Abstract
We summarized over 30 years of research on zebra and quagga mussels in the Laurentian Great Lakes and compared with data from European and North American inland lakes. Invasion dynamics, growth, and reproduction of dreissenids in the Great Lakes are governed by lake morphometry. At < 30 m mussels overshot their carrying capacity and declined within 13–15 years after first detection. At 30–90 m their densities increased more slowly and declined to a lesser extent, while at > 90 m populations continue to increase even after 30 years of invasion. After the proliferation of quagga mussels, benthic wet biomass (including molluscs shells) increased about two orders of magnitude and currently exceeds zooplankton biomass > 40-fold. Strong benthic/pelagic coupling redirects food and energy from the water column to the bottom causing an increase in Secchi depth, decline in phosphorus, chlorophyll, phytoplankton and zooplankton biomass. The abundance of commercially important fishes declined as a result of the dramatic decrease in their main food deep water amphipods Diporeia, which has been outcompeted by exotic mussels. However, the introduction of round goby into the Great Lakes in the 1990s provided an important link between dreissenids and commercially and recreationally valuable fish species, increasing their productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dreissena polymorpha (Pallas), the zebra mussel, and D. rostriformis bugensis (Andrusov), the quagga mussel, both have planktonic larvae and attached adult stages, can form unusually high densities for freshwater bivalves in the invaded waterbodies, and are highly efficient suspension feeders. These life histories are typical of marine mussels, but in North American and most of the European freshwaters they represent a novel ecological type (Johnson & Carlton, 1996; Karatayev et al., 1997, 2007a). Although the two Dreissena species are similar, they are not identical and have a different history of spread, different tolerance to environmental parameters, and different distribution within waterbodies, resulting in dissimilar ecosystem impacts (Nalepa et al., 2010; Karatayev et al., 2011a, 2015a, 2021a; Benson, 2014; bij de Vaate et al., 2014). Zebra mussels started spreading beyond their native range at the beginning of the nineteenth century, when three canals were constructed in Russia (in what is now Belarus) to connect shipping routes between the Black Sea and Baltic Sea basins (reviewed in Kerney & Morton, 1970; Kinzelbach, 1992; Karatayev et al., 2003, 2007a, 2008; Pollux et al., 2010; van der Velde et al., 2010a). By the late 1980s, when zebra mussels finally reached North America, over 2000 papers on their biology, ecology, spread, and control were already published in Europe (reviewed in Karatayev et al., 2015a). The rapid spread of zebra mussels across North American freshwaters, with its significant ecological impacts and damage to industry using raw water, triggered another strong spike in studies (Nalepa & Schloesser, 1993, 2014; Schloesser & Schmuckal, 2012) and facilitated the interest of North American scientists to European publications resulting in several reviews (Strayer, 1991; Ramcharan et al., 1992; Strayer & Smith, 1993; Karatayev et al., 1997, 1998; Molloy et al., 1997). Three decades of zebra mussels’ studies in North America have largely confirmed previous European findings, although they did bring additional valuable information on zebra mussels biology and ecology.
In contrast to zebra mussels, relatively little was known about its congener the quagga mussel before they invaded North America: thus, less than 8% of all papers published in Europe on dreissenids were focused on the quagga mussels (Karatayev et al., 2015a). Originally native to a small area of the Dnieper-Bug Liman (a large shallow productive estuary with variable salinity) in the Dnieper River delta, and lower reaches of the South Bug and Ingulets rivers in Ukraine, quagga mussel started spreading beyond its range only in the middle of the twentieth century, colonizing waterbodies already populated with zebra mussels (reviewed in Zhulidov et al., 2004, 2010; Karatayev et al., 2007b, 2011a, 2015a; van der Velde et al., 2010b). In North America quagga mussels invaded all the Laurentian Great Lakes except Lake Superior, forming densities much higher than that of zebra mussels (Jarvis et al., 2000; Patterson et al., 2005; Watkins et al., 2007; Nalepa et al., 2010; Karatayev et al., 2014a, 2021a).
Over 30 years of research conducted on the Great Lakes revealed novel patterns of population dynamics and ecological impacts of quagga mussels. It was found that lake morphometry governs population dynamics, distribution, and ecological impacts of both dreissenid species, but data on quagga mussels were especially intriguing (reviewed in Karatayev et al., 2015a, 2021a). In shallow basins and embayments of the Great Lakes, both species can colonize the whole bottom, form more or less similar population density, and have comparable ecological impacts. In contrast, in deep lakes (Michigan, Huron, and Ontario) zebra mussels largely occupy a narrow nearshore zone with limited impact on the offshore environment, while quagga mussels form much higher density, colonize the whole lake bottom, and have strong lake-wide impact.
