Abstract
MicroRNAs (miRs) are regulatory RNAs with 18–25 nucleotides lengths involved in various biological processes. Some miRs, including miR-22, play an essential role in regulating neurological disorders. MiR-22 is a brain-enriched regulatory element involved in angiogenesis, energy supply, adjustment of ionic channels, and suppression of malignant cell proliferation, migration, and invasion. This article discusses the protective and therapeutic effects of miR-22 on neurological diseases and injuries, including cerebral ischemia, neurodegenerative diseases, epilepsy, and brain malignancies. We also correlated miR-22 with amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), panic disorders, schizophrenia, neural tube defect (anencephaly), and traumatic brain injury. This work provides a therapeutic perspective for miR-22 as a new approach in treating neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MiRs are expressed in all body organs, including the nervous system [1]. MiRs are believed to regulate approximately 60% of human genes, and 70% of known miRs are expressed in the brain [2]. Since a single miR can target and change the expression of many genes or many other miRs, they have fundamental roles in normal physiological processes and pathological conditions [3]. MiRs have been shown to play fundamental roles in several cellular and molecular mechanisms, including neurodevelopment, brain plasticity, cell maturation, differentiation, and survival [4]. They also play a crucial role in axons and dendrites’ outgrowth and morphology [5].
Unlike most other miRs, which belong to miR families with multiple members, miR-22 belongs to a single member miR family [6]. This miR is an evolutionally conserved miRNA, which its seed sequence is identified from the fruit fly to humans and in the mammalian genome, it is encoded by an exon of the miR-22 host gene (miR-22HG) [6] and located in chromosome 17p13 [7]. MiR-22 is widely expressed in various body tissues, including the brain [8,9,10] and its expression in both neurons and glia has been reported [11]. MiR-22 acts as a potent antioxidant and anti-inflammatory and exerts many protective effects through various mechanisms [12, 13]. Overexpression of miR‐22 increased tissue antioxidant capacity by increasing superoxide dismutase (SOD) level. It also can decrease the reactive oxygen species (ROS) and malondialdehyde (MDA) levels [13], which play a crucial role in neuronal damages. Moreover, miR‐22 has exerted anti-apoptotic effects by inhibiting the increase of the Bax/Bcl‐2, Cl‐Casp‐3/Casp‐3, and Cl‐Casp‐9/Casp‐9 ratios [13]. Therefore, miR-22 can protect cells from diseases and injuries through anti-oxidative, anti-inflammatory, and anti-apoptotic effects.
Therapeutic Potential of miR-22
MiR-22 in Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common neurodegenerative disease clinically characterized by cognitive impairment [14]. AD is the most common cause of dementia and it is estimated to be responsible for approximately 60–70% of memory disorders [15, 16]. The main pathological change in this disease is the formation of extracellular plaques after oligomerization of amyloid β (Aβ) monomer and intracellular neurofibrillary tangles made of tau protein in the different parts of the brain, including the hippocampus, and the pathogenesis of AD is mainly caused by the imbalance between production and elimination of the Aβ [17].
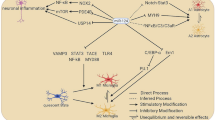
MiR-22 regulates the expression of effector genes related to AD (Fig. 1A) [18]. The expression of miR‐22 is lower in peripheral blood and the brain of AD individuals in association with the onset and development of AD [15]. In contrast, inflammatory factors, including IL‐1β, IL‐18, and TNF‐α, and NLRP3 inflammasome, have been found at higher levels in these patients. Recently, Han et al. [15] found that the expression of inflammatory factors in AD brain is negatively associated with miR‐22 and overexpression of this miR reduces the expression level of inflammatory factors in the hippocampus [15]. Hippocampus is closely involved in cognition [19, 20] and is affected severely in AD. Also, miR‐22 has been found to bind and interact with Gasdermin D (GSDMD), the protein of pyroptosis. MiR‐22 can inhibit pyroptosis by suppressing the expression of GSDMD, which leads to improvement of memory ability [15]. Pyroptosis is a novel inflammatory death pattern and mainly depends on the caspase family, especially caspase‐1, which can mediate the cleavage of GSDMD and pro‐IL‐1β [21]. These oligomers anchor on the cell membrane and result in cell membrane pore formation and increasing cellular osmotic pressure, which lead to membrane rupture and release of inflammatory factors [15].
