Abstract
In our recent study, we observed consistent increases in miR-124-3p levels in exosomes derived from cultured BV2 microglia which was treated with repetitive traumatic brain injury (rTBI) mouse model brain extracts. To clarify the mechanisms underlying increases in microglia-derived exosomal miR-124-3p and their role in regulating neuronal autophagy after TBI, we investigated the impact of exosomal miR-124-3p on neuronal autophagy in scratch-injured HT22 neurons and rTBI mice. We harvested injured brain extracts from rTBI mice at 3 to 21 days post injury (DPI) for the treatment of cultured BV2 microglia in vitro. We observed significant induction of autophagy following TBI in vitro, and that inhibition of activated neuronal autophagy could protect against trauma-induced injury. Our results indicated that co-culture of injured HT22 neurons with miR-124-3p overexpressing BV2 microglia exerted a protective effect by inhibiting neuronal autophagy in scratch-injured neurons. Further research revealed that these effects were achieved mainly via upregulation of exosomal miR-124-3p, and that Focal adhesion kinase family-interacting protein of 200 kDa (FIP200) plays a key role in trauma-induced autophagy. Injection of exosomes into the vena caudalis in in vivo experiments revealed that exosomal miR-124-3p was associated with decreases in the modified neurological severity score (mNSS) and improvements in Morris water maze (MWM) test results in rTBI mice. Altogether, our results indicate that increased miR-124-3p in microglial exosomes following TBI may inhibit neuronal autophagy and protect against nerve injury via their transfer into neurons. Thus, treatment with microglial exosomes enriched with miR-124-3p may represent a novel therapeutic strategy for the treatment of nerve injury after TBI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, making it a significant medical, public health, and societal concern [1, 2]. More than 50 million people are newly diagnosed with TBI internationally each year, with an estimated global economic cost of $US400 billion. Nearly half of the world's population will have experienced one or more TBIs in their lifetime [3]. Road traffic incidents, falls, and violence are the most common causes of TBI [4, 5]. Considering the rapid modernization of society, increases in the number of older adults in the population, and continued warfare, the incidence of TBI increases rapidly each year. Indeed, TBI has been ranked among the top three specific neurological conditions accounting for neurodisability globally, both at present and in projections up to 2030 [6].
TBI initiates a complex series of pathological reactions, which can be mainly divided into two stages. The primary insult is mainly caused by external impact, leading to acute pathological changes including brain contusions, intracerebral hemorrhage, and axonal shearing. Secondary pathological changes—which include oxidative stress, Ca2+ overload, mitochondrial injury, glutamate excitotoxicity, and neuroinflammation—lead to further deterioration of nerve function [7, 8]. Despite the remarkable progress in pathology, there are still no evidence-based therapeutic methods for controlling nerve damage following TBI [9], and the molecular events occurring following TBI remain to be fully elucidated.
Autophagy is an evolutionarily conserved homeostatic process in which parts of the cytoplasm are fused within multi-membraned vesicles termed autophagosomes, following which they are delivered to lysosomes for degradation [10, 11]. Strikingly, increasing evidence has indicated that neuronal autophagy is involved in the pathophysiology of both experimental and clinical TBI [12, 13]. Recent studies and our preliminary work have supported the notion that the autophagy pathway is persistently activated after TBI, which may lead to neurological dysfunction including motor, sensory, learning, and memory impairments [14,15,16,17,18]. Such findings indicate that suppression of neuronal autophagy may represent a novel therapeutic intervention for TBI.
MicroRNAs (miRNAs) are a class of small, non-coding endogenous RNA molecules that regulate a wide variety of gene expression at the post-transcription level by binding to the complementary seed sequences in the 3′-untranslated regions (3′UTRs) of target mRNAs, leading to mRNA degradation or translational repression [19]. Numerous studies have confirmed that miRNAs play an important role in the pathological processes of different diseases, and accumulating evidence has suggested that miRNA dysregulation can be observed in both in vivo and in vitro TBI models [20,21,22]. Since 2009, our research team has investigated the roles of and mechanisms underlying miRNA function in TBI, demonstrating the involvement of different miRNAs in regulating various cellular processes after TBI, including neuronal apoptosis, angiogenesis, the immune–inflammatory response, and blood–brain barrier (BBB) permeability [23,24,25,26,27]. Significantly, an increasing number of miRNAs have been characterized to modulate the major cascades of autophagy, including autophagy induction, vesicle nucleation, vesicle elongation, autophagosome formation, autophagosome retrieval, fusion of the autophagosome to a lysosome/endosome, autolysosome formation, and autolysosome cargo degradation by targeting important molecular complexes or components for autophagy [28,29,30]. However, the role of miRNAs in TBI-induced neuronal autophagy and the contribution of this process to nerve injury after TBI remain to be further elucidated.
Exosomes are small bioactive vesicles (40–100 nm) secreted by various cells in vitro and in vivo under both physiological and pathological conditions. They interact with other cells at the cell surface via a specific receptor or by mixing of their cargos with cellular contents after endocytosis. Proteins and miRNAs can be loaded as cargos into exosomes, and are transferred into target cells by fusing exosomes with live cells to finish the cell-to-cell transmission of biological information, thereby affecting numerous physiological and pathological processes [31]. Moreover, exosomes are convenient for long-term storage and can easily cross the BBB while protecting their enveloped molecules via their bilayer lipid structure [32, 33]. Microglia, the resident immune cells in the brain, play a key role in maintaining homeostasis in the brain. In our previous study, we used a rTBI mouse model and harvested the injured brain extracts from the acute to the chronic phase of TBI to treat cultured BV2 microglia in vitro. Then we conducted a miRNA microarray analysis, observing for the first time that miR-124-3p levels in microglial exosomes increased most apparently in the miRNAs. Further research demonstrated that such increases contribute to neurite outgrowth via the transfer of miR-124-3p into neurons, and are associated with improvements in neurological outcomes in rTBI mice [9]. Nonetheless, the specific mechanisms underlying this process remain unclear.
These observations suggest that increases in miR-124-3p in microglial exosomes play a key role in regulating various pathological processes after TBI, which may include neuronal autophagy. Based on these findings, the present study aimed to clarify whether microglia-derived exosomal miR-124-3p plays a protective role by regulating neuronal autophagy after TBI, and to further explore the potential mechanisms underlying the regulation of neuronal autophagy by microglia-derived exosomal miR-124-3p. The results of this study are expected to provide insight into the development of novel therapeutic strategies for regulating autophagy after TBI using miRNA-manipulated microglial exosomes.
Materials and Methods
All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and approved by the Tianjin Medical University Animal Care and Use Committee.
Controlled Cortical Impact (CCI)-Induced rTBI Model
TBI is mostly caused by external mechanical forces clinically, and CCI-induced TBI model simulates this mechanism very well. Outcomes associated with TBI induced by CCI are consistent with those associated with brain injury in humans, including deficit s in spatial learning and memory, recognition memory, a variety of motor functions and so on [34]. Besides, CCI-induced TBI model has been shown to produce many clinically relevant pathophysiological changes including primary and secondary neuronal death, changes in hippocampal and subventricular zone cell proliferation disruption of the blood–brain-barrier, and evidence of oxidative stress and inflammation [35]. These features make CCI induced TBI model one of the most widely used models in TBI research. In order to clarify the impact of microglial exosomes on neurological outcomes following TBI, we utilized a CCI-induced rTBI model, which has been shown to induce obvious neurological impairments [9, 36]. Adult male C57BL/6 mice (age: 10–12 weeks, weight: 20–25 g) were purchased from the Chinese Academy of Military Science (Beijing, China). The mice were anesthetized with 4.6% isoflurane and then positioned in a stereotaxic frame using ear bars. After a midline scalp incision, a 3.0-mm craniotomy was performed centrally over the right parietal bone. The impounder tip of the injury device (eCCI, model 6.3; American Instruments, Richmond, VA, USA) was then extended to its full impact distance, positioned on the surface of the exposed dura mater, and reset to affect its surface.
The impact parameters were set at a velocity of 3.6 m/s and a deformation depth of 1.2 mm. Repetitive impact was performed four times at 24-h intervals [36]. Any mice with a herniation of the dura mater were eliminated from the group [37]. After each injury, the incision was stitched with interrupted 6–0 silk sutures, and the mice were then placed in a well-heated cage at 37 °C until recovery of consciousness. Mice from the control group underwent the same procedures except for cortical impact. Soaked soft mouse food was prepared for the mice that had undergone surgery.
