, contribute to the cellular damage that occurs in ischemic stroke (Puyal et al. 2013; Zaiman et al. 2011). Increasing neuronal survival and anti-apoptosis could attenuate infarction and improve functional outcomes after stroke. miR-124 has been shown to inhibit neuronal apoptosis in the cerebral ischemic region of rats by activating the Wnt/β-catenin signaling pathway (Che et al. 2019). Injection of miR-124 mimetics significantly reduced cerebral infarction-induced neurological deficits and infarction areas (Che et al. 2019). miR-124 was demonstrated to have anti-apoptotic and neuroprotective effects. Moreover, in a recent study, using RISC immunoprecipitation, researchers identified 98 high-confidence miR-124 targets, some of which directly led to decreased viability and higher apoptotic rates in miR-124-deleted neurons, both of which could be rescued by miR-124 overexpression. In addition, the study demonstrated that miR-124 regulation was essential for the long-term survival of terminally differentiated neurons by targeting a cascade of apoptosis-relevant genes (Kutsche et al. 2018). Thus, these qualities could contribute to the following hypothetical mechanism: induction of K63-linked RIP1 ubiquitination, mediated by UBXN1 repression and inhibition of USP14-dependent RE1 silencing transcriptional factor (REST) degradation (Doeppner et al. 2013; Song et al. 2019c). However, conflicting results of miR-124 on apoptosis after stroke have also been reported in other cell types. By targeting the PI3K/AKT signaling pathway and promoting reactive oxygen species (ROS) production, miR-124 potentially induced apoptosis in brain vascular endothelial cells (Wang et al. 2018b). A recent study also demonstrated that ischemic postconditioning exerts its neuroprotective effect by negatively regulating the PI3K/AKT2 signaling pathway by miR-124. miR-124 significantly decreased the expression of Caspase-3 and BAX and increased the expression of the anti-apoptotic protein Bcl-2. Similarly, the inhibition of miR-124 also inhibits cellular apoptosis and autophagy by increasing the PI3K/AKT/mTOR signaling pathway (Miao et al. 2020). With more research, it is possible that the comprehensive effects of miR-124 on apoptosis and stroke treatment could be clinically applied.
Excessive excitatory glutamate (Glu) neurotransmission is toxic and leads to neuronal cell death. After an ischemic stroke, Glu is released in high levels, while Glu transporters (GLT-1) and reuptake is widely suppressed. miR-124 was reported to attenuate excitotoxicity by upregulating the expression of GLT-1 via the AKT and mTOR pathways in astrocytes injured by OGD/reperfusion (Huang et al. 2019). Angiogenesis can also contribute to recovery after stroke. miR-124 increased angiogenesis in the ischemic striatum 56 days after MCAO, as shown by CD31 immunohistochemistry (Doeppner et al. 2013). In addition to the abovementioned findings, miR-124 also plays an important role in regulating inflammation.
ICH
ICH is usually caused by the rupture of small blood vessels secondary to chronic hypertension or other vasculopathy (Qureshi et al. 2009). Compared to ischemic stroke, ICH has a higher mortality and leads to more severe disability (An et al. 2017b). Primary and secondary ICH have similar underlying pathological changes induced by the pooling of blood in the brain parenchyma and hematoma formation (Steiner et al. 2006). These changes are associated with multiple biological processes, including oxidative damage, inflammation, and edema formation, all of which contribute to an extensive cascade of cellular and molecular alterations in the brain that add to further destruction of brain tissue (Keep et al. 2012). Studies on ICH patients and rodent models have demonstrated that plasma miR-124 was induced by collagenase during the acute injury phase, suggesting that miR-124 is derived from brain injuries. In rodents, these levels are further decreased during the delayed recovery phase, which suggests healing of the brain injury, and it finally returned to baseline levels when the rats fully recover (Wang et al. 2018d). This pattern of miR-124 plasma concentration is a promising candidate biomarker for the early detection and predictive prognosis of human ICH. Given that the cascade of cellular and molecular changes, such as excitotoxicity, inflammation, neuronal apoptosis, and neurodegeneration, are similar to observed in ischemic stroke, miR-124 probably plays a similar function in ICH as that observed in ischemic stroke. Unfortunately, there are limited studies showing that miR-124 ameliorates ICH-induced inflammatory injury, though data indicate that this is achieved by modulating microglial polarization toward the M2 phenotype via C/EBP-α, as well as by significantly attenuating neuronal apoptosis via the Bcl-2/Bcl-xl pathway in vivo and in vitro (Yu et al. 2017). We look forward to more studies that may reveal whether miR-124 plays an important role in ICH.
miR-124 in SCI and Spinal Cord-Associated Neuropathic Pain
Promoting Neuronal Differentiation in SCI
SCI results in the loss of motion and sensory function below the damage plane, with devastating physical, psychosocial, and vocational implications for patients. Epidemiological studies by the World Health Organization (WHO) have shown that the incidence of SCI worldwide averages 10–40 people per million. China and the United States have a high incidence of SCI (more than 40 people per million a year) (Ahuja et al. 2017; Lee et al. 2014; Singh et al. 2014; Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance 2016; Witiw and Fehlings 2015). The pathological process of SCI includes primary and secondary mechanisms, which eventually form glial scars composed of microglia, reactive astrocytes, and secreted inhibitory proteins, such as chondroitin sulfate proteoglycan in the injured area to inhibit axon regeneration and myelination (Okada et al. 2018). A recent study indicated that miRNAs function as gene expression switches in key processes of SCI (Nieto-Diaz et al. 2014). Therefore, understanding how an injury affects miRNA expression and the meaning of these changes in the SCI pathological process will help explore the potential application of miRNAs. Among them, the link between the regulation of miR-124, which is highly expressed in the mammalian CNS, and SCI has attracted much attention (Song et al. 2019a).
A miRNA microarray (miCHIP) study revealed that miR-124 is one of the most highly expressed miRNAs in the rat spinal cord (Brandenburger et al. 2012). Moreover, changes in miR-124 expression are observed in neurons in the peri-lesion area of mice with SCI (Zhao et al. 2015b). In one study, the expression of miR-124 was significantly decreased within 7 days after SCI (Zhao et al. 2015b). Several neurons in the peri-lesion area were NeuN+/miR-124−, but the neurons distal to the peri-lesion area were NeuN+/miR-124+, indicating that miR-124 expression was downregulated after SCI. To date, no direct connection has been shown between miR-124 expression and the severity of SCI, although efforts have been made to explore the specific roles of miR-124 in SCI. Studies have shown that the upregulation of miR-124 dramatically increased the differentiation of NSCs into NeuN+ cells in vitro but reduced the percentage of GFAP+ cells in vivo in rats with SCI (Xu et al. 2012). Moreover, transplantation of bone marrow mesenchymal stem cells overexpressing miR-124 also promoted neuronal differentiation and, thus, accelerated the repair of SCI compared relative to control cells (Song et al. 2017; Zhao et al. 2015a; Zou et al. 2014). This effect may be attributed to miR-124, which was shown to directly target pyridoxal kinase (PDXK) to increase the expression of SCI repair-related proteins, such as TRH, PGI2, and GM (Song et al. 2017).
Attenuating Spinal Cord-Associated Neuropathic Pain
Neuropathic pain, or pain caused by a lesion or disease of the somatosensory nervous system, usually occurs in 40–50% of patients with SCI within the first year following SCI (Chi et al. 2019). miR-124 has been reported to play vital roles in spinal cord-associated neuropathic pain (Willemen et al. 2012). A recent study using microarray-based approaches has demonstrated that miR-124 is involved in pain processing and that it can attenuate inflammatory pain induced by complete Freund’s adjuvant via the inhibition of IL-6R expression in the spinal cord (Liu et al. 2017). Moreover, intrathecal administration of miR-124 completely prevented the transition from acute to persistent IL-1β-induced hyperalgesia in LysM-GRK2+/− mice, presenting evidence for miR-124 to treat persistent inflammatory (Willemen et al. 2012). This is consistent with the finding that miR-124 is a key negative regulator of neuroinflammation, which has been reviewed in another section of this article (MiR-124 in neuroinflammation).
Taken together, the above findings suggest that the neuroprotective and anti-inflammatory effects of miR-124 after SCI or spinal cord disorder make it a promising therapeutic candidate for targeting SCI.
miR-124 in NDDs
NDDs, which include Alzheimer’s disease (AD), PD, ALS, and frontotemporal dementia, are characterized by dysfunction and death of specific neuronal subtypes (Giuliani et al. 2017; Haston and Finkbeiner 2016). Along with the increasing human population, NDDs are becoming a challenge to healthcare systems. Unfortunately, there are still no effective treatment options, as current therapies only relieve symptoms (Mason et al. 2014; Jucker 2010; Mitsumoto et al. 2014; Socias et al. 2018).
In addition to progressive deterioration of neuronal structure and function (Bredesen et al. 2006), evidence derived from genetic, neuropathological, and in vitro or in vivo studies shows that NDDs often display common mechanisms and pathological features (Glass et al. 2010; Jucker 2010; Nainu et al. 2019; Philip et al. 2002). The misfolding, aggregation, and accumulation of proteins in the brain are hallmark events of NDDs at the cellular level. Although distinct NDDs have different protein aggregates, the protein misfolding process is remarkably similar (Bennett 2005; Ross and Poirier 2004; Soto 2003; Soto and Pritzkow 2018). The accumulated misfolded proteins activate resident immune cells, such as microglia and astrocytes, which induce sustained inflammatory responses. Concomitantly, immune cells release a variety of other neurotoxic factors (Brown and Neher 2010; Glass et al. 2010; Sofroniew 2015), which further contribute to the progression of NDDs (Liang et al. 2017). Previous studies have demonstrated that mitochondrial dysfunction and cumulative oxidative stress are common features of several NDDs, such as AD, PD, ALS, and Friedreich’s ataxia (Federico et al. 2012; Gandhi and Abramov 2012; Kim et al. 2015). Oxidative stress may impair the DNA repair system that accelerates the aging process and development of NDDs (Kim et al. 2015). Furthermore, being associated with inflammatory and immune responses, BBB disruption leads to neuronal injury, synaptic dysfunction, and loss of neuronal connectivity (Sweeney et al. 2018; Zlokovic 2008). miR-124 has been reported to play a neuroprotective role in animal models of NDDs, suggesting that some therapeutic approaches involving this particular miRNA may be effective for treating these diseases (Du et al. 2017; Kong et al. 2015; Zhou et al. 2019).
