Abstract
Predicting the ecosystem effects of invasive species and the best control strategies requires understanding population dynamics and population regulation. Invasive bivalves zebra and quagga mussels (Dreissena spp.) are considered the most aggressive invaders in freshwaters and have become major drivers of ecosystem processes in the Laurentian Great Lakes. Combining all lake-wide studies of Dreissena spp. conducted in the Great Lakes, we found that invasion dynamics are largely governed by lake morphometry. Where both species are present, quagga mussels generally become dominant in 8–13 years. Thereafter, zebra mussels remain common in shallow lakes and embayments and lake-wide Dreissena density may remain similar, while in deep lakes quagga led to a near-complete displacement of zebra mussels and an ensuing dramatic increase in overall dreissenid density. In deep lakes, overall Dreissena biomass peaked later and achieved ~ threefold higher levels than in shallow lakes. Comparison with 21 waterbodies in North America and Europe colonized by both dreissenids confirmed that patterns of invasion dynamics found in the Great Lakes are very consistent with other waterbodies, and thus can be generalized to other lakes. Our biophysical model predicted that the long-term reduction in primary producers by mussel grazing may be fourfold less in deep compared to shallow lakes due to thermal stratification and a smaller proportion of the epilimnion in contact with the bottom. While this impact remains greatest in shallow areas, we show that when lakes are vertically well-mixed, dreissenid grazing impact may be greatest offshore, revealing a potentially strong offshore carbon and phosphorus sink.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population dynamics and regulation underpin both the ecological role of threatened and invasive species and successful management strategies (Yokomizo et al. 2009; Jackson et al. 2015; Strayer et al. 2019; White 2019). For new invaders, one of the most well-known hypothesis postulates that an initial slow population growth is followed by a population explosion accelerated by low intra- and interspecific competition, lack of enemies (predators, parasites and diseases), lack of defense mechanisms in native plants and animals (naïve prey), and sometimes by ongoing propagule pressure (continuous introduction of individuals to a new area) (Lockwood et al. 2013) until some equilibrium with the surrounding ecosystem is reached (Zenkevich 1940; Stanzykowska 1978; Ramcharan et al. 1992). Another “boom and bust” hypothesis suggests very high invader density followed by a population collapse (Simberloff and Gibbons 2004; Strayer et al. 2017). Other populations do not have such a distinct pattern but exhibit strong oscillatory dynamics and large temporal variations in population density (reviewed in Parker et al. 1999). Regardless of density pattern, population dynamics and their causative factors are not well understood (e.g., Crooks 2005; Strayer and Malcom 2006; Strayer et al. 2017; Aagaard and Lockwood 2016), preventing generalizations about which patterns are most common for a given invader type or habitat (Burlakova et al. 2006; Strayer et al. 2019). In particular, determining if and when an initially abundant invader declines remains challenging due to environment-specific population dynamics, competition among invaders, and study-specific methodology (Magnuson 1990; Strayer et al. 2019).
Invasive bivalves Dreissena polymorpha (zebra mussel; Pallas 1771) and D. rostriformis bugensis (quagga mussel; Andrusov 1897) are considered the most aggressive invaders in freshwater systems of the Northern hemisphere (reviewed in Nalepa and Schloesser 1993, 2014; Karatayev et al. 2002, 2007, 2015). Both species continue to spread in Europe and North America (Karatayev et al. 2007, 2011; Pollux et al. 2010; Benson 2014) causing large ecological and economic impacts on invaded waterbodies (reviewed in Karatayev et al. 1997, 2002, 2015; Vanderploeg et al. 2002; Hecky et al. 2004; O’Neill 2008; Higgins and Vander Zanden 2010; Nakano and Strayer 2014). It is well established that shortly after their introduction, both dreissenid species spread rapidly across invaded ecosystems in Europe and North America, with zebra mussels exhibiting higher initial rates of spread (reviewed in Karatayev et al. 2011, 2015). However, we do not know if or when Dreissena populations reach their peak abundance and start declining, with recent meta-analysis finding inconclusive results partly due to system-specific methodologies and partly due to system-specific environmental conditions (Strayer et al. 2019). Further, the two dreissenid species differ in habitat requirements, growth, and population dynamics, and the more competitive quagga mussels typically dominate zebra mussels in waterbodies where they have both invaded (reviewed in Karatayev et al. 2011, 2015).
Higher primary production, temperature, and wave stress in shallower areas all represent ubiquitous sources of environmental variation in lake ecosystems (Wetzel 1983; Jónasson 2004). Benthic invertebrates particularly face large gradients in population growth and turnover, with most species limited to a particular depth zone (Brinkhurst 1974). Although zebra and quagga mussels both have a high reproductive potential, planktonic larvae and an attached benthic adult stage, zebra mussels are usually limited to hard substrates in the littoral zone, where the surface mixed layer intersects the bottom and temperature varies dramatically during the year. In contrast, quagga mussels have a higher bioenergetic efficiency, can spawn at lower temperatures, and are more efficient suspension feeders (reviewed in Karatayev et al. 2015). Additionally, quagga mussels can colonize soft sediments of the profundal zone (located below the thermocline) where temperatures are continuously cold and where high-quality food resources (phytoplankton) are generally limited (Karatayev et al. 2018a). Therefore, quagga mussels in the profundal zone have slower growth, longer life span, and different time of reproduction compared to mussels in the shallow well-mixed littoral zone (Nalepa et al. 2010; Karatayev et al. 2015; 2018a). As a result, ecosystem-wide dreissenid distribution, abundance, and ecological impacts may depend on the relative proportions of the littoral and profundal zones (reviewed in Karatayev et al. 2011, 2015).
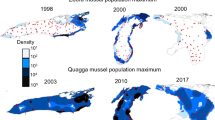
The Laurentian Great Lakes have a unique set of characteristics to address the effect of morphometry on Dreissena spp. invasion dynamics as they are the only freshwater ecosystems in the world that have broad environmental gradients (depth and trophic status), have long-term environmental and biological monitoring data, and have been colonized by both dreissenid species. Both zebra and quagga mussels were found for the first time in Lake Erie (Carlton 2008; Mills et al. 1993) and in the next few years spread into lakes Ontario, Michigan, and Huron (Mills et al. 1993; Marsden et al. 1993; Nalepa et al. 2001) (Fig. 1). Early in the invasion, the Great Lakes were dominated by zebra mussels, however later, they were almost completely replaced by quagga mussels in most of the Great Lakes (Javis et al. 2000; Patterson et al. 2005; Watkins et al. 2007; Nalepa et al. 2010; Karatayev et al. 2011, 2014).
In this study we analyzed all available lake-wide data on distribution, density and biomass of dreissenids in the Great Lakes from 1990 to 2018, and applied a statistical and a mechanistic biophysical model to address the following questions: (1) How different are dreissenid population dynamics across deep Great Lakes and shallow lakes and embayments? (2) How do population distribution, biomass and filter-feeding impacts on phytoplankton progress with time for shallow and deep lakes? (3) Which mussel species will win in competition and will the outcome depend on lake morphometry? and (4) How do all these processes affect dreissenid impacts on ecosystems? Finally, to check if our results can be relevant for shallow and deep lakes around the world, we compared invasion dynamics of Dreissena spp. in the Great Lakes to other North American and European lakes to reveal potential similarities and differences.
Methods
Study area
Shallow lakes and embayments (later termed as “shallow lakes”) of the Great Lakes analyzed in this paper include Lake St. Clair, Saginaw Bay of Lake Huron, and the western basin of Lake Erie. These waterbodies are vertically well-mixed in summer with the entire bottom exposed to a large variation in temperature from about 3–4 °C in winter and to 20–25 °C in summer (Schelske and Roth 1973; Burns 1985; Mortimer 1987; Edsall et al. 1988; Leach 1991; Skubinna et al. 1995) (Table 1). They have short retention time and are among the most productive waterbodies in the Great Lakes due to the large inputs of nutrients (Schelske and Roth 1973; Burns 1985; Barbiero and Tuchman 2004; Edsall et al. 1988; Leach 1991; Nguyen 2014). In contrast, deep lakes and lake basins (later termed as “deep lakes”) include Lake Ontario, Lake Michigan, the Lake Huron main basin, and the eastern basin of Lake Erie. These waterbodies are dimictic, with periods of complete vertical mixing in spring and autumn, and with summer stratification that usually develops in late May or early June (Burns 1985; Rowe et al. 2015). In these lakes most of the bottom area and hypolimnetic waters are located in the deep and consistently cold (temperatures range about 4–10 °C over the year) profundal zone, isolated by the thermocline from the warm productive surface waters for much of the growing season (Table 1). The warm well-mixed epilimnion varies among lakes but usually starts from a few meters after the establishment of stratification and reaches 10–20 m by the end of autumn (Barbiero and Tuchman 2001; Beletsky et al. 2006, 2013; Scofield et al. 2017). The metalimnion thickness is about 8 m, so the hypolimnion usually commences at a depth of about 28–30 m (Schelske and Roth 1973; Burns 1985; Rowe et al. 2015). Maximum surface temperature in summer also varies among lakes and reaches 20–25 °C, while the near bottom temperature during summer is about 4–6 °C and typically does not exceed 10 °C (Schelske and Roth 1973; Beletsky and Schwab 2001; Rowe et al. 2015; Karatayev et al. 2018a). The proportion of the total lake area with a depth zone > 30 m (area roughly located below the thermocline) is very similar in lakes Ontario, Michigan, and Huron (77.6–79.1%). In the eastern basin of Lake Erie, although well stratified in summer, < 36% of the bottom is located below thermocline while > 64% of the bottom is exposed to warm epilimnetic temperatures. Therefore, in our study we considered this basin as an intermediate waterbody between shallow and deep lakes. We omitted the central basin of Lake Erie from our analysis, where Dreissena populations are low and fluctuate predominantly in response to intermittent hypoxia rather than invasion processes (Karatayev et al. 2018b).
