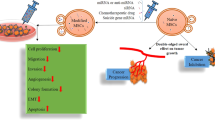
Abstract
MicroRNAs (miRs) are potential therapeutic targets in glioblastoma multiforme (GBM), but the difficulties associated with their delivery to tumor target cells have hampered their widespread use. Mesenchymal stem cells (MSCs) can migrate to the sites of cancers, including GBM and exert anti-tumor effects. In this study, it is shown that Wharton’s jelly-MSCs (WJ-MSCs) have the ability to deliver exogenous miRs to GBM cells and the functional impact of this delivery is characterized. It is found that the labeled miR-124, as an example for miR of interest, can be successfully delivered with WJ-MSCs to U87 GBM cells via dependent or exosome-independent processes. It is demonstrated that the delivered exogenous miR-124 significantly decreases the luciferase activity of the target gene CDK6. In addition, the delivered miR-124 enhances the chemosensitivity of GBM cells to temozolomide and decreases the migration of GBM cells. These results suggest that the use of exogenous miRNA delivery with the derived exosomes from WJ-MSCs may provide a novel approach for miRNA replacement therapy in GBM cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive cancer of the adult central nervous system. Current treatment regimens for GBM include surgery, radiation therapy and chemotherapy [1, 2]. Unfortunately, because of the diffuse nature of malignant gliomas that makes complete surgical resection nearly impossible and because of their resistance to chemo- and radio- therapy, prognosis remains extremely poor and the median survival of the patients have been reported to be between 12 and 14 months [3]. The poor survival is mostly related to delivery failure of therapeutic agents to tumor regions [4]. It is also shown that GBM cells consistently acquire resistance to the commonly used alkylating agents and to the other chemotherapy treatments [5]. Recent evidences suggest that mesenchymal stem cells (MSCs) have a tropism for brain tumors and can be used as delivery vehicles for treatment of GBM [6]. MicroRNAs (miRs) are small 18–22 base pairs long oligonucleotides that regulate gene expression. They suppress the translation by binding to the 3′- and 5′-untranslated regions of the targeted mRNAs [7]. MiRs can regulate numerous processes such as cell proliferation, invasion, differentiation, apoptosis and angiogenesis [8, 9]. Moreover, it is shown that miRs can influence chemoresistance in GBM cells [10]. One of the main mechanisms in GBM pathogenesis is the impairment of the miRs regulatory network in GBM cells [11].
According to previous studies [12, 13], miR-124 can be significantly downregulated in human glioma tissues versus normal brain tissues. MiR-124 is also enriched in brain and improved neuronal differentiation [14], suppressed invasion and migration of GBM cells [15], controlled cell growth through regulation of cyclin dependent kinase 6 [16] and its overexpression increased chemosensitivity of GBM cells to temozolomide [17].
Tropism of stem cells for tumors, has attracted a lot of interest in recent years for delivery of therapeutic miRs to the region of cancer cells [18, 19]. Derivation of stem cells from human umbilical cord Wharton’s jelly (WJ) have numerous advantages. Wharton’s jelly mesenchymal stem cells (WJ-MSCs) can easily be obtained in a large amount with high proliferation rate and when derived after several passages, have stemness characteristics [20, 21].
Since according to the previous reports, miR-124 is downregulated in GBM cells and this miR has the ability of tumor suppression and enhancement of chemosensitivity of GBM cells [13, 17], in this report, we have studied the transfer of miR-124 into the GBM cancer cells by the WJ-MSCs. It is shown that WJ-MSCs delivers miR-124 to glioma cells and the delivered miR-124 regulates gene targeting and impacts their function.
Materials and Methods
Isolation and Growth Conditions of WJ-MSCs
After removing the cord tissue of the infants delivered by cesarean section, isolated WJ was cut into small pieces and plated in the culture medium composed of Dulbecco’s modified Eagle’s medium low-glucose (DMEM-LG) supplemented with fetal bovine serum (FBS, 10%), L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), Fungizone (25 μg/ml), basic fibroblast growth factor (bFGF, 5 ng/ml) and epidermal growth factor (EGF, 5 ng/ml). The first medium change occurred after 4 days and followed by medium changes every 3–4 days until the cells became confluent [22]. The cells were passaged, using trypsin with diamine tetraacetic acid (EDTA), and maintained in the culture medium. Results of the FACS analysis showed that the cells were positive for CD73, CD90 and CD105 (Abcam) but negative for the hematopoietic markers CD31, CD34, CD45 (PE, eBiosciences) and HLA-DR (Abcam). Ability of the different cell types to differentiate to osteoblasts and adipocytes is investigated elsewhere [23, 24] and the protocols for differentiation and the methodology to confirm it are fully described therein. The purity of all the WJ-MSC preparations was over 95%.