Early in the invasion both dreissenid species in deep Great Lakes were limited to the shallow nearshore zone, directly impacting littoral communities. Here dreissenids, associated benthic invertebrates, and algae alter nutrient cycling and retain phosphorus from allochthonous sources in the nearshore, thus impacting offshore pelagic communities through “nearshore phosphorus shunt” (Hecky et al., 2004), but this impact was largely limited to the shallow nearshore environment (Vanderploeg et al., 2010; Barbiero et al., 2018; Karatayev et al., 2021a). The replacement of zebra with quagga mussels in lakes Ontario, Michigan, and Huron in the mid-2000s was associated with a dramatic increase in dreissenid lake-wide population density and a shift in the bulk of mussels from the nearshore to mid-depth zone (30–50 m). It was estimated that the fraction of water column cleared per day in Lake Michigan in this zone in 2007–2008 exceeded phytoplankton growth, encouraging a new “mid-depth carbon and phosphorus sink” hypothesis (Vanderploeg et al., 2010). The expansion of quagga mussel populations in mid-depth zones resulted in increased transparency and silica concentrations, declines in seston, chlorophyll concentrations, phytoplankton, zooplankton, and benthos (excluding dreissenids), and altered the structure of benthic and pelagic communities lake-wide (Fahnenstiel et al., 2010; Bunnell et al., 2014; Barbiero et al., 2018; Burlakova et al., 2018, 2022a). Recently, the maximum of quagga mussels density in all deep Great Lakes has shifted even deeper than in the mid-2000s and is now located at depths 71–90 m in lakes Michigan and Huron (Karatayev et al., 2021a), and at 51–90 m in 2018 Lake Ontario (Karatayev et al., 2022). This shift resulted in a large three to fourfold decline in dreissenid density and a 2–18-fold decline in biomass in the shallowest zone, suggesting that the impact of dreissenids in the nearshore areas of the Great Lakes has diminished (Nalepa et al., 2020; Karatayev et al., 2020, 2021a). Based on these data, a new “offshore carbon and phosphorus sink” hypothesis was suggested, although the relative importance of this and two other previous hypotheses (“nearshore phosphorus shunt” and “mid-depth carbon and phosphorus sink”) depends on the distribution of mussel biomass, their clearance rate, and their access to the epilimnion phytoplankton, and requires further investigation (Karatayev et al., 2021a).
The goal of this study is to review the major findings of Dreissena research conducted over the last 30 years in the Laurentian Great Lakes and to compare these with data from European and North American inland lakes. Here we contrast data from deep stratified Great Lakes (Michigan, Huron, and Ontario) with large fetch, well-mixed littoral zone, and vast profundal zone with data from inland, mostly polymictic, lakes. Results of extensive research on population dynamics and various aspects of ecology from the deep Great Lakes are largely novel and can be very important for other deep stratified lakes recently colonized by both dreissenid species both in North America (e.g. Finger Lakes in New York) and in Europe (e.g. lakes Constance, Geneva, Neuchatel, and Biel in Switzerland). Although large lakes similar to the Laurentian Great Lakes exist in other parts of the world, they are either not colonized by dreissenid species, or have no comparable information on quagga mussels. All lakes and reservoirs used in this study are much smaller than the Great Lakes and therefore we refer to these waterbodies as “inland lakes”, though their actual sizes vary greatly. Relatively shallow and small polymictic lakes, lake basins, and embayments that are part of the Great Lakes system (e. g. Lake St. Clair, western basin of Lake Erie, and Saginaw Bay of Lake Huron) were also grouped with the inland lakes.
Distribution
Zebra mussels
Although zebra and quagga mussels have similar life history, they have different tolerance to environmental parameters defining their distribution within a waterbody (Orlova et al., 2005; Nalepa et al., 2010; Karatayev et al., 2014b, 2015a, 2021a). Zebra mussels are better resistant to dislodgment due to higher initial byssal production rates, attachment strength, and flattened ventral edge (Mackie, 1991; Dermott & Munawar, 1993; Claxton & Mackie, 1998; Peyer et al., 2009, 2010), and thus are better adapted to the unstable environment of the littoral zone, specifically in areas with swift currents and waves. In polymictic inland lakes as well as in shallow areas of the Great Lakes, zebra mussel distribution is driven by the availability of hard substrate for attachment and wave activity, and if appropriate substrates are available, they may colonize the whole bottom (Nalepa et al., 1995, 1996, 2002; Hunter & Simons, 2004; Karatayev et al., 2021a). In the profundal zone with soft sediments, zebra mussels are limited by the lack of hard substrate for attachment and low oxygen (Lyakhnovich et al., 1994; Karatayev & Burlakova, 1995; Karatayev et al., 1998, 2014c, 2021a, 2021b, 2022; Burlakova et al., 2006; Goedkoop et al., 2011; Hetherington et al., 2019), but if suitable substrates are available, zebra mussels may spread deeper, especially in large lakes with deeper thermocline that allow oxygenated water and abundant food to reach the bottom. In large lakes with long fetch, strong wave activity may inhibit mussels in the shallowest areas pushing them deeper. In Lake Garda, zebra mussels were found down to 50 m with maximum density (24,000/m2) at 18 m (Franchini, 1978), and in Bodensee down to 55 m with maximum density at 5 – 15 m (Walz, 1973). However, in stratified lakes below the thermocline, even if suitable substrates are available, zebra mussels never form high densities due to too low temperatures for growth, food and, occasionally, oxygen limitations (Grim, 1971; Walz, 1973, 1978a, b).