Dreissena population dynamics
We compared the timing of zebra and quagga mussel introductions into the Great Lakes, using published data of the first reported year of D. polymorpha and D. r. bugensis invasion (Hebert et al. 1989; Griffiths et al. 1991; Mills et al. 1993; Nalepa et al. 2001; Hunter and Simons 2004; Carlton 2008). The first zebra mussels in North America were found in Lake St. Clair (Hebert et al. 1989) and at several locations in Lake Erie in 1988 (Leach 1993; Garton and Haag, 1993). However, Carlton (2008) later reported that the first D. polymorpha were found in Lake Erie two years earlier. This difference indicates that the timing of the first report in Lake Erie is questionable, but for the purpose of this study we used 1988 as the first year of zebra mussel record in Lake Erie (Fig. 1; Table 2). In the following year zebra mussels had been found in lakes Ontario, Michigan, and Huron (Griffiths et al. 1991). It took longer for quagga mussels to spread from Lake Erie, where they were found for the first time in 1989 (Mills et al. 1993), into lakes Ontario (1990, Mills et al. 1993), Michigan, and Huron (both in 1997, Nalepa et al. 2001) and St. Clair (2001, Hunter and Simons 2004) (Fig. 1). The gap between these two introductions ranged from one year in lakes Erie and Ontario to 8 years in lakes Michigan and Huron, and to 13 years in Lake St. Clair (Table 2).
For assessment of zebra and quagga mussel population dynamics we analyzed all available lake-wide studies on their distribution, density and biomass conducted in the Great Lakes from 1990 to 2018 (Online Resource 1). To increase the spatial resolution of surveys we added data from the U.S. EPA Great Lakes National Program Office (GLNPO) long-term monitoring stations (in cases where these stations were not originally included into sampling designs) to each survey conducted in Lake Ontario starting from 2003 (10 stations per survey) and in Lake Michigan starting from 2005 (14 stations per survey, 2 stations from Green Bay were excluded). It should be mentioned that shallow areas (< 10 m) in all lakes were not sampled during all of these surveys, with the exception of western Erie, Saginaw Bay, and St. Clair.
Detailed sampling protocol could be found in the primary papers (Karatayev et al., 2014, 2018b; 2020; Nalepa et al. 2018, 2020). In short, Dreissena samples were collected from 7 to 175 stations depending on the lake (or lake basin) size and study design (Online Resource 1). At each station, usually three replicate samples were collected using a grab (either regular or petite Ponar, or Ekman). Hard substrates were occasionally sampled by SCUBA divers using a quadrat. Dreissena mussels were identified to species, when possible. All individuals retained in samples elutriated using 500 µm sieve were counted, and data reported as densities (ind.m−2). In shallow lakes densities were calculated as average (± standard error of the mean), while in deep lakes we calculated lake-wide Dreissena spp. density and biomass (± standard error) as a weighted average using means of stations located at 4 depth zones (< 30 m, 31–50 m, 51–90 m, and > 90 m), considering the proportion of the total lake area represented by each zone (Manly 2009).
While Dreissena densities are available for all lake-wide surveys used in this study, mussel weight was not always measured directly (Online Resource 1). In Lake Ontario Dreissena spp. were not weighed from 1990 to 2003, but weight was estimated using an average of 3.34 mg tissue dry weight (TDW) per mussel following Birkett et al. (2015) with some adjustments (Karatayev et al. 2020). In the main basin of Lake Huron, Dreissena biomass was not recorded from 2000 to 2007 (Nalepa et al. 2007, 2018) and was estimated using an average of 5.19 mg ash-free tissue dry weight (AFTDW) per mussel, as calculated from 2012 samples. Although these data may not account for potential differences in relative mussel weights over time, and in some cases by depth, we reconstructed the population dynamics of dreissenid biomass to follow their changes across different lakes and depth zones.
Over the years different authors reported Dreissena biomass in different units. For consistency across studies, all historic and current data on Dreissena biomass were converted into AFTDW (Online Resource 1). In Lake Erie (1992, 1993, 1998, 2009, 2014, reviewed in Burlakova et al. 2017) and St. Clair (2001, Hunter and Simons 2004; 2014, Great Lakes / Huron-Erie Corridor data from Theodore Angradi, U.S. EPA, Angradi.Theodore@epa.gov; 2018), Dreissena was measured as shell and tissue wet weight (STWW). To convert STWW into AFTDW, we used the equation: AFTDW (g) = 0.0274 × STWW (g); to convert TDW into AFTDW, we used the equation: AFTDW (g) = 0.8711 × TDW (g) (both equations are based on Dreissena collected from Lake Michigan in 2014 by A. Elgin following the sites and methods described in Glyshaw et al. 2015). For Lake Michigan 2015, we used the equation AFTDW (g) = 0.01996 × STWW (g) published by Nalepa et al. (2018). Dreissena biomass in Lake Ontario 2008 and 2013, Lake Huron 2012 and 2017, and Lake Michigan pre-2015 were calculated as AFTDW by determining relationships between AFTDW and shell length for different depth zones, and then applying these relationships to size frequency distributions at each station (see Nalepa et al. 2014, 2020; Karatayev et al. 2020 for more detail). We compared the maximum density and biomass of D. polymorpha in each waterbody with the most recent total density and biomass of combined Dreissena spp. using ANCOVA with depth as covariate on log-transformed data.
To visualize lake-wide changes in the spatial distribution of zebra and quagga mussels, average densities from each sampling station were interpolated using ‘Natural Neighbor Interpolation’ within the Spatial Analyst tool in ArcMAP 10.7.1. For a given set of points (i.e. sampling stations), this technique constructs a Voronoi diagram by partitioning the space into cells, one cell for each point. A new Voronoi polygon is constructed around the point to be interpolated. Weights are applied based on the proportion of overlap between the initial and new polygon to calculate the value of the interpolated point (Sibson 1981).
General regression model of population dynamics
We compared the effects of depth, time since the first record, and lake-specific effects on Dreissena biomass by fitting generalized additive models to primary data (1) all shallow lakes and (2) all deep lakes. This approach allowed us to account for non-linear effects of depth or time in each lake type using splines, as well as an interaction between depth and time (e.g., peak biomass moving offshore) using 2-dimensional splines. We accounted for lake-specific effects, by including lake identity as an additive factor representing the difference in average biomass in a lake from the average biomass across all lakes. To evaluate the effects of each factor (lake, depth, time, and depth-time interaction), we compared how well models incorporating each factor explained the data. For this, we used a fully nested set of models and evaluated model performance using the Akaike Information Criterion (AIC, with lower values indicating better performance) to avoid overfitting. Following convention, we considered models with AIC difference > 2 to statistically differ in performance (Burnham and Anderson 2002). Throughout, we fitted models to total Dreissena AFTDW biomass assuming a Tweedy error distribution and used the gam function in the package mgcv 1.8–27 (Wood 2017) in R 3.5.2 (R Core Team 2018) (Online Resource 2). Given the limited number of lakes in our analyses, we constrained the basis dimension k (i.e., smoothness) of fitted splines to 10 in order to avoid over-fitting; however, greater values of k = 100 for depth and depth-time interactions improved AIC values of all models by < 0.5% (authors’ unpublished data). We note that large (> 5 km) distances between adjacent sampling stations limited the potential for spatial autocorrelation among samples.
We visualized the effects of depth and time by plotting their effects estimated in models incorporating all factors. For reference, we plotted the (smoothed) fraction of total Dreissena biomass represented by quagga mussels across years. To quantify the potential impact of observed biomass, we additionally estimated the intensity of dreissenid filter-feeding on phytoplankton during vertically well-mixed spring and fall isothermal periods. The filter-feeding intensity, or fraction of the water column cleared per day by mussel filter feeding, was calculated as clearance rate multiplied by biomass, divided by water column depth, and compared to a benchmark phytoplankton growth rate of 0.06/day (Rowe et al. 2017; Vanderploeg et al. 2010). We used values of biomass and water column depth and moderate estimates of dreissenid clearance rate (10 mL/mg AFTDW/h; Vanderploeg et al. 2010) reflecting temperatures near 4 °C and moderate food quality.
General biophysical model
We applied the biophysical model of Rowe et al. (2017) to investigate the influence of lake bathymetry on the progression of dreissenid biomass over time in a theoretical framework consisting of a phosphorus-limited lower food web and dreissenid bioenergetic model linked to a three-dimensional fine-resolution hydrodynamic model. We applied the finite volume community ocean model (FVCOM) to an idealized circular lake of 50-km radius, centered at the geographic location of southern Lake Michigan (Lon. −87.03, Lat. 42.57). FVCOM (v. 3.1.6) is an unstructured grid, finite-volume, free surface, three-dimensional primitive equation ocean model that solves the momentum, continuity, temperature, salinity (set to zero here), and density equations (Chen et al. 2003). External and internal time steps were 20 s. The unstructured grid consisted of 3019 nodes and 5856 elements, with 20 terrain-following vertical (sigma) layers of uniform thickness. Mean element side lengths 1.2 km (0.73–1.3 km). The lateral boundaries were closed, and ice cover was not simulated. Rowe et al. (2017) described the lower food web in detail. Briefly, the phosphorus-limited lower food web included state variables representing dissolved phosphorus, and biomass of phytoplankton, zooplankton, detritus, and dreissenid mussels, with fixed phosphorus:carbon ratio (0.016). The phytoplankton photosynthesis-irradiance functions were reported by Fahnenstiel et al. (2016) and temperature-dependent dreissenid clearance rates were reported by Vanderploeg et al. (2010). Model simulations were initialized on January 1, at 4 °C, velocities at zero, dissolved P at 3.2 µg/L, detritus at 1.6 mgC/L, phytoplankton and zooplankton at 0.04 mgC/L, Dreissena at 0.02 gAFTDW m−2. Lakewide-mean total P was 7 µg/L and remained constant over the simulations. Hourly meteorologic variables were interpolated from stations surrounding Lake Michigan (Rowe et al. 2017). Meteorology of the year 2010 was repeated over the 20-year simulations.