Culture of HEK T293 and U87 Cells
HEK 293T and U87 cells were obtained from the Pasteur Institute of Iran (Tehran). The HEK 293T and U87 cells were maintained in DMEM supplemented with 10% FBS (FBS, Gibco), penicillin (100 U/mL, Gibco) and streptomycin (0.1 mg/mL, Gibco). The cells were cultured under standard conditions of 95% humidity and 5% CO2 at 37 °C.
Transgene lentiviral vector of miR-124 with the flanking sequences (~ 200 bps at each end) was PCR-amplified from human genomic DNA. The PCR products were cloned into plenti-III-GFP carrying fluorescent dyes and puromycin resistance gene. The cloning was confirmed by using XbaI digestion, PCR and sequencing analysis.
Transfection of miR-124
RNA duplexes corresponding to has-miR-124 were first labeled with Cy3 (Life Technologies), then WJ-MSCs with 3–4 passages were transfected by Lipofectamine 3000 (Life Technologies, Inc.). Fluorescence microscopy indicated that the transfection efficiency was approximately 70%.
Lentiviral Preparation and Transduction of WJ-MSCs
Lentivirus produce with calcium phosphate precipitation method according to manufacture’s protocol. WJ-MSCs with 3–4 passages were transduced with Plenti III-miR-124-GFP and Plenti III-GFP backbone lentiviral vectors at multiplicity of infection (MOI) of 20 in the presence of 4 mg/mL polybrene. U87 cells were transduced with Plex-jRed lentiviral vectors. GFP and j-Red expressions were visualized using a fluorescence microscope.
Preparation of Exosomes
WJ-MSCs that transduced with miR-124-Cy3 and control-miR were cultured in MSC medium using Gibco™ Exosome-Depleted FBS (Thermo Fisher, USA). Following 4 days of incubation, exosomes were isolated from the supernatants of the WJ-MSC cultures using the exosome precipitation solution, ExoQuick, (System Bioscience, Mountain View, CA). The protein coontent was determined using the Micro BCA assay kit.
Co-culture of WJ-MSCs and U87 GBM Cells
The green fluorescence CMFDA CellTracker reagent (Molecular Probes, Invitrogen) was used to label U87 GBM cells according to the manufacturer’s protocol. The labeled U87 GBM cells were added at a ratio of 1:2 to miR-transfected WJ-MSCs plated in 8-well plates (WJ-MSCs-miR-124 and U87 cells dependently contact). After 72 h, the cells were analyzed by fluorescence microscopy and flow cytometer to detect the influence of delivered miR-124 on proliferation of U87 cells.
To examine the delivery of the miR-124 mimic with independent contact, transwell chamber was employed with 0.4 μm-pore diameter filters that do not allow the cell infiltration (WJ-MSCs-miR-124-Cy3 and U87 cells). WJ-MSCs that transfected with miR-124-Cy3 were plated onto the transwell inserts, whereas U87 GBM cells were seeded in the lower well of the transwell chambers. After 72 h, the U87 GBM cells were collected and analyzed with flow cytometer to detect the delivered miR-124-Cy3 in U87 cells. Overview of the adopted co-culture method and the used transwell system is shown in Fig. 1.
Luciferase Assay
U87 cells that transfected with 3′UTR of CDK6 or GAPDH plasmids were seeded at a density of 1 × 105 cells per well in 6-well plates. After 24 h, engineered WJ-MSCs containing miR-124 or control plasmid were seeded on top of a transwell insert. U87 cells were harvested 72 h after transfection and Luciferase assays were performed. Luciferase assays were performed with the dual luciferase reporter assay system (Promega) according to the manufacturer’s instructions. The fold activation was normalized against firefly luciferase.