AD
Approximately 47 million people worldwide have AD, and the prevalence of AD is 15% in those aged 68 years or older in the United States (Arvanitakis et al. 2019). miR-124 has been reported to be downregulated in patients with AD (An et al. 2017a; Burgos et al. 2014; Zhang et al. 2017a). miR-124-1 was found to be hypermethylated in AD brains, which would explain its suppression (Villela et al. 2016). Intracellular aggregates of insoluble tau proteins are one of the characteristics of AD, and one study demonstrated that tau increased the silencing activity of miR-124 through DEAD-box RNA helicase 6 activity (Chauderlier et al. 2018). In addition, downregulation of miR-124 resulted in elevated CAPN1, and CAPN1 induced cleavage of p35 to p25 as well as the formation of the p25/cyclin-dependent kinase 5 (CDK5) complex, which resulted in tau hyperphosphorylation and cellular apoptosis (Zhou et al. 2019). This led to the accumulation of extracellular amyloid plaques, the major cause of AD in vitro. Overexpression of miR-124 suppressed BACE1, an enzyme that plays an indispensable role in the generation of the β-amyloid (Aβ) peptide (An et al. 2017a; Du et al. 2017; Zhao et al. 2019). Abnormal accumulation of Aβ peptide participates in the formation of amyloid plaques (Du et al. 2017). Furthermore, it has been reported that PTBP1 is a target of miR-124 (Mokabber et al. 2019). Through PTBP1, miR-124 was shown to regulate alternative splicing of the amyloid precursor protein (APP) mRNA, and abnormal neuron-specific APP mRNA splicing affects Aβ peptide production (Smith et al. 2011). This suggests that miR-124 could be used in a fine-tuning manner to remedy abnormal neuronal splicing of APP to reduce Aβ peptide production. However, there exists controversial data regarding the alterations of miR-124 in the brains of patients with AD. Wang et al. separately examined different brain regions from AD patients and discovered that miR-124 was increased in the hippocampus and temporal cortex, the first two brain regions damaged in AD (Wang et al. 2018c). In their recent study, abnormally upregulated miR-124 was detected in P301S mice (a well-known model of tau pathology), leading to decreased expression of protein phosphatase 1, accompanied by tau hyperphosphorylation at multiple sites; these findings implied that miR-124 had an impact on the regulation of tau pathology (Hou et al. 2020). Taken together, these findings suggest that disorders involving miR-124 play an important role in various AD pathological processes, and that miR-124 holds therapeutic promise.
PD
Worldwide, the incidence estimates of PD range from 5 to 35 new cases per 100,000 individuals yearly (Twelves et al. 2003). The prevalence increases with age, and it is about 2–3% in those aged 65 years or older (Lappin et al. 2018). One study estimated that the average disease duration until death is between 6.9 and 14.3 years (Jiao et al. 2018). PD is characterized by the selective loss of midbrain dopaminergic (DA) neurons. It has been demonstrated that miR-124 levels are significantly lower in the plasma of patients with PD, PD mouse models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and DA neuronal cell lines treated with 6-hydroxydopamine or methyl phenyl pyridinium (Dong et al. 2018; Kanagaraj et al. 2014; Li et al. 2017; Rosas-Hernandez et al. 2018; Yao et al. 2019).
In the development of PD, upregulation of miR-124 may regulate several pathogenetic events involved in anti-inflammatory effects, decreasing oxidative stress, and anti-apoptotic effects, thus reducing the loss of DA neurons. The roles of miR-124 in inhibiting neuroinflammation, decreasing ROS production and neuronal apoptosis, and improving cell viability are related to the regulation of JAK/STAT3, MEKK3/NF-κB, AnnexinA5 (ANXA5)/ERK, and calpain/CDK5 signals, respectively (Dong et al. 2018; Geng et al. 2017; Kanagaraj et al. 2014; Yao et al. 2018). Moreover, miR-124 targets Bim, a vital protein regulating the apoptosis and autophagy of DA neurons in the pathogenesis of PD, to reduce Bax translocation into the mitochondria and lysosomes. This relationship was implicated in the alleviation of apoptosis and promotion of autophagy in DA neurons (Wang et al. 2016a). P38 and p62 are upregulated in MPTP-induced PD model; therefore, by targeting p38 and p62, overexpression of miR-124 could suppress the secretion of proinflammatory mediators and promote autophagy in the inflammatory response of PD (Yao et al. 2019). Overexpression of death-associated protein kinase 1 (DAPK1) resulted in DA injury and locomotor disabilities in mice with PD; however, miR-124 rescued impairment in PD by targeting DAPK1 (Lu et al. 2020; Su et al. 2019). These results indicate that miR-124 could be a potential therapeutic target for regulating the pathological process in PD.
ALS
In Europe, the incidence of ALS is about 2.6–3.0 cases per 100,000 people, with a prevalence of 5.4 per 100,000 people (Cicero et al. 2017; van Es et al. 2017). ALS is a fatal neurodegenerative disease characterized by progressive motoneuron loss, and most patients with ALS die of respiratory complications (Niedermeyer et al. 2019). miR-124 may be a possible indicator of disease stage/progression in ALS. A recent study using the SOD1 G93A model of ALS showed that the spinal motor neuron-derived exosomal miR-124 and its extracellular localization are increased even at the pre-symptomatic stage; this event occurs before a large number of spinal motor neurons undergo degeneration, indicating that the extracellular change in miR-124 is likely an early pathological event (Yelick et al. 2020). Expression of miR-124 is increased in the brain of ALS model mice in the late stages of the disease, and is significantly upregulated in the hippocampus, subventricular zone, brainstem motor nuclei, and primary motor cortex of 18-week-old ALS mice compared to controls (Marcuzzo et al. 2015). Similarly, miR-124 was increased in differentiating ependymal stem cell progenitors in vitro, particularly in stressed neurons, as well as linked to neurodegeneration in 18-week-old ALS mice (Marcuzzo et al. 2015, 2014). Increased miR-124 levels were also found in mSOD1 NSC-34 cells (an in vitro ALS model) and their derived exosomes (Pinto et al. 2017). The activation of neuroinflammation and neurotoxic effects has been shown to contribute to motoneuron degeneration (Brites and Vaz 2014; Cunha et al. 2018). Overexpression of miR-124 seems to directly or indirectly reduce the differentiation of astrocytes and increase neuronal differentiation (Krichevsky et al. 2006; Marcuzzo et al. 2014). However, alterations in miRNA localization can lead to different effects and outcomes. The ability of neurite development may be lost in the early stages of ALS and thereby contribute to the progressive nature of axonal degeneration. However, miR-124 has been shown to play an important role in promoting neurogenesis, which has been reviewed in another section of this article (Ischemic stroke). These findings suggest a complex mechanism underlying the relationship between miR-124 and ALS.
miR-124 in Epilepsy
Epilepsy is a common, recurrent, and intractable seizure disorder, affecting over 70 million people worldwide (Thijs et al. 2019). It is bimodally distributed with two peaks: infants less than 1 year old and in people over the age of 50 years (Thijs et al. 2019). The pathogenesis of epilepsy is thought to result from disrupted gene expression, leading to an imbalance between excitatory and inhibitory activity within a neuronal network, a disorder in synaptic structure, cell death, and inflammation. Anti-epileptic drugs are effective in less than half of the patients, and specific anti-epileptic drugs are not available (Walker 2018). Emerging evidence indicates that certain miRNAs play important roles in epileptogenesis (Jimenez-Mateos et al. 2012; Tan et al. 2013; Zheng et al. 2016). No compelling evidence has been found to date relating to the risk of epilepsy to mutations or variations in the miR-124 gene (Manna et al. 2016), although significant upregulation of miR-124 was detected in the seizure-related stages in children with mesial temporal lobe epilepsy (Peng et al. 2013). However, Wang et al. (2016a, b) observed that miR-124 was downregulated in epilepsy models injected intraperitoneally with lithium chloride, as intra-hippocampal supplementation with miR-124 inhibited neuronal firing and excitability as well as susceptibility to epileptic seizures, by targeting CREB1. Moreover, Brennan et al. (2016) demonstrated that miR-124 played dual and opposing roles in epilepsy, attenuating epileptogenesis via the neuron restrictive silencer factor, while promoting epilepsy via inflammation, showing that miR-124 might be aberrantly expressed during epileptogenesis and may be a key regulator and promising therapeutic target for epilepsy. However, in a recent study, a combination of anti-miRNA oligonucleotides (AMOs) against miR-124 and miR-137 administered locally to the dentate gyrus normalized neuroblast proliferation and prevented NSCs loss upon non-convulsive seizures in a subpopulation of patients with epilepsy (Bielefeld et al. 2019). In addition, the effects of AMO co-administration against miR-124 and miR-137 were better than individual AMO administration, which supports the theory that the action mediated by miRNA synergy is necessary. There might be more synergistic effects between miR-124 and other miRNAs, and their co-administration could play more functions in the treatment of CNS diseases. In general, because of the complexity of epilepsy, the few studies published to date are insufficient to draw any conclusion on the comprehensive function and mechanism of miR-124.
miR-124 in MS
MS is a chronic inflammatory autoimmune disease of the CNS. Today, 2–3 million people are affected by MS, and almost three out of four people with MS are women (Compston and Coles 2002; Dobson and Giovannoni 2019). MS is affected by many factors, including environmental and genetic factors; according to epidemiological studies, environmental factors are more important than genetic factors. Low levels of vitamin D, smoking, obesity, exposure to sunlight, and Epstein–Barr virus infection play important roles in MS development. At present, more than 150 genes have been associated with MS; among them, the most significant one is the HLA DRB1*15:01 haplotype (Dobson and Giovannoni 2019; Reich et al. 2018).
Evidence indicates that the pathogenesis of MS is related to a series of pathological events, such as activation of microglia and macrophages, neuronal injury, and glial reaction (Thompson et al. 2018). The main symptoms of MS are neuralgia, cognitive impairment, spinal neuritis, and disability, which affect the normal life of young people (Correale et al. 2017). At present, the major treatments for MS are disease-modifying therapies—the use of drugs, such as dimethyl fumarate, natalizumab, and ocrelizumab; however, all these drugs have side effects, presumably since they do not take into account the patient’s disease phenotype, prognostic factors, acceptable risk preferences, etc. (Tintore et al. 2019).
Activated T cells and B cells are found in the CNS of patients with MS. Activated T cells produce cytokines (IL-17, IFN-γ, etc.) to activate microglia and macrophages. Activation of macrophages (mainly type M1, positive expression of MHC II) can improve the antigen-presenting effect of antigen-presenting cells, promote autoimmunity and inflammatory reactions caused by T and B cells, and cause tissue damage (Baecher-Allan et al. 2018; Essandoh et al. 2016). The activated microglia can release proinflammatory factors, ROS, glutamic acid, etc., which further cause inflammatory reactions, demyelination, and neuronal injury (Faissner et al. 2019). miR-124, which is a marker of both anti-inflammation and immune cell quiescence, was strongly downregulated in progressive MS patients, suggesting the therapeutic opportunities of MS by targeting inflammatory reactions (Amoruso et al. 2020).