Relevance to other lakes
For comparison of Dreissena spp. invasion dynamics in the Great Lakes and other waterbodies, we compiled a dataset of 8 lakes from North America and 13 lakes and reservoirs from Europe colonized with both zebra and quagga mussels, including Dnipro-Bug Liman in the native range of Dreissena where the two species have co-existed for thousands of years (Online Resource 3). For each waterbody, whenever possible, we collected data on the surface area, average and maximum depths, year of the first record of quagga and zebra mussel invasion, their density and relative proportion of species from all dreissenids for all available years. From these 21 waterbodies six were deep stratified lakes, two lakes were medium stratified, and the remaining 13 waterbodies were polymictic. All waterbodies were colonized with zebra mussel before quagga mussel invaded, except one: Dnieper-Bug Liman located in the native range of both Dreissena species.
Results
General population dynamics
Dreissena spp. density and biomass dynamics depended on lake, year, and depth in all lakes, but their distribution across depths changed through time only in deep lakes (Table 3, Figs. 2, 3, 4, 5, 6 and 7). Zebra mussels colonized all waterbodies first, quickly reaching their population maximum in shallow lakes and basins and in the littoral zones of deep lakes and basins (Figs. 1, 2 and 3. Table 2). Thereafter, lake- and basin-wide biomass trends diverged as quagga mussels became more common. In shallow lakes, dreissenid biomass changed synchronously across all depths and our best-performing model in shallow lakes did not include an interaction between depth and year. We note that the post-2010 increase at 8–15 m depths in shallow lakes (Fig. 7a) arises predominantly from patterns in Saginaw Bay (Online Resource 4) and reflects the dominance of quagga mussels in Lake Huron.
Conversely, in deep lakes, total Dreissena biomass continued to grow as mussels expanded from shallow to deep regions (Figs. 4, 5, 7b), and our best-performing model (based on AIC score) included lake-specific, year, and depth effects, as well as an interaction between depth and year. Dreissena biomass also increased much more slowly in the profundal zone than in the shallow zone of deep lakes and in shallow lakes (Fig. 7b). Following this, Dreissena lake-wide biomass peaked at least 15 years later in deep lakes compared to shallow lakes. We noted that higher maximum biomass in deeper rather than in shallow lakes in the population model (Fig. 7a, b) arises only from the effect of averaging population trends across shallow lakes, which exhibited greater variation in the timing of peak mussel biomass compared to deep lakes (Online Resource 4).
Long-term dynamics of Dreissena polymorpha (dashed lines, triangles) and D. rostriformis bugensis (black lines, circles) density (ind.m−2) and biomass (g m−2 AFTDW) in lakes Ontario, Michigan, main basin of Lake Huron, and eastern basin of Lake Erie. Vertical bars are standard errors. Lake-wide densities for lakes Ontario, Michigan, and Huron were calculated as weighted averages from 4 depth zones
Interspecific Dreissena competition
Dreissenid competition also depended on lake morphometry (Table 2, Fig. 4). While zebra mussels were initially dominant in all lakes (Table 2, Fig. 4), in deep lakes this species was largely limited to a narrow band in the nearshore zone (> 99% of lake-wide populations at < 50 m depths in Lake Michigan and at < 30 m in Lakes Ontario and Huron, Figs. 5, 6). Subsequently, quagga mussels replaced zebra mussels faster and more completely in deep lakes than in shallow lakes (6–8 years in deep, vs. 8–13 years in shallow). As the species spread across the entire bottom of deep lakes (Fig. 5, 6), quagga mussels reached significantly higher lake-wide presence than zebra mussels (5–17-fold higher biomass and 8–42-fold higher density in recent data in lakes Ontario, Huron, and Michigan; Table 2). As a consequence, quagga mussels in deep lakes caused almost complete extirpation of zebra mussels (Fig. 4), and no zebra mussels were found in lake-wide surveys in Lake Ontario in 2008 and 2013, in Lake Michigan in 2015, or in the Lake Huron main basin in 2017 (although zebra mussels can still occur at low densities in poorly sampled < 10–18 m depths; Karatayev et al. 2013). Conversely, in shallow lakes the outcome of dreissenid competition did not cause significant changes in the lakes-wide average dreissenid density (Table 2), but varied widely across lakes depending on the prevalent substrate (e.g., twofold increase in the western basin of Lake Erie dominated by soft sediments and sevenfold decline in Saginaw Bay with abundant rocky substrates; non-significant changes). Taken together, quagga mussels became dominant in about 8–13 years of dreissenid coexistence, but even so, after 20 + years of coexistence, zebra mussels remain common and comprised 13–33% of all dreissenid density and 6–15% of biomass.
These results from shallow Great Lakes and lake basins are in line with 8 shallow inland North American and European waterbodies, where quagga mussels became dominant in about 4–12 (6.5 ± 0.9) years of coexistence (Oneida Lake: 4y, Lake Balaton: 4y; Lake Müggelsee: 5y; Main Bay of Rybinskoe Reservoir: 6y; Kuybyshev Reservour: 12y; Saratov Reservoir: 9y; Volga Delta: 7y; Lake Eem: 5y; Online Resource 3). In every case, despite their dominance, quagga mussels did not appear to fully replace zebra mussels, with zebra mussels accounting for 13.9 ± 2.1% of all dreissenids (range 4 to 22%) after many years of coexistence (range 6 to 23 years, mean 11.8 years; Online Resource 3). Also paralleling our results, in deep stratified inland lakes (lakes Keuka, Canandaigua, Skaneateles, Constance), the switch from zebra to quagga mussel dominance occurred significantly earlier (after 1.3 ± 0.2 years of coexistence) than in polymictic shallow lakes (4–12, average 6.5 ± 0.9 years of coexistence; P < 0.001, two tail t-test assuming unequal variances). Finally, in other deep lakes with long-term invasion data, quagga mussels almost completely replaced zebra mussels, comprising 99.8% of density of all Dreissena in Lake Cayuga in 2013 and in Lake Seneca in 2010.
Dreissena ecosystem impacts
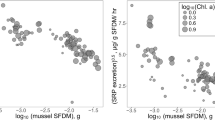
In deep lakes observed shifts in peak biomass from shallow to deep areas translate to a shift in peak impacts of filter-feeding by Dreissena (as estimated by the daily fraction of water column filtered) from shallow (< 30 m) to intermediate (30–90 m) depths. However, in deeper areas filter-feeding intensity remained low relative to the benchmark phytoplankton growth rate (Fig. 7). Conversely, in shallow lakes estimated filtration intensity remained high over all depths but has declined by ~ 50% 10 years after the peak in Dreissena spp. biomass.
Dynamics of Dreissena spp. biomass and the resulting mussel filtering activity during isothermal periods in the Great Lakes across time and space. Colors are predictions of generalized additive models incorporating all effects (Lake, Depth, Year, and Depth-Year interaction, Table 3) fitted to AFTDW biomass a, b and the estimated fraction of the water column cleared per day c, d in each sample. Bottom inset panels in a, b give the fraction of biomass represented by quagga (red) and zebra mussels (gray) across time; note that quagga mussel relative abundance in shallow lakes peaks at 87%. Red contour line in c, d indicates the benchmark phytoplankton growth rate of 0.06 d−1. Early dreissenid biomass, species composition, and impacts (pre-1995) predominantly represent conditions in Lake Ontario, which was invaded and sampled earlier than Michigan and Huron. Note that the depth range for deep lakes in d is truncated for clarity
In the biophysical model simulations, dreissenid biomass grew over time from a uniform initial seed level to approaching a steady-state biomass over a period of years (Fig. 8, see Online Resource 5 for lake specific results). A seasonal cycle in dreissenid growth occurred in the model, with growth occurring when the surface-mixed layer is in contact with the bottom, approximately March–April and November–December, and biomass loss occurring when stratification suppresses turbulent exchange of phytoplankton between the surface-mixed layer and bottom, approximately June–September and January–February, although the exact timing will vary with bathymetry and meteorology. Even though the model simulated dreissenids as a single biomass pool and neglected population dynamics, due to differences in food supply and temperature, the time to reach a steady-state biomass varied among shallow and deep lakes, and by depth zone. Steady-state biomass was reached in less than five years in the shallow lake, in 5–10 years in the < 50-m zones of the intermediate and deep lakes, and was not fully reached after 20 years in the 51–90 and > 90 m zones (Online Resource 5).
Biophysical model simulations showing the influence of three bathymetry scenarios on dreissenid biomass and chlorophyll-a reduction in an idealized circular lake with a phosphorus-limited lower food web and dreissenid bioenergetics model linked to a fine-resolution hydrodynamic model. For each bathymetry scenario a annual mean values of dreissenid biomass b and percent reduction in surface chlorophyll-a, relative to the first year of invasion c are plotted at steady-state, 20 years after invasion, averaged over bathymetric depth zones (< 30, 31–50, 51–90, and > 90 m) and lake-wide (Mean)
Our key model result showed lower Dreissena impacts on lake-wide mean chlorophyll concentrations in deeper lakes compared to shallow lakes even though total Dreissena biomass was greater after 20 years (Fig. 8). Suppression of vertical mixing during thermal stratification reduces filtering impacts of mussels on epilimnetiuc phytoplankton in deeper habitats > 30 m. As a result of decreasing food supply and temperature with depth, both the growth rate and steady-state mussel biomass declined with depth (especially > 50 m) in deeper lakes. Nevertheless, lakes with 36% and 78% of the bottom deeper than the seasonal thermocline (~ 30 m) supported two-times the steady-state lake-wide biomass of the shallow lakes (10 m). Chlorophyll concentration in the < 30 m depth range increased with mean lake depth due to supplement by horizontal transport from deeper offshore waters with less mussel impact. Due to this resource subsidy to shallow areas from the offshore epilimnion, mussel biomass in the < 30 m zones of deep lakes was two to five-times the biomass of shallow lakes (< 30 m). However, our biophysical model did not reproduce the boom-and-bust dynamics of mussel biomass (observed in shallow lakes and < 30 m zones of deep lakes, Fig. 7a, b) and the resulting decline of mussel filtering impacts in the littoral zone (Fig. 7c, d), suggesting that the decline may be caused by processes not included in the model, such as population demographic shifts or predators.