Temozolomide (TMZ) Treatment, Cell Viability and Apoptosis Assay
To explore the effect of miR-124 transfer in sensitivity enhancement of U87 GBM cells, U87 cells were cultivated at 5 × 103 in 96-well plates and incubated at 37 °C for one night. After 24 h, exosomes that were derived from WJ-MSC-miR-124-Cy3 and control-miR, were added to the U87 cells. Delivery of exosomes that contained miR-124 was detected with flow cytometer. After ensuring from delivery of miR-124 to the U87 cells, they were treated with 100 µM Temozolomide (TMZ). In another group and as a control, U87 cells were cultivated with the derived exosomes from WJ-MSCs that were transduced with control miR and treated with TMZ. The apoptosis assay was tested in U87 cells that were treated with transfer miR-124 and TMZ using the TUNEL kits (TMR Red Roche).To examine the viability of the U87 cells that were treated with miR-124 and TMZ, MTT assay was performed 24, 48 and 72 h after incubation of the cells with miR-124 and TMZ.
Real-time Quantitative PCR Analysis
Total RNA was extracted using QIAzol reagent (Qiagen) and complementary DNA (cDNA) synthesis was carried out with reverse transcriptase (Fermentas) and random hexamers for gene primers. Real-time PCR was performed with SYBR Premix Dimer EraserTM (TaKaRa) and analyzed using the Rotor-Gene 6.1 (Corbett) and the REST software. All the reactions were performed in triplicate. Expression data of mRNA and miRNA were normalized to GAPDH and SNORD 47 (U47) reference genes, respectively. Data concerning the specific primers are listed in Table 1.
Transwell Migration Assay
U87 labeled cells cultured with exosomes from WJ-MSC-miR-124 for 48 h and then were plated on the upper compartment of the 0.8 transwell chamber and were allowed to migrate to the underside of the top chamber for 6–8 h. The migration of the fluorescently labeled U87 labeled cells was evaluated using a fluorescence microscope. Each experiment was done in triplicates. The data were analysed by Statistical Package for the Social Sciences (SPSS software, v.16 for windows; SPSS Inc., Chicago, IL). The P values of ≤ 0.05 were considered as statistically significant. All the data are presented as mean ± SE.
Results
The purpose of the study was to determine whether WJ-MSCs can be used for delivery of miR-124 to GBM cells and providing treatment to this disease. In this section the results of treating U87 GBM cells by WJ-MSCs are provided in a stepwise manner.
Characterization of WJ-MSCs
WJ-MSCs were isolated from the WJ tissue of human umbilical cords. Our analyses confirmed consistent expression of CD73 and CD105 and CD90 in the cells. As can be verified in Fig. 2a–c, WJ-MSCs express mesenchymal antigens, lack expression of hematopoietic markers and are negative for CD31, CD34, HLA-DR and CD45 (Fig. 2d–g). The adipogenic and osteogenic differentiation in WJ-MSCs confirm the multipotency of these cells (Fig. 2h, i).
Delivery of miR-124 to U87 GBM Cells by WJ-MSCs
MiR-124 delivery to U87 GBM cells by WJ-MSC-miR-124 was investigated. MSCs can intracellularly communicate with other cells through gap junctional intercellular Green CellTracker communication (direct contacts) or secretion of vesicles and soluble factors (indirect contacts) [25, 26]. Recent studies indicate that miRs release from MSCs in form of exosomes [27, 28]. In this work and following 2 days of co-culture, the WJ-MSC-miR-124-cy3 and U87 cells labeled with Green CellTracker were viewed under a fluorescence microscope (Fig. 3a, b). As presented in Fig. 3c, d, the merged images indicate that miR-124 is efficiently delivered by the transfected WJ-MSCs into the U87 GBM cells. Also the transfer of Cy3-miR-124 was studied using two-channel flow cytometry. Figure 3e shows that WJ-MSCs were transfected with miR-124-Cy3. Transfer was confirmed by detection of Cy3 in U87 GBM cells. In transwell-cultured WJ-MSCs, Cy3 was also detected in U87 labeled GBM cells (Fig. 3f). In co-cultured cells, detection of miR-124-Cy3 shows that U87 cells are positive for Cy3 and, as such, miR-124-Cy3 is transferred from WJ-MSCs to U87 cells (Fig. 3g). These data demonstrate that miR-124 transfers with the derived exsosomes from WJ-MSCs to GBM cells. Percentage of the marked cells in both the co-culture and the transwell experiments are shown in the two-color flow cytometry dot plots in Fig. 3. In the co-culture experiment, both the interacellular and the MSC-derived exosomes deliver miR-124-Cy3 to GBM cells. Therefore, a greater percentage of cells express both Cy3 and the Green CellTracker.