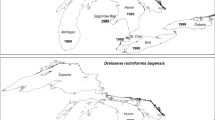
In the deep Great Lakes with large littoral zone and availability of suitable substrates, zebra mussels may spread much deeper than in inland lakes. In Lake Ontario the maximum density of zebra mussels in 1995 was recorded at 18–38 m with a few mussels found down to 109 m (Watkins et al., 2007); in Lake Michigan in 2000, zebra mussels were occasionally present down to 128 m (Nalepa et al., 2014) with maximum density recorded at 27–46 m (Fleischer et al., 2001); and in Lake Huron zebra mussels in 2003 spread down to 80 m with the maximum at 20–30 m (Nalepa et al., 2007). These unusually deep early records of maximum depth for zebra mussel distribution, however, could be results of wrongly identified quagga mussels. Nevertheless, the bulk of zebra mussel population in the deep Great Lakes was always limited to a relatively narrow band in the nearshore zone (> 99% of lake-wide populations at < 50 m depths in Lake Michigan and at < 30 m in lakes Ontario and Huron) (Fig. 1). In spite of the tendency of zebra mussels to spread deeper in the Great Lakes at areas with the appropriate substrates, high zebra mussel densities (> 20,000/m2) were often recorded in the depth range 2–7 m, similar to inland lakes (Stewart & Haynes, 1994; Chase & Bailey, 1999). It should be mentioned that these shallow areas were largely under sampled during lake-wide Dreissena surveys in the Great Lakes, as depths < 18 m in the main basin of Lake Huron, and depths < 10 m in lakes Michigan and Ontario were not sampled (reviewed in Karatayev et al., 2021a).
Quagga mussels
In contrast to zebra, quagga mussels are able to colonize the cold profundal zone due to their higher filtration rate at low food concentrations, greater assimilation efficiency (Diggins, 2001; Baldwin et al., 2002; Stoeckmann, 2003), and greater plasticity in shell production allowing more energy allocation to growth and reproduction (Mills et al., 1999; Karatayev et al., 2011b; Nalepa et al., 2010; Pryanichnikova, 2012). In shallow polymictic lakes and littoral zones of deep lakes exposed to currents and wave actions, quagga, similar to zebra mussels, are limited by hard substrate for attachment and have extremely patchy distributions with large multilayer aggregations next to bare sediments with few or no mussels (Nalepa et al., 2010; Burlakova et al., 2012).
In the early invasion of the Great Lakes, quagga mussels quickly colonized nearshore (< 30 m) environments, but later they spread down to maximum depths that have never been colonized by zebra mussels (Fig. 1, for details see “Population dynamics”) (Watkins et al., 2007; French et al., 2009; Nalepa et al., 2010, 2014; Karatayev et al., 2021a). In Lake Michigan in 2015 quagga mussels were common at 196 m (maximum depth sampled; Nalepa et al., 2020; Karatayev et al., 2021a); in the main basin of Lake Huron in 2017 mussel density at 142 m exceeded 5000/m2 (Karatayev et al., , 2020); and in Lake Ontario in 2018 mussels were found down to 211 m (Karatayev et al., 2021a, 2021b, 2022).
Recent lake-wide studies conducted using videography revealed their spatial distribution at different spatial scales (Karatayev et al., 2018a, 2020, 2021b). It was estimated that quagga mussels’ bottom coverage among deep Great Lakes was the highest in Lake Ontario, and the lowest in Lake Huron, and ranged from 0.6 to 25.3% at < 30 m zone, 13.6–58% at 30–100 m zone, and 6.3–16.1% in the deepest zone (> 100 m) (Fig. 2; Karatayev et al., 2018a, 2020, 2021b). In nearshore areas with abundant food supplies, quagga mussels are limited by physical disturbance (wave and currents) to areas with suitable substrate for attachment (e.g. gravel, rocks, bedrock). Here they typically exhibit heterogeneous distribution with large multilayer aggregations on stable rocky substrates bordering areas with little or no mussels on unconsolidated sediments (Fig. 2). In the mid-depth zone where food is still available but physical disturbance is lower, mussels usually form the largest aggregations. However, these are mostly single layer aggregations with less habitat complexity compared to the shallowest zone. In the deepest zone, quagga mussels densities are usually the lowest, and single mussels or very small druses are almost evenly distributed on the surface of bottom sediments. This distribution pattern is likely beneficial to mussels in the profundal zone because it reduces food competition where resources are scarce. In this deepest zone, mussels form sizable aggregations only along ridges, trenches, or on rocks emerging above the sediment surface (Fig. 3). These irregularities in the bottom floor create turbulence that can deliver additional food to the area, thus supporting higher densities of mussels than the flat bottom areas (Karatayev et al., 2018a, 2020, 2021b). Similarly in marine environments, the presence of enclosure walls enhanced Mercenaria mercenaria (a suspension feeding bivalve that is common across a variety of estuarian habitats along the Atlantic and Gulf coasts of North America) growth by 15–21%, perhaps because hydrodynamic roughness of projecting walls increased mixing into the otherwise depleted bottom waters (Peterson & Beal, 1989).
Dreissena percent coverage (expressed as mean z-score) along depth gradient in lakes Michigan in 2015 (red), Huron in 2017 (blue), Ontario in 2018 (green), and eastern basin of Lake Erie in 2019 (black) (A) and same data excluding Lake Huron where Dreissena density was very low (B). Dashed lines denote 30 m and 100 m depth ranges. Upper photos represent typical Dreissena coverage in depth zone < 30 m, 30 – 100 m, and > 100 m
These patterns of quagga mussel distribution in deep Great Lakes were not possible to predict based on European or North American studies from inland lakes as virtually no such research is known in deep waterbodies. Later, however, similar high quagga mussel densities were reported from the profundal zone of several deep stratified North American inland lakes, including Cayuga, Seneca, Skaneateles, and Canandaigua (NY, USA) (Watkins et al., 2012), confirming that results obtained on the Great Lakes are relevant to inland lakes with similar morphometry. Overall, in all deep stratified lakes, quagga mussels may form higher maximum densities and spread deeper than zebra mussels.