Resveratrol was found to affect the apoptosis of T cells by regulating the miR-124/SK1 pathway in EAE. Resveratrol upregulated miR-124 by targeting SK1, thus affecting the development of T cells (Gandy et al. 2019). T helper (Th) 1 and Th17, two mature Th effector cells, are important in the process of MS, since they can release IFN-α and IL-17A (Baecher-Allan et al. 2018). miR-124 can inhibit the expression of cytokine signal transduction inhibitor 5, and thereby promote the expression of STAT1 and STAT3, which are very important for the differentiation of Th1 and Th17 cells, and subsequently promote the maturation and differentiation of CD4+ cells (Jiang et al. 2014). It has been shown that miR-124 can inhibit the production of IL-6 and TNF-α by targeting STAT3 and TACE, which can further reduce the release of LPS-induced cytokines (Sun et al. 2013; Wang et al. 2017). In addition, the role of miR-124 in M2 polarization might shed light on the therapy of MS symptoms (Mikita et al. 2011; Weng et al. 2019).
In addition, Glu-mediated excitotoxicity is an important process in the pathogenesis of MS, leading to neuronal death by overstimulation of glutaminergic receptors and increased axonal calcium (Ciccarelli et al. 2014). miR-124 interacted with Glutamate Ionotropic Receptor AMPA Type Subunit 2 (GRIA2, receptors mediated excitatory neurotransmission) mRNA and downregulated its translation in the hippocampus (Ho et al. 2014). In contrast, it was found that following hippocampal demyelination in mice, miR-124 levels in the hippocampus increased, and it was associated with the downregulation of GRIA2 content, resulting in a decrease in synaptic plasticity (Dutta et al. 2013). These results suggest that the different expression patterns of miR-124 might be closely related to the progression of MS, and changes in miR-124 levels could address memory decline in patients with MS.
miR-124 in GBM
The grade IV type tumor arising from astrocytes, which is infiltrative and undifferentiated from other normal cells, is called glioblastoma, also known as glioblastoma multiforme (GBM). GBM is the most common malignant gliomas of the CNS, accounting for approximately 60–70% of all malignant gliomas, with the highest incidence rate (3.19 per 100,000 before 2009) (Louis et al. 2007). The incidence of GBM increases dramatically after the age of 54 years, with a peak incidence of 15.24 per 100,000 at the age of 75–84 years. The median overall survival of GBM patients is only about 12–15 months (Ostrom et al. 2015). Thus, GBM are widely noted for their high incidence and low five-year survival rate (no more than 5%) (Dolecek et al. 2012). The role of angiogenesis and the high invasiveness of GBM, which are the main causes of poor prognosis and therapeutic tolerance, are particularly pivotal and involve a variety of molecular mechanisms and signaling pathways. Hypoxia, which acts as the starting signal, prompts the expression of hypoxia-inducible factor 1, resulting in an increase in vascular endothelial growth factor (VEGF) (Jain 2014). In addition, the activation of mitogen signaling pathways (such as PI3K and MAPK) (Jayson et al. 2016), the secretion of extracellular matrix proteins (MMP-2, TWIST1) (Florczyk et al. 2013), and altered cellular metabolism (Beyer et al. 2017) contribute to the progression of GBM. Owing to their multiple pathogeneses, clinical symptoms, like progressive headaches, focal neurologic deficits, and seizures, are complex and intractable (Huse et al. 2011). Although there have been improvements in the clinical therapy of glioblastomas, such as surgical excision combined with DNA methylation agent-mediated chemotherapy and radiotherapy, the prognosis and overall survival of patients have not changed significantly (Gittleman et al. 2018; Xue et al. 2020). Therefore, research on targeted molecular therapy is warranted.
Recent studies and evidence have shown that different miRNA expression profiles play distinct roles in the progression of GBM (Fowler et al. 2011). Quantitative RT-PCR was used to investigate the expression profiles of 192 miRNAs in primary brain tumor and non-tumor brain tissues; the results showed that miR-124 was downregulated in GBM (Silber et al. 2008; Yang et al. 2013). In addition, the expression of miR-124 decreased progressively in glioma tissues from WHO grades II to IV (Deng et al. 2019). Another study that examined 119 clinical GBM patients found that those with lower levels of miR-124 had shorter survival times (Fowler et al. 2011). At present, the mechanisms that suppress miR-124 in GBM remain unclear. The following two mechanisms are recognized. The first mechanism is the overexpression of the transcriptional repressor RE1 silencing transcriptional factor (REST), which represses miR-124 to regulate the oncogenic properties of high-REST GBM stem-like cells (HR-GSCs) in humans. The study showed that REST-miR-124 pathways could regulate self-renewal, apoptosis, and invasion in GSCs (Marisetty et al. 2017). The second mechanism is growth factor signaling. In a mouse NSC culture without growth factors, miR-124 expression is increased, indicating that growth factors may promote GBM formation by repressing miR-124 (Silber et al. 2008).
miR-124 regulates cell growth, differentiation, invasion, and apoptosis by targeting multiple genes or proteins. A previous study showed that miR-124 inhibits the proliferation of C6 glioma cells by targeting Smad4 (Zhang et al. 2017b). Transfection of miR-124 induced the arrest of GBM cells in the G0/G1 phase, thus inhibiting the growth and proliferation of GBM (Chen et al. 2014; Silber et al. 2008; Skalsky and Cullen 2011). One study showed that increasing the expression of miR-124 affects the ability of GBM cells to survive and absorb nutrients and oxygen. Moreover, miR-124 overexpression improved the survival rate of tumor-bearing mice (Mucaj et al. 2015). In addition, overexpression of miR-124 significantly inhibited the invasion of U87-124 and U373-124 cells through a Matrigel membrane in vitro (Xia et al. 2012). At present, several targets, associated with the abovementioned behaviors, have been identified.
Upregulation of miR-124 in glioma cancer stem cells inhibited immunosuppression mediated by the IL-6/JAK/STAT3 signaling pathway, indicating that miR-124 functions through downregulation of STAT3 (Johnson et al. 2018; Wei et al. 2013). Transfection of T cells along with miR-124 administration in patients with GBM affected the expression of cytokines (IL-2, IFN-1, and TNF-α), implying that the therapeutic effect of miR-124, which targets STAT3, relies on the T cell-mediated immune response, which is a potential immunotherapeutic agent (Bo et al. 2013). The miR-124/SOX9 axis also exerts an effect on stem cells differentiating into neurons. Overexpression of miR-124 was shown to silence SOX9 in patient-derived GBM cells, reducing the radiation resistance and tumorigenicity of patients with GBM (Sabelström et al. 2019). miR-124 has been reported to directly target the Ras viral oncogene homolog (R-Ras) and the neuroblastoma Ras viral oncogene homolog (N-Ras) in glioma cells, showing a negative correlation (Shi et al. 2014). Furthermore, Akt, ERK1/2, and VEGF, which are downstream effectors of R-Ras and N-Ras pathways in cell proliferation and survival, are suppressed by miR-124 in GBM cells (Chappell et al. 2011; Shi et al. 2014). A recent study focused on the functions of p62 and demonstrated that the accumulation of p62 could promote glioma progression by regulating autophagy, proliferation, migration, apoptosis, TMZ resistance, and the NF-κB signaling pathway, and its functions could be partially reversed by miR-124 overexpression (Deng et al. 2019). In view of the unique benefits of miR-124 in negatively regulating the proliferation and invasiveness of GBM involving various molecular mechanisms, targeting the delivery of miR-124 to GBM tumor cells may be therapeutically valuable for GBM treatment.
Methods for the Delivery of miR-124-Based Drugs to the CNS
The basis of drug development is a deep understanding of the molecular mechanisms of pathological processes. The development of miRNA-related drugs requires systematic analysis of patient samples to clarify the pathogenesis and biological relationship between miRNAs and diseases through in vivo and in vitro models. Although miR-124 is identified as a promising therapeutic for treating CNS diseases and injuries, the BBB essentially restricts the entry of therapeutic drugs into the brain or spinal cord. The challenges, described below, include the design of an miR-124 delivery system that crosses the BBB and enables tissue/cell-specific targeting. Several methods have been developed for the delivery of miR-124 or other miRNAs to the CNS for the treatment of inflammation, stroke, PD, and GBM.
Exosome Delivery System
Exosomes are 40–100 nm vesicles released as a consequence of multivesicular endosome fusion with the plasma membrane (Raposo and Stoorvogel 2013). The exosome is a well-studied vesicle, which can cross the BBB and carry membrane and cytosolic proteins, lipids, and RNA cargos to mediate brain remodeling after diseases and injuries (Haney et al. 2015; Valadi et al. 2007; Xin et al. 2014). Several miRNA mimetics and inhibitors have been effectively delivered to the brain using exosomes, and could protect against CNS injury (Alvarez-Erviti et al. 2011; Kim et al. 2020; Lakhal and Wood 2011; Xin et al. 2012). Other than promoting microglial M2 polarization, miR-124 is also detected to be abundant in the M2-microglia-derived exosomes, which can be administered intravenously to inhibit neuronal inflammation and neuronal autophagy, enhance neurogenesis, and improve neurological outcome following traumatic brain injury in mice (Ge et al. 2020; Huang et al. 2018; Li et al. 2019c; Yang et al. 2019). Mesenchymal stem cells can migrate to cancer sites, including GBM, and exert anti-tumor and neuroprotective effects (Otero-Ortega et al. 2019; Sharif et al. 2018). In addition, it has been demonstrated that mesenchymal stem cell exosomes loaded with miR-124 significantly inhibit the activity of CDK6, enhance the chemosensitivity of GBM cells to temozolomide, and decrease the migration of GBM cells (Sharif et al. 2018). Exosomal miR-124 from M2-microglia also promoted neurogenesis and protected the brain from ischemic stroke or ischemia–reperfusion injury (Song et al. 2019b). Therefore, identification of the cellular source of exosomes seems important; the native cargos might play a synergistic role with miR-124 to achieve better effects. To achieve neuron-specific targeting, miR-124-loaded exosomes could also be modified. Exosomes modified by RVG-Lamp2b and injected after brain ischemia have been shown to promote cortical neuronal progenitor cell differentiation, cortical neurogenesis, and protect against ischemic brain injury (Yang et al. 2017). The challenges in exosomal miR-124 application might include identifying the best exosome-secreted cells and developing appropriate exosome modification.