Preparation of Brain Extracts
To prepare the brain extracts, mice were anesthetized and euthanized via transcardiac perfusion with cold PBS at 3, 7, 14, or 21 d after the last brain injury (n = 6 animals per time point) [38]. The injured hemispheres of the brain were dissected and isolated on ice. Brain tissue from each time point was homogenized by adding neurobasal medium containing 2% B27 and 1% glutamine (Thermo Fisher Scientific) at a concentration of 100 mg/ml. The homogenate was centrifuged at 12,000 g for 20 min at 4 °C. The supernatant from brain tissue extracts was collected and stored at − 80 °C.
BV2 Microglia Culture and Treatment with rTBI Brain Extracts
BV2 microglial cells were purchased from China Infrastructure of Cell Line Resources (Beijing, China). For the experiments, cells were seeded into 6-well plates at a density of 5 × 105/cm2, following which they were cultured in DMEM/F12 culture medium containing 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Thermo Fisher Scientific) at 37 °C. Pure BV2 microglia were identified via immunofluorescence staining for Iba-1. Microglia were then washed twice with PBS and cultured in neurobasal medium prior to treatment with the brain extracts. The brain extracts from rTBI or control mice were added to the culture medium at a ratio of 1:10 (extracts/culture medium). After 24 h of treatment, the culture medium containing the brain extracts was removed, and the microglia were washed twice with PBS to avoid any interference of FBS on the exosomes. Microglia were cultured for another 48 h in serum-free neurobasal medium before subsequent isolation of exosomes [38].
Microglial Exosome Isolation, Identification, and Labeling
In order to isolate microglial exosomes, the supernatant of the microglia culture medium was collected into 50-ml polypropylene tubes and centrifuged at 300 g for 10 min at 4 °C to remove the free cells. The supernatant was then transferred into a fresh centrifuge tube and was centrifuged at 2000 g for 10 min at 4 °C to remove cell debris, following which it was centrifuged again at 10,000 g for 30 min at 4 °C to further remove the cell particles. Then, the supernatant was filtered to remove dead cells and particles larger than 200 nm using a 0.22 mm filter (Millipore-Sigma). Next, ultracentrifugation was performed at 100,000 g for 70 min at 4 °C to collect the exosomes. The supernatant was discarded, and the precipitates were stored at 4 °C for further experiments.
To identify microglial exosomes, transmission electron microscopy (TEM, HT7700; Hitachi, Tokyo, Japan) was used to observe the shape and size of particles in the precipitates. Briefly, the precipitates were diluted in distilled water (1 mg/ml) and mixed with same amount of 4% paraformaldehyde. Twenty microliters of each sample were placed on a glow-discharged, carbon-coated formvar film attached to a metal specimen grid. The grid was incubated for 5 min with 50 ml 1% glutaraldehyde at room temperature, and washed eight times with 100 ml distilled water (2 min each time). After it was dried for 30 min, an equal volume of 10% uranyl acetate was added to the grid for 5 min at room temperature, followed by 50 ml methyl cellulose-uranyl acetate (5 ml 4% uranyl acetate and 45 ml 2% methyl cellulose) for 10 min at 4 °C. The samples were then dried and examined via TEM after blotting the redundant solution. Moreover, the size distribution of particles in the pellets was measured and analyzed using Nano Sight (Particle Metrix, Meerbusch, Germany), in accordance with the manufacturer’s protocol. Western blot analyses were used to examine levels of the exosome biomarkers CD9 and CD63.
Exosomes were labeled with PKH67 dye (Sigma-Aldrich), in accordance with the manufacturer’s instructions. Briefly, an exosome suspension in diluent C was mixed with 4 μL PKH67 and incubated for 10 min at room temperature. Then, 20 ml of chilled PBS was added to stop the labeling reaction. Labeled exosomes were ultra-centrifuged at 100,000 g for 70 min, washed with PBS, and ultra-centrifuged again at 100,000 g, following which the precipitates were resuspended in PBS.
Transfection of miR-124-3p Mimic and Inhibitor
To investigate the role of miR-124-3p, an miR-124-3p mimic and inhibitor (Gene Pharma, Shanghai, China) were transfected into microglia and neurons as previously described (Table 1) [27, 39]. Briefly, the miR-124-3p mimic or inhibitor was diluted to an accurate concentration of 20 mM. Then, 5 ml miR-124-3p mimic or inhibitor was mixed with 5 ml Lipofectamine-3000 (Thermo Fisher Scientific) in 500 ml serum-free DMEM/F12 and incubated for 20 min at room temperature. This transfection solution was washed twice with PBS and then added to the culture plates. After 48 h of transfection, the microglia or neurons were prepared for subsequent experiments. To evaluate transfection efficacy, real-time PCR was performed to detect the change in level of miR-124-3p in microglia and their exosomes.
HT22 Cell Culture Studies and Establishment of a Neuronal Scratch-Injury Model
Since hippocampal neurons are more easily to be injured in TBI, a mouse hippocampal neuron cell line HT22 was used in this study. HT22 cell does not express cholinergic and glutamate receptors like mature hippocampal neurons in vivo. HT22 cells were purchased from China Infrastructure of Cell Line Resources (Beijing, China). Cells were cultured in DMEM/F12 culture medium containing 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin (Thermo Fisher Scientific) in a 37 °C incubator with 5% CO2. The purity of cultured HT22 neurons was determined via immunofluorescence staining for microtubule-associated protein 2 (MAP-2).
To investigate the impact of traumatic injury on neurons in vitro, a scratch-injury was developed as previously described [27]. Using a 10-ml pipette tip, cultured HT22 neurons were scratched across the cell surface vertically and horizontally with a 4-mm space between each line, and floated cells were washed away using culture medium.
Target Prediction and Luciferase Reporter Assay
For the dysregulated miR-124-3p, target predictions were made using Target Scan (https://www.targetscan.org/).
A luciferase reporter assay was performed to clarify whether miR-124-3p directly targeted FIP200 (rb1cc1) mRNA in HT22 neurons [40]. Luciferase reporter constructs were made by ligating FIP200 3′UTR fragments containing the predicted binding sites (TargetScan, https://www.targetscan.org/cgi-bin/targetscan/vert_71/view_gene.cgi?rs=ENST00000025008.5&taxid=9606&showcnc=0&shownc=0&shownc_nc=&showncf1=&showncf2=&subset=1#miR-124-3p.1) into the luciferase reporter vector pGL3. Briefly, the fragment was amplified from mouse genomic DNA via PCR. The pGL3-FIP200-3′UTR construct was then inserted into the pGL3 control vector containing SV40 promoter (Promega, Madison, WI, USA), using XbaI enzyme. In addition, a mutant type (Mut) luciferase reporter was generated by deleting the original binding site for miR-124-3p.
For the reporter assay, HT22 neurons were cultured in 96-well plates. Wild-type (WT) or Mut FIP200-3′UTR was co-transfected with 200 pmol miR-124-3p mimic or scrambled oligonucleotides (Gene Pharma) in neurons using Lipofectamine-3000. After incubation for 48 h, the cells were harvested, and the luciferase activity was detected using a dual-luciferase reporter system (Promega, Madison, WI, USA), in accordance with the manufacturer’s protocol.
Lentivirus Transfection and RNA Interference (RNAi)
To demonstrate the precise effect of miR-124-3p on autophagy signaling by targeting FIP200, a recombinant lentiviral vector that overexpresses FIP200 was used. For specific gene knockdown on FIP200 mRNA, the lentiviral particles of siRNA targeting FIP200 (si-FIP200) or a scrambled siRNA Control (si-Control) was introduced into the lentiviral vector (GenePharma, Shanghai, China). They were transfected into HT22 neurons in accordance with the manufacturer’s instructions. And the expression levels of FIP200 were detected by Western blot analysis after 72 h.
In Vitro HT22 Neuron Cultures, Treatment, and Grouping Methods
In order to investigate the level of autophagy activation following TBI and its effect on nerve injury, HT22 neurons were divided into three groups: normal cells (Control), scratch-injury treatment (Injury), scratch-injury + chloroquine (CQ) treatment (Injury + CQ). CQ was prepared in 3-mM stocks prior to use, respectively, and diluted in medium to the indicated final concentrations. HT22 cells were treated with CQ (30 μM) for 48 h. The other groups were supplemented with an equal amount of PBS as a control. After 24 h incubation, cell detection was performed as described later.
