Abstract
Approximately, 16 million strokes occur worldwide each year, causing 6 million deaths and considerable disability, implying an enormous social, individual health, and economic burden. Due to this high incidence, strategies to promote stroke recovery are urgently needed. Research into new therapeutic approaches for stroke has determined that intravenous administration of mesenchymal stem cells (MSCs) is a good strategy to improve recovery by amplifying mechanisms implicated in brain plasticity. Recent studies have demonstrated the efficacy of MSCs in stroke, with no need for them to reach the area of brain injury. Although the mechanisms by which they mediate restorative effects are still unknown, the evidence suggests that MSCs might use specialised communication by sending and receiving biological information included in elements called exosomes. Exosomes are nanosized extracellular vesicles released into physical environments, and they have recently been suggested to mediate restorative stem cell effects. Moreover, after stroke, exosomes can also be synthesised and released from brain cells, passing through the blood-brain barrier (BBB), and can be detected in peripheral blood or in cerebrospinal fluid. Thus, exosomes could possibly be biomarkers that reflect pathological progress and promote stroke recovery. This review discusses the translational aspects of MSC-derived exosomes and their various roles in brain repair and as circulating biomarkers in stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization, stroke is the leading cause of death and disability worldwide [1]. Approximately, 6 million people die due to stroke each year; 80% of these deaths occur in low- and middle-income countries. Pathophysiological responses after stroke are complex, and there is currently no therapy to repair the damage after stroke [1]. Specifically, for ischaemic stroke, only intravenous thrombolysis (tissue plasminogen activator), endovascular treatment, and their management in a stroke unit are effective therapies to treat the injury [2]. In the case of intracerebral haemorrhage, specific treatment approaches, including early diagnosis and haemostasis, management of blood pressure, and minimally invasive surgery to remove intraventricular blood promise to reduce mortality and increase functional survival [3]. Due to the high incidence of this disease, strategies to promote recovery in stroke are urgently needed. Along these lines, cell-based therapy has emerged as a novel key element of regenerative medicine therapies for stroke treatment. Mesenchymal stem cells (MSCs) have been extensively investigated for their reparative properties after stroke. MSCs participate in processes, such as neurogenesis, synaptogenesis, oligodendrogenesis, axonal connectivity, and myelin formation, showing efficacy not only in grey matter affectation, but also white matter injury [4,5,6,7,8,9,10,11]. Although the mechanisms by which stem cell therapy act are still unknown, recent evidence has suggested they might be related to long-distance cell-to-cell communication by paracrine function through secretory factors in the extracellular environment [5]. Intercellular communication between stem cells and the damaged organ was thought to be regulated via the release of free molecules that transmit the signal by binding to a receptor. With the discovery of exosomes that contain many molecules, a new frontier of signal transduction was opened. Exosomes have complex functions in intercellular communication and compound exchange, although their physiological roles are still poorly understood.
After stroke, exosomes can also be synthesised and released from brain cells responding to stroke, are able to pass through the blood brain barrier (BBB), and can be detected in peripheral blood or in the cerebrospinal fluid [12,13,14]. Moreover, exosomes are released into the blood stream from blood cells and endothelial cells responding to stroke [15]. Together, these exosomes can be detected in blood and can be used as biomarkers reflecting the stroke’s pathological progress and promoting recovery.
The present review summarises the main mechanisms involved in stroke regarding the related therapeutic approaches proposed, focusing on MSC-derived exosomes. Moreover, the nature and characteristics of exosomes, and their role in treatment for preclinical models of stroke and as a biomarker are discussed, elucidating how and why these vesicles could provide novel opportunities in stroke treatment and diagnosis.