Nanoparticles
Delivery systems for miRNA precursors or antagonists can also be approached with nanoparticles, which also have the potential to bypass the BBB and cell-specific delivery after modifications. Nanoparticles encapsulate and protect miRNAs from degradation, enhancing circulation time and targeted accumulation (Blanco et al. 2015). The nanoparticles used for therapeutic miRNA delivery have a size range of 1–500 nm (Boca et al. 2020). In addition to size, the shape, surface chemistry, and charge of nanoparticles affect their capability to specifically target cells and the way cells internalize them (Behzadi et al. 2017). On this basis, the choice of the biomaterial that constitutes the nanoparticles is extremely important, as it dictates the final properties and structure of the nanoparticles. Multiple nanoparticles for miRNA delivery has been extensively investigated, due to their efficient cargo release within the cell cytoplasm and their easy synthesis and functionalization, including liposomes, synthetic polymers, natural polymers, as well as inorganic nanoparticles, such as gold, calcium phosphate, silica, and iron oxides, which has been well-reviewed elsewhere (Lee et al. 2019). miR-124-loaded nanoparticles were shown to reverse tumor-mediated immune suppression and prolong survival in a murine glioma model system by regulating the intracellular signaling pathway (Yaghi et al. 2017). This delivery approach is also applicable to other malignancies, such as non-small cell lung, breast, and ovarian cancers (Lin et al. 2016; Seviour et al. 2016). Intracerebroventricular injection of miR-124-loaded nanoparticles reduced inflammatory cytokine levels and enhanced brain repair in PD (Gan et al. 2019; Saraiva et al. 2016). However, intravenous injections of miR-124-loaded nanoparticles increased NSCs survival and neuronal differentiation in vitro but did not contribute to stroke outcome in vivo (Saraiva et al. 2018). Additional studies might concentrate on achieving the targeted distribution and reducing toxic and off-target effects.
Adeno-Associated Viruses (AAV)
AAV capsids are a rapidly emerging gene therapy delivery system for the treatment of neurological diseases. AAVs have an unprecedented ability to transfer genes to the CNS and achieve efficient and stable therapeutic levels of miRNAs (Deverman et al. 2018a). AAVs are 25 nm non-enveloped viruses with a single-stranded genome of about 4800 nucleotides that replicate only in the presence of several proteins complemented by adenoviruses, hence the name (Schaffer et al. 2008). Currently, more than 100 natural AAV variants comprising at least 8 serotypes have been identified from vertebrates (Gao et al. 2003). Noteworthy, many of them, such as AAV1, AAV2, AAV5, AAV9, AAVrh.10, and AAV-DJ8, have been evaluated to spread broadly in the CNS and transduce cells with high efficiency (Deverman et al. 2018b). The AAV genome contains two open reading frames (ORF) flanked by inverted terminal repeat elements (ITR), which are the minimal cis-acting elements necessary for viral genome integration, replication, and packaging into particles (Srivastava et al. 1983). The first ORF (rep) encodes four rep proteins that are involved in the replication of the viral genome, whereas the second ORF (cap) encodes three structural proteins (VP1, VP2, and VP3). The VP proteins self-assemble to form the viral capsid, into which the viral genome then loads, and cap, therefore, plays a great role in the viral gene transduction properties (Schaffer et al. 2008). To generate a recombinant AAV for gene delivery, rep and cap are excised from between the ITRs, replaced with the therapeutic transgenes and promoters in their place, and the two viral ORFs are supplied as helper genes in trans to package the transgenes inside the capsid (Kwon and Schaffer 2008). In the CNS, most cells are post-mitotic, and many chronic neurological diseases necessitate long-term transgene expression, which further contributes to the application prospects of AAVs. Transgenes that encode therapeutic genes have been successfully delivered to a variety of tissues and cell types within the CNS with AAVs, as reviewed elsewhere (Deverman et al. 2018b; Ojala et al. 2015). Engineered artificial anti-C9orf72-targeting miRNA AAV was intrathecally injected in an ALS mouse model to reduce the repeat-containing transcripts and showed a significant reduction in the toxicity caused by C9orf72 transcripts after treatment (Martier et al. 2019). Cappella et al. reviewed a series of studies in detail showed that intrathecal AAV-mediated miRNAs delivery could be used as a potential treatment for SOD1-linked ALS, although additional studies and further improvements are required to determine whether the approach is safe and above all efficient (Cappella et al. 2021).
Conclusions and Perspectives
The cellular and molecular changes underlying CNS injuries provide substantial potential biomarkers and therapeutic targets. miRNA research has become a rapidly growing field since their discovery more than two decades ago. Based on their one-to-many molecular regulatory properties, miRNAs contribute to diverse physiological and pathophysiological functions in a global pattern. In this review, we have discussed the significant roles of miR-124 in pathological processes involved in CNS disorders (Table 1). Although some disease-specific alterations of miR-124 in the CNS await further investigation, the biological significance and utility of miR-124 in CNS disease is becoming evident. The challenge lies in the productive exploitation of miRNA-based therapeutics, including designing a miR-124 delivery system that can cross the BBB. It will also be necessary to enable tissue/cell-specific targeting, while conferring higher stability and avoiding potential off-target effects, as well as determining therapeutic windows and modes of treatment (injected intravenously or positionally) according to individual features.
Another emerging area of research is the systematic pharmacokinetics of novel miRNA drugs. At present, little is known about the therapeutic combination of miRNAs, and delivery systems have pharmacokinetic characteristics, defined by the target cell, half-life, biological distribution, metabolic pathway, and organ effects. In general, miRNA-related therapies require interdisciplinary studies and are promising in providing more practical insights into current biomedical problems. With miRNAs as novel biological targets, we look forward to future progress of miR-124-based therapies in CNS diseases and injuries in the next decade.
Abbreviations
- AAV:
-
Adeno-associated viruses
- Aβ:
-
β-Amyloid
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- AMO:
-
Anti-miRNA oligonucleotide
- ANXA5:
-
AnnexinA5
- APP:
-
Amyloid precursor protein
- BBB:
-
Blood–brain barrier
- CDK:
-
Cyclin-dependent kinase
- CNS:
-
Central nervous system
- DA:
-
Dopaminergic
- DAPK1:
-
Death-associated protein kinase 1
- EAE:
-
Experimental autoimmune encephalomyelitis
- EV:
-
Extracellular vesicle
- GBM:
-
Glioblastoma
- ICH:
-
Intracerebral hemorrhage
- ITR:
-
Inverted terminal repeat elements
- LPS:
-
Lipopolysaccharide
- MCAO:
-
Middle cerebral artery occlusion
- miRNA:
-
microRNA
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS:
-
Multiple sclerosis
- NDDs:
-
Neurodegenerative diseases
- NSC:
-
Neuronal stem cells
- OGD:
-
Oxygen–glucose deprivation
- ORF:
-
Open reading frame
- PD:
-
Parkinson’s disease
- RISC:
-
RNA-induced silencing complex
- ROS:
-
Reactive oxygen species
- SCI:
-
Spinal cord injury
- STAT3:
-
Signal transducer and activator of transcription 3
- TACE:
-
TNF-α converting enzyme
- Th:
-
T helper
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
References
Adamczak J et al (2017) Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis. Neurobiol Dis 99:47–57. https://doi.org/10.1016/j.nbd.2016.12.015
Ahuja CS et al (2017) Traumatic spinal cord injury-repair and regeneration. Neurosurgery 80:S9-s22. https://doi.org/10.1093/neuros/nyw080
Akerblom M, Jakobsson J (2014) MicroRNAs as neuronal fate determinants. Neuroscientist 20:235–242. https://doi.org/10.1177/1073858413497265
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355. https://doi.org/10.1038/nature02871
Amoruso A et al (2020) Immune and central nervous system-related miRNAs expression profiling in monocytes of multiple sclerosis patients. Sci Rep 10:6125. https://doi.org/10.1038/s41598-020-63282-3
An F, Gong G, Wang Y, Bian M, Yu L, Wei C (2017a) MiR-124 acts as a target for Alzheimer’s disease by regulating BACE1. Oncotarget 8:114065–114071. https://doi.org/10.18632/oncotarget.23119
An SJ, Kim TJ, Yoon BW (2017b) Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 19:3–10. https://doi.org/10.5853/jos.2016.00864
Arvanitakis Z, Shah RC, Bennett DA (2019) Diagnosis and management of dementia: review. JAMA 322:1589–1599. https://doi.org/10.1001/jama.2019.4782
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. https://doi.org/10.1038/nm747
Baecher-Allan C, Kaskow BJ, Weiner HL (2018) Multiple sclerosis: mechanisms and immunotherapy. Neuron 97:742–768. https://doi.org/10.1016/j.neuron.2018.01.021
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Behzadi S et al (2017) Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev 46:4218–4244. https://doi.org/10.1039/c6cs00636a
Bennett MC (2005) The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther 105:311–331. https://doi.org/10.1016/j.pharmthera.2004.10.010
Beyer S, Fleming J, Meng W, Singh R, Haque SJ, Chakravarti A (2017) The role of miRNAs in angiogenesis, invasion and metabolism and their therapeutic implications in gliomas. Cancers. https://doi.org/10.3390/cancers9070085
Bhalala OG, Srikanth M, Kessler JA (2013) The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol 9:328–339. https://doi.org/10.1038/nrneurol.2013.67
Bielefeld P et al (2019) Co-administration of anti microRNA-124 and -137 oligonucleotides prevents hippocampal neural stem cell loss upon non-convulsive seizures. Front Mol Neurosci 12:31. https://doi.org/10.3389/fnmol.2019.00031
Bi Y, Liu G, Yang R (2009) MicroRNAs: novel regulators during the immune response. J Cell Physiol 218:467–472. https://doi.org/10.1002/jcp.21639
Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33:941–951. https://doi.org/10.1038/nbt.3330
Boca S et al (2020) Nanoscale delivery systems for microRNAs in cancer therapy. Cell Mol Life Sci 77:1059–1086. https://doi.org/10.1007/s00018-019-03317-9
Bo Y, Guo G, Yao W (2013) MiRNA-mediated tumor specific delivery of TRAIL reduced glioma growth. J Neurooncol 112:27–37. https://doi.org/10.1007/s11060-012-1033-y