Overview of miR-22 corresponding paths in nervous system disorders. The upregulated (green) or dysregulated miR-22 (red) might be involved in a broad spectrum of neurological conditions, including Alzheimer’s disease (A), ischemic injuries (B), Parkinson’s disease (C), Huntington’s disease (D), epilepsy (E), and brain malignancy (F). Potential interactions with signaling pathways are depicted and create conditions in which the up-regulation of miR-22 may prevent neuronal and microglia damage, abnormal angiogenesis and neurogenesis, inflammation, oxidative stress, and uncontrolled cancer promotion. In this scheme, miR-22 would describe the rationale of combinatorial therapies in nervous system disorders. BDNF: brain-derived neurotrophic factor; GSDMD: gasdermin D; IL: interleukin; TNF-α: Tumour Necrosis Factor Alpha; Rcor1: REST corepressor 1; HDAC4: histone deacetylase 4; RGS2: G-protein signaling 2; Htt: huntingtin; MAPK14: Mitogen-Activated Protein Kinase 14; TP53inp1: tumor protein p53-inducible nuclear protein 1; PGE2: prostaglandin E2; MIP-2: macrophage inflammatory protein; COX-2: cyclooxygenase-2; Ang-1: Angiopoietin-1; VEGF: Vascular endothelial growth factor; PKC: Protein kinase C; ERK: extracellular signal-related kinase; TRPM7: Transient receptor potential melastatin 7; PAPST1: 3ʹ- Phosphoadenosine 5ʹ-Phosphosulfate Transporter; SIRT1: sirtuin 1; MMP9: Matrix metallopeptidase 9; EGFR: Epidermal growth factor receptor
Intraventricular injection of miR-22 showed neuroprotective effects against AD. A study by Wang et al. revealed that intraventricular injection of miR-22 rescues disruption of the synaptic structures in the hippocampus of AD animals [22]. The work demonstrated that after miR-22 injection, the number of glial cells in the hippocampus of AD animals was reduced, whereas the number of Nissl bodies and the expression level of brain-derived neurotrophic factor (BDNF) in the hippocampal neurons were increased [22]. The overall result of the miR-22 injection was a reduction in the number of hippocampal apoptotic neurons by lowering the Bax/Bcl2 ratio in AD rats, which had led to learning and memory improvement [22]. Further research on the regulatory mechanisms of miR-22 on BDNF gene expression and optimizing the clinical administration locus/approach seem to be essential steps for future developments.
MiR-22 in Ischemic Injuries
Stroke is one of the most common neurological disorders with a high mortality and disability rate. Inflammation amplifies neural damages in ischemia associated with miR-22 downregulation (Fig. 1B). Downregulation of miR‑22 after ischemic stroke lets inflammatory factors upregulate, including interleukin 1β (IL-1β), IL‑6, IL‑18 and tumor necrosis factor‑α (TNF-α) [12]. It also induces the expression of cyclooxygenase‑2 (COX‑2), inducible NO synthase (iNOS), prostaglandin E2 (PGE2), and macrophage inflammatory protein (MIP‑2). Downregulation of miR-22 has also been shown to induce the expression of phosphorylated‑p38 (p‑p38), mitogen‑activated protein kinase (MAPK), and nuclear factor kappa B (NF‑κB) [12]. Following a stroke, inflammatory cells, including microglial cells, astrocytes, neutrophils, and lymphocytes, are activated and induce the release of inflammatory chemokines and cytokines [12, 23]. As inflammation plays a crucial role in the pathogenesis of ischemic brain injuries, modulation via miR-22 can alleviate stroke injuries and potent protective factors. A previous study found that miRNA-22 reduces IL-1β, IL-6, IL-18, and TNF-α expression and inhibits PGE2 and MIP-2 expression in the ischemic stroke model [12].
Cao et al. [8] found that miR-22 downregulation after ischemia is associated with cZNF292 (a circular RNA). They reported that cZNF292 is upregulated in ischemia conditions and results in the downregulation of miR-22. In other words, miR-22 expression negatively is regulated by cZNF292 [8]. With miR-22 downregulation after ischemia, Bax, cleaved-poly ADP-ribose polymerase (PARP) and cleaved-caspase-3 expression and frequency of apoptotic cells increases in neural stem cells (NSCs) [8]. Also, downregulation of miR-22 results in Wnt3a and β-catenin reduction. Since Wnt3a and β-catenin are involved in growth-associated processes, their reduction causes neuronal damage. cZNF292 silencing activates Wnt/β-catenin and PKC/ERK pathways and upregulates miR-22 expression [8]. Therefore, cells can be protected from ischemic damages by silencing cZNF292.
The protective effects of miR-22 against cerebral ischemia are not limited to its anti-inflammatory and anti-apoptotic properties. A recent study found a relationship between miR-22 and angiogenesis in the brain following ischemia. Wang et al. [10] observed a significant increase in the CD34+ cells and vascular endothelial growth factor+ (VEGF+) microvessels in the cortex and serum Ang-1 and VEGF levels in ischemic rats [10]. They also found that the angiogenic function of miR-22 is associated with PI3K/Akt signaling pathway.