We then investigated the effect of microglial exosomes with overexpression of miR-124-3p on nerve injury after TBI. A co-culture was established by plating HT22 cells and microglia in a Transwell permeable support system (0.4 μm pore size, Corning, NY, USA). The miR-124-3p-upregulated microglia were seeded onto the upper chamber (UC) of the Transwell system, while scratch-injured neurons were seeded onto the lower chamber (LC). The exosome inhibitor, GW4869, was used to inhibit exosome release. The neurons were divided into four groups: normal cells (Control), scratch-injury treatment (Injury), scratch-injury + miR-124-3p-upregulated micoglia co-culture (Injury + co-culture), scratch-injury treatment + miR-124-3p-upregulated microglial exosome + GW4869 (Injury + co-culture + GW4869). The cells were further incubated for 24 h prior to the experiment.
To confirm the role of miR-124-3p from microglial exosomes on neuronal autophagy, we divided neurons into four groups: normal cells (Control), scratch-injury treatment (Injury), scratch-injury + miR-124-3p-upregulated microglial exosome treatment (Injury + Exo-124+), scratch-injury + microglial exosome treatment (Injury + Exo). The cells were further incubated for 24 h prior to the experiment.
To investigate the specific mechanisms underlying increases in miR-124-3p transfer to neurons and their effects on FIP200 and autophagy after TBI, we divided neurons into five groups: normal cells (Control), scratch-injury treatment (Injury), scratch-injury + miR-124-3p mimic treatment (Injury + miR-124 mimic), scratch-injury + negative control (NC) mimic treatment (TBI + NC mimic), scratch-injury + miR-124-3p inhibitor treatment (Injury + miR-124 inhibitor), scratch-injury + NC inhibitor treatment (Injury + NC inhibitor). The cells were further incubated for 24 h after injury.
We then investigated the contribution of FIP200 to the anti-autophagy effect of miR-124-3p in TBI models in vitro. A functional rescue experiment was performed, and scratch-injured neurons were divided into four groups: Lentiviral miR-124-3p mimic + lentiviral vector treatment (miR-124-3p + Vector), lentiviral miR-124-3p mimic + lentiviral vector with FIP200 overexpression (miR-124-3p + FIP200), normal cells + lentiviral vector (NC + Vector), normal cells + lentiviral vector with si-FIP200 (NC + si-FIP200). LV-FIP200 and LV-si-FIP200 were transfected into neurons 48 h before injury, and the neurons were further cultured for 24 h after injury.
Immunofluorescence Staining
Immunofluorescence staining was performed to identify the cultured cells and measure levels of autophagy. The cells were fixed in 4% PFA for 15 min at room temperature, following which they were treated with 3% BSA for 30 min at 37 °C to block nonspecific staining. They were then incubated overnight at 4 °C with primary antibodies: Iba-1 (1:200), MAP-2 (1:200) (both from Abcam, Cambridge, United Kingdom), LC3 (1:100) (from Cell Signaling Technology, Danvers, MA, USA). The next day, they were rinsed with PBS, following which they were incubated with secondary antibodies for 1 h at room temperature. The nuclei were counterstained with DAPI (Abcam). The cells were then evaluated under an Olympus microscope (cellSens system) and an Olympus confocal microscope (FV 1200).
To confirm the neuronal uptake of exosomes, BV2 microglia derived exosomes were labeled with the dye PKH67. Then, the labeled exosomes were added to the HT22 neurons and incubated at 37 °C for 12 h, following which immunofluorescence staining for MAP-2 was performed.
In Vivo Neurological Outcome assay
To clarify the impact of miR-124-3p overexpression in microglial exosomes on neurological outcomes after TBI, several in vivo experiments were performed. The mice were randomly divided into five groups: sham surgery (Control), rTBI + PBS (rTBI), rTBI + untransfected microglial exosomes (rTBI + EXO), rTBI + miR-124-3p-upregulated microglial exosomes (rTBI + EXO-124+), and rTBI + miR-124-3p-downregulated microglial exosomes (rTBI + EXO-124−). First, an miR-124-3p mimic/inhibitor was transfected into cultured microglia with Lipofectamine-3000. Then, exosomes generated from these miR-124-3p-upregulated or -downregulated cells or non-transfected microglia were harvested and intravenously injected via tail vein into rTBI mice (30 μg total protein of exosome precipitate in 200 μl PBS/mouse) at 1 h after the first impact [41].
As previously described, post-traumatic neurological impairments were assessed using the modified neurological severity score (mNSS), which is based on the results of motor, sensory, reflex, and balance tests [25, 42]. Tests were conducted by an observer who was blinded to the experimental conditions and treatments. Tests were performed before injury and at 1, 3, 7, 14, and 21 DPI to study the effect of different treatments on neurological function. Neurological function was graded on a scale of 0–18, where a total score of 18 points indicates severe neurological impairment and a score of 0 indicates normal performance.
The Morris water maze (MWM) test was used to evaluate spatial learning and memory after TBI, as previously described [24, 43]. The spatial acquisition trial was performed in the daylight period at 21–25 DPI, and the reference memory probe trial was conducted 24 h after the last spatial acquisition training (26 DPI). The experiment was monitored using a tracking system (Ethovision 3.0; Noldus Information Technology, Wageningen, The Netherlands) to record the latency to reach the platform, as well as the time spent in the goal quadrant.
In addition, the rotarod test was used to evaluate transient motor deficits in different rTBI mouse groups [44]. The rotarod (Ugo Basile, Comerio, Italy) test was performed in accelerating rotational mode (4–40 rpm) 1 day prior to the first impact, Mice first underwent a training trial for at least 200 s, following which five trials were performed at 1, 3, 7, 14, and 21 DPI. The average latency to fall from the rotating rod (rotarod latency) was recorded, and mice unable to grasp the rod were assigned a latency of 0 s.
Real-Time PCR
Total RNA was extracted from cultured microglia, microglial exosomes, cultured parental and treated neurons, and the injured hemispheres using Trizol reagent (Thermo Fisher Scientific). The RNA concentration and quality were evaluated using a Nanodrop Spectrophotometer (ND-2000; Thermo Fisher Scientific). Furthermore, cDNA generation and quantitative RT-PCR were performed using the Hairpin-it miR-124-3p/mRNA RT-PCR Quantitation kit (Gene Pharma) with corresponding primers (Table 2), in accordance with the manufacturer’s protocols. U6 served as the internal control for miR-124-3p, and GAPDH was used as the internal control for FIP200. The Ct was acquired using the CFX Connect RT-PCR system (Bio-Rad, Hercules, CA USA). The data were analyzed using the 2−∆∆Ct formula.
Western Blot Analysis
Western blot analyses were performed at 24 h after scratch injury and other treatments. An 8% SDS acrylamide gel was used to detect FIP200 (1:1000; CST). SDS–polyacrylamide gels (10%) were used to detect Beclin-1 (1:1000; CST) and P62 (1:1000; CST). SDS–polyacrylamide gels (12%) were used to detect CD9 (1:2000) and CD63 (1:1000; both from Abcam Inc.), as well as cleaved caspase 3 (1:1000), Bcl-2 (1:1000), and LC3B (1:1000) (all from Cell Signaling Technology). β-Actin (1:1000; Cell Signaling Technology) was used as the internal control. The ChemiDoc XRS + Imaging System (Bio-Rad) was used for densitometry. The mean pixel density of each band was measured using Quantity One software (Bio-Rad). Detailed antibody information is presented in Table 3.
Statistical Analysis
All data represent the results of at least three independent experiments (biologically repeated) and are expressed as means ± SD, except for the data from the spatial acquisition trials of the MWM test, which are expressed as means ± SEM. Data from the spatial acquisition trials of the MWM test and mNSS were analyzed using repeated-measures analyses of variance (ANOVA). Other statistical comparisons were performed using one-way ANOVA followed by least-significant difference post hoc analyses or Student’s t-tests. P values less than 0.05 were considered significant.
Results
Levels of miR-124-3p Expression were Increased in BV2 Microglia and Their Exosomes After Treatment with rTBI Mouse Brain Extracts
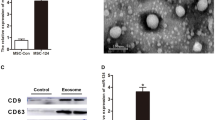
In order to determine the level of miR-124-3p expression in microglial exosomes after TBI, we first cultured BV2 cells—a common, commercially available mouse microglial cell line. Pure BV2 microglia were identified via immunofluorescence staining for Iba-1 (Fig. 1a, b), following which an rTBI mouse model was prepared. Cultured microglia were treated with extracts of the injured brain tissue at 3, 7, 14, and 21 DPI. To characterize the exosomes produced by microglia, we isolated the microglia-generated particles from supernatants of the culture medium. TEM was performed to observe the collected exosomes, which were round particles with a double membrane ranging from 40 to 100 nm in size (Fig. 1c). Nano Sight analysis further revealed that particle diameter was heterogeneous, and that the peak diameter was 80.0 ± 0.7 nm (Fig. 1d, e). In addition, Western blot analyses revealed abundant expression of CD9 and CD63 (Fig. 1f). Our results indicated that these particles were mainly composed of exosomes. Total RNA was then extracted from microglia and their exosomes, and the level of miR-124-3p expression was detected via real-time PCR. Our results reconfirmed that miR-124-3p levels were upregulated in both microglia and microglial exosomes from all rTBI groups (Fig. 1g, h).