Discussion
Morphometry determines Dreissena population dynamics
We found that Dreissena biomass increases at a slower rate in deeper lakes and is characterized by a gradual shift in dreissenid species present from shallow to deeper areas. In shallow lakes and in shallow zones of deep lakes, dreissenids (predominantly zebra mussels) reached maximum density soon after initial colonization (see Initial invasion below) and then declined (Figs. 2, 4). However, in intermediate zones of deep lakes (30–50 m), Dreissena densities (predominantly quagga mussels) increased more slowly, peak later (13–15 years since initial invasion), and subsequently declined from their maximum levels to a lesser extent (Fig. 4). In the deepest zone (> 90 m) quagga mussel density and biomass continued to increase in all deep lakes which, coupled with population declines in shallow areas earlier in the invasion, results in the overall shift of dreissenid presence toward deeper areas. Given a peak at the deepest depths has yet to occur, this shift will likely become more pronounced over time.
The delayed increase in Dreissena spp. at greater depths can arise from poor growth conditions during thermal stratification and reduced recruitment. Mussels were virtually absent at > 130 m depth in any of the deep lakes before 2003 and 2007 (Fig. 4), suggesting low propagule supply. Concomitantly, lower food availability might cause starvation mortality of young mussels; this process can explain the absence of recruitment over the past decade at > 40 m depths in eastern Lake Erie (Karatayev et al. 2018a). Lower food availability and consistently low temperatures at greater depths reduce growth rates of established mussels, an effect that was reproduced by our biophysical model (Fig. 8). Greater longevity of established mussels in deeper areas (due to reduced temperature, wave stress, and perhaps predation) allows mussels to reach larger individual sizes (Karatayev et al. 2018a). This is especially evident when biomass is measured directly: for instance, the mean weight of an individual mussel increased fivefold in Lake Michigan (2000–2015) and tenfold at > 30 m in the profundal zone of eastern Lake Erie (1998–2014), where increasing biomass and no recruitment have been observed since 2009 (Karatayev et al. 2018a). Slow individual growth and high supply of propagules from shallow areas might allow many mussels to establish before biomass (proportional to filtration intensity) becomes high and inhibits recruitment by depleting food, creating density dependence that ultimately limits dreissenid abundance (Karatayev et al. 2018a). Thus, slower individual growth at greater depths may explain why peak mussel biomass (over all years) was observed in deep (50–100 m) rather than shallow areas (Fig. 7b).
Over time, a larger biomass is formed by fewer but substantially larger mussels, and the increasing individual size in deep areas leads to the observed discrepancy between biomass and density trends (e.g., 4.5-fold biomass growth but continuous density decline in eastern Erie from 1998 to 2014). As individuals grow slowly and live for many years, biomass changes in our data follow density dynamics with a large time lag. As a result, Dreissena biomass continues to increase in almost all deep lakes and across all depth zones (except the shallowest 0–30 m zone; Fig. 4) despite decreasing densities, while in shallow lakes and shallow zones of deep lakes biomass has declined over the last 15 years (Figs. 2, 4, 7a, b). Observed boom-and-bust trends in biomass in shallow areas and in density in deep areas suggest that lake-wide Dreissena biomass in deep lakes may eventually decline as well. However, our biophysical model suggested that at steady-state, deeper lakes might support greater lake-wide dreissenid biomass than shallower lakes (given the same trophic status, i.e. total P concentration; Fig. 8), likely due to the food supplement by horizontal transport from deeper offshore waters with less mussel impact.
Morphometry determines the outcome of Dreissena competition
We found that the outcome of zebra and quagga mussel competition depends on lake morphometry and is dramatically different in shallow vs. deep lakes (Figs. 2, 3, 4, 5, 6 and 7). In shallow lakes both zebra and quagga mussels over time colonize the entire bottom (although zebra mussel distribution may be more heterogeneous as they usually do not form high density on soft substrates (reviewed in Karatayev et al. 1998), and, most importantly, the two species can coexist (Table 2). In general, quagga mussels became dominant 8–13 years after coexistence, but zebra mussels remain common, comprising 13–33% of Dreissena spp. combined density and 6–15% of biomass. The percentage of zebra mussels in a particular lake increases with the availability of hard substrates in hydrologically active zones where they survive better and achieve higher densities due to their better resistance to dislodgment (as a result of higher initial byssal production rates, attachment strength, and flattened ventral edge, Mackie 1991; Dermott and Munawar 1993; Claxton and Mackie 1998; Peyer et al. 2009, 2010). For instance, on hard substrates in Saginaw Bay zebra mussel average density during their population maximum in 1992 was 22 times higher (although non-significant due to high variation in data) than quagga mussel density recorded in 2017, although on soft substrates quagga mussel density was always higher than that of zebra mussels (Fig. 2). This shows that quagga mussel dominance in shallow waterbodies can homogenize dreissenid distribution across space, but do not result in significant changes in lake-wide dreissenid density (Table 2).
Our findings on Dreissena competition in the Great Lakes parallel findings in other North American and European waterbodies. In other shallow, polymictic lakes quagga mussels became dominant in about 4–12 years of coexistence but do not appear to completely replace zebra mussels (Online Resource 3). However, multiple system-specific factors can influence the outcome of competition, including selective predation by fish (e.g. higher predation pressure on quagga mussels, Bowers et al. 2005; Casper and Johnson 2010; Zhulidov et al. 2006, 2010; Rudstam and Gandino 2020). In Oneida Lake quagga mussels became dominant in 2009, four years after the first record, reached 97% of density of all dreissenids by 2015 (Hetherington et al. 2019), but then declined to 78% by 2019, most likely due to the selective predation by another Ponto-Caspian invader, the round goby (Barton et al. 2005; Patterson et al. 2005; Hetherington et al. 2019). Within their native range in the Dnieper River Delta and Dnieper-Bug Liman where dreissenids coexisted for thousands of years, the two species have been dominant in different areas of waterbodies or in different years in the same waterbody (Markovskiy 1954; Moroz and Alekseenko 1983). In agreement to our results from the Great Lakes, quagga replaced zebra mussels in other deep stratified lakes significantly earlier (1–2 years, average 1.3 ± 0.2 years of coexistence) than in polymictic shallow lakes (Online Resource 3) and the competition leads to the virtually complete displacement of zebra mussels.
A suite of demographic and physiological traits allows quagga mussels to colonize the large cold profundal zone of deep lakes unsuitable for zebra mussels, including the ability to colonize soft substrates, higher filtration rate at low food concentrations, greater assimilation efficiency (Diggins 2001; Baldwin et al. 2002; Stoeckmann 2003), and greater plasticity in shell production allowing more energy be allocated to growth and reproduction (Mills et al. 1999; Karatayev et al. 2010; Nalepa et al. 2010; Pryanichnikova 2012). Quagga mussels can also reproduce at lower temperatures, a demographic advantage in the profundal zone where zebra mussels will not reproduce (Roe and MacIsaac 1997; Claxton and Mackie 1998; Nalepa et al. 2010). Furthermore, indirect competition with quagga mussels via lake-wide food depletion may contribute to the loss of shallow, dense zebra mussel reefs (prominent early in the invasion, Dermott et al. 1993; Nalepa et al. 1995; Chase and Bailey 1999) and accelerate the net shift in Dreissena presence offshore. Alternatively, other factors like fish predation may also cause decline in zebra mussel densities in shallow areas (Bowers et al. 2005; Casper and Johnson 2010; Zhulidov et al. 2006, 2010; Rudstam and Gandino 2020). We should mention, however, that although zebra mussels were not recorded during recent lake-wide studies that rarely sample < 10 m depths, they are still present in very shallow areas of deep lakes, marinas, river mouths, and on navigational buoys (Karatayev et al. 2013, 2018a; Burlakova et al. 2014).
Morphometry determines Dreissena ecosystem impacts
Our biophysical model highlighted a much lower impact of dreissenids on phytoplankton concentrations in deep lakes due to two factors. First, in deeper areas greater biomass is required to filter the same fraction of the water column due to greater volume per unit bottom habitat. Thus, during well-mixed spring and fall periods, we show that mussel filtering had the greatest local impact in shallow lakes (Fig. 7c) and in shallow areas of deep lakes early in the invasion (Fig. 7d), despite that the highest biomass was observed most recently at 30–90 m (Fig. 7b). Second, in deep regions thermal stratification further limits the impact of filtration by mussels on phytoplankton in the epilimnion by preventing mixing of the entire water column and thereby denying bottom-dwelling mussels access to such phytoplankton (Rowe et al. 2015; Waples et al. 2017; Karatayev et al. 2018a). Combining the effects of these features over time, our biophysical model suggested that the decline in lake-wide primary producers is fourfold greater in shallow lakes compared to deep lakes (chlorophyll-a declines 0.2 μgL−1 in deep and 0.9 μgL−1 in shallow lakes, Fig. 8).