MiR-22 in Parkinson’s Disease
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta [24,25,26]. Symptoms of PD include not only movement disabilities but also behavioral and memory disorders [24, 25], and therefore patients’ lives are severely affected.
Changes in miR-22 expression are closely associated with PD and it has been shown that its expression is downregulated in 6-hydroxy dopamine (6-OHDA)-treated cells (Fig. 1C). MiR-22 downregulation has also been observed in the cerebrospinal fluid (CSF) of PD patients [27]. Augments in ROS and oxidative stress play a crucial role in dopaminergic neuron death, leading to PD onset and progression [25, 26]. On the other hand, as the preclinical studies show, miR-22 overexpression can decrease ROS production, oxidative stress, and caspase-3 activity in 6-OHDA-treated cells [27]. MiR-22 plays its protective effect against PD via transient receptor potential melastatin 7 (TRPM7) [27]. TRPM7 is a direct target of miR-22 and is downregulated by miR-22 overexpression [27]. TRPM7 is activated by ROS and plays an important role in neuronal death by inducing toxic Ca2 influx into neurons [27, 28]. Therefore, miR-22 promotes cell survival and proliferation in 6-OHDA-induced PD by targeting TRPM7.
MiR-22 in Huntington’s Disease
Huntington’s disease (HD) is a lethal neurodegenerative disease caused by a mutation in exon 1 of the Huntingtin (Htt) gene [29] and clinically manifests with abnormal involuntary choreiform movements and mood and personality changes [30]. The protective effect of miR-22 (Fig. 1D) against HD was revealed by Jovicic et al. [29]. They induced HD on the cortical and striatal cultured neurons by exposing them to a mutated human huntingtin fragment (Htt171-82Q) and evaluated the protective effects of miR-22. The results revealed that overexpression of the miR-22 could decrease neuronal degeneration and, conversely, elevate neuronal viability through various mechanisms, including reduction of caspase activation and downregulation of MAPK14/p38 and tumor protein p53-inducible nuclear protein 1 (Tp53inp1) [29]. MiR-22 protects neurons against HD by suppressing apoptotic pathways and affecting specific HD-related markers, including Rcor1, HDAC4, Rgs2, and Htt [29]. Therefore, the protective role of miR-22 in slowing down HD progression is worthy of (pre)clinical trials.
MiR-22 and Epilepsy
Epilepsy is a brain disease with neurobiological, psychological, cognitive, and social consequences that affects more than 70 million people worldwide [31, 32]. Finding the appropriate treatment for it is a medical priority.
MiR-22 has recently been proven to have protective effects (Fig. 1E) against the development of epileptogenic networks by suppressing neuroinflammatory signaling [33]. In knockdown miR-22 animals, accelerated and exacerbated epilepsy has been reported. MiR-22 deficiency results in sooner, more prolonged, and more frequent spontaneous seizures in epileptic conditions [33]. MiR-22 is also an important regulator of newly formed neuron morphogenesis in adults and plays an essential role in suppressing aberrant neurogenesis associated with epilepsy [34]. Following status epilepticus, adult hippocampal neurogenesis increases and remains high for up to 6 weeks [35]. Aberrant hippocampal neurogenesis involves the dysregulation of cell division, maturation, morphology, and migration of newly formed neurons and their electrophysiological properties and functional integration into existing neuronal circuits [34, 36, 37]. MiR-22 is demonstrated to regulate epilepticus-induced aberrant hippocampal neurogenesis, dendritic arborization, and migration of newly formed neurons [34]. Taken together, miR-22 seems to be a valuable therapeutic marker to reduce the symptoms and injuries in temporal lobe epilepsy, as the most common form of drug-refractory acquired epilepsy. Further investigations are required to drive a clear conclusion.
MiR-22 Against Brain Malignancy
MiR-22 downregulation in glioblastoma [38] can encourage researchers to investigate the protective effects of miR-22 overexpression on this aggressive malignancy (Fig. 1F). Glioblastoma is the most malignant and common brain tumor that affects both the elderly and the young [39, 40]. According to recent reports, this tumor has a high mortality rate and survival of fewer than two years [41]. Therefore, identifying therapy is an emergency.
Studies revealed that miR-22 mimics downregulating the sirtuin 1 (SIRT1) expression and inhibits the expression of matrix metallopeptidase 9 (MMP9) and epidermal growth factor receptor (EGFR), which leads to a decrease in proliferation, migration, and invasion of tumoral cells [38].
Recently, Zhang et al. [42] reported that miR-22 overexpression increases apoptosis in glioma cells and, conversely, reduces cell proliferation by arresting cells at G2/M of the cell cycle [42]. It is demonstrated that induction of cell cycle arrest by overexpression of miR-22 is associated with depletion of cyclin B1 expression. Another critical point is that miR-22 overexpression can sharply elevate the sensitivity of glioma cells to cisplatin [42], which can increase the chance of cure. It has been revealed by TargetScan analysis that SNAIL-1 is a target of miR-22 and some essential protective effects of miR-22 are exerted through SNAIL-1 [42]. SNAIL-1 is increased in glioma cells and is associated with cell proliferation and survival. MiR-22 overexpression suppresses SNAIL-1 and results in the reduction of cell viability [42].