Identification of BV2 microglial exosomes and levels of miR-124-3p expression in BV2 microglia and their exosomes after treatment with extracts of the rTBI mouse brain tissue at 3, 7, 14, and 21 DPI. a Cultured BV2 microglia under the transmission light microscope. b Immunofluorescence staining for Iba-1 to identify microglia, revealing a pure culture. c TEM image of exosomes isolated from the microglia culture medium. The exosomes exhibited a round shape and double-layer membrane, with a size range of 40–100 nm. d, e Nano Sight analysis revealed that the diameter of the particles was heterogeneous, and that the peak diameter was 80.0 ± 0.7 nm. f Western blot analysis revealed that CD9 and CD63 were more highly expressed in the microglia-generated particles (par) than in the microglial supernatant (sup), indicating that these particles were mainly composed of exosomes. g, h Real-time PCR results reconfirmed that miR-124-3p levels were upregulated in both microglia and microglial exosomes from all rTBI groups (n = 6 mice/time point). Data were expressed as mean ± SD; N = 3 for Nano Sight, N = 3 for Western blot, N = 3 for Real-time PCR. ***P < 0.001 versus control group. rTBI repetitive traumatic brain injury, TEM transmission electron microscopy
Inhibition of Activated Neuronal Autophagy Protected Against Trauma-Induced Injury In Vitro
To further investigate the role of autophagy in TBI pathology, scratch-injury experiments were performed using cultured HT22 neurons. Pure HT22 neurons were identified via immunofluorescence staining for MAP-2, following which they served as in vitro models of TBI (Fig. 2a–c). Western blot analyses were performed to detect LC3-II/I, P62, Beclin-1, cleaved caspase 3, and Bcl-2 expression in neurons after scratch injury. Our findings indicated that TBI led to an increase in neuronal levels of LC3, Beclin-1, cleaved caspase 3, and Bcl-2, as well as decreases in levels of P62 (Fig. 2d–i). Immunofluorescence staining revealed that LC3, a well-established marker of autophagy, was highly expressed in the injured neurons (Fig. 2j, k). These results suggest that scratch injury can activate neuronal autophagy and lead to neuronal apoptosis.
Neuronal autophagy was significantly induced after TBI in vitro, and inhibition of activated neuronal autophagy protected against trauma-induced injury. a Cultured HT22 neurons under the transmission light microscope. b Cultured HT22 neurons with a scratch injury under the transmission light microscope. c Immunofluorescence staining for MAP-2 to identify HT22 neurons, revealing a pure culture. d, e Western blot analysis revealed that LC3 II/I expression was upregulated in cultured HT22 neurons after scratch injury, further increasing following treatment with CQ. f, g Beclin-1 expression was upregulated after scratch injury and downregulated after treatment with CQ, while P62 expression was downregulated after scratch injury and upregulated after treatment with CQ. h, i Western blot analysis revealed that cleaved caspase 3 and Bcl-2 levels were increased in the scratch-injured neurons, although CQ treatment attenuated such increases. j, k Immunofluorescence staining revealed upregulation of LC3 puncta in HT22 neurons after scratch injury, which increased further after treatment with CQ. Data were expressed as mean ± SD; N = 4–5 for Western blot, N = 3 for Immunofluorescence staining. **P < 0.01, ***P < 0.001 versus control group; #P < 0.05, ###P < 0.001 versus injury group. TBI traumatic brain injury, MAP-2 microtubule-associated protein 2, CQ chloroquine
To further clarify whether inhibition of neuronal autophagy can protect against trauma-induced injury, the injured neurons were treated with CQ, a commonly used inhibitor of autophagy. We then measured levels of LC3, cleaved caspase 3, and Bcl-2 via Western blot analysis, and further examined LC3 levels via immunofluorescence staining. Our results indicated that levels of cleaved caspase 3 and Bcl-2 were lower in the CQ-treated group than in the injury group, while levels of LC3 were higher (Fig. 2d, e, h–k). Taken together, these results clearly demonstrate that autophagy is significantly induced following TBI in vitro. Furthermore, induction of autophagy following TBI may be responsible for neuronal damage, and inhibition of activated neuronal autophagy may protect against trauma-induced injury.
Exosomes from miR-124-3p-Overexpressing Microglia Exerted a Protective Effect in Injured Neurons by Inhibiting Neuronal Autophagy
To identify the effects of miR-124-3p-overexpressing BV2 microglia and their exosomes on neuronal autophagy after TBI in vitro, we developed a scratch-injury model using cultured pure HT22 neurons. We first transfected an miR-124-3p mimic into the cultured microglia. Real-time PCR revealed that miR-124-3p levels were significantly upregulated following transfection (Fig. 3a). Then, the injured neurons were co-cultured with miR-124-3p-overexpressing microglia, or with miR-124-3p-overexpressing microglia conditioned with GW4869, which inhibits the generation of exosomes (Fig. 3b). Trauma-induced increases in autophagy-related proteins (LC3, Beclin-1) were significantly attenuated by microglia treatment, which also significantly increased P62 expression (Fig. 3c, d). In addition, increases in levels of apoptosis mediators (cleaved caspase 3 and Bcl-2) in the injury group were significantly attenuated in the co-cultured group (Fig. 3e, f). These results indicate that co-culture of neurons with miR-124-3p-overexpressing microglia may suppress trauma-induced autophagy and exert protective effects in injured neurons. In contrast, following GW4869 treatment, changes in protein levels of LC3, Beclin-1, P62, cleaved caspase 3, and Bcl-2 were alleviated, indicating that an exosome inhibitor may reverse the inhibition of autophagy and the protective effects induced by co-culture of neurons with miR-124-3p-overexpressing microglia (Fig. 3c–f). Furthermore, to demonstrate that microglia-derived exosomes can enter neurons, exosomes isolated from the cultured microglia were labeled with PKH67 and incubated with neurons. Immunofluorescence staining experiments revealed that the labeled exosomes were taken into the neurons (Fig. 3g). Taken together, these findings suggest that co-culture of injured neurons with miR-124-3p-overexpressing microglia exerts a protective effect by inhibiting neuronal autophagy in scratch-injured neurons. These effects were likely achieved via microglial exosomes.
Exosomes from miR-124-3p-overexpressing microglia exerted a protective effect on injured neurons by inhibiting neuronal autophagy. a Real-time PCR analyses revealed that miR-124-3p expression was significantly upregulated in the cultured microglia after transfection with an miR-124-3p mimic. b The co-culture system of neurons and microglia. Neurons and microglia were cultured together in a Transwell system. c, d Western blot analysis of autophagy-related proteins (LC3, P62, Beclin-1) revealed that autophagy was inducted in the cultured neurons after scratch injury. Activation of neuronal autophagy was inhibited by exosomes derived from the co-cultured miR-124-3p-overexpressing microglia. This inhibitory effect was alleviated by GW4869 treatment. e, f Western blot analysis revealed that levels of apoptosis-related proteins (cleaved caspase 3, Bcl-2) were increased in the scratch-injured neurons and reduced after treatment with exosomes derived from the co-cultured miR-124-3p-overexpressing microglia. This inhibitory effect was also alleviated by GW4869 treatment. g Immunofluorescence staining revealed that the labeled microglial exosomes were taken into neurons. Data were expressed as mean ± SD; N = 3 for Real-time PCR, N = 4–5 for Western blot. ***P < 0.001 versus control group; ###P < 0.001 versus injury group; ∆P < 0.05, ∆∆P < 0.01, ∆∆∆P < 0.001 versus I + Co-culture group
Microglia-Derived Exosomal miR-124-3p Inhibited the Activation of Neuronal Autophagy by Increasing miR-124-3p Expression in the Injured Neurons
Real-time PCR analyses revealed that exosomes harvested from BV2 microglia transfected with an miR-124-3p mimic exhibited increases in miR-124-3p levels (Fig. 4a). To demonstrate the impact of exosomal miR-124-3p on neuronal autophagy induced by trauma injury in neurons, the scratch-injured neurons were treated with normal exosomes or miR-124-3p-overexpressing exosomes. Real-time PCR analysis revealed that scratch injury increased levels of miR-124-3p expression in neurons, and that such expression was remarkably upregulated following treatment with miR-124-3p-overexpressing exosomes. However, no significant changes in miR-124-3p expression were observed in injured neurons treated with normal exosomes (Fig. 4b). We then measured neuronal expression of autophagy-related proteins including LC3, Beclin-1, and P62 following injury and exosome treatment. Our data demonstrated that treatment with microglial exosomes inhibited the expression of LC3 and Beclin-1 while promoting the expression of P62, especially in neurons treated with miR-124-3p-overexpressing exosomes (Fig. 4c–f). These results indicated that inhibition of activated neuronal autophagy in the injured neurons was due to microglia-derived exosomal miR-124-3p.