Extracellular Vesicles
Extracellular vesicles (EVs) are released by almost all cell types and appear as membrane-surrounded vesicles in all body fluids [16]. According to their origin, various EV types can be distinguished: (i) ectosomes or shedding microvesicles; (ii) apoptotic bodies; and (iii) exosomes. A common feature of all three vesicle subtypes is a lipid bilayer membrane that includes proteins, RNA, DNA, and other molecules [17]. Microvesicles have diameters of 100–1000 nm and apoptotic bodies have sizes of 500 nm to several micrometres [16]. Exosomes are thought to be approximately 30–150 nm in diameter. These small membranous vesicles are formed by inward budding of endosomal membranes, resulting in the progressive accumulation of intraluminal vesicles within large multivesicular bodies that can either travel to lysosomes for degradation or release their contents into the extracellular space as “exosomes” [17]. Exosomes have complex functions in intercellular communication and compound exchange, although their physiological roles are still poorly understood. They are considered important transfer vectors for intercellular communication and can excite target areas, stimulating their biological functions [18]. Specifically, exosomes derived from stem cells have recently been suggested to mediate restorative stem cell effects and prevent postischaemic immunosuppression [19]; they could be an interesting resource for therapeutic applications in the field of regenerative medicine in stroke [20,21,22,23].
Isolation of Exosomes
Several strategies have been used to isolate exosomes from MSCs. Each approach harnesses a specific feature, such as size, density, shape, and the specific proteins in the surface [24]. The most common and accepted technique is differential ultracentrifugation. It is believed to provide better results, with low contamination of the samples, but requires a long time and specific and expensive instruments [25]. Possibly, the most functional technique to isolate exosomes is the precipitation by polymers like polyethylene glycol (PEG). Based on this, commercial kits are available making the process easier. It is an easy method that allows the use of common technologies that is also scalable to large samples sizes [26]. However, commercial exosome isolation kits are commonly associated with more impurities than ultracentrifugation [27]. Other techniques used are sequential ultrafiltration or size exclusion chromatography (SEC) [28], magneto-immunocapture [29], and acoustic nanofilter [30]. These approaches have proven effective for obtaining exosomes but demand the use of specialised equipment managed by skilled employees.
Exosome Markers and Content
Exosomes are typically enclosed in a lipid bilayer membrane, which is used for transport and serves to protect the luminal cargo against damage from the severe extracellular environment. This lipid bilayer also contains polysaccharide and glycan signatures on their lipid surface, predominantly comprised of polylactosamine, α-2,3- and α-2,6-sialic acids, mannose, and complex N-linked glycans [31]. In addition, this bilayer contains proteins, some of which have been considered specific exosome markers. Keerthikumar et al. have identified CD9, Alix, CD63, and TSG101 in exosomes [32]. All these markers, together with CD81, can be used to identify exosomes; otherwise, they could be mistaken for other extracellular vesicles [17, 33].
Exosomes are also comprised of luminal cargo (e.g., RNA, DNA, proteins, peptides, and lipid-derivatives) inside the lipid bilayer membrane:
Regarding RNA and DNA, exosome formation by invagination of the multivesicular body’s membrane also sequesters a large amount of cytosol, including the therein-contained RNA. In 2007, Valadi et al. were the first to confirm the presence of RNA inside exosomes, including mRNA, miRNA, and some noncoding RNA [34]. In addition, exosomes were shown to carry single-stranded DNA, double-stranded DNA, amplified oncogene sequences, transposable elements, and mitochondrial DNA [35,36,37].
Exosomes have also been shown to carry proteins and peptides. Previous studies from our group using proteomics analyses of the MSC-derived exosomes have identified 2416 proteins that are implicated in three global functions: molecular function regulation, catalytic activity, and binding. Moreover, exosomes contain proteins involved in brain repair functions, including synaptic transmission, neuronal differentiation from neural stem cells, angiogenesis, neuronal projections, neurite outgrowth, neurite fasciculation, and axonal growth [22, 38]. Whilst exosomes contain a common set of proteins irrespective of the cell type (some of which are presumably involved in exosome biogenesis) and exosomes could present a tissue/cell type-specific signature [17], it is unclear how these proteins are targeted to exosomes. More studies are needed to unravel any sorting/packaging signals in exosomes and to address the question of selectivity versus randomness.
Apart from RNA, DNA, and proteins, exosomes are also enriched in certain lipids, primarily ceramide, cholesterol, phosphatidylserine, and sphingolipids [39,40,41].