Brandenburger T et al (2012) Expression of spinal cord microRNAs in a rat model of chronic neuropathic pain. Neurosci Lett 506:281–286. https://doi.org/10.1016/j.neulet.2011.11.023
Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443:796–802. https://doi.org/10.1038/nature05293
Brennan GP et al (2016) Dual and opposing roles of MicroRNA-124 in epilepsy are mediated through inflammatory and NRSF-dependent gene networks. Cell Rep 14:2402–2412. https://doi.org/10.1016/j.celrep.2016.02.042
Brites D, Vaz AR (2014) Microglia centered pathogenesis in ALS: insights in cell interconnectivity. Front Cell Neurosci 8:117. https://doi.org/10.3389/fncel.2014.00117
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41:242–247. https://doi.org/10.1007/s12035-010-8105-9
Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248. https://doi.org/10.1016/j.neuron.2013.12.034
Burgos K et al (2014) Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS ONE 9:e94839. https://doi.org/10.1371/journal.pone.0094839
Cappella M, Pradat PF, Querin G, Biferi MG (2021) Beyond the traditional clinical trials for amyotrophic lateral sclerosis and the future impact of gene therapy. J Neuromuscul Dis 8:25–38. https://doi.org/10.3233/JND-200531
Chappell WH et al (2011) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2:135–164. https://doi.org/10.18632/oncotarget.240
Chauderlier A et al (2018) Tau/DDX6 interaction increases microRNA activity. Biochim Biophys Acta 1861:762–772. https://doi.org/10.1016/j.bbagrm.2018.06.006
Che QQ, Huang T, Zhang YD, Qian XJ (2019) Effect of miR-124 on neuronal apoptosis in rats with cerebral infarction through Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci 23:6657–6664. https://doi.org/10.26355/eurrev_201908_18556
Cheng LC, Pastrana E, Tavazoie M, Doetsch F (2009) miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 12:399–408. https://doi.org/10.1038/nn.2294
Chen Q, Lu G, Cai Y, Li Y, Xu R, Ke Y, Zhang S (2014) MiR-124-5p inhibits the growth of high-grade gliomas through posttranscriptional regulation of LAMB1. Neuro-oncology 16:637–651. https://doi.org/10.1093/neuonc/not300
Chen Y, Sun JX, Chen WK, Wu GC, Wang YQ, Zhu KY, Wang J (2019) miR-124/VAMP3 is a novel therapeutic target for mitigation of surgical trauma-induced microglial activation. Signal Transduct Target Ther 4:27. https://doi.org/10.1038/s41392-019-0061-x
Chi B, Chau B, Yeo E, Ta P (2019) Virtual reality for spinal cord injury-associated neuropathic pain: systematic review. Ann Phys Rehabil Med 62:49–57. https://doi.org/10.1016/j.rehab.2018.09.006
Chivero ET, Liao K, Niu F, Tripathi A, Tian C, Buch S, Hu G (2020) Engineered extracellular vesicles loaded with miR-124 attenuate cocaine-mediated activation of microglia. Front Cell Dev Biol 8:573. https://doi.org/10.3389/fcell.2020.00573
Ciccarelli O et al (2014) Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol 13:807–822. https://doi.org/10.1016/s1474-4422(14)70101-2
Cicero CE et al (2017) Metals and neurodegenerative diseases. A systematic review. Environ Res 159:82–94. https://doi.org/10.1016/j.envres.2017.07.048
Collaborators GBDN (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Compston A, Coles A (2002) Multiple sclerosis. The Lancet (London, England) 359:1221–1231. https://doi.org/10.1016/s0140-6736(02)08220-x
Correale J, Gaitan MI, Ysrraelit MC, Fiol MP (2017) Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain 140:527–546. https://doi.org/10.1093/brain/aww258
Cunha C et al (2018) Downregulated glia interplay and increased miRNA-155 as promising markers to track ALS at an early stage. Mol Neurobiol 55:4207–4224. https://doi.org/10.1007/s12035-017-0631-2
Deng D et al (2019) p62 acts as an oncogene and is targeted by miR-124-3p in glioma. Cancer Cell Int 19:280. https://doi.org/10.1186/s12935-019-1004-x
Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY (2018a) Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov 17:641–659. https://doi.org/10.1038/nrd.2018.110
Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY (2018b) Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov 17:767. https://doi.org/10.1038/nrd.2018.158
Dobson R, Giovannoni G (2019) Multiple sclerosis—a review. Eur J Neurol 26:27–40. https://doi.org/10.1111/ene.13819
Doeppner TR et al (2013) MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol 126:251–265. https://doi.org/10.1007/s00401-013-1142-5
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology 14(Suppl 5):v1–v49. https://doi.org/10.1093/neuonc/nos218
Dong RF, Zhang B, Tai LW, Liu HM, Shi FK, Liu NN (2018) The neuroprotective role of MiR-124-3p in a 6-hydroxydopamine-induced cell model of Parkinson’s disease via the regulation of ANAX5. J Cell Biochem 119:269–277. https://doi.org/10.1002/jcb.26170
Dutta R et al (2013) Hippocampal demyelination and memory dysfunction are associated with increased levels of the neuronal microRNA miR-124 and reduced AMPA receptors. Ann Neurol 73:637–645. https://doi.org/10.1002/ana.23860
Du X, Huo X, Yang Y, Hu Z, Botchway BOA, Jiang Y, Fang M (2017) miR-124 downregulates BACE 1 and alters autophagy in APP/PS1 transgenic mice. Toxicol Lett 280:195–205. https://doi.org/10.1016/j.toxlet.2017.08.082
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269. https://doi.org/10.1038/nrc1840
Essandoh K, Li Y, Huo J, Fan GC (2016) MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock 46:122–131. https://doi.org/10.1097/shk.0000000000000604
Faissner S, Plemel JR, Gold R, Yong VW (2019) Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-019-0035-2
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322:254–262. https://doi.org/10.1016/j.jns.2012.05.030
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8:355–369. https://doi.org/10.1016/S1474-4422(09)70025-0
Feigin VL, Norrving B, Mensah GA (2017) Global burden of stroke. Circ Res 120:439–448. https://doi.org/10.1161/CIRCRESAHA.116.308413
Florczyk SJ et al (2013) Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 34:10143–10150. https://doi.org/10.1016/j.biomaterials.2013.09.034
Fowler A et al (2011) miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. Eur J Cancer (Oxford England: 1990) 47:953–963. https://doi.org/10.1016/j.ejca.2010.11.026
Gan L, Li Z, Lv Q, Huang W (2019) Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int J Pharm 567:118449. https://doi.org/10.1016/j.ijpharm.2019.118449
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012:428010. https://doi.org/10.1155/2012/428010
Gandy KAO, Zhang J, Nagarkatti P, Nagarkatti M (2019) Resveratrol (3,5,4′-trihydroxy-trans-stilbene) attenuates a mouse model of multiple sclerosis by altering the miR-124/sphingosine kinase 1 axis in encephalitogenic T cells in the brain. J Neuroimmune Pharmacol 14:462–477. https://doi.org/10.1007/s11481-019-09842-5
Gao G et al (2003) Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA 100:6081–6086. https://doi.org/10.1073/pnas.0937739100
Gao C, Shen J, Meng ZX, He XF (2019) Sevoflurane inhibits glioma cells proliferation and metastasis through miRNA-124-3p/ROCK1 axis. Pathol Oncol Res. https://doi.org/10.1007/s12253-019-00597-1
Gauthier BR, Wollheim CB (2006) MicroRNAs: “ribo-regulators” of glucose homeostasis. Nat Med 12:36–38. https://doi.org/10.1038/nm0106-36
Ge X et al (2020) Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol Ther 28:503–522. https://doi.org/10.1016/j.ymthe.2019.11.017
Gebauer K et al (2013) Hsa-mir-124-3 CpG island methylation is associated with advanced tumours and disease recurrence of patients with clear cell renal cell carcinoma. Br J Cancer 108:131–138. https://doi.org/10.1038/bjc.2012.537
Geng L, Liu W, Chen Y (2017) miR-124-3p attenuates MPP(+)-induced neuronal injury by targeting STAT3 in SH-SY5Y cells. Exp Biol Med (Maywood) 242:1757–1764. https://doi.org/10.1177/1535370217734492
Gittleman H, Boscia A, Ostrom QT, Truitt G, Fritz Y, Kruchko C, Barnholtz-Sloan JS (2018) Survivorship in adults with malignant brain and other central nervous system tumor from 2000–2014. Neuro-oncology 20:vii6–vii16. https://doi.org/10.1093/neuonc/noy090
Giuliani D et al (2017) Multiple beneficial effects of melanocortin MC4 receptor agonists in experimental neurodegenerative disorders: therapeutic perspectives. Prog Neurobiol 148:40–56. https://doi.org/10.1016/j.pneurobio.2016.11.004
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934. https://doi.org/10.1016/j.cell.2010.02.016
Gong G, Gu Y, Zhang Y, Liu W, Li L, Li J (2020) Retraction notice to “Tanshinone IIA alleviates oxidative damage after spinal cord injury in vitro and in vivo through up-regulating miR-124” [Life Sci. 216 (2019) 147–155]. Life Sci. https://doi.org/10.1016/j.lfs.2020.117497
Gu X et al (2016) MicroRNA124 regulated neurite elongation by targeting OSBP. Mol Neurobiol 53:6388–6396. https://doi.org/10.1007/s12035-015-9540-4
Gu X et al (2018) CBX2 inhibits neurite development by regulating neuron-specific genes expression. Front Mol Neurosci 11:46. https://doi.org/10.3389/fnmol.2018.00046
Guarnieri DJ, DiLeone RJ (2008) MicroRNAs: a new class of gene regulators. Ann Med 40:197–208. https://doi.org/10.1080/07853890701771823
Hamzei Taj S et al (2016a) Dynamic modulation of microglia/macrophage polarization by miR-124 after focal cerebral ischemia. J Neuroimmune Pharmacol 11:733–748. https://doi.org/10.1007/s11481-016-9700-y
Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M (2016b) MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 91:151–165. https://doi.org/10.1016/j.biomaterials.2016.03.025
Haney MJ et al (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
Haston KM, Finkbeiner S (2016) Clinical trials in a dish: the potential of pluripotent stem cells to develop therapies for neurodegenerative diseases. Annu Rev Pharmacol Toxicol 56:489–510. https://doi.org/10.1146/annurev-pharmtox-010715-103548
Hatfield S, Ruohola-Baker H (2008) microRNA and stem cell function. Cell Tissue Res 331:57–66. https://doi.org/10.1007/s00441-007-0530-3
He XW et al (2019) Increased plasma levels of miR-124-3p, miR-125b-5p and miR-192-5p are associated with outcomes in acute ischaemic stroke patients receiving thrombolysis. Atherosclerosis 289:36–43. https://doi.org/10.1016/j.atherosclerosis.2019.08.002
Ho VM et al (2014) GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons. Mol Cell Neurosci 61:1–12. https://doi.org/10.1016/j.mcn.2014.04.006
Hou TY et al (2020) Correcting abnormalities in miR-124/PTPN1 signaling rescues tau pathology in Alzheimer’s disease. J Neurochem 154:441–457. https://doi.org/10.1111/jnc.14961
Huang S et al (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32:512–528. https://doi.org/10.1096/fj.201700673R