Population dynamics
Zebra mussels
Usually in inland lakes in Europe and North America it takes between 1 – 4 years (on average 2.5 ± 0.2 years, mean ± standard error, Karatayev et al., 2015a) for zebra mussels to reach their population maximum after being first detected in a waterbody. Observations from the local shallow areas of deep Great Lakes suggest that zebra mussels have population dynamics similar to those in the inland lakes. In shallow areas of Lake Ontario (Stewart & Haynes, 1994) and Lake Michigan (Marsden et al., 1993) zebra mussels reached high density in 1991 and 1992, respectively, two to three years after they were first reported from these lakes (Griffiths et al., 1991). However, it took much longer for zebra mussels to reach lake-wide population maximum in these lakes. Thus, zebra mussel lake-wide population maximum in Lake Ontario was recorded in 1995 and in lakes Michigan and Huron in 2000, respectively 6 and 11 years after they were first found in these lakes (Nalepa et al., 2007; 2009a, 2010; Watkins et al., 2007; Karatayev et al., 2021a, 2022). It should be mentioned, however, that the low frequency of the lake-wide surveys (usually every 5 years) precludes establishing accurate timing of population maximum. In addition, shallow areas suitable for zebra mussels were largely under sampled during lake-wide Dreissena surveys in all Great Lakes (Karatayev et al., 2021a). Another caveat is that in all deep Great Lakes zebra mussel population densities declined right after the initial peak (Nalepa et al., 1995, 2001, 2003, 2007, 2009a, 2010; Watkins et al., 2007; Karatayev et al., 2014a, 2021a, 2022), as they were outcompeted by quagga mussels (See “Interspecific competition”) preventing analysis of long-term zebra mussels’ dynamics alone, without the impact of quagga mussels.
Quagga mussels
The average time required for quagga mussels from the first record in a waterbody to population maximum is 6 – 19 years (average 12.2 ± 1.5 years), about fivefold longer than that for zebra mussels (reviewed in Karatayev et al., 2011a, 2015a; Ginn et al., 2018). In deep stratified inland lakes, quagga mussels spread much deeper than zebra mussels (Watkins et al., 2012; Ginn et al., 2018), however there is no sufficient long-term data to reveal the pattern of quagga mussels’ population dynamics in these lakes. Extensive studies conducted in the Great Lakes over the last 30 years have greatly improved our knowledge of distribution and population dynamics of quagga mussels in deep lakes (Watkins et al., 2007; Nalepa et al., 2010, 2020; Karatayev et al., 2021a, 2021c).
Analysis of the long-term population dynamics of quagga mussels in the deep Great Lakes reveal remarkably similar depth-wise changes (Fig. 4). In the shallow (< 30 m) regions of all lakes, quagga mussels overshot their carrying capacity and began to decline within 13 – 15 years after first detection. However, after 20 years there was another increase in density suggesting boom and bust dynamics in the shallow polymictic zone (Karatayev et al., 2021b) where quagga mussels are characterized by fast growth and short longevity (Elgin et al., 2022). This recent increase, however, was recorded so far only in Lake Ontario, and thus requires further confirmation. In the intermediate zones (> 30–50 and > 50–90 m), quagga mussel densities increase more slowly, peak later, and subsequently decline from their maximum levels, but to a lesser extent. In the deepest and coldest zone (> 90 m) where mussel growth is the lowest but longevity is the highest, quagga mussel populations continue to increase in all deep lakes. This population growth in deep areas coupled with population declines in shallow areas results in the overall shift of dreissenid presence toward greater depths (Nalepa et al., 2010, 2020; Karatayev et al., 2020, 2021a, 2022). This is an ongoing process indicating that the maximum of densities at the greater depths has yet to occur, and that the shift will likely become more pronounced over time. Although mussels may already have reached population maximum in the nearshore areas, due to the ongoing increase in deep areas as well as lake-wide, quagga mussel populations still have not reached lake-wide maximum even 20–30 years after colonization, making these population trends dramatically different from those in shallow lakes. Again, these patterns of quagga mussel dynamics were unexpected from previous European experience due to relatively recent quagga mussel invasion and the lack of studies on the lake-wide population dynamics in deep waterbodies.
Population dynamics of quagga mussels at different depth zones in lakes Huron, Michigan, and Ontario. Lines denote splines of z-score changes in mussels density (x-axes). Line colors denote significant increases in red, significant decreases in blue, and no significant change in black. Gray shading denotes 95% confidence intervals of the mean
It is not entirely clear what mechanisms allowed quagga mussels in the profundal zone to prevail over the nearshore population, or how deep the bulk of mussel population will eventually spread in the Great Lakes. Early in the invasion the majority of Dreissena occurred in the well mixed littoral zone where abundant food supply coupled with warm temperature during the growing season resulted in high mussel growth rates, short longevity, and fast population growth (Karatayev et al., 2018b, 2021a; Elgin et al., 2022). In addition, mussels in the nearshore area require hard substrates and need to produce enough byssus threads for attachment to prevent dislodgement by waves. Both the high growth rate and the high rate of byssus production are costly. In the profundal zone mussels live in a much more stable environment with no wave disturbance and at constantly low temperature, resulting in slow growth and long-life span (Karatayev et al., 2018b; Elgin et al., 2022). These mussels exhibit slow population growth but require less energy and may survive at lower food concentrations than nearshore mussels.