The development of ExoCarta (available online: http://www.exocarta.org), a manually curated database that lists proteins, RNA, and lipids identified in exosomes [42], and Vesiclepedia (available online: http://microvesicles.org), a community annotation compendium for all exosomes [43], have allowed researchers to successively add identified constituents of exosomes and provide a general overview of their molecular composition [42]. These two databases are supplemented with contributions from several authors working in the exosome field. Furthermore, ExoCarta also provides annotations with International Society of Extracellular Vesicles standards, thereby aiding researchers in comprehending the characterisation performed on the exosomes.
Mesenchymal Stem Cell-Derived Exosomes as Treatment for Stroke
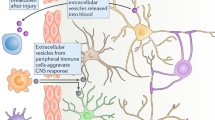
Multiple studies have found MSC-derived exosomes to be good candidates for the treatment of stroke due to their ability to mediate restorative effects and to play a role in the neural plasticity mechanisms involved in stroke (Fig. 1). Ten studies have examined the effects of MSC-derived exosomes for their ability to induce restorative effects in stroke models [19, 21, 22, 38, 44,45,46,47,48,49], which are described below:
Role of exosomes as a treatment and biomarker after stroke. a Exosomes as a treatment in experimental animal models of stroke. MSC-derived exosomes are administered by the systemic route in experimental animal models of stroke. MSC-derived exosomes increase neurogenesis, angiogenesis, neurite remodelling, axonal sprouting, and oligodendrogenesis. Moreover, MSC-derived exosomes decrease apoptosis and immune system response. b Content of exosomes as a biomarker in preclinical and clinical studies. Different levels of miRNAs and proteins have been associated with stroke not only in experimental animal models but also in patients
Functional Recovery
Recent studies using MSC-derived exosomes have been found to reduce neurological impairment. Intravenously administered exosomes achieved better results in foot-fault and modified neurological severity score tests in a model of transient intraluminal middle cerebral artery occlusion [21]. Moreover, in the only mouse study performed, intravenously administered MSC-derived exosomes using the same model reduced postischaemic motor coordination impairment as shown on the rotarod, tightrope, and corner turn tests [19]. In another study, MSC+ exosomes enhanced recovery of neurological function, according to results from the corner test [44]. Intravenous administration of exosomes also improved functional recovery, as shown by the beam walking test, the modified Rogers scale, and the rotarod test in an experimental animal model of subcortical stroke [38] and in an experimental animal model of intracerebral haemorrhage [22].
Long-term Brain Protection
Intravenously administered MSC-derived exosomes have not been shown on magnetic resonance imaging to reduce the lesion volume [21]. However, exosomes have been demonstrated to induce protection inside the core of the lesion. MSC+ exosomes have decreased the expression of cleaved caspase 3 and cleaved PARP, two indicators of apoptosis in the lesion zone. Moreover, the protein expressions of γ-H2AX, a DNA damage marker, and cytosolic cytochrome C, a mitochondrial damage marker, were both decreased in the MSC+ exosome-treated animals [44].
Grey Matter Repair
Exosomes have been demonstrated to promote grey matter repair and recovery, showing an increase in neurogenesis (increased densities of doublecortin and NeuN+ neurons) in the ischaemic boundary zone [21]. Moreover, CD31+ BrdU+ cells were also elevated, indicating that new endothelial cells had formed [19]. Exosomes also increased the cellular expression of the vWF marker and the number of small vessels, indicating higher endothelial function integrity and angiogenesis [44].
Peripheral Immune Response
Exosome administration attenuates the effects of focal cerebral ischaemia on the cellular composition of the peripheral blood system and the activation state of its cells, indicating that exosomes reverse stroke-induced peripheral immunosuppression. Thus, MSC+ exosomes attenuated the twofold increase in the activation of CD4 and CD8 T lymphocytes and the decrease in dendritic cells that occurred in saline-treated mice [19]. In another study, MSC+ exosomes decreased the expression of MMP-9, IL-1β, tumour necrosis factor (TNF)α, RANTES, PAI-1, NF-κB, iNOS CD11, and CD68, indicating a decrease in inflammation [44].