Huang WY et al (2019) miR-124 upregulates astrocytic glutamate transporter-1 via the Akt and mTOR signaling pathway post ischemic stroke. Brain Res Bull 149:231–239. https://doi.org/10.1016/j.brainresbull.2019.04.013
Huse JT, Phillips HS, Brennan CW (2011) Molecular subclassification of diffuse gliomas: seeing order in the chaos. Glia 59:1190–1199. https://doi.org/10.1002/glia.21165
Jacobs AH, Tavitian B, consortium IN (2012) Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab 32:1393–1415. https://doi.org/10.1038/jcbfm.2012.53
Jain RK (2014) Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer cell 26:605–622. https://doi.org/10.1016/j.ccell.2014.10.006
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflamm 16:142. https://doi.org/10.1186/s12974-019-1516-2
Jayson GC, Kerbel R, Ellis LM, Harris AL (2016) Antiangiogenic therapy in oncology: current status and future directions. The Lancet (London, England) 388:518–529. https://doi.org/10.1016/s0140-6736(15)01088-0
Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39:959–966. https://doi.org/10.1161/STROKEAHA.107.500736
Jha MK, Jo M, Kim JH, Suk K (2019) Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist 25:227–240. https://doi.org/10.1177/1073858418783959
Ji Q et al (2016) Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS ONE 11:e0163645. https://doi.org/10.1371/journal.pone.0163645
Jiang S et al (2014) MeCP2 reinforces STAT3 signaling and the generation of effector CD4+ T cells by promoting miR-124-mediated suppression of SOCS5. Sci Signal 7:ra25. https://doi.org/10.1126/scisignal.2004824
Jiang D et al (2020) Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnol 18:105. https://doi.org/10.1186/s12951-020-00665-8
Jiang L, Lin T, Xu C, Hu S, Pan Y, Jin R (2016) miR-124 interacts with the Notch1 signalling pathway and has therapeutic potential against gastric cancer. J Cell Mol Med 20:313–322. https://doi.org/10.1111/jcmm.12724
Jiao S, Liu Y, Yao Y, Teng J (2018) miR-124 promotes proliferation and neural differentiation of neural stem cells through targeting DACT1 and activating Wnt/β-catenin pathways. Mol Cell Biochem 449:305–314. https://doi.org/10.1007/s11010-018-3367-z
Jimenez-Mateos EM et al (2012) Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 18:1087–1094. https://doi.org/10.1038/nm.2834
Jin K et al (2006) Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA 103:13198–13202. https://doi.org/10.1073/pnas.0603512103
Johnson DE, O’Keefe RA, Grandis JR (2018) Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 15:234–248. https://doi.org/10.1038/nrclinonc.2018.8
Jucker M (2010) The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med 16:1210–1214. https://doi.org/10.1038/nm.2224
Juzwik CA et al (2019) microRNA dysregulation in neurodegenerative diseases: a systematic review. Prog Neurobiol 182:101664. https://doi.org/10.1016/j.pneurobio.2019.101664
Kanagaraj N, Beiping H, Dheen ST, Tay SS (2014) Downregulation of miR-124 in MPTP-treated mouse model of Parkinson’s disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience 272:167–179. https://doi.org/10.1016/j.neuroscience.2014.04.039
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11:720–731. https://doi.org/10.1016/s1474-4422(12)70104-7
Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24:325–340. https://doi.org/10.5607/en.2015.24.4.325
Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M (2020) Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release 317:273–281. https://doi.org/10.1016/j.jconrel.2019.11.009
Kong Y, Wu J, Zhang D, Wan C, Yuan L (2015) The role of miR-124 in Drosophila Alzheimer’s disease model by targeting delta in notch signaling pathway. Curr Mol Med 15:980–989. https://doi.org/10.2174/1566524016666151123114608
Krichevsky AM, Sonntag KC, Isacson O, Kosik KS (2006) Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24:857–864. https://doi.org/10.1634/stemcells.2005-0441
Kutsche LK et al (2018) Combined experimental and system-level analyses reveal the complex regulatory network of miR-124 during human neurogenesis. Cell Syst 7:438-452.e438. https://doi.org/10.1016/j.cels.2018.08.011
Kwon I, Schaffer DV (2008) Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res 25:489–499. https://doi.org/10.1007/s11095-007-9431-0
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294:853–858. https://doi.org/10.1126/science.1064921
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12:735–739. https://doi.org/10.1016/s0960-9822(02)00809-6
Lakhal S, Wood MJ (2011) Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays 33:737–741. https://doi.org/10.1002/bies.201100076
Lappin JM, Darke S, Farrell M (2018) Methamphetamine use and future risk for Parkinson’s disease: evidence and clinical implications. Drug Alcohol Depend 187:134–140. https://doi.org/10.1016/j.drugalcdep.2018.02.032
Laterza OF et al (2009) Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 55:1977–1983. https://doi.org/10.1373/clinchem.2009.131797
Lee SWL et al (2019) MicroRNA delivery through nanoparticles. J Control Release 313:80–95. https://doi.org/10.1016/j.jconrel.2019.10.007
Lee BB, Cripps RA, Fitzharris M, Wing PC (2014) The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 52:110–116. https://doi.org/10.1038/sc.2012.158
Leung LY et al (2014) Comparison of miR-124-3p and miR-16 for early diagnosis of hemorrhagic and ischemic stroke. Clin Chim Acta 433:139–144. https://doi.org/10.1016/j.cca.2014.03.007
Li N, Pan X, Zhang J, Ma A, Yang S, Ma J, Xie A (2017) Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol Sci 38:761–767. https://doi.org/10.1007/s10072-017-2841-9
Li AD, Tong L, Xu N, Ye Y, Nie PY, Wang ZY, Ji LL (2019a) miR-124 regulates cerebromicrovascular function in APP/PS1 transgenic mice via C1ql3. Brain Res Bull 153:214–222. https://doi.org/10.1016/j.brainresbull.2019.09.002
Li C et al (2019b) Long noncoding RNA LINC00511 induced by SP1 accelerates the glioma progression through targeting miR-124-3p/CCND2 axis. J Cell Mol Med 23:4386–4394. https://doi.org/10.1111/jcmm.14331
Li D et al (2019c) Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem Res 44:1903–1923. https://doi.org/10.1007/s11064-019-02825-1
Li R, Zhao K, Ruan Q, Meng C, Yin F (2020) Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther 22:75. https://doi.org/10.1186/s13075-020-2146-x
Lian H et al (2015) NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85:101–115. https://doi.org/10.1016/j.neuron.2014.11.018
Liang Z et al (2017) Impact of aging immune system on neurodegeneration and potential immunotherapies. Prog Neurobiol 157:2–28. https://doi.org/10.1016/j.pneurobio.2017.07.006
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Lin J, Xu K, Wei J, Heimberger AB, Roth JA, Ji L (2016) MicroRNA-124 suppresses tumor cell proliferation and invasion by targeting CD164 signaling pathway in non-small cell lung cancer. J Gene Ther. https://doi.org/10.13188/2381-3326.1000006
Liu XS et al (2011) MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS ONE 6:e23461. https://doi.org/10.1371/journal.pone.0023461
Liu CC, Cheng JT, Li TY, Tan PH (2017) Integrated analysis of microRNA and mRNA expression profiles in the rat spinal cord under inflammatory pain conditions. Eur J Neurosci 46:2713–2728. https://doi.org/10.1111/ejn.13745
Louis DN et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Lu Y, Gong Z, Jin X, Zhao P, Zhang Y, Wang Z (2020) LncRNA MALAT1 targeting miR-124-3p regulates DAPK1 expression contributes to cell apoptosis in Parkinson’s disease. J Cell Biochem. https://doi.org/10.1002/jcb.29711
Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27:435–448. https://doi.org/10.1016/j.molcel.2007.07.015
Manakov SA, Morton A, Enright AJ, Grant SG (2012) A neuronal transcriptome response involving stress pathways is buffered by neuronal microRNAs. Front Neurosci 6:156. https://doi.org/10.3389/fnins.2012.00156
Manna I et al (2016) An SNP site in pri-miR-124, a brain expressed miRNA gene, no contribution to mesial temporal lobe epilepsy in an Italian sample. Neurol Sci 37:1335–1339. https://doi.org/10.1007/s10072-016-2597-7
Marcuzzo S et al (2014) Altered miRNA expression is associated with neuronal fate in G93A-SOD1 ependymal stem progenitor cells. Exp Neurol 253:91–101. https://doi.org/10.1016/j.expneurol.2013.12.007
Marcuzzo S et al (2015) Up-regulation of neural and cell cycle-related microRNAs in brain of amyotrophic lateral sclerosis mice at late disease stage. Mol Brain 8:5. https://doi.org/10.1186/s13041-015-0095-0
Marisetty AL, Singh SK, Nguyen TN, Coarfa C, Liu B, Majumder S (2017) REST represses miR-124 and miR-203 to regulate distinct oncogenic properties of glioblastoma stem cells. Neuro Oncol 19:514–523. https://doi.org/10.1093/neuonc/now232
Martier R et al (2019) Targeting RNA-mediated toxicity in C9orf72 ALS and/or FTD by RNAi-based gene therapy. Mol Ther Nucleic Acids 16:26–37. https://doi.org/10.1016/j.omtn.2019.02.001
Mason AR, Ziemann A, Finkbeiner S (2014) Targeting the low-hanging fruit of neurodegeneration. Neurology 83:1470–1473
Matsubara H et al (2007) Apoptosis induction by antisense oligonucleotides against miR-17-5p and miR-20a in lung cancers overexpressing miR-17-92. Oncogene 26:6099–6105. https://doi.org/10.1038/sj.onc.1210425
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435. https://doi.org/10.1038/nature07201
Miao W et al (2020) Ischemic postconditioning exerts neuroprotective effect through negatively regulating PI3K/Akt2 signaling pathway by microRNA-124. Biomed Pharmacother 126:109786. https://doi.org/10.1016/j.biopha.2019.109786
Mikita J et al (2011) Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler (Houndmills, Basingstoke, England) 17:2–15. https://doi.org/10.1177/1352458510379243
Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T (2007) RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res 1131:37–43. https://doi.org/10.1016/j.brainres.2006.11.035
Mitsumoto H, Brooks BR, Silani V (2014) Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol 13:1127–1138. https://doi.org/10.1016/s1474-4422(14)70129-2
Mokabber H, Najafzadeh N, Mohammadzadeh Vardin M (2019) miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J Cell Physiol 234:8941–8950. https://doi.org/10.1002/jcp.27563
Mori T, Tan J, Arendash GW, Koyama N, Nojima Y, Town T (2008) Overexpression of human S100B exacerbates brain damage and periinfarct gliosis after permanent focal ischemia. Stroke 39:2114–2121. https://doi.org/10.1161/STROKEAHA.107.503821
Morris JK et al (2015) Decrease in levels of the evolutionarily conserved microRNA miR-124 affects oligodendrocyte numbers in Zebrafish, Danio rerio. Invert Neurosci 15:4. https://doi.org/10.1007/s10158-015-0180-1