In shallow lakes, Dreissena spp. typically has homogeneous ecological impacts across space because shallow lakes experience weaker thermal stratification and shallow depths allow abundant mussels to easily filter the entire water column (Fig. 7c). As a result, the change in dominance from zebra to quagga mussels had little effect on lake-wide population density and ecosystem impact (Fig. 2; Table 4). Conversely, in deep lakes ecological impacts of Dreissena spp. gradually shift offshore throughout the invasion (Fig. 7d). Early in the invasion dreissenids were limited to the shallow zone (< 30 m; Figs. 5, 6), directly impacting littoral communities throughout the growing season (reviewed in Karatayev et al. 1997, 2002, 2015; Hecky et al. 2004; Fig. 8), but having less impact on offshore areas (Fahnenstiel et al. 1995; Vanderploeg et al. 2010; Fig. 8). Also, littoral communities of dreissenids and associated benthic invertebrates and algae alter nutrient cycling and retain phosphorus from allochthonous sources in the nearshore and hence impact offshore pelagic communities through the process known as a “nearshore phosphorus shunt” (Hecky et al. 2004). Therefore, early in the invasion dreissenid effect on profundal and epilimnetic communities could be characterized as remote (horizontal, i.e., nearshore to offshore) and continuous during the entire growing season (Table 4). The most dramatic consequence of this remote impact was the sharp decline in amphipod Diporeia in profundal areas of all deep Great Lakes long before these areas were colonized with dreissenids (reviewed in Vanderploeg et al. 2010; Karatayev et al. 2015; Burlakova et al. 2018; Barbiero et al. 2018), although other reasons for the decline of Diporeia were also suggested (Barbiero et al. 2011; Nalepa et al. 2006; Watkins et al. 2007; Hewson et al. 2013).
The replacement of zebra with quagga mussels in deep Great Lakes in mid 2000s was associated with an order of magnitude increase in lake-wide population density and biomass and a shift in the bulk of Dreissena spp. from the nearshore zone to depths > 30 m (Figs. 5, 6 and 7). Similarly to the Great Lakes, in Lake Skaneateles just one year after the first record of quagga mussels, the combined density of all dreissenids was already eightfold and in Lake Canandaigua 13-fold higher than density of zebra mussels (Online Resource 3).
Our analysis indicated that during the spring and fall isothermal periods, filter-feeding intensity is more likely to exceed the benchmark phytoplankton growth rate at 30–90 m depths than at < 30 m depths (Fig. 7d). Accordingly, Vanderploeg et al. (2010) estimated that the fraction of water column cleared per day in Lake Michigan in the 30–50 m zone in 2007–2008 greatly exceeded phytoplankton growth and suggested a hypothesis of a mid-depth carbon and phosphorus sink caused by quagga mussel activity that also affected the offshore region “downstream” of the mid-depth zone. Thus, the expansion of quagga mussel populations in mid-depth zone in the mid-2000s was associated with increased transparency and silica concentrations, decreases in seston, chlorophyll, phytoplankton, zooplankton, and benthos (excluding dreissenids), and altered community structure (Fahnenstiel et al. 2010; Bunnell et al. 2014; Barbiero et al. 2018; Burlakova et al. 2018). Conversely, the impact of profundal dreissenids on epilimnetic waters, in contrast to littoral mussels, is remote and likely limited to pulses during turnover events (Table 4), while the effect on benthos is continuous and direct.
Although it was suggested (Vanderploeg et al. 2010) that further expansion of mussels into deep water would be constrained by the extremely low phytoplankton biomass and productivity, the most recent data clearly indicate that the maximum of quagga mussels density in all deep Great Lakes has shifted much deeper in the last 5 years and is located now in lakes Michigan and Huron at 71–90 m and in Lake Ontario at 51–70 m depth intervals (Fig. 5). This shift was concurrent with a large 3–4 fold decline of dreissenid density and 2–18 fold decline in biomass in the shallowest zone, suggesting that the direct and continuous impact of dreissenids in the nearshore areas of the Great Lakes has diminished, while the role of profundal mussels has increased. Hence, because of this shift in lakes Ontario and Michigan, dreissenid filter feeding has the potential to strongly suppress phytoplankton abundance during isothermal periods in the 30–90 m depth zones. Conversely, this impact in the littoral zones has declined from its peak (Fig. 7d) and is likely lower in Lake Huron compared to other lakes due to lower Dreissena biomass (Fig. 4). We hypothesize that mussel populations will likely continue to grow in even deeper areas calling for further investigation to understand the ecosystem impact of this shift. We thus suggest a new “offshore carbon and phosphorus sink” hypothesis. We should mention that two previously described mechanisms of Dreissena spp. impacts on the ecosystem (“nearshore phosphorus shunt” and “mid-depth carbon and phosphorus sink”) still exist (Fig. 7d), and the relative importance of all these mechanisms depends on the distribution of mussels biomass, their clearance rate, and their access to the epilimnion phytoplankton.
Biophysical model limitations
The most distinctive difference between the model and actual observations was the model’s failure to show a decline in mussel biomass that was observed in shallow areas later in the invasion. This difference arose because we model instantaneous mussel biomass growth and omit mussel size/age structure (Caswell 2001). In reality, young individuals take time to grow (e.g., years for profundal dreissenids, Karatayev et al. 2018a) and initially have very low individual weight. Early in the invasion, most individuals are small, total biomass and competition are low, and recruitment is high. With time, high densities translate to high biomass (the ‘boom’) that can inhibit recruitment by depleting food; poor recruitment then leads to population decline (the ‘bust’; e.g., Fig. 4; Caswell 2001). This population dynamic represents cohort cycles and can occur in any species including Dreissena spp., where high biomass might inhibit recruitment by depleting food (Karatayev et al. 2018a). Our biophysical model simulated slower biomass increase in deep areas due to colder temperatures and food limitation, consistent with observations, but by omitting size/age structure and recruitment processes, our biophysical model did not reproduce effects of density-dependent limitations on recruitment.
Alternative features missing from the model that might explain observed biomass fluctuations include predation by the invading round gobies and declines in mussel carrying capacity over time. In addition to mortality rates and recruitment, lake trophic status may influence the dreissenid carrying capacity, but total P was constant across our modeled bathymetry scenarios. The total P concentration in the biophysical model (7 µg/L) was similar to eastern and central Lake Erie and Lake Ontario (6–8 µg/L), but greater than Lakes Michigan and Huron (3–7 µg/L) around 2010 (Chapra and Dolan 2012). In contrast, mean total P of western Erie and Saginaw Bay was 15.5 and 16.5 µg/L, respectively, in 2010 (Chapra and Dolan 2012). The mean total P concentration of Lake Michigan declined from 4.6 to 3.0 µg/L over the course of the quagga mussel invasion (2000–2010), which may represent increased transfer of P to the sediment as a result of mussel filter feeding (Rowe et al. 2017), and cause a negative feedback on dreissenid carrying capacity, a process that was not represented in our model. However, changes in primary production and goby abundance are unlikely to be as strong and simultaneous across all lakes as observed declines in density and (in shallow lakes and zones) biomass.
A second key difference between our model and data is that in deep lakes simulated biomass was greatest in the < 30 m zone while observed biomass was greatest in the 30–100 m zone (Figs. 4, 7b). This difference can arise from multiple reasons. As suggested earlier in the Discussion, greater biomass in mid-depth areas might arise from density-dependent recruitment and size/age structure (combined with propagule supply from shallow areas). Littoral mussel biomass might also be lower in reality than in the model due to greater mussel mortality (here modeled as spatially homogeneous) in shallow areas from greater goby predation, wave stress, or temperatures. Unstable bottom conditions caused by wave action and sediment movement might also explain rapid declines in mussel populations < 30 m (Nalepa et al. 2010). Both zebra and quagga mussels also experienced greater mortality when kept in Lake Erie water at a warmer temperature representative of littoral zone conditions relative to colder temperatures representative of hypolimnetic conditions (Karatayev et al. 2010). Lower water temperatures may also contribute to the success of quagga mussels in the 30–90 m zone, as respiration rates decrease with temperature (Stoeckmann 2003), thus a lower food intake is required to maintain biomass at low temperature. Finally, greater light penetration in deeper areas (due to lower sediment resuspension) might increase carrying capacity in mid-depth zones, but this contradicts reduced growth of profundal compared to littoral mussels in in situ experiments (Karatayev et al. 2018a).
Our model results also suggested that littoral zones of deep lakes supported greater biomass than shallow lakes, owing to horizontal transport of phytoplankton from offshore production that was protected from local benthic filter feeders by stratification and a deep water column (Rowe et al. 2015; Waples et al. 2017). Rowe et al. (2015) showed satellite remote sensing evidence of reduced phytoplankton abundance in Lake Michigan in areas of high filter-feeding intensity during the spring isothermal period; these locations were surrounded by areas of higher phytoplankton abundance, which likely supplement the depleted regions by horizontal transport. Waples et al. (2017) documented particulate organic carbon fluxes to Lake Michigan mussel beds in mid-depth regions that were up to ~ 6 times greater than pelagic primary production, further indicating supplement to these regions by lateral transport. Unfortunately, we were unable to test the prediction that shallow areas of deep lakes support higher dreissenid densities than shallow lakes using our data due to differences in dreissenid carrying capacity (e.g., lake trophic status), mortality, and sampling coverage between these habitats.