Also, miR-22 is protective against medulloblastoma, the most frequent malignant central nervous system (CNS) tumor in children. Its overexpression induces apoptosis and reduces cell proliferation in medulloblastoma via miR-22 target, PAPST1 [43].
The clinical value of miR-22 against malignancy is apparent in cases with complicated surgery and/or progressive malignancy and is worthy of investigation.
MiR Delivery to CNS
The specificity and a large number of targets give priority to miRs for translation to the clinics. Recent experiences suggest a high safety level for RNA therapeutics [44, 45]. However, safe delivery of the miR of interest to the target tissue is a matter of current development. This is more visible for miR-22 with functions in different tissues. The blood–brain barrier (BBB) and the unique functions of RNAs in CNS can be considered challenges towards (pre)clinical development of miR-22. Therefore, delivery of a functional miR into the specific CNS cells is critical. To date, several methods have been developed for microRNA delivery to CNS.
Intranasal Administration
This type of administration is a common approach and non-invasive pathway for miR-based drugs delivery to bypass the BBB and allow access to the brain [44,45,46]. In addition to being non-invasive, another advantage of intranasal delivery is that the drug does not undergo changes in circulation and directly enters the brain. MiRs can be loaded on the nanoparticles and administered intranasally [47]. Although no intranasal administration of miR-22 has been reported to treat neurological diseases, several studies have reported the therapeutic effects of intranasal administration of other microRNAs [44, 45, 47, 48]. Therefore, this method can be an appropriate approach for delivering miR-based drugs to CNS.
Intracerebroventricular Injection
Intracerebroventricular (ICV) injection is another way to bypass the BBB and deliver miR-based drugs to the brain. Unlike intranasal administration, this method is invasive and usually is used in experimental animal models [11, 22, 33]. Also, miRs can be genetically deleted or overexpressed by this method [33]. As experimental studies show, ICV injection of miRs can be a potent therapeutic approach. For example, during the first few days after status epilepticus, ICV injection of miR-22 in mice reduces spontaneous seizures [11]. Also, ICV injection of miR-22 in AD rats has increased BDNF expression, inhibited neuronal apoptosis, and improved cognition performance [22]. This approach can give the locus specificity for the delivery of miRs, which can be considered in the case of malignancy.
Intrathecal Injection
Due to the extensive contact between CSF and CNS, intrathecal injection is an attractive method to deliver drugs and cells to CNS, especially the spinal cord, in extensive diseases such as ALS and ischemia [49,50,51,52]. This method is an appropriate and safe route for drug delivery and is easily performable by lumbar puncture [49].
Exosome-Mediated Delivery
Exosome-mediated delivery is a novel method, which made it possible to cross the BBB and transmit miRs to the brain by even intravenous injection [53]. Exosomes are 30 to 100 nm cell-secreted vesicles in diameter that can cross the BBB and carry miRs to the brain [54]. Several studies have been reported therapeutic effects of exosomal miRs against neurological diseases [53, 55, 56]. Nevertheless, limiting the exosomes to be brain-specific is rarely possible, and for miRs like miR-22 can be challenging.
Viral and Nonviral Vectors
Vectors could efficiently transfer miRs into target tissues and cells and are classified into two main categories: viral and nonviral [57]. Viral vectors are formed from retroviruses, lentiviruses, and adenoviruses and provide high transfection efficiency. Although nonviral vectors provide lower transfection efficiency, they are much less toxic and immunogenic [57].
Studies have shown that miRs can be transferred into the CNS by vectors to alleviate the symptoms in neurological diseases, including AD [58], PD [59], HD [60], and ALS [61]. However, the long-term clinical perspective of this approach is controversial.
Conclusions and Future Perspectives
MiR-22 is a potent protective agent against many neurological disorders, including AD, PD, HD, epilepsy, cerebral ischemia, and brain malignancies, including glioblastoma, glioma, and medulloblastoma (Fig. 1). This miR exerts its protectivity through various mechanisms, including suppressing the overproduction of ROS and inflammatory factors, inhibiting normal cell apoptosis, and some other molecular mechanisms. It also exerts its effects via some known targets such as TRPM7, SNAIL-1, and PAPST1. Future studies should search and find the other possible targets.
Furthermore, we have found a possible correlation between miR-22 and other neurological disorders (Table 1). However, further investigation is needed to conclude.