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. https://doi.org/10.1038/nri2448
Mucaj V et al (2015) MicroRNA-124 expression counteracts pro-survival stress responses in glioblastoma. Oncogene 34:2204–2214. https://doi.org/10.1038/onc.2014.168
Nainu F, Salim E, Asri RM, Hori A, Kuraishi T (2019) Neurodegenerative disorders and sterile inflammation: lessons from a Drosophila model. J Biochem 166:213–221. https://doi.org/10.1093/jb/mvz053
Niedermeyer S, Murn M, Choi PJ (2019) Respiratory failure in amyotrophic lateral sclerosis. Chest 155:401–408. https://doi.org/10.1016/j.chest.2018.06.035
Nieto-Diaz M et al (2014) MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Front Cell Neurosci 8:53. https://doi.org/10.3389/fncel.2014.00053
Ojala DS, Amara DP, Schaffer DV (2015) Adeno-associated virus vectors and neurological gene therapy. Neuroscientist 21:84–98. https://doi.org/10.1177/1073858414521870
Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y (2018) Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res 126:39–43. https://doi.org/10.1016/j.neures.2017.10.004
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665. https://doi.org/10.1111/bph.13139
Ostrom QT et al (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17(Suppl 4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos M, Fuentes B, Diekhorst L, Diez-Tejedor E, Gutierrez-Fernandez M (2019) Role of exosomes as a treatment and potential biomarker for stroke. Transl Stroke Res 10:241–249. https://doi.org/10.1007/s12975-018-0654-7
Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F (2013) Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci: MN 50:291–297. https://doi.org/10.1007/s12031-013-9953-3
Philip C, Wong HC, Borchelt DR (2002) Genetically engineered mousemodels of neurodegenerativediseases. Nat Neurosci 5:633–639
Pinto S, Cunha C, Barbosa M, Vaz AR, Brites D (2017) Exosomes from NSC-34 cells transfected with hSOD1-G93A are enriched in miR-124 and drive alterations in microglia phenotype. Front Neurosci 11:273. https://doi.org/10.3389/fnins.2017.00273
Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med 17:64–70. https://doi.org/10.1038/nm.2266
Ponomarev ED, Veremeyko T, Weiner HL (2013) MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61:91–103. https://doi.org/10.1002/glia.22363
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Cell 179:292–311. https://doi.org/10.1016/j.cell.2019.08.053
Puyal J, Ginet V, Clarke PG (2013) Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog Neurobiol 105:24–48. https://doi.org/10.1016/j.pneurobio.2013.03.002
Qian D et al (2019) Blocking Notch signal pathway suppresses the activation of neurotoxic A1 astrocytes after spinal cord injury. Cell Cycle (Georgetown, Tex) 18:3010–3029. https://doi.org/10.1080/15384101.2019.1667189
Qureshi AI, Mendelow AD, Hanley DF (2009) Intracerebral haemorrhage. The Lancet 373:1632–1644. https://doi.org/10.1016/S0140-6736(09)60371-8
Rainer TH, Leung LY, Chan CPY, Leung YK, Abrigo JM, Wang D, Graham CA (2016) Plasma miR-124-3p and miR-16 concentrations as prognostic markers in acute stroke. Clin Biochem 49:663–668. https://doi.org/10.1016/j.clinbiochem.2016.02.016
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. https://doi.org/10.1083/jcb.201211138
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378:169–180. https://doi.org/10.1056/NEJMra1401483
Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A (2004) Identification of mammalian microRNA host genes and transcription units. Genome Res 14:1902–1910. https://doi.org/10.1101/gr.2722704
Rosas-Hernandez H, Chigurupati S, Raymick J, Robinson B, Cuevas E, Hanig J, Sarkar S (2018) Identification of altered microRNAs in serum of a mouse model of Parkinson’s disease. Neurosci Lett 687:1–9. https://doi.org/10.1016/j.neulet.2018.07.022
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10-17. https://doi.org/10.1038/nm1066
Sabelström H et al (2019) Driving neuronal differentiation through reversal of an ERK1/2-miR-124-SOX9 axis abrogates glioblastoma aggressiveness. Cell Rep 28:2064-2079.e2011. https://doi.org/10.1016/j.celrep.2019.07.071
Sacco S, Marini C, Toni D, Olivieri L, Carolei A (2009) Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 40:394–399. https://doi.org/10.1161/STROKEAHA.108.523209
Sano M, Ohtaka M, Iijima M, Nakasu A, Kato Y, Nakanishi M (2017) Sensitive and long-term monitoring of intracellular microRNAs using a non-integrating cytoplasmic RNA vector. Sci Rep 7:12673. https://doi.org/10.1038/s41598-017-12847-w
Sanuki R et al (2011) miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat Neurosci 14:1125–1134. https://doi.org/10.1038/nn.2897
Saraiva C, Paiva J, Santos T, Ferreira L, Bernardino L (2016) MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease. J Control Release 235:291–305. https://doi.org/10.1016/j.jconrel.2016.06.005
Saraiva C, Esteves M, Bernardino L (2017) MicroRNA: basic concepts and implications for regeneration and repair of neurodegenerative diseases. Biochem Pharmacol 141:118–131. https://doi.org/10.1016/j.bcp.2017.07.008
Saraiva C, Talhada D, Rai A, Ferreira R, Ferreira L, Bernardino L, Ruscher K (2018) MicroRNA-124-loaded nanoparticles increase survival and neuronal differentiation of neural stem cells in vitro but do not contribute to stroke outcome in vivo. PLoS ONE 13:e0193609. https://doi.org/10.1371/journal.pone.0193609
Sasidharan V et al (2017) The miR-124 family of microRNAs is crucial for regeneration of the brain and visual system in the planarian Schmidtea mediterranea. Development 144:3211–3223. https://doi.org/10.1242/dev.144758
Schaffer DV, Koerber JT, Lim KI (2008) Molecular engineering of viral gene delivery vehicles. Annu Rev Biomed Eng 10:169–194. https://doi.org/10.1146/annurev.bioeng.10.061807.160514
Sessa F et al (2019) Human brain injury and miRNAs: an experimental study. Int J Mol Sci. https://doi.org/10.3390/ijms20071546
Seviour EG et al (2016) Functional proteomics identifies miRNAs to target a p27/Myc/phospho-Rb signature in breast and ovarian cancer. Oncogene 35:801. https://doi.org/10.1038/onc.2015.177
Sharif S, Ghahremani MH, Soleimani M (2018) Delivery of exogenous miR-124 to glioblastoma multiform cells by Wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev Rep 14:236–246. https://doi.org/10.1007/s12015-017-9788-3
Shi Z et al (2014) MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro-oncology 16:1341–1353. https://doi.org/10.1093/neuonc/nou084
Shimada IS, Peterson BM, Spees JL (2010) Isolation of locally derived stem/progenitor cells from the peri-infarct area that do not migrate from the lateral ventricle after cortical stroke. Stroke 41:e552-560. https://doi.org/10.1161/STROKEAHA.110.589010
Silber J et al (2008) miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6:14. https://doi.org/10.1186/1741-7015-6-14
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG (2014) Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 6:309–331. https://doi.org/10.2147/clep.s68889
Skalsky RL, Cullen BR (2011) Reduced expression of brain-enriched microRNAs in glioblastomas permits targeted regulation of a cell death gene. PLoS ONE 6:e24248. https://doi.org/10.1371/journal.pone.0024248
Smith P, Al Hashimi A, Girard J, Delay C, Hebert SS (2011) In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J Neurochem 116:240–247. https://doi.org/10.1111/j.1471-4159.2010.07097.x
Socias SB et al (2018) Exploiting the therapeutic potential of ready-to-use drugs: repurposing antibiotics against amyloid aggregation in neurodegenerative diseases. Prog Neurobiol 162:17–36. https://doi.org/10.1016/j.pneurobio.2017.12.002
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. https://doi.org/10.1016/j.tins.2009.08.002
Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16:249–263. https://doi.org/10.1038/nrn3898
Song JL, Zheng W, Chen W, Qian Y, Ouyang YM, Fan CY (2017) Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Exp Mol Med 49:e332. https://doi.org/10.1038/emm.2017.48
Song M et al (2019a) MiR-124 improves spinal cord injury in rats by activating the Wnt/β-catenin signaling pathway. Panminerva Med. https://doi.org/10.23736/s0031-0808.19.03656-5
Song Y et al (2019b) M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9:2910–2923. https://doi.org/10.7150/thno.30879
Song YK et al (2019c) Productive transcription of miR-124-3p by RelA and RNA polymerase II directs RIP1 ubiquitination-dependent apoptosis resistance during hypoxia. Exp Cell Res 378:21–31. https://doi.org/10.1016/j.yexcr.2019.03.004
Sorensen SS, Nygaard AB, Carlsen AL, Heegaard NHH, Bak M, Christensen T (2017) Elevation of brain-enriched miRNAs in cerebrospinal fluid of patients with acute ischemic stroke. Biomark Res 5:24. https://doi.org/10.1186/s40364-017-0104-9
Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4:49–60. https://doi.org/10.1038/nrn1007
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21:1332–1340. https://doi.org/10.1038/s41593-018-0235-9
Spinal Cord Injury (SCI) (2016) Facts and figures at a glance. J Spinal Cord Med 39:493–494
Srivastava A, Lusby EW, Berns KI (1983) Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol 45:555–564. https://doi.org/10.1128/JVI.45.2.555-564.1983
Steiner T, Rosand J, Diringer M (2006) Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke 37:256–262. https://doi.org/10.1161/01.STR.0000196989.09900.f8
Su W, Aloi MS, Garden GA (2016) MicroRNAs mediating CNS inflammation: Small regulators with powerful potential. Brain Behav Immun 52:1–8. https://doi.org/10.1016/j.bbi.2015.07.003
Su LN, Song XQ, Xue ZX, Zheng CQ, Yin HF, Wei HP (2018) Network analysis of microRNAs, transcription factors, and target genes involved in axon regeneration. J Zhejiang Univ Sci B 19:293–304. https://doi.org/10.1631/jzus.B1700179
Su Y et al (2019) MicroRNA-26a/death-associated protein kinase 1 signaling induces synucleinopathy and dopaminergic neuron degeneration in Parkinson’s disease. Biol Psychiatry 85:769–781. https://doi.org/10.1016/j.biopsych.2018.12.008
Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF, Liu X (2013) MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res 23:1270–1283. https://doi.org/10.1038/cr.2013.116
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150. https://doi.org/10.1038/nrneurol.2017.188
Takase Y et al (2019) Insulinoma-associated protein 1 expression in pancreatic neuroendocrine tumours in endoscopic ultrasound-guided fine-needle aspiration cytology: an analysis of 14 patients. Cytopathology 30:194–200. https://doi.org/10.1111/cyt.12640
Tan CL et al (2013) MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science (New York, NY) 342:1254–1258. https://doi.org/10.1126/science.1244193