Conclusions
We provided strong evidence that lake morphometry determines invasion dynamics of both zebra and quagga mussels. In shallow lakes dreissenids exhibit a classic boom-and-bust dynamic but remain common across all years and water depths. In deep lakes a rapid colonization of shallow areas by zebra mussels is followed by a much slower expansion of quagga mussels to deeper areas where there is less food and lower temperatures. Overall, the net effect in deep lakes is a dramatic increase in ecosystem-wide dreissenid density, and with time a shift in Dreissena spp. biomass to deep areas. We also found that where both Dreissena species co-occur, quagga mussels generally become dominant in 8–13 years but morphometry affects the outcome of competition: while zebra mussels are almost completely displaced in deep lakes, they remain common in shallow lakes. Comparison with 21 waterbodies in North America and Europe colonized by both dreissenids confirmed that patterns of invasion dynamics found in the Great Lakes are very consistent with other lakes and reservoirs, and thus can be generalized to other lakes. Our biophysical model reproduced major differences in Dreissena invasion between shallow and deep lakes and predicted that the long-term reduction in primary producers by mussel grazing may be fourfold less in deep lakes compared to shallow lakes due to greater water volume and thermal stratification which reduces relative food availability. Whilst the impact of mussel grazing remains greatest in shallow areas, we showed that when lakes are well-mixed during the isothermal period in spring and fall, dreissenid grazing impact may be greatest offshore at 30–90 m depths; this reveals a novel, potentially strong offshore carbon and phosphorus sink.
Data availability
All primary data and model code are included in Supporting Information, Online Resources 1–5.
Code availability
Included in Supporting Information, Online Resource 2.
References
Aagaard K, Lockwood JL (2016) Severe and rapid population declines in exotic birds. Biol Invasions 18:1667–1678
Baldwin BS, Mayer MS, Dayton J, Pau N, Mendilla J, Sullivan M, Moore A, Ma A, Mills EL (2002) Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Can J Fish Aquat Sci 59:680–694
Barbiero RP, Tuchman ML (2001) Results from the U.S. EPA’s biological open water surveillance program of the Laurentian Great Lakes: II. Deep chlorophyll maxima. J Great Lakes Res 27:155–166
Barbiero RP, Tuchman ML (2004) Long-term dreissenid impacts on water clarity in Lake Erie. J Great Lakes Res 30:557–565
Barbiero RP, Schmude K, Lesht BM, Riseng CM, Warren GJ, Tuchman ML (2011) Trends in Diporeia populations across the Laurentian Great Lakes, 1997–2009. J Great Lakes Res 37:9–17
Barbiero RP, Lesht BM, Warren GJ, Rudstam LG, Watkins JM, Reavie ED, Kovalenko KE, Karatayev AY (2018) A comparative examination of recent changes in nutrients and lower food web structure in Lake Michigan and Lake Huron. J Great Lakes Res 44:573–589. https://doi.org/10.1016/j.jglr.2018.05.012
Barton DR, Johnson RA, Campbell L, Petruniak J, Patterson M (2005) Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. J Great Lakes Res 31(S2):252–261
Beletsky D, Schwab D (2001) Modeling circulation and thermal structure in Lake Michigan: annual cycle and interannual variability. J Geophys Res 106:19745–19771
Beletsky D, Schwab D, McCormick M (2006) Modeling the 1998–2003 summer circulation and thermal structure in Lake Michigan. J Geophys Res 111:1–18
Beletsky D, Hawley N, Rao Y (2013) Modeling summer circulation and thermal structure of Lake Erie. J Geophys Res 118:1–15
Benson AJ (2014) Chronological history of zebra and quagga mussels (Dreissenidae) in North America, 1988–2010. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 9–32
Birkett K, Lozano SJ, Rudstam LG (2015) Long-term trends in Lake Ontario’s benthic macroinvertebrate community from 1994–2008. Aquat Ecosys Health Manag Soc 18:78–88
Bowers R, Sudomir JC, Kershner MW, de Szalay FA (2005) The effects of predation and unionid burrowing on bivalve communities in a Laurentian Great Lake coastal wetland. Hydrobiologia 545:93–102
Brinkhurst RO (1974) The Benthos of Lakes. Macmillan, London
Bunnell DB, Barbiero RP, Ludsin SA, Madenjian CP, Warren GJ, Dolan DM, Brenden TO, Briland R, Gorman OT, He Ji X, Johengen TH, Lantry BF, Lesht BM, Nalepa TF, Riley SC, Riseng CM, Treska TJ, Tsehaye I, Walsh MG, Warner DM, Weidel BC (2014) Changing ecosystem dynamics in the Laurentian Great Lakes: bottom-up and top-down regulation. Bioscience 64:26–39
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Burlakova LE, Karatayev AY, Padilla DK (2006) Changes in the distribution and abundance of Dreissena polymorpha within lakes through time. Hydrobiologia 571:133–146
Burlakova LE, Tulumello BL, Karatayev AY, Krebs RA, Schloesser DW, Paterson WL, Griffith TA, Scott MW, Crail T, Zanatta DT (2014) Competitive replacement of invasive congeners may relax impact on native species: interactions among zebra, quagga, and native unionid mussels. PLoS ONE 9(12):E114926. https://doi.org/10.1371/journal.pone.0114926
Burlakova LE, Karatayev AY, Mehler K, Daniel S (2017) Lake Erie Survey within Cooperative Science and Monitoring Initiative. Chapter 1. In: Lake Erie and Lake Michigan Benthos: Cooperative Science and Monitoring Initiative. Final Report. USGSGLRI G14AC00263. Great Lakes Center, SUNY Buffalo State, Buffalo, NY. Available at: http://greatlakescenter.buffalostate.edu/sites/greatlakescenter.buffalostate.edu/files/uplo ads/Documents/Publications/LakeErieandMichiganBenthos_CSMI2014–2015_FinalReport.pdf
Burlakova LE, Barbiero RP, Karatayev AY, Daniel SE, Hinchey EK, Warren GJ (2018) The benthic community of the Laurentian Great Lakes: analysis of spatial gradients and temporal trends from 1998–2014. J Great Lakes Res 44:600–617
Burns NM (1985) Erie, the lake that survived. Rowman and Allanheld Publishers, USA
Carlton JT (2008) The zebra mussel Dreissena polymorpha found in North America in 1986 and 1987. J Great Lakes Res 34:770–773
Casper AF, Johnson LE (2010) Contrasting shell/tissue characteristics of Dreissena polymorpha and Dreissena bugensis in relation to environmental heterogeneity in the St. Lawrence River. J Great Lakes Res 36:184–189
Caswell H (2001) Matrix Population Models. Sinauer Assoc. Inc., Sunderland, MA
Chapra SC, Sonzogni WC (1979) Great Lakes total phosphorus budget for the mid-1970’s. J Water Pollut Control Fed 51:2524–2533
Chapra SC, Dolan DM (2012) Great Lakes total phosphorus revisited: 2. Mass balance modeling. J Great Lakes Res 38:741–754. https://doi.org/10.1016/j.jglr.2012.10.002
Chase ME, Bailey RC (1999) The ecology of the zebra mussels (Dreissena polymorpha) in the lower Great Lakes of North America: I. Population dynamics and growth. J Great Lakes Res 25:107–124
Chen C, Liu H, Beardsley RC (2003) An unstructured grid, finite-volume, three-dimensional, primitive equations ocean model: Application to coastal ocean and estuaries. J Atmo Oceanic Technol 20:159–186. https://doi.org/10.1175/1520-0426(2003)020%3c0159:AUGFVT%3e2.0.CO;2
Claxton WT, Mackie GL (1998) Seasonal and depth variations in gametogenesis and spawning of Dreissena polymorpha and Dreissena bugensis in eastern Lake Erie. Can J Zool 76:2010–2019
Crooks JA (2005) Lag times and exotic species: the ecology and management of biological invasions in slow motion. Ecoscience 12:316–329
Dermott R, Mitchell J, Murray I, Fear E (1993) Biomass and production of zebra mussel (Dreissena polymorpha) in shallow waters of northeastern Lake Erie. In: Nalepa TF, Schloesser DW (eds) Zebra Mussels: Biology, impacts, and control. Lewis Publishers, Ann Arbor, Michigan, pp 399–413
Dermott R, Munawar M (1993) Invasion of Lake Erie offshore sediments by Dreissena, and its ecological implications. Can J Fish Aquat Sci 50:2298–2304
Diggins TP (2001) A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. J Great Lakes Res 27:457–466
Edsall TA, Manny BA, Raphael N (1988) The St. Clair River and Lake St. Clair: An ecological profile. U.S. Fish and Wildlife Serv., Biol. Rept. No. 85 (7.3)
Fahnenstiel GL, Lang GA, Nalepa TF, Johengen TH (1995) Effects of zebra mussel (Dreissena polymorpha) colonization on water quality parameters in Saginaw Bay, Lake Huron. J Great Lakes Res 21:435–448
Fahnenstiel G, Pothoven S, Nalepa T, Vanderploeg H, Klarer D, Scavia D (2010) Recent changes in primary production in the offshore region of southeastern Lake Michigan. J Great Lakes Res 36(Supplement 3):20–29
Fahnenstiel GL, Sayers MJ, Shuchman RA, Yousef F, Pothoven SA (2016) Lake-wide phytoplankton production and abundance in the Upper Great Lakes: 2010–2013. J Great Lakes Res 42:619–629. https://doi.org/10.1016/j.jglr.2016.02.004
Garton D, Haag W (1993) Seasonal reproductive cycles and settlement patterns of Dreissena polymorpha in western Lake Erie. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: Biology, impacts, and control. Lewis Publishers, Ann Arbor, Michigan, pp 111–128