MiR-22, with the mentioned acts and potential, can be an ideal therapeutic target/agent for neurological disorders. The interplay of miR-22 with environmentally originated stimuli [62] should be clarified. The regulatory mechanisms effective on miR-22, in a pathological context, need further investigation. A better understanding of the signaling pathways/feedback loops that can modulate the expression and action of miR-22 can help further therapeutic development. Regarding the therapeutic potential of miR-22, we suggest the design of (pre)clinical trials to use this RNA as a biological therapeutic agent in brain disorders and possibly other organs. Either delivery of the synthetic miR-22 to the CNS or blocking its inhibitors via small molecules can exhibit neuroprotective effects in different neuropathological conditions. The roles of miR-22 in axon regeneration and suppressing malignancy are particularly interesting to explore. Using smart nanoparticles can guide us to a superior therapeutic capability with lesser off-targets.
References
Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C (2016) Distribution of miRNA expression across human tissues. Nucleic Acids Res 44(8):3865–3877. https://doi.org/10.1093/nar/gkw116
Kou X, Chen D, Chen N (2020) The Regulation of microRNAs in Alzheimer’s disease. Front Neurol 11:288. https://doi.org/10.3389/fneur.2020.00288
Reddy KB (2015) MicroRNA (miRNA) in cancer. Cancer Cell Int 15:38. https://doi.org/10.1186/s12935-015-0185-1
Tonacci A, Bagnato G, Pandolfo G, Billeci L, Sansone F, Conte R, Gangemi S (2019) MicroRNA cross-involvement in autism spectrum disorders and atopic dermatitis: a literature review. J Clin Med 8(1):88. https://doi.org/10.3390/jcm8010088
Ma D, Qiao J, Qu Q, He F, Chen W, Yu B (2020) Weighted gene co-expression network analysis to investigate the key genes implicated in global brain ischemia/reperfusion injury in rats. Adv Clin Exp Med. 29(6):649–659. https://doi.org/10.17219/acem/121918
Huang ZP, Wang DZ (2014) miR-22 in cardiac remodeling and disease. Trends Cardiovasc Med 24(7):267–272. https://doi.org/10.1016/j.tcm.2014.07.005
Wang J, Li Y, Ding M, Zhang H, Xu X, Tang J (2017) Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review). Int J Oncol 50(2):345–355. https://doi.org/10.3892/ijo.2016.3811
Cao Y, Liu H, Zhang J, Dong Y (2020) Circular RNA cZNF292 silence alleviates OGD/R-induced injury through up-regulation of miR-22 in rat neural stem cells (NSCs). Artif Cells Nanomed Biotechnol 48(1):594–601. https://doi.org/10.1080/21691401.2020.1725536
Hu J, Zhou W, Zhou Z, Yang Q, Xu J, Dong W (2020) miR-22 and cerebral microbleeds in brainstem and deep area are associated with depression one month after ischemic stroke. Braz J Med Biol Res 53(5):e9162. https://doi.org/10.1590/1414-431x20209162
Wang X, Shi C, Pan H, Meng X, Ji F (2020) MicroRNA-22 exerts its neuroprotective and angiogenic functions via regulating PI3K/Akt signaling pathway in cerebral ischemia-reperfusion rats. J Neural Transm (Vienna) 127(1):35–44. https://doi.org/10.1007/s00702-019-02124-7
Jimenez-Mateos EM, Arribas-Blazquez M, Sanz-Rodriguez A, Concannon C, Olivos-Ore LA, Reschke CR et al (2015) microRNA targeting of the P2X7 purinoceptor opposes a contralateral epileptogenic focus in the hippocampus. Sci Rep 5:17486. https://doi.org/10.1038/srep17486
Dong H, Cui B, Hao X (2019) MicroRNA-22 alleviates inflammation in ischemic stroke via p38 MAPK pathways. Mol Med Rep 20(1):735–744. https://doi.org/10.3892/mmr.2019.10269
Tang Q, Len Q, Liu Z, Wang W (2018) Overexpression of miR-22 attenuates oxidative stress injury in diabetic cardiomyopathy via Sirt 1. Cardiovasc Ther. 36(2):e12318. https://doi.org/10.1111/1755-5922.12318
Soria Lopez JA, González HM, Léger GC (2019) Alzheimer’s disease. Handb Clin Neurol 167:231–255. https://doi.org/10.1016/B978-0-12-804766-8.00013-3
Han C, Guo L, Yang Y, Guan Q, Shen H, Sheng Y, Jiao Q (2020) Mechanism of microRNA-22 in regulating neuroinflammation in Alzheimer’s disease. Brain Behav 10(6):e01627. https://doi.org/10.1002/brb3.1627
Mantzavinos V, Alexiou A (2017) Biomarkers for Alzheimer’s disease diagnosis. Curr Alzheimer Res 14(11):1149–1154. https://doi.org/10.2174/1567205014666170203125942