Early in the invasion food resources are usually sufficient to maintain a large nearshore mussel population, as well as to support a slow but steady population growth of offshore mussels. Mussels in the nearshore environment retain phosphorous and carbon from offshore and allochthonous sources at the expense of the offshore communities (see “Ecological Impacts” below). With time, the population in the mid-depth zone (> 30–50 m) reaches high enough densities to deplete food resources in the entire lake to a level that is neither sufficient for the zebra mussels, nor for the fast-growing nearshore populations of quagga mussels. Mid-depth mussels are competing for food resources with both nearshore and deeper populations, but the deep mussels that exhibit even slower individual and population growth and can survive at lower food concentrations than mid-depth mussels can eventually win the competition.
Growth and longevity
Zebra mussels
There is abundant literature on the zebra mussel’s growth in inland lakes (Walz, 1978a, b; Lvova, 1980; Smit et al., 1992, 1993; Sprung, 1992, 1995; Dorgelo, 1993; Dall & Hamburger, 1996; Bitterman et al., 1994; Burlakova, 1998; Allen et al., 1999; Horvath & Lamberti, 1999; Yu & Culver, 1999; Garton & Johnson, 2000; Karatayev et al., 2011b). In contrast to inland lakes, few studies were conducted on the growth of zebra mussels in the Great Lakes (MacIsaac, 1994; Stoeckmann & Garton, 1997; Karatayev et al., 2011b) and, to our knowledge, there is no data available on longevity of quagga mussels that live below the thermocline of stratified Great Lakes. Based on seasonal observation of zebra mussels’ growth in temperate regions, it was established that mussel growth stops in the winter and resumes in the spring after water temperatures warm up to 10 °C, suggesting that this is a lower temperature threshold for growth (Morton, 1969a, b; Alimov, 1974; Karatayev, 1983; Mackie, 1991; Jantz & Neumann, 1992). However, these conclusions were based on field observations during the winter, when low temperatures are coincident with low phytoplankton abundance and contradict with the occasional field records of zebra mussels from depths far below the thermocline: 50 m in Garda Lake (Franchini, 1978), 55 m in Bodensee Lake (Walz, 1973), 80 m in Lake Huron (Nalepa et al., 2007), 109 m in Lake Ontario (Watkins et al., 2007), and 128 m in Lake Michigan (Nalepa et al., 2014). Although near-bottom temperature at these depths never reaches 10 °C, zebra mussels grew to the adult stage, though the growth rate was very slow.
The importance of food versus temperature limitation on dreissenid growth was studied in the lab experiment where zebra and quagga musses were kept in flow-through tanks for 289 days with Lake Erie surface water at two temperature treatments, including ambient temperature that mimicked the littoral temperature regime (range 4–25 °C, average 11.9 ± 0.6 °C), and chilled water (range 5–8 °C, average 6.2 ± 0.6 °C) that mimicked hypolimnion conditions of Lake Erie (Karatayev et al., 2011b). Neither species grew during winter in both treatments when cold water coincided with low food supply. However, during the growing season both species showed significant positive growth both at the littoral regime (zebra mussels grew an average of 46% and quagga mussels 106%) and at the profundal regime (27 and 77%, respectively), suggesting that the lack of food, rather than low temperatures per se, limits the growth of both dreissenid species during winter. It is an open question which factor—temperature, or food, or a combination of both—largely limits zebra mussels to the well-mixed areas, preventing establishment of a sizable population in the cold profundal zone, though exceptions do occur. Another open question is what constitutes the lowest temperature threshold for zebra mussels growth, as it became clear that mussels may grow way below 10 °C if sufficient food is available.
In shallow areas of the lower Great Lakes, zebra mussels’ longevity was very similar to inland lakes (3–5 years) (Lvova, 1980; Jantz & Neumann, 1992; Burlakova, 1998; Garton & Johnson, 2000; Karatayev et al., 2006), and ranged from 2 to > 4 years (Chase & Bailey, 1999). Since the early 2010s, zebra mussels in all deep Great Lakes were virtually outcompeted by the quagga mussels, preventing further study on the growth and longevity in profundal environment on these lakes.
Quagga mussel
Less studies were done on quagga mussels’ growth and longevity compared to the zebra mussels (Karatayev et al., 2014b). Outside deep Great Lakes, quagga mussels’ growth was studied in the western basin of Lake Erie (MacIsaac, 1994; Stoeckmann & Garton, 1997; Stoeckmann, 2003), in Lake Mead (Wong et al., 2012), in the St. Lawrence River (Casper et al., 2014), in the Meuse River (Marescaux et al., 2015), as well as in laboratory conditions (Baldwin et al., 2002; Karatayev et al., 2011b; Metz et al., 2018). In all cases, except Lake Mead colonized by quagga mussels alone, authors compared both Dreissena species, and all reported faster growth in quagga mussels. Although there are no data on quagga mussel longevity, based on their faster growth rate and similarity to zebra mussels’ maximum sizes, we may suggest that their typical longevity in polymictic waters is about 3–4 years. The longevity, however, may vary depending on the environmental conditions like food, temperature, oxygen, etc. (Karatayev et al., 2006).