Microglia derived exosomal miR-124-3p inhibited the activation of neuronal autophagy by increasing miR-124-3p expression in the injured neurons. a Real-time PCR revealed that miR-124-3p expression was significantly upregulated in microglial exosomes after transfection with an miR-124-3p mimic. b Real-time PCR revealed that miR-124-3p expression was upregulated in HT22 neurons after scratch injury, further increasing significantly after treatment with miR-124-3p-overexpressing exosomes. c, d, e, f Western blot analysis of autophagy-related proteins (LC3, P62, Beclin-1) revealed that autophagy was induced in the cultured neurons after scratch injury. Activation of autophagy was inhibited by microglial exosomes, especially miR-124-3p-overexpressing exosomes. Data were expressed as mean ± SD; N = 3 for Real-time PCR, N = 5 for Western blot. **P < 0.01, ***P < 0.001 versus control group; ##P < 0.01, ###P < 0.001 versus injury group; ∆P < 0.05 versus I + Exo 124+ group
miR-124-3p Targeted FIP200 and Suppressed Activated Neuronal Autophagy in Injured Neurons
To explore the mechanism underlying the suppression of trauma-induced neuronal autophagy by miR-124-3p, we predicted the potential target genes of miR-124-3p using bioinformatics analysis (https://www.targetscan.org/). Our results indicated that the 3′ UTR of FIP200 mRNA has unique complementary binding sites for miR-124-3p, indicating that FIP200 may be a target of miR-124-3p (Fig. 5a). To identify whether miR-124-3p could directly target the 3′UTR of FIP200 mRNA, we performed a dual-luciferase reporter assay. The fragment of the 3′UTR of FIP200 mRNA containing the putative miR-124-3p binding site or its mutant 3′UTR was cloned into luciferase reporter vector pGL3 to construct pGL3-FIP200-3′UTR WT or pGL3-FIP200-3′UTR Mut, and then co-transfected with miR-124-3p mimics or scrambled oligonucleotides into neurons (Fig. 5b). Our results indicated that relative luciferase activity was significantly decreased in the neurons co-transfected with pGL3-FIP200-3′UTR WT with miR-124-3p, whereas no apparent difference was observed in the neurons co-transfected with pGL3-FIP200-3′UTR Mut with miR-124-3p, relative to levels observed in the control group (Fig. 5c). These findings confirmed that miR-124-3p can specifically target and suppress the 3′UTR of FIP200.
miR-124-3p targeted FIP200 and suppressed activation of neuronal autophagy in injured neurons. a Schematic representation of the potential binding sites for miR-124-3p in the FIP200 3′UTR, which were obtained using publicly available algorithms. b The WT (FIP200 WT 3′UTR) and mutant type [FIP200 mutant (mut) 3′UTR] luciferase reporter constructs exhibited intact and mutated seed sequences (underlined), respectively, in the miR-124-3p binding site. c The relative luciferase activity of the WT and mut reporter constructs, which were co-transfected with either the miR-124-3p mimic or scrambled oligonucleotides. Data are presented as the ratio of luciferase activity from the scrambled oligonucleotides versus miR-124-3p mimic-transfected HT22 neurons. Our findings indicate that miR-124-3p inhibited the luciferase activity of the WT, but not the mut type, 3′UTR reporter construct. Furthermore, miR-124-3p directly targeted FIP200 and downregulated its expression by binding to the 3′UTR sites. d, e Western blot analysis revealed that FIP200 expression was upregulated in the cultured HT22 neurons after scratch injury. When compared with the injury group, expression of FIP200 was inhibited in the I + miR-124 mimic group and increased in the I + miR-124 inhibitor group. f FIP200 mRNA levels in neurons were determined via real-time PCR in different groups. No obvious differences in FIP200 mRNA expression were observed among the transfected groups. g, h Western blot analysis of autophagy-related proteins (LC3, P62, Beclin-1) revealed that trauma-induced neuronal autophagy was inhibited in the I + miR-124 mimic group and promoted in I + miR-124 inhibitor group. Data were expressed as mean ± SD; N = 3 for Relative luciferase activity, N = 4–5 for Western blot, N = 3 for Real-time PCR. ***P < 0.001 versus control group; ##P < 0.01, ###P < 0.001 versus injury group; ∆∆∆P < 0.001 versus I + miR-124 mimic group. WT wild-type
To further confirm the effect of miR-124-3p on FIP200 expression in the injured neurons, we respectively transfected an miR-124-3p mimic, miR-124-3p inhibitor, negative control mimic, and inhibitor directly into cultured HT22 neurons. Overexpression of miR-124-3p significantly decreased the protein expression of FIP200, while low expression of miR-124-3p produced the opposite result (Fig. 5d, e). However, among these transfected groups, there were no obvious changes in levels of FIP200 mRNA expression (Fig. 5f). These results suggest that miR-124-3p mainly suppress the translation of FIP200, rather than its transcription. In addition, we examined the expression of autophagy-related proteins to evaluate the direct effect of miR-124-3p on autophagy in injured neurons. As expected, our results indicated that autophagy was inhibited in injured neurons transfected with the miR-124-3p mimic, while it was slightly enhanced in injured neurons transfected with the miR-124-3p inhibitor (Fig. 5g, h). These results demonstrated that miR124-3p had induced autophagy inhibition in the injured neurons, possibly by targeting FIP200.
Overexpression of miR-124-3p Mainly Suppressed FIP200-Mediated Autophagy in Injured Neurons
To further demonstrate the contribution of FIP200 to the inhibition of neuronal autophagy, a functional rescue experiment was performed by overexpressing FIP200 in injured neurons. LV-FIP200 and LV-miR-124-3p were co-transfected into the neurons prior to scratch-injury. In addition, we transfected LV-si-FIP200 and an LV-si-negative control into the neurons, respectively. Western blot analyses revealed that overexpression of miR-124-3p inhibited FIP200 protein expression, and that co-transfection of LV-FIP200 reversed this inhibitory effect (Fig. 6a, b). Moreover, neuronal expression of FIP200 was silenced by LV-si-FIP200 (Fig. 6a, b). Furthermore, our results indicated that the inhibitory effects of miR-124-3p overexpression on autophagic activation were significantly blocked by FIP200 overexpression, and that silencing of FIP200 via siRNA mimicked the effects of miR-124-3p in neurons subjected to scratch injury (Fig. 6c–f). These findings further verified that overexpression of FIP200 can eliminate the inhibitory effects of miR-124-3p on autophagy. These results suggest that FIP200, as the target of miR-124-3p, plays a key role in trauma-induced autophagy and may promote neuroprotective effects in the injured neurons.