White Matter Repair
Exosome treatment produces a significant enhancement of white matter repair. In one study, these improvements were associated with neurite remodelling in the ischaemic boundary zone: accumulation of phosphorylated neurofilament and synaptophysin-positive areas were significantly increased along the ischaemic boundary zone of the cortex and striatum [21]. In other studies, diffusion tensor imaging tractography data showed that exosomes significantly improved mean axial diffusivity, indicating an enhancement in fibre tract integrity [22, 38]. Along these lines, the authors observed an increase in axonal density in the cortex area, indicating that exosome treatment produces significant axonal sprouting from the cortex to the striatum. Moreover, oligodendrogenesis-associated markers (2′,3′-cyclic-nucleotide 3′-phosphodiesterase [CNPase], A2B5, and myelin oligodendrocyte glycoprotein) were increased in those animals receiving treatment. Lastly, these authors found myelin restoration after exosome administration [38].
Biodistribution
Intravenously administered, labelled MSC-derived exosomes were found in brain tissue and in peripheral organs, such as the lung, liver, and spleen at 24 h after administration. The authors also found colabelling between exosomes with vascular endothelial growth factor, NeuN, CNPase, and Iba-1 at 24 h after exosome administration [22, 38].
Altogether, these preclinical studies indicate the possible beneficial roles of exosomes in stroke (Table 1).
Endogenous Drug Delivery Nanosystem
Some authors have demonstrated that exosomes hold great promise as an endogenous drug delivery nanosystem for the treatment of cerebral ischaemia given their unique properties, including high delivery efficiency and the ability to cross the BBB. Researchers have attached molecules to the exosome surface or content to improve their efficacy. Along these lines, modified exosomes could efficiently deliver miR-17-92 [45], miR-124 [46], microRNA 133b [47, 48], and RGDyK peptide [49], obtaining significantly enhanced outcomes compared with naïve MSC-derived exosome treatment.
These studies suggest that exosomes can be used therapeutically for the targeted delivery of gene drugs to the brain; thus, there is great potential for clinical applications.
Therapeutic Characteristics of Exosomes
The use of exosomes derived from MSCs is potentially translational because they have the following characteristics:
-
Exosomes derived from MSCs have been demonstrated to mediate restorative effects in experimental animal models of stroke [19, 21, 22, 38, 44].
-
Exosomes resolve several safety considerations, potentially associated with the transplantation of living cells, including immune compatibility and formation of tumours and emboli [50].
-
Exosomes mimic “nature’s delivery systems”, allowing for the delivery of their biological molecules, which participate in brain repair mechanisms [22, 51].
-
Due to the exosomes’ small size, they can avoid phagocytosis or degradation by macrophages and can circulate for extended periods of time within the body [51].
-
Storage can be performed without the application of potentially toxic cryopreservative agents for a long period [50].
-
The use of MSC-sourced exosomes is economical and practical for clinical use because it prevents invasive cell collection procedures, and they can be immediately available for treatment of the acute phase of stroke.
-
Selective manipulation of exosome cargo expression might lead to an enhancement of therapeutic efficiency, leading to individualised medicine [45,46,47,48,49].
-
Quantification and analysis of circulating exosomes can serve as biomarkers in patients with stroke [13,14,15].
Comparison Between MSC-derived Exosomes and Other Sources of Exosomes Tested for Stroke
Exosomes from various cell types have been tested as a treatment for stroke in addition to MSCs, such as embryonic stem cells (ESCs), neural stem cells (NSCs), and mononuclear cells (MNCs).
All these exosome sources have shown efficacy in experimental animal models of stroke. ESC-derived exosomes showed improvement in neurological scores and similarly showed reduction in lesion volume and brain water content. Moreover, ESC-derived exosomes not only considerably reduced glial fibrillary acidic protein expression but also rescued NeuN-positive neurons to a significant extent [52]. NSC-derived exosomes enhanced sensorimotor function and significantly decreased tissue loss and lesion volume [53]. Along these lines, MNC-derived exosomes have been shown to improve neurological outcome and affect a mean decrease of 36% in total infarct volume [54]. In comparison, intravenously administered MSC-derived exosomes reduced postischaemic motor coordination impairment in various experimental animal models of stroke, such as transient intraluminal middle cerebral artery occlusion [21], focal cerebral ischaemia [19], left middle cerebral artery occlusion [44], and subcortical stroke [38]. MSC-derived exosomes have been demonstrated to promote grey matter repair and recovery, showing an increase in neurogenesis and angiogenesis in the ischaemic boundary zone [19, 21, 44].