Glyshaw PW, Riseng CM, Nalepa TF, Pothoven SA (2015) Temporal trends in condition and reproduction of quagga mussels (Dreissena rostriformis bugensis) in southern Lake Michigan. J Great Lakes Res 41:16–26. https://doi.org/10.1016/j.jglr.2015.08.006
Griffiths RW, Schloesser DW, Leach JH, Kovalak WP (1991) Distribution and dispersal of the zebra mussel (Dreissena polymorpha) in the Great Lakes region. Can J Fish Aquat Sci 48:1381–1388
Hebert PDN, Muncaster BW, Mackie GL (1989) Ecological and genetic studies on Dreissena polymorpha (Pallas): a new mollusc in the Great Lakes. Can J Fish Aquat Sci 46:1587–1591
Hecky RE, Smith REH, Barton DR, Guildford SJ, Taylor WD, Charlton MN, Howell ET (2004) The near shore phosphorus shunt: A consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can J Fish Aquat Sci 61:1285–1293
Hetherington A, Rudstam LG, Schneider RL, Holeck KT, Hotaling CW, Cooper JE, Jackson JR (2019) Invader invaded: population dynamics of zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena rostriformis bugensis) in polymictic Oneida Lake, NY, USA (1992–2013). Biol Invasions 21:1529–1544
Hewson I, Eaglesham JB, Höök TO, LaBarre BA, Sepúlveda MS, Thompson PD, Watkins JM, Rudstam LG (2013) Investigation of viruses in Diporeia spp. from the Laurentian Great Lakes and Owasco Lake as potential stressors of declining populations. J Great Lakes Res 39:499–506. https://doi.org/10.1016/j.jglr.2013.06.006
Higgins SN, Vander Zanden MJ (2010) What a difference a species makes: A meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol Monogr 80:179–196
Hunter RD, Simons KA (2004) Dreissenids in Lake St. Clair in 2001: Evidence for population regulation. J Great Lakes Res 30:528–537
Jackson MC, Ruiz-Navarro A, Britton JR (2015) Population density modifies the ecological impacts of invasive species. Oikos 124:880–887
Jarvis P, Dow J, Dermott R, Bonnell R (2000) Zebra (Dreissena polymorpha) and quagga mussel (Dreissena bugensis) distribution and density in Lake Erie, 1992–1998. Can Tech Rep Fish Aquat Sci 2304. Fisheries and Oceans Canada
Jónasson PM (2004) Benthic invertebrates. In: O’Sullivan PE, Reynolds CS (eds) The Lakes Handbook, vol 1. Limnology and Limnetic Ecology. Blackwell Publishing, Oxford, pp 341–416
Karatayev AY, Burlakova LE, Padilla DK (1997) The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in eastern Europe. J Shellfish Res 16:187–203
Karatayev AY, Burlakova LE, Padilla DK (1998) Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). J Shellfish Res 17(4):1219–1235
Karatayev AY, Burlakova LE, Padilla DK (2002) Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In: Leppakoski E, Gollach S, Olenin S (eds) Invasive aquatic species of Europe: Distribution, impacts and management. Kluwer Academic Publishers, Dordreicht, The Netherlands, pp 433–446
Karatayev AY, Padilla DK, Minchin D, Boltovskoy D, Burlakova LE (2007) Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biol Invasions 9:161–180
Karatayev AY, Mastitsky SE, Padilla DK, Burlakova LE, Hajduk MM (2010) Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia 668:183–194
Karatayev AY, Burlakova LE, Mastitsky SE, Padilla DK, Mills EL (2011) Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. J Shellfish Res 30:923–931
Karatayev VA, Karatayev AY, Burlakova LE, Padilla DK (2013) Lakewide dominance does not predict the potential for spread of dreissenids. J Great Lakes Res 39:622–629
Karatayev AY, Burlakova LE, Pennuto C, Ciborowski J, Karatayev VA, Juette P, Clapsadl M (2014) Twenty-five years of changes in Dreissena spp. populations in Lake Erie. J Great Lakes Res 40:550–559
Karatayev AY, Burlakova LE, Padilla DK (2015) Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746:97–112
Karatayev AY, Karatayev VA, Burlakova LE, Rowe MD, Mehler K, Clapsadl MD (2018a) Food depletion regulates the demography of invasive dreissenid mussels in a stratified lake. Limnol Oceanogr 63:2065–2079
Karatayev AY, Burlakova LE, Mehler K, Bocaniov SA, Collingsworth PD, Warren G, Kraus RT, Hinchey EK (2018b) Biomonitoring using invasive species in a large lake: Dreissena distribution maps hypoxic zones. J Great Lakes Res 44:639–649
Karatayev AY, Burlakova LE, Mehler K, Rudstam LG, Watkins JM, Wick M (2020) Dreissena in Lake Ontario 30 years post-invasion. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2020.11.010
Leach JH (1991) Biota of Lake St. Clair: habitat evaluation and environmental assessment. Hydrobiologia 219:187–202
Leach JH (1993) Impacts of the zebra mussel (Dreissena polymorpha) on water quality and fish spawning reefs in western Lake Erie. In: Nalepa TF, Schloesser DW (eds) Zebra mussels. Biology, impacts, and control. Lewis Publishers, Ann Arbor, Michigan, pp 381–397
Lockwood JL, Hoopes MF, Marchetti MP (2013) Invasion Ecology, 2nd edn. Wiley-Blackwell, West Sussex
Mackie GL (1991) Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair Hydrobiologia 219:251–268
Magnuson JJ (1990) Long-term ecological research and the invisible present. Bioscience 40(7):495–501
Manly BFJ (2009) Statistics for Environmental Science and Management, 2nd edn. CRC Press, Boca Raton, FL
Markovskiy YM (1954) Fauna of invertebrates of the lower reaches of the Ukrainian rivers, living conditions, and their potential use. Part II. Dnieper-Bug Liman. Kiev: Academy of Sciences of the Ukrainian SSR Press (in Russian)
Marsden JE, Trudeau N, Keniry T (1993) Zebra mussel study of Lake Michigan. Aquat Ecol technical report 93/14. Illinois Natural History Survey
Mills EL, Dermott RM, Roseman EF, Dustin D, Mellina E, Corm DB, Spidle AP (1993) Colonization, ecology, and population structure of the “quagga” mussel (Bivalvia: Dreissenidae) in the lower Great Lakes. Can J Fish Aquat Sci 50:2305–2314
Mills EL, Chrisman JR, Baldwin B, Owens RW, O’Gorman R, Howell T, Roseman E, Raths MK (1999) Changes in the dressenid community in the lower Great Lakes with emphasis on southern Lake Ontario. J Great Lakes Res 25:187–197
Moroz TG, Aleksenko TL (1983) Benthos of the Dnieper-Bug Liman following the regulation of the Dnieper River run-off. Gidrobiol Zh 19:33–40 (in Russian with English summary)
Mortimer CH (1987) Fifty years of physical investigations and related limnological studies on Lake Erie, 1928–1977. J Great Lakes Res 13:407–435
Nakano D, Strayer DL (2014) Biofouling animals in fresh water: ecology, impacts, and ecological engineering. Front Ecol Environ 12:167–175
Nalepa TF, Schloesser DW (1993) Zebra Mussels Biology, Impacts, and Control. Lewis Publishers, Ann Arbor, Michigan
Nalepa TF, Wojcik JA, Fanslow DL, Lang GA (1995) Initial colonization of the zebra mussel (Dreissena polymorpha) in Saginaw Bay, Lake Huron: population recruitment, density, and size structure. J Great Lakes Res 21:417–434
Nalepa TF, Schloesser DW, Pothoven SA, Hondorp DW, Fanslow DL, Tuchman ML, Fleischer GW (2001) First finding of the amphipod Echinogammarus ischnus and the mussel Driessena bugensis in Lake Michigan. J Great Lakes Res 27:384–391
Nalepa TF, Fanslow DL, Foley AJ III, Lang GA, Eadie BJ, Quigley MA (2006) Continued disappearance of the benthic amphipod Diporeia spp. in Lake Michigan: is there evidence of food limitation? Can J Fish Aquat Sci 63:872–890
Nalepa TF, Fanslow DL, Pothoven SA, Foley AJ III, Lang GA. Mozley SC, Winnell MW (2007) Abundance and distribution of benthic macroinvertebrate populations in Lake Huron in 1972 and 2000–2003. NOAA Technical Memorandum GLERL-140. Great Lakes Environmental Research Laboratory, Ann Arbor, MI
Nalepa TF, Fanslow DL, Pothoven SA (2010) Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. J Great Lakes Res 36:5–19
Nalepa TF, Schloesser DW (2014) Quagga and zebra mussels: Biology, impacts, and control, 2nd edn. CRC Press, Boca Raton
Nalepa TF, Fanslow DL, Lang GA, Mabrey K, Rowe M (2014) Lake-wide benthic surveys in Lake Michigan in 1994–95, 2000, 2005, and 2010: Abundances of the amphipod Diporea spp. and abundances and biomass of the mussels Dreissena polymorpha and Dreissena rostriformis bugensis. NOAA Technical Memorandum GLERL-164
Nalepa TF, Riseng CM, Elgin AK, Lang GA (2018) Abundance and distribution of benthic macroinvertebrates in the Lake Huron system: Saginaw Bay, 2006–2009, and Lake Huron, including Georgian Bay and North Channel, 2007 and 2012. NOAA Technical Memorandum GLERL-172. Great Lakes Environmental Research Laboratory, Ann Arbor, MI
Nalepa TF, Burlakova LE, Elgin AK, Karatayev AY, Lang GA, Mehler K (2020) Abundance and Biomass of Benthic Macroinvertebrates in Lake Michigan in 2015, with a Summary of Temporal Trends. NOAA Technical Memorandum GLERL-175. NOAA, Great Lakes Environmental Research Laboratory, Ann Arbor, MI. doi: https://doi.org/10.25923/g0d3-3v41
Nguyen TD (2014) Circulation and exchange in the Saginaw Bay-Lake Huron system: observations and numerical modeling. Dissertation, Michigan State University
O’Neill CR Jr (2008) The Silent Invasion: Finding Solutions to Minimize the Impacts of Invasive Quagga Mussels on Water Rates, Water Infrastructure and the Environment. Hearing of the U.S. House of Representatives Committee on Natural Resources – Subcommittee on Water and Power, Washington, D.C. Available at: http://naturalresources.house.gov/uploadedfiles/oneilltestimony06.24.08.pdf.