Fluorescence microscopy images representing miR-124 delivery to U87 GBM cells by WJ-MSCs. WJ-MSCs were transfected with Cy3-labeled miR-124. After 24 h, U87 cells labeled with Green CellTracker CMFDA were added to the WJ-MSCs cultures and the expression of the fluorescent miR-124 in U87 cells was analyzed 24 h later with a fluorescence microscope a WJ-MSCs-miR-124-Cy3, b U87-CMFDA, c merged cells (Scale bar = 50 µM), and d Merged cells (original magnification × 20). Flow cytometry results indicating transfer of miR-124-Cy3 from WJ-MSCs to U87-CMFDA cells. e WJ-MSCs were transfected with miR-124-Cy3 f WJ-MSC-derived exosomes were added to GBM cells with transwell, left panel: the GBM cells alone; middle panel: analysis of the GBM cells for CMFDA and Cy3; right panel: Cy3 alone in the GBM cells. g U87 GBM cells co-cultured with WJ-MSC-miR-124-Cy3, left panel: the GBM cells alone; middle panel: analysis of the GBM cells and WJ-MSCs for CMFDA and Cy3; right panel: Cy3 in co-cultured cells
Downregulation of CDK6 Gene Expression in U87 GBM Cells by WJ-MSCs-Derived miR-124
To detect the delivery of miR-124 to U87 GBM cells, the function of miR-124 on GBM cells was examined. MiR-124 has been shown to be involved in regulation of proliferation and targeting CDK6 in neuronal cells that inhibit proliferation of U87 cells [29]. To ensure that miR-124 downregulates CDK6 in U87 GBM cells, its expression was detected with RT-PCR method in U87 GBM cells that were transduced with miR-124. The results of the RT-PCR analysis confirmed that the level of CDK6 decreased in U87 cell that overexpressed miR-124 (see Fig. 4a). Next, WJ-MSC-miR-124 was plated onto the chamber of 0.4 µm-pore diameter transwell inserts and U87 cells that transfected with CDK6-luciferase reporter, were seeded in the lower well of the chamber (indirect contact). After 72 h, the luciferase activity of the cells was measured. As shown in Fig. 4b, after delivery of miR-124 to U87 GBM cells by WJ-MSCs, the luciferase activity of CDK6 in the U87 cells decreased 38% compared with that in the U87 cells that co-cultured by WJ-MSCs and transduced with control miR.
The qRT-PCR analysis results indicating that WJ-MSC-delivered miR-124 downregulated the expression of CDK6 in GBM cells. a The expression level of U87 normal cells (U87-NC) and the U87 cells that were transfected with control miR (U87-Con-miR) or miR-124 (U87-miR-124). U87 cells were transfected with a CDK6 3′-UTR-luciferase plasmid followed by transfection with Con-miR or miR-124. In parallel, U87 cells expressing this plasmid were co-cultured with WJ-MSCs that were transfected with either Con-miR or miR-124. b The luciferase activity of the cells was determined after 72 h of transfection or co-culture. The results are the means ± SE of three different experiments *(p < 0.005)
Enhancement of Chemosensitivity of GBM Cells to TMZ After Delivery of miR-124 by WJ-MSCs
In order to examine whether the transferred miR-124 from WJ-MSC-miR-124 may have a potential role in chemotherapy, the U87 cells were treated with exosomes of WJ-MSC-miR-124 cells and TMZ. Cell viability in the presence of TMZ (100 μM) was assayed with the MTT method. The results showed that the transfer of miR-124 and its overexpression in U87 cells decreases the cell proliferation. As shown in the light microscopic images (Fig. 5a, b), overexpression of miR-124 in U87 cells increased their chemosensitivity to TMZ treatment. Furthermore, MTT test revealed that overexpression of miR-124 decreased the proliferation of U87 cells (Fig. 5c).