Independently of its source, recovery mediated by exosomes could be due, at least in part, to their immunomodulatory effects. In this sense, ESC-derived exosomes decreased levels of reactive oxygen species and levels of TNFα mRNA, downregulated the NR1 mRNA level, reduced malondialdehyde, and improved glutathione levels in the brain [52]. NSC-derived exosomes significantly promoted macrophage polarisation towards an anti-inflammatory M2 phenotype and increased the regulatory T cell population, resulting in the downregulation of proinflammatory effector Th17 cells [53]. In comparison, MSC-derived exosomes also have been demonstrated to mediate immunomodulatory effects, having been shown to produce a twofold decrease in the activation of CD4 and CD8 T lymphocytes, a decrease of CD4+ CD8+ T cells expressing the activation marker CD69, and an increase in the content of dendritic cells [19]. Moreover, exosomes from MSCs also decreased the expression of indicators of inflammation, such as MMP-9, IL-1β, TNFα, RANTES, PAI-1, NF-κB, and iNOS [44].
Regarding enhancement of white matter repair mechanisms, no significant improvements in diffusivity or white matter integrity were observed using NSC-derived exosomes [53]. However, MSC-derived exosome treatment produces a significant enhancement of white matter repair, increasing neurite remodelling in the ischaemic boundary zone and accumulation of phosphorylated neurofilament and synaptophysin-positive areas in the cortex and striatum [21]. Moreover, MSCs significantly improved mean axial diffusivity, indicating an enhancement in fibre tract integrity. The authors observed a significant axonal sprouting from the cortex to the striatum using MSC-derived exosomes as treatment [38].
One of the characteristics that makes exosomes an attractive treatment for stroke is that they are able to cross the BBB. In this sense, exosomes from various sources, such as ESCs, NSCs, and MSCs, when administered intravenously, reach not only different brain cellular compartments (astrocytes, neurons, and vessels) but also peripheral organs (lungs, liver, and spleen) [38, 52, 53].
Moreover, MSC-derived exosomes present several advantages in clinical applications for stroke compared with other cell-derived exosomes. MSC-derived exosomes are easy to obtain because the cells from which they are derived are obtained from healthy donors, without invasive surgery [55], compared with ESCs and NSCs. MSC-derived exosomes also present relatively low immunogenicity [50] compared with NSC-, MNC-, and ESC-derived exosomes. Moreover, using MSCs to obtain exosomes does not present ethical concerns, given they are adult stem cells [55], in comparison with ESCs (obtained from the inner cell mass of the blastocyst) and NSCs (obtained from the crest), which involves ethical and legal considerations. All these advantages mean that MSC-derived exosomes present a great opportunity for the treatment of diseases such as stroke.
Exosomes as Biomarkers
After stroke, exosomes can be synthesised and released from brain cells, passing through the BBB, and can be detected in the peripheral blood or in the cerebrospinal fluid [13, 14, 42]. Moreover, exosomes are released into the blood stream from blood cells and endothelial cells responding to stroke [15]. Together, these circulating exosomes could possibly be ideal biomarkers to reflect the pathological progress of stroke and promote recovery. Several clinical studies have examined circulating exosomal contents, including functional proteins and various nucleic acid species as biomarkers for stroke (Fig. 1).
Regarding protein content, exosome protein levels of cystatin C and CD14 have been related to an elevated risk of vascular events in patients with coronary arterial diseases. Moreover, these two proteins have also been proven to be associated with the progression of cerebral atrophy in patients with manifest vascular disease [12]. Similarly, in another study, exosome protein levels of myelin basic protein, integrin alpha-IIb, talin-1, filamin-A, and proteins of the coagulation cascade (fibrinogen alpha chain and fibrinogen beta chain) were upregulated, whereas albumin was downregulated in patients with recurrent vascular events or cognitive decline without any recurrence of vascular events [56].