Overexpression of miR-124-3p mainly suppressed FIP200-mediated autophagy in injured neurons. a, b Western blot analyses revealed that overexpression of FIP200 reversed the miR-124-3p-induced inhibition of FIP200, and that silencing of FIP200 by siRNA suppressed FIP200 expression in the scratch-injured neurons. c–f Overexpression of FIP200 blocked miR-124-3p-induced inhibition of neuronal autophagy, and silencing of FIP200 by siRNA mimicked the effects of miR-124-3p in the scratch-injured neurons. Data were expressed as mean ± SD; N = 5 for Western blot. ***P < 0.001 versus miR-124-3p + Vector group; ###P < 0.001 versus NC + Vector group
Overexpression of miR-124-3p in Microglial Exosomes Ameliorated TBI-Induced Neurological Outcomes In Vivo
To clarify the impact of miR-124-3p overexpression in microglial exosomes on TBI-induced neurological outcomes in vivo, mice were subjected to various behavioral tests (mNSS, rotarod test, MWM test). The mNSS test was performed from 1 to 21 DPI, in order to evaluate long-term neurological function. Our results indicated that, in the rTBI group, the recovery of mNSS began at 3 DPI and continued until 21 DPI. Furthermore, mNSS was significantly lower in the rTBI + miR-124-3p-upregulated microglial exosomes (rTBI + EXO-124+) group and significantly higher in the rTBI + miR-124-3p-downregulated microglial exosomes (rTBI + EXO-124−) group at 7, 14, and 21 DPI than scores observed in the rTBI group (Fig. 7a). The rotarod test was used to evaluate motor performance. After 4 weeks, control animals exhibited improvements in rotarod test performance, while rTBI animals exhibited noticeable impairments. In addition, when compared with the rTBI group, the rTBI + Exo-124+ group exhibited significant improvements in rotarod test performance, while the opposite results were observed in the rTBI + Exo-124− group, at 7, 14, and 21 DPI. (Fig. 7b). Furthermore, in the spatial navigation trials of the MWM test, escape latency gradually decreased from 21 to 25 DPI, indicating that spatial memory had been established. Probe trials were performed at 26 DPI to examine retrograde reference memory, where more time spent in the goal quadrant demonstrates better memory. Our results indicated that rTBI led to obvious neurological impairments, reflected by increased escape latency and decreased time spent in the goal quadrant. When compared with the rTBI group, the rTBI + Exo-124+ group exhibited decreased escape latency and increased time spent in the goal quadrant, while the opposite results were observed in the rTBI + Exo-124− group (Fig. 7c, d). Taken together, these results demonstrated that treatment with miR-124-3p-upregulated microglial exosomes can ameliorate TBI-induced neurological outcomes.
Overexpression of miR-124-3p in microglial exosomes ameliorated TBI-induced neurological outcomes in vivo. The neurological function of rTBI mice was evaluated using a mNSS, b the rotarod test, and c, d the MWM test. a Results for the mNSS test indicated that miR-124-3p mimic (or inhibitor) treatment decreased (or increased) neurological scores from 7 to 21 DPI. b Rotarod test results indicated that miR-124-3p mimic (or inhibitor) treatment increased (or decreased) the rotarod latency from 7 to 21 DPI. c, d Escape latency for the spatial acquisition trial was shortened, and time spent in the goal quadrant for the probe trial was improved by miR-124-3p mimic treatment, whereas the opposite results were observed following miR-124-3p inhibitor treatment. n = 6–8mice/group. Data were expressed as mean ± SD a, b, d or mean ± SEM c; N = 3 independent experiments. *P < 0.05, **P < 0.01 versus rTBI group. rTBI repetitive traumatic brain injury, mNSS modified Neurological Severity Score, MWM Morris water maze, DPI days post injury
Discussion
In the present study, we reconfirmed that there was a consistent increase in miR-124-3p levels in exosomes derived from cultured BV2 microglia which was treated with rTBI mouse model brain extracts. Furthermore, our results demonstrated that microglial exosomes enriched with miR-124-3p may exert protective effects in both in vivo and in vitro models of TBI: Increases in miR-124-3p in microglial exosomes significantly reduced neuronal injury by suppressing scratch injury-induced autophagy in HT22 neurons. Moreover, such increases improved neurological outcomes in rTBI mice. Further research revealed that miR-124-3p specifically binds with the 3′UTR of FIP200 mRNA to modulate its translation. As FIP200 is a key protein of the autophagy signaling pathway, this result sheds light on the molecular mechanisms underlying anti-autophagy induced by miR-124-3p. Finally, our results indicate that miR-124-3p may represent a therapeutic target for intervention in TBI-induced neuronal autophagy. A summary diagram that relates the different strands of the experiment and explains the inter-relationship between the different experiments is provided in Fig. 8. Indeed, our findings may aid in the development of novel therapeutic strategies for TBI via intravenous administration of miRNA-manipulated microglial exosomes.
Exosomes are endogenous micro-vesicles that play pivotal roles in intercellular signaling by transporting functional cargos such as RNA, lipids, and proteins from one cell to another [45]. In the central nervous system, the comprehensive connection between neurons and glia (including microglia, the immune cells of the brain) takes advantage of secreted exosomes, which mediate intercellular communication over long distances [46]. Emerging evidence supports a critical role for exosomes in mediating complex and coordinated communication among microglia and neurons, suggesting that they regulate pathologic changes in various neurological disorders [47,48,49]. Microglia-derived exosomes have been reported to harbor contents similar to those of exosomes originating from B-cells and dendritic cells, consistent with their roles in the immune system [50]. Microglial exosomes may physiologically modulate neuronal synaptic activity by promoting neuronal production of ceramide and sphingosine [51]. In addition, microglial exosomes can stimulate type-1 cannabinoid receptors (CB1) expressed by GABAergic neurons and inhibit presynaptic transmission by carrying N-arachidonoylethanolamine on their surface [52]. Recent studies have reported that recombinant treatment of primary microglia increases exosome secretion via a GSK3-independent mechanism, and in turn stimulates the release of exosomes containing Wnt3 [53]. This discovery indicates that microglial exosomes regulate the pathologic development of diseases such as Alzheimer’s disease (AD), since the Wnt signaling pathway is closely related to the development of neurodegenerative diseases. In addition, microglial exosomes have been proven to promote amyloid clearance in AD models by increasing the activity of exosome-associated insulin degrading enzyme (IDE), a zinc metallopeptidase known to efficiently degrade amyloid-β protein (Aβ) [54]. Moreover, recent studies have demonstrated that microglial exosomes play a role in tau dissemination, reporting that a certain proportion of tau is released from neurons via exosomes in AD [55]. Accumulating evidence has indicated that microglial exosomes also take part in other CNS diseases, such as infection and neuroinflammation. Our previous study was the first to report that there was a consistent increase in miR-124-3p levels in exosomes derived from cultured BV2 microglia which was treated with rTBI mouse model brain extracts. Further research demonstrated that microglial exosomes enriched with miR-124-3p can contribute to neurite outgrowth via its transfer into neurons, and that such increases are associated with improved neurological outcomes in rTBI mice [9]. Therefore, in the present study, we focused on the precise mechanisms underlying this process.
Autophagy is a self-catabolic process for the degradation of superfluous or aberrant cytoplasmic components, representing an essential cell survival mechanism. Autophagy has been confirmed to be critical for various biological activities. Several recent studies have demonstrated that autophagy is significantly induced after TBI [15,16,17,18]. However, the role of TBI-induced neuronal autophagy remains controversial. Some early studies proposed that TBI induced neuronal autophagy may be responsible for eliminating aberrant cellular components and maintaining cellular homeostasis, thus serving as a protective mechanism [17, 56]. More recent reports have suggested that inhibition of excessive autophagy can attenuate brain injury and improve neurological outcomes post-TBI [12,13,14,15, 57]. Previous studies have consistently reported that microRNAs can regulate autophagy by acting on different targets involved in the major autophagy cascades, which include autophagy induction, vesicle nucleation, vesicle elongation, retrieval, fusion, autolysosome cargo degradation, and various signaling pathways related to autophagy cascades [28, 58,59,60]. For instance, growth signals, energy status, genotoxic stress, hypoxic stress, endoplasmic reticulum (ER) stress, and reactive oxygen species (ROS) elicit a series of signaling pathways that initiate or repress autophagy [61,62,63,64]. In our previous work, we observed that miR-124-3p levels increase from the acute to chronic phase of TBI [9]. Other studies have reported that decreased miR-124-3p expression promotes the progression of breast cancer in human cells mainly by targeting beclin-1 [65]. Furthermore, loss of miR-124-3p may accelerate autophagy and promote the activation of cell survival networks in the aggressive mesenchymal subtype of KRAS mutant non-small cell lung cancer (NSCLC) [66]. These findings indicate that increased miR-124-3p expression in microglia-derived exosomes may inhibit TBI-induced autophagy and influence the pathological progression of TBI.