The results indicating that the delivered miR-124 by WJ-MSCs enhanced the chemosensitivity of GBM cells to TMZ. Phase contrast micrographs of U87 cells that were treated with a the combination of delivered miR-124 + TMZ induced more apoptosis compared with b the cells that are treated just with TMZ (Scale bar = 50 µM). c The cell proliferation in 100 µM TMZ treated cells were tested with MTT assay every 24 h and d TUNEL staining of the delivered miR-124 induced significant cell death 24 h after exposure, TUNEL-positive nuclei indicate that the majority of cells were undergoing apoptosis. U87 cells were cultivated with the derived exosomes from WJ-MSCs-Con miR (U87-WJ-MSCs-Con-miR) and treated with TMZ (U87-WJ-MSCs-Con-miR + TMZ). U87 cells that were cultivated with the derived exosomes from WJ-MSCs-miR-124 and treated with TMZ (U87-WJ-MSCs-miR-124 + TMZ) and U87 cells that transduced with miR-124 directly and treated by TMZ (U87-miR-124 + TMZ) (Scale bar = 50 µM)
In order to test whether the combination of miR-124 with TMZ could play a role in apoptosis, the TUNEL assay was performed. Figure 5d shows the TUNEL assay results and confirms that the combination treatment of miR-124 with TMZ induced the cell apoptosis. It is further found that the transferred miR-124 + TMZ significantly increased apoptosis relative to the sole treatment with miR-124 or TMZ. These results indicate that delivery of miR-124 by WJ-MSCs to U87 cells and subsequent treatment with TMZ, increases the potential of treatment of GBM cancer.
Decrease of GBM Migration by Transfer of miR-124
Previous reports indicate that overexpression of miR-124 in U87 GBM cells decreases their migration [15]. In this work and in order to further examine whether delivery of miR-124 by WJ-MSCs can regulate the function of U87 GBM cells, the transwell migration assay was performed for U87-jRed cells that were cultivated with the derived exosomes from WJ-MSC-miR-124. As presented in Fig. 6, after 4–8 h of plating, the exhibited fluorescence of U87-jRed cells that were cultured with the derived exosomes from WJ-MSC-miR-124 was significantly decreased compared with the cells that were cultured with control. This means that the delivered miR-124 decreased the migration of U87 cells.
The delivered miR-124 by WJ-MSCs decreased the migration of GBM cells. U87-jRed cells that cultivated with the derived exosomes from WJ-MSC-miR-124, after 4–8 h of plating and using transwell migration assay, exhibited fluorescence that is significantly lower than the fluorescence of the cells that are cultured with control. a Data are presented as the number of migrated cells. The results are the means ± SE of three different experiments, ̽ (P < 0.05). b Fluorescence microscopic pictures of the migrated U87-jRed cells (Scale bar = 50 µM)
Discussion
In this study, it is demonstrated that WJ-MSCs have the ability to migrate toward GBM cells in vitro and act as delivery vehicles for miR-124. It is further shown that delivery of miR-124 to GBM cells has the consequent effect of increasing the sensitivity of U87 cells to TMZ.
Problems with accurate delivery of therapeutic agents to the target GBM brain tumors are the main reasons for the poor outcome of treatment. Currently used vehicles for delivery of miRs, like liposomes and viral vectors, are unsuitable due to their low efficiency and safety [30]. As it is indicated in previous studies [6, 31], MSCs have inherent tumor tropism and can target tumor sites by migrating to and localizing to GBM bulk tumors in vivo. Therefore, engineered MSCs can be used as delivery vehicles for gene therapy of tumors [32,33,34]. Recent reports indicated that MSCs are also able to deliver miRs to tumor cells [35]. MiR-based therapy, depending on the function of miRs in the specific diseased tissues, is considered as one of the best therapeutic approaches for the treatment of cancer [36, 37]. MiR-124 is a brain-enriched miR that is critical for regulating neuronal development and differentiation [38, 39]. According to the previous reports [15, 16], miR-124 is significantly downregulated in GBM cells and inhibits their proliferation and decreases their migration.