In addition, distinct miRNA expression patterns in the circulating exosomes have been reported in various pathogenic stroke processes [57]. In a recent study, serum exosomal miR-9 and miR-124 levels were found to be significantly higher in patients with stroke compared with individuals without stroke. These two exosomal miRNAs were also positively correlated with the National Institutes of Health Stroke Scale scores, infarct volumes, and serum IL-6 level [58]. In another study, increased exosomal miR-223 was associated with acute ischaemic stroke occurrence, stroke severity, and short-term outcomes [59]. Lastly, miR-199b-3p, miR-27b-3p, miR-130a-3p, miR-221-3p, and miR-24-3p presented significantly higher expression in those patients with asymptomatic carotid stenosis progression, and this stenosis was associated with stroke development [60].
Future Directions: Exosomes’ Clinical Value
As Therapeutic Application
MSC-derived exosomes have a future potential clinical application mediating restorative effects, and they meet several safety considerations regarding immune compatibility with no tumour or emboli formation due to their small size [50]. Selective manipulation of exosome cargo expression might lead to an enhancement of therapeutic efficiency leading to individualised medicine in future clinical applications [45,46,47,48,49].
However, many aspects, such as the therapeutic window, the most effective administration route, and a dose-response study still need to be determined before translating the treatment to the clinic. Regarding optimum time of administration, acute delivery should be considered if the primary treatment target is concentrated on the protective mechanism; however, chronic delivery would be recommend if the main goal is centred on the repair mechanism. Taking into account that exosome administration promotes brain recovery, it should ideally be administered during the early phase to help inhibit the first steps of the ischemic cascade and to enhance mechanisms not only of protection but also of cerebral plasticity [61]. In this regard, therapies such as exosomes derived from MSCs can present an important clinical advantage because they can be administered immediately upon the diagnosis of stroke. Exosomes can be stored for long periods of time without degradation [62]. Storage at below − 70 °C is the most favourable condition for long-term preservation of fresh exosomes for clinical application [63]. Under this condition, they can be stored in hospitals and can be immediately available for treatment of the acute phase of stroke. Therefore, this therapy would mean an improvement in the patient’s deficit, a shorter hospitalisation time, and a consequent earlier hospital discharge. All this would suppose a decrease in the current health care costs of patients with stroke.
Another important aspect is administration route. Currently, no preclinical comparative studies exist to determine the route of administration that maximises the therapeutic benefits of exosomes. However, the experience derived from cell therapy has shown that the delivery route could determine the timing of administration. A less invasive approach, such as systemic routes (intravenous or intra-arterial) might be ideal in the early time window from 24 h to a month after stroke onset. In contrast, intracerebral injection would be chosen in the chronic phase, when the inflammatory response has ceased [64].
Another aspect of exosome therapy that to our knowledge has not been evaluated in preclinical studies is to analyse whether repetitive therapeutic doses might potentiate the regenerative effect better than monotherapy, to identify the optimal effective dose that could enhance protection, brain repair, and recovery after stroke. In the absence of studies, lessons could be learned from cell therapy and other pathologies such as cancer. The dosing of exosomes in past studies has varied greatly, ranging from 1 to 250 μg per in vivo injection [65]; and if large doses are to be administered in clinical settings, it is important that we fully characterise the composition of these exosomes to determinate their potential as treatment for future clinical applications.
To our knowledge, this is currently the only clinical trial in phases 1 and 2 involving exosomes in stroke. The aim is to assay the administration of MSC-derived exosomes enriched by miR-124 on improvement of disability of patients with acute ischaemic stroke, NCT03384433 [66].
As Biomarker
Importantly, exosomes can be synthesised and released from brain cells, passing through the BBB, and be detected in peripheral blood [50]; other exosomes are released into the blood from blood cells responding to stroke [15], possibly making them ideal biomarkers to reflect the pathological progress of stroke. The content of the blood circulating exosomes could play a role as biomarker in clinical studies, which represents an important clinical application for exosomes in stroke.
Limitations
Treatment with exosomes derived from MSCs is still an incipient therapy. As we have previously noted, many aspects remain to be resolved before being transferred to the clinic as a typical practice. The time of administration, the most effective route, and a dose-response study are aspects that still need to be determined before translating the treatment to the clinic. Moreover, more studies are needed to evaluate the long-term biological safety, possible adverse effects, and efficacy of exosome administration in patients with stroke.