Based on these findings, we focused our efforts on exploring the impact of miR-124-3p on TBI-induced autophagy, in order to reveal the protective effects of microglial exosomes on neuronal injury after TBI. In this study, we used brain extracts harvested from rTBI model mice, which were then added to cultured BV2 microglia to imitate the microenvironment of TBI in vitro. Exosomes can act as mediators for cross-talk between cells, such as microglia and neurons, and are carriers for functional miRNA delivery [48]. We reconfirmed that miR-124-3p levels are increased in exosomes released from microglia exposed to TBI brain extracts at 3, 7, 14, and 21 DPI. It is worth mentioning that a recent study demonstrated that there was a chronic downregulation of miR-124-3p after brain injury [67]. Different brain injury models (rats with severe TBI induced by lateral fluid-percussion injury and status epilepticus induced by electrical amygdala stimulation) and different test samples (dentate gyrus) might be reasons for the difference between this study and our research results. More importantly, they focused on the chronic phase (3 months) after brain injury, which was also quite different from the time point we selected for detection. Accumulating evidence demonstrates that miR-124-3p may play a protective role in several diseases of the CNS. Such studies have reported that miR-124-3p induces neuroprotection and functional improvement after focal cerebral ischemia [68] and may act as target for AD by regulating BACE1, leading to neuroprotective effects [69]. Another study revealed that miR-124-3p plays a neuroprotective role in a 6-hydroxydopamine-induced cell model of Parkinson's Disease (PD) via the regulation of ANAX5 [70]. One recent report suggested that miR-124-3p functions as a protector of dopaminergic neurons during PD via the modulation of cell apoptosis and autophagy by regulating the AMPK/mTOR pathway [71]. Our early studies also indicated that miR-124-3p exerts protective effects in neurons post-TBI. In accordance with these previous findings, our present results demonstrate that inhibition of neuronal autophagy using CQ can protect injured neurons in vitro. Further results revealed that miR-124-3p-enriched microglial exosomes are taken up by neurons, resulting in inhibition of autophagic activity. Thus, these results suggest that the increased miR-124-3p in microglial exosomes can be transferred to the injured neurons to regulate excessive neuronal autophagy, thereby exerting protective effects against neuronal injury following TBI.
FIP200 encodes an evolutionarily conserved protein characterized by a large coiled-coil region containing a leucine zipper motif, which is also known as retinoblastoma coiled coil protein 1 (RB1CC1) [65]. Previous studies have reported that FIP200 can interact with and regulate the activity of several different proteins involved in proliferation, apoptosis, and cell cycle progression [72, 73]. Recent studies have also demonstrated that FIP200, which is a crucial component of the ULK complex, plays an important role in the regulation of autophagy in mammalian cells [28]. The ULK complex—which is composed of ULK1, ULK2, ATG13, ATG101, and FIP200—is critical for autophagosome formation [30]. Therefore, autophagy activation is closely related to cellular levels of FIP200. However, few studies have investigated the role of FIP200 in pathological disease processing, mainly focusing on oncology. In the present study, we identified FIP200 as a potential target gene of miR-124-3p using bioinformatics analysis. We subsequently confirmed that miR-124-3p binds directly to the 3′UTR region of FIP200 mRNA via a luciferase reporter assay. Notably, overexpression of FIP200 via lentivirus abrogated the neuroprotective effects of miR-124-3p on neuronal injury post-TBI, further supporting the notion that miR-124-3p protects against TBI-induced neuronal injury by suppressing FIP200-mediated neuronal autophagy. Altogether, these findings suggest that increased levels of miR-124-3p in microglial exosomes can be transferred to the injured neurons to regulate excessive neuronal autophagy by suppressing FIP200, thereby exerting protective effects against neuronal injury post-TBI.
In future studies, we intend to demonstrate the specific mechanisms underlying the protective effects induced by increases in miR-124-3p in microglial exosomes against nerve injury in vivo, and to clarify whether these mechanisms are also associated with the inhibition of autophagy. We further intend to examine the effects of exosomes on various cell types in nervous system diseases, including TBI. Future studies should also aim to clarify whether the same mechanisms protect against nerve injury caused by other types of nervous system diseases. Our current work mainly focuses on the microglial exosomes enriched with miR-124-3p and their protective effect on injured neurons, it is speculated that exosomes derived from other types of cells may have similar effects, such as neurons, astrocytes and so on. A series of studies on mesenchymal stem cell (MSC)-derived exosomes for TBI treatment have been reported recently, indicating that they could improve the neurologic outcome by promoting endogenous angiogenesis and neurogenesis [74, 75]. It can be inferred that up-regulating the miR-124-3p level in MSC-derived exosomes might be a therapeutic approach for TBI. But these speculations still need to be further verified. In addition, our previous study proved that transfection of miR-124-3p mimic alone to injured neurons could also provide the same neuroprotective effects. However, in order to facilitate clinical application and to prevent degradation of miRNAs by RNase, gene delivery tools like exosomes are necessary.
In conclusion, our results demonstrate that miR-124-3p levels in microglial exosomes increase from the acute to chronic phase of TBI. Furthermore, our findings indicate that microglial exosomes enriched with miR-124-3p can inhibit the activity of FIP200-mediated neuronal autophagy signaling by targeting FIP200, thus attenuating trauma-induced, autophagy-mediated nerve injury in vitro (Fig. 9). Our study also suggests that treatment with microglial exosomes enriched with miR-124-3p represents a promising therapeutic strategy for the treatment of nerve injury after TBI. Microglial exosomes manipulated with miRNA may thus represent a novel therapy for TBI and other neurologic diseases.
References
Johnson WD, Griswold DP (2017) Traumatic brain injury: a global challenge. Lancet Neurol 16:949–950
Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, Maas A (2016) Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health 1:e76–e83
Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Soderberg J, Stanworth SJ, Stein MB, von Steinbuchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K, In TP Investigators (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16:987–1048
Jiang JY, Chinese Head Trauma Study C (2013) Head trauma in China. Injury 44:1453–1457
Gao GY, Jiang JY (2012) Chinese head trauma data bank: effect of gender on the outcome of patients with severe traumatic brain injury. J Neurotrauma. https://doi.org/10.1089/neu.2011.2134
WHO (2017) Neurological disorders: public health challenges. https://www.who.int/mental_health/neurology/neurological_disorders_report_web.pdf. Accessed Sept 20 2017
Levin H, Smith D (2013) Traumatic brain injury: networks and neuropathology. Lancet Neurol 12:15–16
Shlosberg D, Benifla M, Kaufer D, Friedman A (2010) Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6:393–403
Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, Chen F, Wang H, Zhang J, Lei P (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32:512–528
Klionsky DJ (2004) Cell biology: regulated self-cannibalism. Nature 431:31–32
Levine B, Kroemer G (2008) SnapShot: macroautophagy. Cell 132: e161
Lin CJ, Chen TH, Yang LY, Shih CM (2014) Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis 5:e1147
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM (2014) Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 10:2208–2222
Sun L, Liu A, Zhang J, Ji W, Li Y, Yang X, Wu Z, Guo J (2018) miR-23b improves cognitive impairments in traumatic brain injury by targeting ATG12-mediated neuronal autophagy. Behav Brain Res 340:126–136
Sun L, Zhao M, Wang Y, Liu A, Lv M, Li Y, Yang X, Wu Z (2017) Neuroprotective effects of miR-27a against traumatic brain injury via suppressing FoxO3a-mediated neuronal autophagy. Biochem Biophys Res Commun 482:1141–1147
Sun L, Gao J, Zhao M, Cui J, Li Y, Yang X, Jing X, Wu Z (2015) A novel cognitive impairment mechanism that astrocytic p-connexin 43 promotes neuronic autophagy via activation of P2X7R and down-regulation of GLT-1 expression in the hippocampus following traumatic brain injury in rats. Behav Brain Res 291:315–324
Liu CL, Chen S, Dietrich D, Hu BR (2008) Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab 28:674–683
Au AK, Aneja RK, Bayir H, Bell MJ, Janesko-Feldman K, Kochanek PM, Clark RSB (2017) Autophagy biomarkers beclin 1 and p62 are increased in cerebrospinal fluid after traumatic brain injury. Neurocrit Care 26:348–355
Fineberg SK, Kosik KS, Davidson BL (2009) MicroRNAs potentiate neural development. Neuron 64:303–309
Meissner L, Gallozzi M, Balbi M, Schwarzmaier S, Tiedt S, Terpolilli NA, Plesnila N (2016) Temporal profile of microrna expression in contused cortex after traumatic brain injury in mice. J Neurotrauma 33:713–720