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Patterson MWR, Ciborowski JJH, Barton DR (2005) The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie. J Great Lakes Res 31(Suppl. 2):223–237
Peyer SM, McCarthy AJ, Lee CE (2009) Zebra mussels anchor byssal threads faster and tighter than quagga mussels in flow. J Exp Biol 212:2026–2035
Peyer SM, Hermanson JC, Lee CE (2010) Developmental plasticity of shell morphology of quagga mussels from shallow and deep-water habitats of the Great Lakes. J Exp Biol 213:2602–2609
Pryanichnikova EG (2012) Structure and function of dreissenid populations in Rybinsk Reservoir. Dissertation, Papanin Institute for Biology of Inland Waters. Russian Academy of Sciences (in Russian)
Pollux BJA, van der Velde G, bij de Vaate A (2010) A perspective on global spread of Dreissena polymorpha: a review on possibilities and limitations, In: van der Velde G, Rajagopa S, bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, The Netherlands, pp 45–58
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ramcharan CW, Padilla DK, Dodson SI (1992) Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Can J Fish Aquat Sci 49:2611–2620
Roe SL, MacIsaac HJ (1997) Deepwater population structure and reproductive state of quagga mussels (Dreissena bugensis) in Lake Erie. Can J Fish Aquat Sci 54:2428–2433
Rowe MD, Obenour DR, Nalepa TF, Vanderploeg HA, Yousef F, Kerfoot WC (2015) Mapping the spatial distribution of the biomass and filter-feeding effect of invasive dreissenid mussels on the winter-spring phytoplankton bloom in Lake Michigan. Freshw Biol 60:2270–2285
Rowe MD, Anderson EJ, Vanderploeg HA, Pothoven SA, Elgin AK, Wang J, Yousef F (2017) Influence of invasive quagga mussels, phosphorus loads, and climate on spatial and temporal patterns of productivity in Lake Michigan: A biophysical modeling study. Limnol Oceanogr 62(6):2629–2649
Rudstam LG, Gandino CJ (2020) Zebra or quagga mussel dominance depends on trade-offs between growth and defense - Field support from Onondaga Lake, NY. PLoS ONE 15(6):e0235387. https://doi.org/10.1371/journal.pone.0235387
Schelske CL, Roth JC (1973) Limnological survey of lakes Michigan, Superior, Huron, and Erie. Great Lakes Research Division. Publication No 17. The University of Michigan, Ann Arbor, Michigan
Scofield AE, Watkins JM, Weidel BC, Luckey FJ, Rudstam LG (2017) The deep chlorophyll layer in Lake Ontario: extent, mechanisms of formation, and abiotic predictors. J Great Lakes Res 43:782–794
Sibson R (1981) A Brief Description of Natural Neighbor Interpolation. In: Barnett V (ed) Interpolating Multivariate Data. John Wiley & Sons, New York, pp 21–36
Simberloff D, Gibbons L (2004) Now you see them, now you don’t! – population crashes of established introduced species. Biol Invasions 6:161–172
Skubinna JP, Coon TG, Batters TR (1995) Increased abundance and depth of submersed macrophytes in response to decreased turbidity in Saginaw Bay, Lake Huron. J Great Lakes Res 21:476–478
Stanzykowska A (1978) Occurrence and dynamics of Dreissena polymorpha (Pall.) (Bivalvia). Verh Internat Verein Limnol 20:2431–2434
Stoeckmann A (2003) Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Can J Fish Aquat Sci 60:126–134
Strayer DL, Malcom HM (2006) Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshwater Biol 51:117–130
Strayer DL, D’Antonio CM, Essl F, Fowler MS, Geist J, Hilt S, Jarić I, Jöhnk K, Jones CG, Lambin X, Latzka AW, Pergl J, Pyšek P, Robertson P, von Schmalensee M, Stefansson RA, Wright J, Jeschke JM (2017) Boom-bust dynamics in biological invasions: towards an improved application of the concept. Ecology Letters 20:1337–1350
Strayer DL, Adamovich BV, Adrian R, Aldridge DC, Balogh C, Burlakova LE, Fried-Petersen H, G.-Tóth L, Hetherington AL, Jones TS, Karatayev AY, Madill JB, Makarevich OA, Marsden JE, Martel AL, Minchin D, Nalepa TF, Noordhuis R, Robinson TJ, Rudstam LG, Schwalb AN, Smith DR, Steinman AD, Jeschke JM (2019) Long-term population dynamics of dreissenid mussels (Dreissena polymorpha and D. rostriformis): a cross-system analysis. Ecosphere. https://doi.org/10.1002/ecs2.2701
The Great Lakes an Environmental Atlas and Resource Book (1995) U.S. Environmental Protection Agency and Environment Canada. 3rd edition. Chicago, IL; Toronto, Ontario
Vanderploeg HA, Nalepa TF, Jude DJ, Mills EL, Holeck KT, Liebig JR, Grigorovich IA, Ojaveer H (2002) Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can J Fish Aquat Sci 59:1209–1228
Vanderploeg HA, Liebig JR, Nalepa TF, Fahnenstiel GL, Pothoven SA (2010) Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. J Great Lakes Res 36:50–59
Waples JT, Bootsma HA, Klump JV (2017) How are coastal benthos fed? Limnol Oceanogr Lett 2(1):18–28
Watkins JM, Dermott R, Lozano SJ, Mills EL, Rudstam LG, Scharold JV (2007) Evidence for remote effects of dreissenids mussels on the amphipod Diporea: analysis of Lake Ontario benthic surveys, 1997–2003. J Great Lakes Res 33:642–657
Wetzel RG (1983) Limnology, 2nd edn. Saunders College Publishing, Philadelphia
White ER (2019) Minimum time required to detect population trends: the need for long-term monitoring programs. Bioscience 69(1):40–46
Wood SN (2017) Generalized Additive Models: An Introduction with R, 2nd edn. Chapman and Hall/CRC, USA
Yokomizo H, Possingham HP, Thomas MB, Buckley YM (2009) Managing the impact of invasive species: the value of knowing the density-impact curve. Ecol Appl 19:376–386
Zenkevich LA (1940) About acclimatization in the Caspian Sea new invertebrates as a food for fishes and theoretical background for this. Bulletin of Moscow Society of Naturalists. Biological series. Moscow 39(1):18–30 (in Russian)
Zhulidov AV, Kozhara AV, Scherbina GH, Nalepa TF, Protasov A, Afanasiev SA, Pryanichnikova EG, Zhulidov DA, Gurtovaya TY, Pavlov DF (2010) Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biol Invasions 12:1923–1940
Zhulidov AV, Nalepa TF, Kozhara AV, Zhulidov DA, Gurtovaya TY (2006) Recent trends in relative abundance of two dreissenid species, Dreissella polymorpha and Dreissella bugellsis in the lower Don River system, Russia. Arch Hydrobiol 165:209–220
Acknowledgements
Research supported by U.S. Geological Survey, Department of the Interior, under USGS Agreement No. G14AC000263, “Lake Erie and Lake Michigan Benthos: Cooperative Science and Monitoring Initiative” and by an agreement with Cornell University, Department of Natural Resources under Prime Agreement Award GL00E02254 from the U.S. EPA “Great Lakes Long-Term Biological Monitoring Program 2017-2022”. The authors would like to thank the many technicians and vessel crew members who helped to collect and produce the data presented here, most recently: S. Daniel, E. Hartnett, K. Hastings, B. Tulumello, J. Fisher, B. Haas (Great Lakes Center), graduate and undergraduate students (SUNY Buffalo State), as well as G. Carter, P. Glyshaw, K. Mabrey, and M. Wensman (NOAA GLERL and CIGLR). We are thankful to two anonymous reviewers for suggestions that helped to improve our manuscript. The authors declare that there is no conflict of interest regarding the publication of this article. This article has not been subjected to U.S. EPA review. The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. EPA. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. EPA. This is NOAA GLERL Contribution No. 1974.
Funding
Research supported by U.S. Geological Survey, Department of the Interior, under USGS Agreement No. G14AC000263, “Lake Erie and Lake Michigan Benthos: Cooperative Science and Monitoring Initiative” and by an agreement with Cornell University, Department of Natural Resources under Prime Agreement Award GL00E02254 from the U.S. EPA “Great Lakes Long-Term Biological Monitoring Program 2017–2022”.
Author information
Authors and Affiliations
Contributions
AYK: conception and design, acquisition of data, data analysis and interpretation, drafting the article and revising; VAK, LEB: conception and design, data analysis and interpretation, drafting the article and revising; KM, MDR, AKE, TFN: acquisition of data, data analysis and interpretation; article revising.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Online Resource 1
Excel file with primary data on Great Lakes Dreissena spp. densities and biomass for 1990–2018 (XLSX 220 KB)
Online Resource 2
Script for general population model (R 7 KB)
Online Resource 3
Excel file with data on Dreissena spp. invasion dynamics from 21 North American and European waterbodies (XLSX 29 KB)
Online Resource 4
Lake-specific dynamics of Dreissena spp. log AFTDW biomass in shallow lakes across time and space in generalized additive models incorporating all effects (DOCX 141 KB)
Online Resource 5
Dreissena spp. filter-feeding intensity, or fraction of the water column cleared per day under vertically well-mixed conditions (DOCX 93 KB)
Rights and permissions
About this article
Cite this article
Karatayev, A.Y., Karatayev, V.A., Burlakova, L.E. et al. Lake morphometry determines Dreissena invasion dynamics. Biol Invasions 23, 2489–2514 (2021). https://doi.org/10.1007/s10530-021-02518-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02518-3