In addition, many aspects are yet to be resolved about the production of exosomes for routine use in clinical practice; e.g., new techniques are required to obtain large-scale production of MSC-derived exosomes, and the experimental protocol for extracting exosomes from MSCs should be standardised [51].
Exosome content needs to be studied intensively. Many studies have determined the content with microarray and proteomics techniques. However, exosome content might differ according to the origin of the MSCs or the conditions of their culture in vitro.
It is also important to highlight the relevance of studying the mechanisms of MSC-derived therapy and its effects on all components of the brain that might be affected after stroke.
Conclusion
MSCs from various tissue sources have recently been demonstrated to enhance functional recovery in both ischaemic and haemorrhagic experimental models of stroke, and they are demonstrating safety in clinical trials. Despite these findings, the difficulty of treating patients in the acute disease phase and the high cost of maintaining active stem cells could limit the use of MSCs. In this context, MSC-derived exosome therapies might represent a novel strategy as a treatment and biomarker for patients with stroke. In the above paragraphs, we have reviewed the possible applications of MSC-derived exosomes in stroke. Together, these findings suggest that MSC-derived exosomes have a therapeutic potential for treating neurological disease by enhancing brain repair mechanisms and exerting immunomodulatory activities in preclinical studies and have a biomarker role in preclinical and clinical studies.
References
World Health Organization. Neurological disorders [http://www.who.int/mental_health/neurology/neurodiso/en/].
Alonso de Leciñana M, Gutiérrez-Fernández M, Romano M, et al. Strategies to improve recovery in acute ischemic stroke patients: Iberoamerican Stroke Group Consensus. Int J Stroke. 2014;9:503–13.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44.
Leu S, Lin YC, Yuen CM, Lin YC, Yuen CM, Yen CH, et al. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med. 2010;8:63.
Ikegame Y, Yamashita K, Hayashi S, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–85.
Gutiérrez-Fernández M, Rodríguez-Frutos B, Alvarez-Grech J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405.
Zhang HT, Liu ZL, Yao XQ, Yang ZJ, Xu RX. Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: a comparative study. Cytotherapy. 2012;14:1203–14.
Otero-Ortega L, Gutiérrez-Fernández M, Ramos-Cejudo J, Rodríguez-Frutos B, Fuentes B, Sobrino T, et al. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Stem Cell Res Ther. 2015;6:121.
Otero L, Zurita M, Bonilla C, et al. Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral haemorrhage. Cytotherapy. 2012;13:562–7.
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–86.
Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100.
Kanhai DA, de Kleijn DPV, Kappelle LJ, et al. Extracellular vesicle protein levels are related to brain atrophy and cerebral white matter lesions in patients with manifest vascular disease: the SMART-MR study. BMJ Open. 2014;4:3824.
Fruhbeis C, Frohlich D, Kuo WP, Kramer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182.
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–79.
Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 1862;2016:403–10.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Kalra H, Drummen G, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170.
Pegtel DM, Peferoen L, Amor S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369:516.
Doeppner TR, Herz J, Gorgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–43.
Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cell secretes immunologically active exosomes. Stem Cells Dev. 2014;23:1233–44.
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–5.
Otero-Ortega L, Gómez de Frutos MC, Laso-García F. et al. Exosomes promote restoration after an experimental animal model of intracerebral haemorrhage. J Cereb Blood Flow Metab 2017; 0: 1–13.
Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Investig. 2016;126:1190–7.
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804.
Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1. https://doi.org/10.3402/jev.v1i0.18397.
Rider MA, Hurwitz SN, Meckes DG. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. 2016;6:23978.
Han Y, Jia L, Zheng Y, Li W. Salivary exosomes: emerging roles in systemic disease. Int J Biol Sci. 2018;14:633–43.
Sokolova V, Ludwig AK, Hornung S, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B. 2011;1:146–50.
Mathivanan S, Lim JWE, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9:197–208.
Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–7.
Batista BS, Eng WS, Pilobello KT, Hendricks-Muñoz KD, Mahal LK. Identification of a conserved glycan signature for microvesicles. J Proteome Res. 2011;10:4624–33.