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine 14:5541–5554. https://doi.org/10.2147/IJN.S200490
Cheng XR, Cui XL, Zheng Y, Zhang GR, Li P, Huang H et al (2013) Nodes and biological processes identified on the basis of network analysis in the brain of the senescence accelerated mice as an Alzheimer’s disease animal model. Front Aging Neurosci 5:65. https://doi.org/10.3389/fnagi.2013.00065
Baradaran R, Khoshdel-Sarkarizi H, Kargozar S, Hami J, Mohammadipour A, Sadr-Nabavi A, Peyvandi Karizbodagh M, Kheradmand H et al (2020) Developmental regulation and lateralisation of the α7 and α4 subunits of nicotinic acetylcholine receptors in developing rat hippocampus. Int J Dev Neurosci 80(4):303–318. https://doi.org/10.1002/jdn.10026
Rastegar-Moghaddam SH, Mohammadipour A, Hosseini M, Bargi R, Ebrahimzadeh-Bideskan A (2019) Maternal exposure to atrazine induces the hippocampal cell apoptosis in mice offspring and impairs their learning and spatial memory. Toxin Reviews 38(4):298–306. https://doi.org/10.1080/15569543.2018.1466804
Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277(1):61–75. https://doi.org/10.1111/imr.12534
Wang Y, Zhao L, Kan B, Shi H, Han J (2018) miR-22 exerts anti-alzheimic effects via the regulation of apoptosis of hippocampal neurons. Cell Mol Biol 64(15):84–89
Anrather J, Iadecola C (2016) Inflammation and stroke: an overview. Neurotherapeutics 13(4):661–670. https://doi.org/10.1007/s13311-016-0483-x
Bigham M, Mohammadipour A, Hosseini M, Malvandi AM, Ebrahimzadeh-Bideskan A (2021) Neuroprotective effects of garlic extract on dopaminergic neurons of substantia nigra in a rat model of Parkinson’s disease: motor and non-motor outcomes. Metab Brain Dis 36(5):927–937. https://doi.org/10.1007/s11011-021-00705-8
Mohammadipour A, Haghir H, EbrahimzadehBideskan A (2020) A link between nanoparticles and Parkinson’s disease Which nanoparticles are most harmful? Rev Environ Health 35(4):545–556. https://doi.org/10.1515/reveh-2020-0043
Heidari Z, Mohammadipour A, Haeri P, Ebrahimzadeh-Bideskan A (2019) The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran J Basic Med Sci. 22(7):745–751. https://doi.org/10.22038/ijbms.2019.33611.8018
Yang CP, Zhang ZH, Zhang LH, Rui HC (2016) Neuroprotective Role of MicroRNA-22 in a 6-hydroxydopamine-induced cell model of Parkinson’s disease via regulation of its target gene TRPM7. J Mol Neurosci 60(4):445–452. https://doi.org/10.1007/s12031-016-0828-2
Sun Y, Sukumaran P, Schaar A, Singh BB (2015) TRPM7 and its role in neurodegenerative diseases. Channels (Austin) 9(5):253–261. https://doi.org/10.1080/19336950.2015.1075675
Jovicic A, Zaldivar Jolissaint JF, Moser R, Silva Santos Mde F, Luthi-Carter R (2013) MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington’s disease-related mechanisms. PLoS ONE 8(1):e54222. https://doi.org/10.1371/journal.pone.0054222
Cepeda C, Tong XP (2018) Huntington’s disease: from basic science to therapeutics. CNS Neurosci Ther 24(4):247–249. https://doi.org/10.1111/cns.12841
Fei Y, Shi R, Song Z, Wu J (2020) Metabolic control of epilepsy: a promising therapeutic target for epilepsy. Front Neurol 11:592514. https://doi.org/10.3389/fneur.2020.592514
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701. https://doi.org/10.1016/S0140-6736(18)32596-0
Almeida Silva LF, Reschke CR, Nguyen NT, Langa E, Sanz-Rodriguez A, Gerbatin RR (2020) Genetic deletion of microRNA-22 blunts the inflammatory transcriptional response to status epilepticus and exacerbates epilepsy in mice. Mol Brain 13(1):114. https://doi.org/10.1186/s13041-020-00653-x
Beamer EH, Jurado-Arjona J, Jimenez-Mateos EM, Morgan J, Reschke CR, Kenny A et al (2018) MicroRNA-22 Controls aberrant neurogenesis and changes in neuronal morphology after status epilepticus. Front Mol Neurosci 11:442. https://doi.org/10.3389/fnmol.2018.00442
Jessberger S, Parent JM (2015) Epilepsy and adult neurogenesis. Cold Spring Harb Perspect Biol 7(12):a020677. https://doi.org/10.1101/cshperspect.a020677
Kelly T, Beck H (2017) Functional properties of granule cells with hilar basal dendrites in the epileptic dentate gyrus. Epilepsia 58(1):160–171. https://doi.org/10.1111/epi.13605