In the deep Great Lakes, the growth rate of quagga mussels declines with increasing depth, following declines in food availability and temperature (Karatayev et al., 2018b; Elgin et al., 2022). The annual linear growth of 12 mm quagga mussels in cage experiments in Lake Ontario declined 1.7-fold from 10.2 ± 0.8 mm at 15 m depth to 5.9 ± 0.2 mm at 45 m, and 15-fold (to 0.7 ± 0.4 mm) at 90 m depth (Elgin et al., 2022). In addition, it is likely that quagga mussels’ growth depends on the time since invasion. It was suggested (Karatayev et al., 2018b) that early in the invasion food resources in the profundal zone of stratified lakes are abundant and mussel growth is higher. Dreissenids are known to reduce food resources (e.g., seston and chlorophyll concentrations) through their feeding and filtering activities both lake-wide (reviewed in Karatayev et al., 1997, 2007b; Higgins & Vander Zanden, 2010; Pothoven & Fahnenstiel, 2013; Rowe et al., 2015) and locally, within lake basins during thermal stratification (MacIsaac et al., 1992; Ackerman et al., 2001; Edwards et al., 2005), and especially below the thermocline (Karatayev et al., 2018b, 2021a). In the eastern basin of Lake Erie seston concentrations early in the invasion (before 2006) were similar in the surface and near bottom layers across all stations at > 40 m depths, coinciding with the similar mean mussel length in littoral and profundal habitats [with bulk of mussels in 9–13 mm size range and abundant size group of 3–5 mm, Roe & MacIsaac (1997)], and abundant recruitment in both zones (Karatayev et al., 2018b). After 2006, there was a three-fold difference between surface and bottom summer seston concentrations, suggesting a strong depletion of food resources in the hypolimnion during stratification, coinciding with a strong increase in average mussel length in the profundal zone due to the lack of young (< 5 mm) mussels. Since 2009, no successful recruitment and growth of young-of-the-year mussels were recorded in the deep profundal (Karatayev et al., 2018b, authors unpublished data), and the length-frequency distributions between 2009 and 2019 were remarkably similar (with majority of 20–30 mm mussels) with no noticeable growth or mortality. According to Karatayev et al. (2018b), quagga mussels with an initial length of 6.1 mm (average of 139 mussels) in cages installed in the eastern basin of Lake Erie at > 50 m depth grew during the growing season (May–November) on average 1 mm. Similarly, the low mussel growth rate recorded at 90 m (0.7 mm/year) in Lake Ontario in 2018–2019 (Elgin et al., 2022). Based on these data Elgin et al. (2022) suggested that the largest mussels found in the lake in 1992 may have been present in the lake a decade earlier than being first reported in 1990 (Mills et al., 1993). Obviously, these estimations of the initial colonization of the Great Lakes contradict with the well-established time of the introduction of quagga mussels to North America (late 1980s). It is much more likely that, early in the invasion, mussels grew faster, but depleted food resources greatly slowed down both the growth and recruitment, and increased mussel longevity.
Reproduction
As already mentioned above, almost all lakes with quagga mussels were originally colonized by zebra mussels, with the exception of several inland waterbodies in the western USA. This makes it very hard, if not impossible, to study quagga mussel larvae dynamics in plankton, as larvae of the two dreissenid species are morphologically undistinguishable, and genetic analysis is needed to separate them. There are some indications, however, that quagga mussel larvae, in contrast to zebra mussels, can be present in plankton of deep European lakes year-round (Piet Spaak, personnel communications). Studies in inland lakes colonized by only quagga mussels in California, where water temperature was always > 12 °C, found veligers present year-round, but their densities somewhat correlated with the temperature, being the lowest during winter, and the highest when epilimnetic temperature exceeded 22 °C (Reid et al., 2010; Gerstenberger et al., 2011).
In the profundal zone of the Great Lakes quagga mussels have been found to spawn at colder water temperatures (Roe & MacIsaac, 1997; Claxton & Mackie, 1998; Nalepa et al., 2010; Glyshaw et al., 2015). In the eastern basin of Lake Erie, Roe and MacIsaac (1997) reported evidence of gonad development and spawning at 55 m depth at temperature 4.8 °C in July 1996. In Lake Michigan in 2008 at 25 m where the mean bottom temperature was 10 °C (max 19.7 °C recorded in early September), all females had mature oocytes from April through August, and the spawning occurred from early September to November (Nalepa et al., 2010). In contrast, at 45 m (average temperature 5.6 °C, max 11.2 °C in early November) the spawning occurred three months earlier: spent females were observed in early June, and all females were spent by early August (Nalepa et al., 2010). In 2013 in Lake Michigan at 25 m, mussels did not have ripe gametes until later in the season, while mature mussels at 45 m and 93 m were found in April (the mean bottom temperatures were respectively 6.8 °C at 25 m (range 3.9–9.1 °C), 5.0 °C at 45 m (range 2.4–5.9 °C), and 4.0 °C at 93 m (range 2.5–4.5 °C)) (Glyshaw et al., 2015).