In this study, it is found that WJ-MSCs efficiently deliver miR-124 to the co-cultured U87 GBM cells by localization of the Cy3-labeled miR-124 in these cells. In addition, it is demonstrated that when indirectly co-cultured, exosome delivery of miR-124 downregulates the gene expression of CDK6 as its target gene. Therefore, consistent with a previous report [40], downregulation of CDK6 expression by the delivered miR-124 decreases the proliferation of U87 GBM cells. It is also found that the delivered miR-124 decreases the migration of the U87 GBM cells. MiR-124 regulates the cell migration through targeting protein 1 (IQGAP1), laminin c1 (LAMC1) and integrin b1 (ITGB1) that each of these three targets plays a major role in GBM migration and invasion [15]. It is previously shown that delivery of miRs with MSCs may be intervened by the gap-junction-dependent and independent mechanisms and through the secretion of exosomes [28]. In this study, it is found that exosome secretion of WJ-MSCs-miR-124 facilitates the delivery of miR-124 to U87 cells and decreases their migration. This result is consistent with the reported results in a number of previous studies that the derived exosomes from MSCs are able to deliver miRs to recipient cells [40,41,42].
It is recently shown that MiR-124 governs the sensitivity of GBM cells to TMZ treatment and a combination treatment of miR-124 and TMZ induces apoptotic effects by targeting of R-Ras and N-Ras in the cells [17]. R-Ras and N-Ras are two members of the Ras family and as it is reported, their expression level is related to the malignant degree of cancers [43, 44]. In the current work, the effect of delivery of miR-124 by WJ-MSCs in the sensitization of U87 GBM cells to TMZ is also studied. The results show that the combination treatment of U87 cells with miR-124 and TMZ, compared with the U87 GBM cells that are treated just with TMZ, induces more apoptosis and indicates miR-124 functionality as a tumor suppressor. Munoz et al. report that delivery of anti-miR-9 from MSCs to the GBM cells reverses the expression of the multidrug transporter and sensitizes the GBM cells to TMZ [42]. Similarly, in our study, delivery of miR-124 by WJ-MSCs to U87 GBM cells makes them more sensitive to TMZ treatment and induces apoptosis (see Fig. 4). These results suggest the potential role of WJ-MSCs in the functional delivery of miR-124 to GBM cells.
As it is already reported [45], MSCs provide a valuable vehicle for gene therapy in a site-specific manner with low immunogenicity. Several clinical studies indicate that there are potential benefits of MSC-based therapies for the treatment and transplantation to solid organs without the need for high concentration of immunosuppressive drugs [46]. In a recent study [20], it is indicated that WJ-MSCs, in comparison with other sources of MSCs, exhibit a greater expansion capability, a higher proliferation rate, enhanced neurotrophic factors and a stronger potency to default neural lineage differentiation. Furthermore, these cells exert exceptionally low immunogenicity but with high neuroprotective potential and are a superior source for MSCs application without ethical limitations [21, 47, 48]. Therefore, WJ-MSCs not only act as vectors for delivery of miR-124 but also have the capability of inducing neural differentiation and immunogenicity. Based on this capabilities, we believe that delivery of miR-124 with WJ-MSCs can suggest a new trend of treatment of GBM cancer cells.
In a recent study [49], it is shown that GBM cells exposed to conditioned media from human umbilical cord perivascular cells, present higher cellular viability, proliferation and migration and their resistance to temozolomide chemotherapy does not significantly change. In contrast, our study shows that delivery of miR-124 by the derived exosomes from WJ-MSCs to GBM cells decreases their migration and increases their chemosensitivity to TMZ which is consistent with a number of previous studies [42, 50, 51]. These results suggest that in future research, several other aspects, such as functions of exosomes derived from MSCs as cell free vehicles for treatment of GBM and effect of MSC secretions on inducing cancer progression or suppression should also be studied to firmly confirm the MSCs potential for cancer treatments.
In summary, the field of MSC delivery of RNAs for therapeutic applications has yet to identify a specific approach for GBM cancer cells and much more studies need to be done in this area. In this report, it is shown that WJ-MSCs can effectively deliver exogenous miR-124 to GBM cells, impact the cell migration, reduce the proliferation and increase the sensitivity to TMZ in GBM cells. Therefore, the combination treatment of WJ-MSCs with the delivered miR-124 and TMZ can be a new effective treatment for GBM cancer. Our results suggest that the use of exogenous miRs delivered by WJ-MSCs may provide a novel approach for miR replacement therapy for GBM cancers or other types of tumors.
It must be noted that the present study has been performed in vitro, but Specific characteristics of exosomes, including their indigenous nature and the ability to cross biological barriers can be used in future studies for in vivo delivery of miRs. Also other types of miRs can be used with WJ-MSCs in exosome-based therapy of GBM cancer.