Keerthikumar S, Gangoda L, Liem M. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015;6:15375–96.
Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteome. 2010;73:1907–20.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett. 2009;583:1792–9.
Baluska F, Volkmann D, Barlow PW. Cell bodies in a cage. Nature. 2004;428:371.
Carrington JC. RNA silencing: moving targets. Nature. 2000;408:150–1.
Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos MC, et al. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep. 2017;7:44433.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7.
Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:202–12.
Wubbolts R, Leckie RS, Veenhuizen P.T Veenhuizen PTM, Schwarzmann G, Möbius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes: potential implications for their function and multivesicular body formation. J Biol Chem 2003; 278:10963–10972.
Simpson RJ, Kalra H and Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1.
Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:170.
Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–56.
Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, et al. Cluster microRNA cluster miR-17-92 in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–53.
Yang J, Zhang X, Chen X, et al. Exosome mediated delivery of miR-124 Promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–87.
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–46.
Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017;26:243–57.
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–49.
Vizoso FJ, Eiro N, Cid S, et al. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–96.
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–9.
Webb RL, Kaiser EE, Scoville S et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2017;In press. https://doi.org/10.1007/s12975-017-0599-2.
Altmann P, Mildner M, Haider T, et al. Secretomes of apoptotic mononuclear cells ameliorate neurological damage in rats with focal ischemia. Version 2. F1000Res. 2014;3:131.
Gutiérrez-Fernández M, Rodríguez-Frutos B, Otero-Ortega L, Ramos-Cejudo J, Fuentes B, Díez-Tejedor E. Adipose tissue-derived stem cells in stroke treatment: from bench to bedside. Discov Med. 2013;16:37–43.
Koomen JM, Datta A, Chen CP, Sze SK. Discovery of prognostic biomarker candidates of lacunar infarction by quantitative proteomics of microvesicles enriched plasma. PLoS One. 2014;9:94663.
Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, et al. A unique microRNA signature associated with plaque instability in humans. Stroke. 2011;42:2556–63.
Ji Q, Ji Y, Peng J, et al. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11:163645.
Chen Y, Song Y, Huang J, et al. Increased circulating exosomal miRNA-223 is associated with acute ischemic stroke. Front Neurol. 2017;27:57.
Dolz S, Górriz D, Tembl JI, Sánchez D, Fortea G, Parkhutik V, et al. Circulating microRNAs as novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke. 2017;48:10–6.
Gutiérrez-Fernández M, Fuentes B, Rodríguez-Frutos B, Ramos-Cejudo J, Vallejo-Cremades MT, Díez-Tejedor E. Trophic factors and cell therapy to stimulate brain repair after ischaemic stroke. J Cell Mol Med. 2012;16:2280–90.
Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M, et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles. 2017;6:1359478.
Chen J, Chopp M. Exosome therapy for stroke. Stroke. 2018;49:1083–90.
Meamar R, Dehghani L, Ghasemi M, Khorvash F, Shaygannejad V. Stem cell therapy in stroke: a review literature. Int J Prev Med. 2013;4:S139–46.
Johnsen KB, Gudbergsson JM, Skov MN, et al. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 1846;2014:75–87.
ClinicalTrials.gov, U.S. National Library of Medicine–Homepage. [www.clinicaltrials.gov].[Last accessed July 2018].
Acknowledgements
We greatly appreciate the support of ServingMed.com for editing assistance.
Funding
This study was supported by the RESSTORE project (www.resstore.eu), funded by the European Commission under the H2020 program (grant number 681044) and the European Regional Development Fund (FEDER Funding), Miguel Servet (CP15/00069, to María Gutiérrez-Fernández), and a Sara Borrell postdoctoral fellowship (CD12/00706, to Laura Otero-Ortega), a predoctoral fellowship (FI17/00188 to MariCarmen Gomez-de Frutos), PS15/01318, INVICTUS PLUS network (RD16/0019/0005) from the Research Institute Carlos III, Ministry of Science and Innovation of Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Otero-Ortega, L., Laso-García, F., Gómez-de Frutos, M. et al. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl. Stroke Res. 10, 241–249 (2019). https://doi.org/10.1007/s12975-018-0654-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0654-7