The seasonal trends in veliger density also varied along the depth gradient, but with somewhat contradicting results. Veliger density in Lake Michigan at 15 m depth peaked in mid-summer and then declined. At 45 and 110 m density tended to be greater in the fall with maximum in December (although the seasonal peaks in veliger abundance were less apparent), and no veligers were found at all three depths from mid-March to late May (Glyshaw et al., 2015). However, veligers in small quantities are routinely found in April in Long-Term Monitoring samples in lakes Ontario and Michigan (GLNPO data, Watkins personal communications), suggesting year-round presence of quagga mussel larvae in plankton of Great Lakes.
It is unclear what triggers spawning in cold profundal zone with relatively stable temperatures. According to Glyshaw et al. (2015), spawning at 45 m and 93 m in Lake Michigan could be triggered by even a slight temperature increase in spring followed by stable temperatures, while at 25 m the wide temperature fluctuations may have delayed spawning until later in the season. Alternatively, the increase in food during spring mixing can potentially trigger the spawning in deep-water mussels living at constant temperature. It is obvious that more work is needed to determine spawning cues in quagga mussels, especially those found at different water depths.
Another critically important piece of missing information is quagga mussel fecundity. While zebra mussel fecundity was estimated directly (275,000–300,000 eggs/year, Lvova, 1980; up to 1,000,000 eggs/year, Sprung, 1991), we still do not have estimations of quagga mussel fecundity either in shallow or deep environments. Considering the many differences in general biology and ecology of these congeners, it will be too speculative to assume that their fecundity is similar.
Interspecific competition
The introduction of quagga mussels into lakes and reservoirs previously colonized by zebra mussels often caused dramatic decline of zebra mussel populations (Orlova et al., 2004, 2005; Karatayev et al., 2011a, 2015a; Heiler et al., 2013; Noordhuis et al., 2016; Balogh et al., 2018; Hetherington et al., 2019; Strayer et al., 2019). The outcome of this competition in the Great Lakes depends chiefly on lake morphometry (Karatayev et al., 2015a, 2021a). In shallow polymictic Great Lakes basins and embayments quagga mussels became dominant 4–12 years after coexistence but did not fully replace zebra mussels even after 30 years of coexistence (Karatayev et al., 2015a, 2021a, c). The actual outcome of zebra vs. quagga mussel competition may depend on the prevalent substrate in a waterbody as zebra mussels require hard substrate for their attachment, while quagga mussels may colonize soft sediments (Karatayev et al., 2021a). Zebra mussel population in the shallow Saginaw Bay of Lake Huron declined dramatically in 1997 when the bay was colonized by quagga mussels, and by 2017 the combined dreissenid density was eightfold lower than in 1992, but zebra mussels still comprised ~ 41% density of all dreissenids after 20 years of coexistence. Similarly in Lake St. Clair, after 16 years of coexistence with quagga mussels, zebra mussels are still common and comprise about 33% density of all dreissenids. The combined zebra and quagga mussel density in this lake in 2018 (3579 ± 984/m2) was very similar to 1994 (3243 ± 845/m2), when only zebra mussels were present in the lake. In the western basin of Lake Erie, quagga exceeded zebra mussel density in 1998, eight years after the coexistence, and was dominant for about 20 years until, in 2019, zebra mussels reached 72% of the combined dreissenid density, suggesting that results of interspecific competition could be reversed (Karatayev et al., 2021c). In this basin over 98% of all Dreissena spp. were < 18 mm in length (e.g., < 3 years old) due to mortality events most likely caused by periodic massive die-offs that occur once every 2–3 years and followed by recolonization from adjacent shallow areas unaffected by hypoxia, or from upstream tributaries (e.g. Detroit River) (Karatayev et al., 2018c, 2021c).
In contrast to shallow, in deep Great Lakes quagga mussels became dominant faster at greater depths, form much higher density, and drive zebra mussels to virtual extirpation (Nalepa et al., 2010; Madenjian et al., 2015; Karatayev et al., 2021a). In Lake Ontario in 1997, seven years after species coexistence, D. r. bugensis lake-average population density was already exceeding that of D. polymorpha (338 vs. 292/m2), and by 1999 quagga mussels formed 99% of combined dreissenid density. After 13 years of invasion quagga mussels reached the maximum lake-wide population density (4477/m2 in 2003) which was 6.6-fold higher than that of zebra mussels during their population maximum in 1995, and no zebra mussels were found during lake-wide surveys since 2008.
In Lake Michigan in 2005, after eight years of coexistence, zebra mussels were almost completely outcompeted by quagga mussels that represented > 98% of the lake-wide dreissenid density (Nalepa et al., 2009a). By 2010, 13 years after the initial discovery, quagga mussels reached lake-wide population maximum (7862/m2), which was 15 times higher than zebra mussels population maximum in 2000, and zebra mussels were virtually extirpated from the lake. In the main basin of Lake Huron, quagga mussels became dominant (82% of all dreissenids) six years after coexistence, in 2003, and zebra mussels were not recorded in surveys since 2007.
We should mention, however, that although zebra mussels were not recorded during lake-wide surveys in lakes Ontario, Michigan, and Huron since the late 2000s, they are still present in very shallow areas, navigational buoys, the mouths of inflowing rivers, bays, and upper littoral zone of open shores where water motion gives them an advantage over quagga mussels due to higher attachment strengths (Karatayev et al., 2013, 2018b; Burlakova et al., 2014). Location of many marinas in the river mouths and bays allow zebra mussels to attach to resident boats, and they survive better than quagga mussels on boat hauls. Thus, despite the overwhelming dominance of quagga mussels in deep Great Lakes, they continue to be a potential source for the spread of zebra mussels (Karatayev et al., 2013).