References
Furnari, F. B., Fenton, T., Bachoo, R. M., et al. (2007). Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes & Development, 21, 2683–2710.
Desjardins, A., Rich, J. N., Quinn, J. A., et al. (2005). Chemotherapy and novel therapeutic approaches in malignant glioma. Frontiers in Bioscience, 10, 2645–2668.
Giese, A., Bjerkvig, R., Berens, M., & Westphal, M. (2003). Cost of migration: invasion of malignant gliomas and implications for treatment. Journal of Clinical Oncology, 21, 1624–1636.
Louis, D., Posner, J., Jacobs, T., & Kaplan, R. (2000). Report of the brain tumor progress review group. Leesburg: National Institute of Neurological Disorders and Stroke, pp. 1–96.
Yamada, R., & Nakano, I. (2012). Glioma stem cells: their role in chemoresistance. World Neurosurgery, 77, 237–240.
Nakamizo, A., Marini, F., Amano, T., et al. (2005). Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Research, 65, 3307–3318.
Abba, M., Mudduluru, G., & Allgayer, H. (2012). MicroRNAs in cancer: small molecules, big chances. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 12, 733–743.
Li, Y., Guessous, F., Zhang, Y., et al. (2009). MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Research, 69, 7569–7576.
Godlewski, J., Nowicki, M. O., Bronisz, A., et al. (2008). Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Research, 68, 9125–9130.
Bao, L., Hazari, S., Mehra, S., Kaushal, D., Moroz, K., & Dash, S. (2012). Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. The American Journal of Pathology, 180, 2490–2503.
Lawler, S., & Chiocca, E. A. (2009). Emerging functions of microRNAs in glioblastoma. Journal of Neuro-Oncology, 92, 297–306.
An, L., Liu, Y., Wu, A., & Guan, Y. (2013). microRNA-124 inhibits migration and invasion by down-regulating ROCK1 in glioma. PLoS One, 8, e69478.
Wei, J., Wang, F., Kong, L. Y., et al. (2013). MiR-124 inhibits STAT3 signaling to enhance T cell–mediated immune clearance of glioma. Cancer Research, 73, 3913–3926.
Chen, S. M., Chou, W. C., Hu, L. Y., et al. (2015). The effect of MicroRNA-124 over expression on anti-tumor drug sensitivity. PLoS One, 10, e0128472.
Fowler, A., Thomson, D., Giles, K., et al. (2011). miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. European Journal of Cancer, 47, 953–963.
Silber, J., Lim, D. A., Petritsch, C., et al. (2008). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Medicine, 6, 14.
Shi, Z., Chen, Q., Li, C., et al. (2014). MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro-Oncology, 16(10), 1341–1353.
Shah, K. (2012). Mesenchymal stem cells engineered for cancer therapy. Advanced Drug Delivery Reviews, 64, 739–748.
Sun, X. Y., Nong, J., Qin, K., Warnock, G. L., & Dai, L. J. (2011). Mesenchymal stem cell-mediated cancer therapy: a dual-targeted strategy of personalized medicine. World Journal Stem Cells, 3, 96–103.
Kim, D. W., Staples, M., Shinozuka, K., Pantcheva, P., Kang, S. D., & Borlongan, C. V. (2013). Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. International Journal of Molecular Sciences, 14, 11692–11712.
Hsieh, J. Y., Wang, H. W., Chang, S. J., et al. (2013). Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PloS One, 8, e72604.
Liu, S., Hou, K. D., Yuan, M., et al. (2014). Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. Journal of Bioscience and Bioengineering, 117, 229–235.
Ng, F., Boucher, S., Koh, S., et al. (2008). PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood, 112, 295–307.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143–147.
Greco, S. J., & Rameshwar, P. (2012). Mesenchymal stem cells in drug/gene delivery: implications for cell therapy. Therapeutic Delivery, 3, 997–1004.
Matuskova, M., Hlubinova, K., Pastorakova, A., et al. (2010). HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Letters, 290, 58–67.
Mittelbrunn, M., Gutiérrez-Vázquez, C., Villarroya-Beltri, C., et al. (2011). Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications, 2, 282.