Bielefeld P, Durá I, Danielewicz J, Lucassen PJ, Baekelandt V, Abrous DN et al (2019) Insult-induced aberrant hippocampal neurogenesis: functional consequences and possible therapeutic strategies. Behav Brain Res 372:112032. https://doi.org/10.1016/j.bbr.2019.112032
Chen H, Lu Q, Fei X, Shen L, Jiang D, Dai D (2016) miR-22 inhibits the proliferation, motility, and invasion of human glioblastoma cells by directly targeting SIRT1. Tumour Biol 37(5):6761–6768. https://doi.org/10.1007/s13277-015-4575-8
Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N (2018) Current challenges and opportunities in treating glioblastoma. Pharmacol Rev 70(3):412–445. https://doi.org/10.1124/pr.117.014944
Soomro SH, Ting LR, Qing YY, Ren M (2017) Molecular biology of glioblastoma: classification and mutational locations. J Pak Med Assoc 67(9):1410–1414
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70(4):299–312. https://doi.org/10.3322/caac.21613
Zhang Y, Tu L, Zhou X, Li B (2020) MicroRNA-22 regulates the proliferation, drug sensitivity and metastasis of human glioma cells by targeting SNAIL1. J BUON 25(1):491–496
Xu QF, Pan YW, Li LC, Zhou Z, Huang QL, Pang JC et al (2014) MiR-22 is frequently downregulated in medulloblastomas and inhibits cell proliferation via the novel target PAPST1. Brain Pathol 24(6):568–583. https://doi.org/10.1111/bpa.12136
Wang K, Kumar US, Sadeghipour N, Massoud TF, Paulmurugan R (2021) A microfluidics-based scalable approach to generate extracellular vesicles with enhanced therapeutic MicroRNA loading for intranasal delivery to mouse glioblastomas. ACS Nano. https://doi.org/10.1021/acsnano.1c07587
Mai H, Fan W, Wang Y, Cai Y, Li X, Chen F et al (2019) Intranasal administration of miR-146a agomir rescued the pathological process and cognitive impairment in an AD mouse model. Mol Ther Nucleic Acids 18:681–695. https://doi.org/10.1016/j.omtn.2019.10.002
Zhu YQ, Liao B, Liu YH, Wang Z, Zhu XH, Chen XB, Wang MQ (2019) MicroRNA-155 plays critical effects on Th2 factors expression and allergic inflammatory response in type-2 innate lymphoid cells in allergic rhinitis. Eur Rev Med Pharmacol Sci. 23(10):4097–4109. https://doi.org/10.26355/eurrev_201905_17911
Sukumar UK, Bose RJC, Malhotra M, Babikir HA, Afjei R, Robinson E et al (2019) Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 218:119342. https://doi.org/10.1016/j.biomaterials.2019.119342
Su Y, Sun B, Gao X, Dong X, Fu L, Zhang Y et al (2020) Intranasal delivery of targeted nanoparticles loaded with miR-132 to brain for the treatment of neurodegenerative diseases. Front Pharmacol 11:1165. https://doi.org/10.3389/fphar.2020.01165
Kalkowski L, Golubczyk D, Kwiatkowska J, Holak P, Milewska K, Janowski M et al (2021) Two in one: use of divalent manganese ions as both cross-linking and MRI contrast agent for intrathecal injection of hydrogel-embedded stem cells. Pharmaceutics 13(7):1076. https://doi.org/10.3390/pharmaceutics13071076
Fang H, Yang M, Pan Q, Jin HL, Li HF, Wang RR et al (2021) MicroRNA-22-3p alleviates spinal cord ischemia/reperfusion injury by modulating M2 macrophage polarization via IRF5. J Neurochem 156(1):106–120. https://doi.org/10.1111/jnc.15042
Jin Y, Xu L, Xu Y (2021) Effect of intrathecal injection of miRNA-138 on neuropathic pain in rats undergoing partial sciatic nerve ligation and its underlying mechanism. Ann Palliat Med. 10(6):6873–6882. https://doi.org/10.21037/apm-21-669
Baumert B, Sobuś A, Gołąb-Janowska M, Ulańczyk Z, Paczkowska E, Łuczkowska K et al (2020) Local and systemic humoral response to autologous lineage-negative cells intrathecal administration in ALS patients. Int J Mol Sci 21(3):1070. https://doi.org/10.3390/ijms21031070
Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W (2019) Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res 11(2):780–792
Yang J, Zhang X, Chen X, Wang L, Yang G (2017) Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7:278–287. https://doi.org/10.1016/j.omtn.2017.04.010
Zhang Y, Liu J, Su M, Wang X, Xie C (2021) Exosomal microRNA-22-3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/BMF axis. Stem Cell Res Ther 12(1):111. https://doi.org/10.1186/s13287-020-02091-x