Liu L, Sun T, Liu Z, Chen X, Zhao L, Qu G, Li Q (2014) Traumatic brain injury dysregulates microRNAs to modulate cell signaling in rat hippocampus. PLoS ONE 9:e103948
Martinez B, Peplow PV (2017) MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury. Neural Regen Res 12:1749–1761
Lei P, Li Y, Chen X, Yang S, Zhang J (2009) Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res 1284:191–201
Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, Li SH, Jiang RC, Kang CS, Zhang JN (2014) miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep 4:6718
Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J (2015) MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain Res 1603:150–157
Ge X, Huang S, Gao H, Han Z, Chen F, Zhang S, Wang Z, Kang C, Jiang R, Yue S, Lei P, Zhang J (2016) miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res 1650:31–40
Han Z, Chen F, Ge X, Tan J, Lei P, Zhang J (2014) miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res 1582:12–20
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015) MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget 6:8474–8490
Legakis JE, Yen WL, Klionsky DJ (2007) A cycling protein complex required for selective autophagy. Autophagy 3:422–432
Frankel LB, Lund AH (2012) MicroRNA regulation of autophagy. Carcinogenesis 33:2018–2025
Thompson AG, Gray E, Heman-Ackah SM, Mager I, Talbot K, Andaloussi SE, Wood MJ, Turner MR (2016) Extracellular vesicles in neurodegenerative disease—pathogenesis to biomarkers. Nat Rev Neurol 12:346–357
Zheng T, Pu J, Chen Y, Mao Y, Guo Z, Pan H, Zhang L, Zhang H, Sun B, Zhang B (2017) Plasma exosomes spread and cluster around beta-amyloid plaques in an animal model of Alzheimer's disease. Front Aging Neurosci 9:12
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Osier N, Dixon CE (2016) The controlled cortical impact model of experimental brain trauma: overview, research applications, and protocol. Methods Mol Biol 1462:177–192
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14:128–142
Gao H, Han Z, Bai R, Huang S, Ge X, Chen F, Lei P (2017) The accumulation of brain injury leads to severe neuropathological and neurobehavioral changes after repetitive mild traumatic brain injury. Brain Res 1657:1–8
Ge X, Li W, Huang S, Yin Z, Yang M, Han Z, Han Z, Chen F, Wang H, Lei P, Zhang J (2019) Increased miR-21-3p in injured brain microvascular endothelial cells after traumatic brain injury aggravates blood-brain barrier damage by promoting cellular apoptosis and inflammation through targeting MAT2B. J Neurotrauma 36:1291–1305
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30:1556–1564
Han Z, Ge X, Tan J, Chen F, Gao H, Lei P, Zhang J (2015) Establishment of lipofection protocol for efficient miR-21 transfection into cortical neurons in vitro. DNA Cell Biol 34:703–709
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, Wang B, Wang G, Jia Z, Pu P, Zhang W, Kang C (2010) Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest 90:144–155
Kim J, Jeong D, Nam J, Aung TN, Gim JA, Park KU, Kim SW (2015) MicroRNA-124 regulates glucocorticoid sensitivity by targeting phosphodiesterase 4B in diffuse large B cell lymphoma. Gene 558:173–180
Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32:2682–2688
Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ (2016) Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA 113:170–175
Ge X, Yu J, Huang S, Yin Z, Han Z, Chen F, Wang Z, Zhang J, Lei P (2018) A novel repetitive mild traumatic brain injury mouse model for chronic traumatic encephalopathy research. J Neurosci Methods 308:162–172
Osier N, Motamedi V, Edwards K, Puccio A, Diaz-Arrastia R, Kenney K, Gill J (2018) Exosomes in acquired neurological disorders: new insights into pathophysiology and treatment. Mol Neurobiol 55:9280–9293
Paolicelli RC, Bergamini G, Rajendran L (2018) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157
Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO (2014) Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34:15482–15489
Budnik V, Ruiz-Canada C, Wendler F (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17:160–172
Kramer-Albers EM, Hill AF (2016) Extracellular vesicles: interneural shuttles of complex messages. Curr Opin Neurobiol 39:101–107
Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L (2005) Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol 175:2237–2243
Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C (2012) Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 31:1231–1240
Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C (2015) Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep 16:213–220
Hooper C, Sainz-Fuertes R, Lynham S, Hye A, Killick R, Warley A, Bolondi C, Pocock J, Lovestone S (2012) Wnt3a induces exosome secretion from primary cultured rat microglia. BMC Neurosci 13:144
Tamboli IY, Barth E, Christian L, Siepmann M, Kumar S, Singh S, Tolksdorf K, Heneka MT, Lutjohann D, Wunderlich P, Walter J (2010) Statins promote the degradation of extracellular amyloid {beta}-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J Biol Chem 285:37405–37414
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, Ikezu T (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18:1584–1593
Ban BK, Jun MH, Ryu HH, Jang DJ, Ahmad ST, Lee JA (2013) Autophagy negatively regulates early axon growth in cortical neurons. Mol Cell Biol 33:3907–3919
Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L (2018) Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem 47:864–878
Wu H, Wang F, Hu S, Yin C, Li X, Zhao S, Wang J, Yan X (2012) MiR-20a and miR-106b negatively regulate autophagy induced by leucine deprivation via suppression of ULK1 expression in C2C12 myoblasts. Cell Signal 24:2179–2186
Menghini R, Casagrande V, Marino A, Marchetti V, Cardellini M, Stoehr R, Rizza S, Martelli E, Greco S, Mauriello A, Ippoliti A, Martelli F, Lauro R, Federici M (2014) MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis 5:e1029
Korkmaz G, le Sage C, Tekirdag KA, Agami R, Gozuacik D (2012) miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 8:165–176
Zhao G, Zhang JG, Liu Y, Qin Q, Wang B, Tian K, Liu L, Li X, Niu Y, Deng SC, Wang CY (2013) miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKalpha1. Mol Cancer Ther 12:83–93
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, Moriyama M (2010) MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res 70:2339–2349
Liao WT, Ye YP, Zhang NJ, Li TT, Wang SY, Cui YM, Qi L, Wu P, Jiao HL, Xie YJ, Zhang C, Wang JX, Ding YQ (2014) MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. J Pathol 232:415–427
Wang F, Xiong L, Huang X, Zhao T, Wu LY, Liu ZH, Ding X, Liu S, Wu Y, Zhao Y, Wu K, Zhu LL, Fan M (2013) miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res 11:657–667
Li S, Qiang Q, Shan H, Shi M, Gan G, Ma F, Chen B (2016) MiR-20a and miR-20b negatively regulate autophagy by targeting RB1CC1/FIP200 in breast cancer cells. Life Sci 147:143–152
Mehta AK, Hua K, Whipple W, Nguyen MT, Liu CT, Haybaeck J, Weidhaas J, Settleman J, Singh A (2017) Regulation of autophagy, NF-kappaB signaling, and cell viability by miR-124 in KRAS mutant mesenchymal-like NSCLC cells. Sci Signal 10(4):eaam6291
Vuokila N, Lukasiuk K, Bot AM, van Vliet EA, Aronica E, Pitkanen A, Puhakka N (2018) miR-124-3p is a chronic regulator of gene expression after brain injury. Cell Mol Life Sci 75:4557–4581
Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M (2016) MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 91:151–165
An F, Gong G, Wang Y, Bian M, Yu L, Wei C (2017) MiR-124 acts as a target for Alzheimer's disease by regulating BACE1. Oncotarget 8:114065–114071
Dong RF, Zhang B, Tai LW, Liu HM, Shi FK, Liu NN (2018) The neuroprotective role of MiR-124-3p in a 6-hydroxydopamine-induced cell model of Parkinson's disease via the regulation of ANAX5. J Cell Biochem 119:269–277
Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X, Lu G, Zhang S (2016) miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson's disease. Am J Transl Res 8:2127–2137
Abbi S, Ueda H, Zheng C, Cooper LA, Zhao J, Christopher R, Guan JL (2002) Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol Biol Cell 13:3178–3191
Wang D, Olman MA, Stewart J Jr, Tipps R, Huang P, Sanders PW, Toline E, Prayson RA, Lee J, Weil RJ, Palmer CA, Gillespie GY, Liu WM, Pieper RO, Guan JL, Gladson CL (2011) Downregulation of FIP200 induces apoptosis of glioblastoma cells and microvascular endothelial cells by enhancing Pyk2 activity. PLoS ONE 6:e19629
Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, Wu D, Zhang ZG (2017) Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol Neurobiol 54:2659–2673
Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y (2017) Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int 111:69–81
Acknowledgements
This research was supported by Grants from the National Natural Science Foundation of China (Grant Nos. 81772060, 81471252), Tianjin Science Foundation (Grant Nos. 16JCYBJC27200, 16JCQNJC11000, 16JCYBJC26900, 18ZXDBSY00090), Tianjin Medical University General Hospital Youth Cultivation Foundation (Grant Nos. ZYYFY2016001, ZYYFY2014025). The authors appreciate Li Liu, Weiyun Cui and Lei Zhou from Tianjin Neurological Institute for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and approved by the Tianjin Medical University Animal Care and Use Committee (Permissions No. 011/2017).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, D., Huang, S., Yin, Z. et al. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem Res 44, 1903–1923 (2019). https://doi.org/10.1007/s11064-019-02825-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02825-1