Kosaka, N., Iguchi, H., Yoshioka, Y., Takeshita, F., Matsuki, Y., & Ochiya, T. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. Journal of Biological Chemistry, 285, 17442–17452.
Collino, F., Deregibus, M. C., Bruno, S., et al. (2010). Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One, 5, e11803.
Broderick, J. A., & Zamore, P. D. (2011). MicroRNA therapeutics. Gene Therapy, 18, 1104–1110.
Karussis, D., Kassis, I., Kurkalli, B. G. S., & Slavin, S. (2008). Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. Journal of the Neurological Sciences, 265, 131–135.
Mohr, A., Lyons, M., Deedigan, L., et al. (2008). Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. Journal of Cellular and Molecular Medicine, 12, 2628–2643.
Amano, S., Li, S., Gu, C., et al. (2009). Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. International Journal of Oncology, 35, 1265.
Park, S. A., Ryu, C. H., Kim, S. M., et al. (2011). CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. International Journal of Oncology, 38, 97.
Lim, P. K., Bliss, S. A., Patel, S. A., et al. (2011). Gap junction–mediated import of MicroRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Research, 71, 1550–1560.
Bader, A. G., Brown, D., & Winkler, M. (2010). The promise of microRNA replacement therapy. Cancer Research, 70, 7027–7030.
Nana-Sinkam, S. P., & Croce, C. M. (2011). MicroRNAs as therapeutic targets in cancer. Translational Research, 157, 216–225.
Yoo, A. S., Sun, A. X., Li, L., et al. (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature, 476, 228–231.
Cheng, L. C., Pastrana, E., Tavazoie, M., & Doetsch, F. (2009). miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nature Neuroscience, 12, 399–408.
Lee, H. K., Finniss, S., Cazacu, S., et al. (2013). Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget, 4, 346–361.
Katakowski, M., Buller, B., Zheng, X., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335, 201–204.
Munoz, J. L., Bliss, S. A., Greco, S. J., Ramkissoon, S. H., Ligon, K. L., & Rameshwar, P. (2013). Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy—Nucleic Acids, 2, e126.
Blum, R., Jacob-Hirsch, J., Amariglio, N., Rechavi, G., & Kloog, Y. (2005). Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1α, causing glycolysis shutdown and cell death. Cancer Research, 65, 999–1006.
Zou, C., Xu, Q., Mao, F., et al. (2012). MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle, 11, 2137–2145.
Ryan, J. M., Barry, F. P., Murphy, J. M., & Mahon, B. P. (2005). Mesenchymal stem cells avoid allogeneic rejection. Journal of Inflammation, 2, 8.
Pileggi, A., Xu, X., Tan, J., & Ricordi, C. (2013). Mesenchymal stromal (stem) cells to improve solid organ transplant outcome: lessons from the initial clinical trials. Current Opinion in Organ Transplantation, 18, 672.
Wang, H. S., Hung, S. C., Peng, S. T., et al. (2004). Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells, 22, 1330–1337.
Drela, K., Lech, W., Figiel-Dabrowska, A., et al. (2016). Enhanced neuro-therapeutic potential of Wharton’s Jelly–derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy, 18, 497–509.
de Castro, J. V., Gomes, E. D., Granja, S., et al. (2017). Impact of mesenchymal stem cells’ secretome on glioblastoma pathophysiology. Journal of Translational Medicine, 15, 200.
Wang, B., Yao, K., Huuskes, B. M., et al. (2016). Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Molecular Therapy, 24, 1290–1301.
Li, X., Liu, L., Yang, J., et al. (2016). Exosome derived from human umbilical cord mesenchymal stem cell mediates mir-181c attenuating burn-induced excessive inflammation. EBioMedicine, 8, 72–82.
Acknowledgements
This work is a part of PhD thesis of Samaneh Sharif that is financially supported by the Department of Molecular Medicine, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
The authors indicate no potential conflicts of interest. All the authors have materially participated in the research and the article preparation and have approved the final article.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sharif, S., Ghahremani, M.H. & Soleimani, M. Delivery of Exogenous miR-124 to Glioblastoma Multiform Cells by Wharton’s Jelly Mesenchymal Stem Cells Decreases Cell Proliferation and Migration, and Confers Chemosensitivity. Stem Cell Rev and Rep 14, 236–246 (2018). https://doi.org/10.1007/s12015-017-9788-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9788-3