Lee ST, Im W, Ban JJ, Lee M, Jung KH, Lee SK et al (2017) Exosome-based delivery of miR-124 in a Huntington’s disease model. J Mov Disord. 10(1):45–52. https://doi.org/10.14802/jmd.16054
Yang N (2015) An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig 5(4):179–181. https://doi.org/10.4103/2230-973X.167646
Paul S, Bravo Vázquez LA, Pérez Uribe S, Roxana Reyes-Pérez P, Sharma A (2020) Current status of microRNA-based therapeutic approaches in neurodegenerative disorders. Cells 9(7):1698. https://doi.org/10.3390/cells9071698
Khodr CE, Becerra A, Han Y, Bohn MC (2014) Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects. Brain Res 1550:47–60. https://doi.org/10.1016/j.brainres.2014.01.010
Pfister EL, DiNardo N, Mondo E, Borel F, Conroy F, Fraser C et al (2017) Artificial miRNAs reduce human mutant huntingtin throughout the striatum in a transgenic sheep model of Huntington’s disease. Hum Gene Ther 29(6):663–673. https://doi.org/10.1089/hum.2017.199
Borel F, Gernoux G, Cardozo B, Metterville JP, Toro Cabrera GC, Song L et al (2016) Therapeutic rAAVrh10 mediated SOD1 silencing in adult SOD1(G93A) mice and nonhuman primates. Hum Gene Ther 27(1):19–31. https://doi.org/10.1089/hum.2015.122
Shahba S, Mehrzad J, Malvandi AM (2021) Neuroimmune disruptions from naturally occurring levels of mycotoxins. Environ Sci Pollut Res Int 28:32156–32176. https://doi.org/10.1007/s11356-021-14146-4
Liguori M, Nuzziello N, Introna A, Consiglio A, Licciulli F, D’Errico E, Scarafino A, Distaso E, Simone IL (2018) Dysregulation of MicroRNAs and target genes networks in peripheral blood of patients with sporadic amyotrophic lateral sclerosis. Front Mol Neurosci 11:288. https://doi.org/10.3389/fnmol.2018.00288
Ma J, Shui S, Han X, Guo D, Li T, Yan L (2016) microRNA-22 attenuates neuronal cell apoptosis in a cell model of traumatic brain injury. Am J Transl Res 8(4):1895–1902
Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, Singh N, Nagarkatti M, Nagarkatti P (2014) Expression, regulation and function of microRNAs in multiple sclerosis. Int J Med Sci 11(8):810–818. https://doi.org/10.7150/ijms.8647
Siegel SR, Mackenzie J, Chaplin G, Jablonski NG, Griffiths L (2012) Circulating microRNAs involved in multiple sclerosis. Mol Biol Rep 39(5):6219–6225. https://doi.org/10.1007/s11033-011-1441-7
Muiños-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipilä T, Maron E et al (2011) Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry 69(6):526–533. https://doi.org/10.1016/j.biopsych
Zhang WD, Yu X, Fu X, Huang S, Jin SJ, Ning Q, Luo XP (2014) MicroRNAs function primarily in the pathogenesis of human anencephaly via the mitogen-activated protein kinase signaling pathway. Genet Mol Res 13(1):1015–1029. https://doi.org/10.4238/2014.February.20.3
Yu S, Zeng YJ, Sun XC (2018) Neuroprotective effects of p53/microRNA 22 regulate inflammation and apoptosis in subarachnoid hemorrhage. Int J Mol Med 41(4):2406–2412. https://doi.org/10.3892/ijmm.2018.3392
Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM (2011) Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry 69(2):188–193. https://doi.org/10.1016/j.biopsych.2010.09.039
Acknowledgements
We express our sincere gratitude for the Vice Chancellor’s support for Research, Mashhad University of Medical Sciences, Mashhad, Iran. The authors acknowledge Prof. Giovanni Lombardi for his kind revision of this article.
Funding
This study is funded by the Mashhad University of medical sciences. AMM received support from funds from ricerca corrente to IRCCS Istituto Ortopedico Galeazzi.
Author information
Authors and Affiliations
Contributions
SHRM, AEB, and SS contributed to the drafting of the manuscript. AMM and AM contributed to overall conceptual design, drafting, and final edits and approval.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rastegar-moghaddam, S., Ebrahimzadeh-Bideskan, A., Shahba, S. et al. MicroRNA-22: a Novel and Potent Biological Therapeutics in Neurological Disorders. Mol Neurobiol 59, 2694–2701 (2022). https://doi.org/10.1007/s12035-022-02769-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02769-8