Tang SL, Huang QH, Wu LG, Liu C, Cai AL (2018) MiR-124 regulates osteoblast differentiation through GSK-3beta in ankylosing spondylitis. Eur Rev Med Pharmacol Sci 22:6616–6624. https://doi.org/10.26355/eurrev_201810_16136
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet (London, England) 393:689–701. https://doi.org/10.1016/s0140-6736(18)32596-0
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. The Lancet 391:1622–1636. https://doi.org/10.1016/s0140-6736(18)30481-1
Tian F et al (2014) Core binding factor beta (Cbfbeta) controls the balance of chondrocyte proliferation and differentiation by upregulating Indian hedgehog (Ihh) expression and inhibiting parathyroid hormone-related protein receptor (PPR) expression in postnatal cartilage and bone formation. J Bone Miner Res 29:1564–1574. https://doi.org/10.1002/jbmr.2275
Tintore M, Vidal-Jordana A, Sastre-Garriga J (2019) Treatment of multiple sclerosis—success from bench to bedside. Nat Rev Neurol 15:53–58. https://doi.org/10.1038/s41582-018-0082-z
Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O (2014) Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 34:1573–1584. https://doi.org/10.1038/jcbfm.2014.130
Twelves D, Perkins KS, Counsell C (2003) Systematic review of incidence studies of Parkinson’s disease. Mov Disord 18:19–31. https://doi.org/10.1002/mds.10305
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. https://doi.org/10.1038/ncb1596
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, van den Berg LH (2017) Amyotrophic lateral sclerosis. The Lancet 390:2084–2098. https://doi.org/10.1016/s0140-6736(17)31287-4
Varley J, Brooks DJ, Edison P (2015) Imaging neuroinflammation in Alzheimer’s disease and other dementias: recent advances and future directions. Alzheimers Dement 11:1110–1120. https://doi.org/10.1016/j.jalz.2014.08.105
Villela D et al (2016) Differential DNA methylation of MicroRNA genes in temporal cortex from Alzheimer’s disease individuals. Neural Plast 2016:2584940. https://doi.org/10.1155/2016/2584940
Walker MC (2018) Pathophysiology of status epilepticus. Neurosci Lett 667:84–91. https://doi.org/10.1016/j.neulet.2016.12.044
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M (2007) Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106:436–443. https://doi.org/10.1097/00000542-200703000-00007
Wang H et al (2016a) MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson’s disease by targeting to bim. Brain Pathol (Zurich, Switzerland) 26:167–176. https://doi.org/10.1111/bpa.12267
Wang W et al (2016b) The microRNA miR-124 suppresses seizure activity and regulates CREB1 activity. Expert Rev Mol Med 18:e4. https://doi.org/10.1017/erm.2016.3
Wang D et al (2017) Activation of PPARgamma inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem Biophys Res Commun 486:726–731. https://doi.org/10.1016/j.bbrc.2017.03.106
Wang R et al (2018a) EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer 17:166. https://doi.org/10.1186/s12943-018-0911-0
Wang SW, Deng LX, Chen HY, Su ZQ, Ye SL, Xu WY (2018b) MiR-124 affects the apoptosis of brain vascular endothelial cells and ROS production through regulating PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci 22:498–505. https://doi.org/10.26355/eurrev_201801_14201
Wang X et al (2018c) A novel MicroRNA-124/PTPN1 signal pathway mediates synaptic and memory deficits in Alzheimer’s disease. Biol Psychiatry 83:395–405. https://doi.org/10.1016/j.biopsych.2017.07.023
Wang Z et al (2018d) Plasma miR-124 is a promising candidate biomarker for human intracerebral hemorrhage stroke. Mol Neurobiol 55:5879–5888. https://doi.org/10.1007/s12035-017-0808-8
Wang J, Wang W, Zhai H (2019) MicroRNA-124 enhances dopamine receptor expression and neuronal proliferation in mouse models of Parkinson’s disease via the Hedgehog signaling pathway by targeting EDN2. Neuroimmunomodulation 26:174–187. https://doi.org/10.1159/000501339
Wei J et al (2013) miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res 73:3913–3926. https://doi.org/10.1158/0008-5472.CAN-12-4318
Wei C, Ren L, Li K, Lu Z (2018) The regulation of survival and differentiation of neural stem cells by miR-124 via modulating PAX3. Neurosci Lett 683:19–26. https://doi.org/10.1016/j.neulet.2018.05.051
Weng H et al (2011) Plasma miR-124 as a biomarker for cerebral infarction. Biomed Res 32:135–141
Weng Q et al (2019) Phenotypic screening-based identification of 3,4-disubstituted piperidine derivatives as macrophage M2 polarization modulators: an opportunity for treating multiple sclerosis. J Med Chem 62:3268–3285. https://doi.org/10.1021/acs.jmedchem.8b01635
Willemen HL, Huo XJ, Mao-Ying QL, Zijlstra J, Heijnen CJ, Kavelaars A (2012) MicroRNA-124 as a novel treatment for persistent hyperalgesia. J Neuroinflamm 9:143. https://doi.org/10.1186/1742-2094-9-143
Witiw CD, Fehlings MG (2015) Acute spinal cord injury. J Spinal Disord Tech 28:202–210. https://doi.org/10.1097/bsd.0000000000000287
Wu Y, Yao J, Feng K (2020) miR-124-5p/NOX2 axis modulates the ROS production and the inflammatory microenvironment to protect against the cerebral I/R injury. Neurochem Res 45:404–417. https://doi.org/10.1007/s11064-019-02931-0
Xia H et al (2012) Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem 287:9962–9971. https://doi.org/10.1074/jbc.M111.332627
Xin H et al (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30:1556–1564. https://doi.org/10.1002/stem.1129
Xin H, Li Y, Chopp M (2014) Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci 8:377. https://doi.org/10.3389/fncel.2014.00377
Xu W, Li P, Qin K, Wang X, Jiang X (2012) miR-124 regulates neural stem cells in the treatment of spinal cord injury. Neurosci Lett 529:12–17. https://doi.org/10.1016/j.neulet.2012.09.025
Xue L, Liu H, Chen Y, Wei L, Hong J (2020) Computational analysis and verification of molecular genetic targets for glioblastoma. Biosci Rep. https://doi.org/10.1042/BSR20201401
Yaghi NK et al (2017) Immune modulatory nanoparticle therapeutics for intracerebral glioma. Neuro Oncol 19:372–382. https://doi.org/10.1093/neuonc/now198
Yang S, Liu X, Li X, Sun S, Sun F, Fan B, Zhao S (2013) MicroRNA-124 reduces caveolar density by targeting caveolin-1 in porcine kidney epithelial PK15 cells. Mol Cell Biochem 384:213–219. https://doi.org/10.1007/s11010-013-1800-x
Yang J, Zhang X, Chen X, Wang L, Yang G (2017) Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7:278–287. https://doi.org/10.1016/j.omtn.2017.04.010
Yang Y, Ye Y, Kong C, Su X, Zhang X, Bai W, He X (2019) MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res 44:811–828. https://doi.org/10.1007/s11064-018-02714-z
Yao L, Ye Y, Mao H, Lu F, He X, Lu G, Zhang S (2018) MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J Neuroinflamm 15:13. https://doi.org/10.1186/s12974-018-1053-4
Yao L et al (2019) MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J 33:8648–8665. https://doi.org/10.1096/fj.201900363R
Yelick J, Men Y, Jin S, Seo S, Espejo-Porras F, Yang Y (2020) Elevated exosomal secretion of miR-124-3p from spinal neurons positively associates with disease severity in ALS. Exp Neurol 333:113414. https://doi.org/10.1016/j.expneurol.2020.113414
Yong HYF, Rawji KS, Ghorbani S, Xue M, Yong VW (2019) The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol 16:540–546. https://doi.org/10.1038/s41423-019-0223-3
Yu A et al (2017) MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-alpha pathway in intracerebral hemorrhage. Immunol Lett 182:1–11. https://doi.org/10.1016/j.imlet.2016.12.003
Yun SP et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24:931–938. https://doi.org/10.1038/s41591-018-0051-5
Zaiman AL et al (2011) A critical role for the protein apoptosis repressor with caspase recruitment domain in hypoxia-induced pulmonary hypertension. Circulation 124:2533–2542. https://doi.org/10.1161/CIRCULATIONAHA.111.034512
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. https://doi.org/10.1523/JNEUROSCI.6221-11.2012
Zhang X, Huang X, Fang C, Li Q, Cui J, Sun J, Li L (2017a) miR-124 regulates the expression of BACE1 in the hippocampus under chronic cerebral hypoperfusion. Mol Neurobiol 54:2498–2506. https://doi.org/10.1007/s12035-016-9845-y
Zhang Z, Gong Q, Li M, Xu J, Zheng Y, Ge P, Chi G (2017b) MicroRNA-124 inhibits the proliferation of C6 glioma cells by targeting Smad4. Int J Mol Med 40:1226–1234. https://doi.org/10.3892/ijmm.2017.3088
Zhao Y, Jiang H, Liu XW, Xiang LB, Zhou DP, Chen JT (2015a) MiR-124 promotes bone marrow mesenchymal stem cells differentiation into neurogenic cells for accelerating recovery in the spinal cord injury. Tissue Cell 47:140–146. https://doi.org/10.1016/j.tice.2015.01.007
Zhao Y et al (2015b) Loss of microRNA-124 expression in neurons in the peri-lesion area in mice with spinal cord injury. Neural Regen Res 10:1147–1152. https://doi.org/10.4103/1673-5374.156983
Zhao MY, Wang GQ, Wang NN, Yu QY, Liu RL, Shi WQ (2019) The long-non-coding RNA NEAT1 is a novel target for Alzheimer’s disease progression via miR-124/BACE1 axis. Neurol Res 41:489–497. https://doi.org/10.1080/01616412.2018.1548747
Zheng H et al (2016) MiR-219 protects against seizure in the kainic acid model of epilepsy. Mol Neurobiol 53:1–7. https://doi.org/10.1007/s12035-014-8981-5
Zhou Y, Deng J, Chu X, Zhao Y, Guo Y (2019) Role of post-transcriptional control of calpain by miR-124-3p in the development of Alzheimer’s disease. J Alzheimers Dis 67:571–581. https://doi.org/10.3233/JAD-181053
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. https://doi.org/10.1016/j.neuron.2008.01.003
Zou D, Chen Y, Han Y, Lv C, Tu G (2014) Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells. Neural Regen Res 9:1241–1248. https://doi.org/10.4103/1673-5374.135333
Acknowledgements
The authors would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China [#81571199]; the National Natural Science Foundation of China [#81870974]; and the Fundamental Research Funds for the Central Universities, JLU.
Author information
Authors and Affiliations
Contributions
JX and YZ conceived this review article and participated in preparing all drafts of the manuscript. LW, YL, and XW participated in part of the original draft preparation, and final editing and approval of the final draft was performed by YL and GC. All the authors read the article and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, J., Zheng, Y., Wang, L. et al. miR-124: A Promising Therapeutic Target for Central Nervous System Injuries and Diseases. Cell Mol Neurobiol 42, 2031–2053 (2022). https://doi.org/10.1007/s10571-021-01091-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01091-6