Ecological impacts
Ecological impacts of dreissenids on invaded waterbodies are well documented both locally and lake-wide (reviewed in Karatayev et al., 1994, 1997, 2002, 2007a, 2007b; Higgins & Vander Zanden, 2010; Kelley et al., 2010). The system-wide effects of Dreissena spp. are associated with their role as suspension feeders (Karatayev et al., 1997, 2002, 2007a, b, 2015b; Beekey et al., 2004; Higgins & Vander Zanden, 2010; Kelley et al., 2010; Burlakova et al., 2012). Suspension feeding not only affects planktonic communities, it also transfers materials from the water column to the benthos, enhancing the coupling between planktonic and benthic components of the ecosystem, which can trigger a suite of changes that increase the relative importance of the benthic community—a process referred to as "benthification" (Mayer et al., 2014)—resulting in redistribution of invertebrate biomass and secondary production from pelagic to benthic environment (reviewed in Karatayev et al., 1997, 2002). Most studies agree that the Dreissena spp. introduction is associated with the increase in water transparency and reduction in the concentration of seston, phosphorous, chlorophyll, and phytoplankton (reviewed in Karatayev et al., 1997, 2002, 2022; Ibelings et al., 2007; Kelly et al., 2010; Higgins & Vander Zanden, 2010; Goedkoop et al., 2011; Mayer et al., 2014; Noordhuis et al., 2016). Improved water transparency enhances macrophyte biomass and coverage as macrophytes grow deeper in the lake (reviewed in Karatayev et al., 1997, 2002; Higgins & Vander Zanden, 2010; Zhu et al., 2006; Ibelings et al., 2007; Mayer et al., 2014; Noordhuis et al., 2016; Wegner et al., 2019). The intensity and extent of system-wide effects depend on many factors, including mussel population density and distribution in a waterbody, mussel dominant species, water mixing rates, retention time, lake morphology, and time since the initial invasion (Karatayev et al., 1997, 2002, 2015a, 2021a; Reed-Andersen et al., 2000; Kelly et al., 2010).
Impact on pelagic communities
Early in the invasion in the 1990s when mussel densities in lakes Michigan and Huron were low and dreissenids were almost exclusively represented by zebra mussels, their impacts in the deep Great Lakes were restricted to a narrow nearshore zone and shallow embayments (Fahnenstiel et al., 1995; Johengen et al., 1995; Skubinna et al., 1995; Vanderploeg et al., 2010). Thus, no substantial lake-wide changes in water clarity, total phosphorous, and silica concentrations were recorded in the 1990s in lakes Michigan and Huron when these lakes were colonized by zebra mussel alone (Fahnenstiel et al., 2010; Kerfoot et al., 2010; Mida et al., 2010; Pothoven & Fahnenstiel, 2014; Barbiero et al., 2018; Pothoven & Vanderploeg, 2020), suggesting very limited system-wide effects. We should mention, however, that because the zebra mussel period was of short duration, its effect on benthic and other communities in the Great Lakes was largely overlooked (Karatayev et al., 2021a; Burlakova et al., 2022a) making it difficult to separate the effects of zebra and quagga mussels.
After the expansion of quagga mussels into deeper areas, the average lake-wide dreissenid biomass in lakes Michigan and Huron has increased 22–45-fold (Karatayev et al., 2021a). Before Dreissena spp. invasion, the wet biomass of plankton invertebrates in the Great Lakes exceeded benthic biomass on average almost sixfold (Fig. 5). After the proliferation of quagga mussels, benthic wet biomass (including dreissenids shells, comprising 30 – 50% of the whole mussel wet weight) increased about two orders of magnitude and it currently exceeds zooplankton biomass > 40-fold.
This dramatic change in the ratio of planktonic to benthic biomass due to expansion of extremely powerful populations of benthic filter-feeder caused pronounced and continuous impacts on the ecosystems of lakes Michigan and Huron, including increase in Secchi depth (Mida et al., 2010; Barbiero et al., 2012, 2018; Pothoven & Fahnenstiel, 2014; Dove & Chapra, 2015; Pothoven & Vanderploeg, 2020) and a decline in total phosphorus (Mida et al., 2010; Barbiero et al. 2012, 2018; Pothoven & Fahnenstiel, 2014; Dove & Chapra, 2015; Pothoven & Vanderploeg, 2020), chlorophyll (Barbiero et al., 2012, 2018; Pothoven & Fahnenstiel, 2014; Pothoven & Vanderploeg, 2020), phytoplankton primary production (Pothoven & Fahnenstiel, 2014), phytoplankton (Barbiero et al., 2018) and zooplankton biomass (Barbiero et al., 2012, 2018). The nearly complete loss of winter and spring diatom blooms (Fahnenstiel et al., 2010; Kerfoot et al., 2010; Barbiero et al., 2012) caused an increase in silica concentrations (Barbiero et al., 2012, 2018; Dove & Chapra, 2015). Changes in the phytoplankton community of Lake Michigan in 2007–2008 as compared to 1983–1987 (pre-Dreissena) and 1995–1998 (when lake was colonized by zebra mussel alone) included the dramatic reduction in all phytoplankton groups, with the exception of cyanobacteria and chlorophytes during spring isothermal period along with the decline in the deep chlorophyll layer (Fahnenstiel et al., 2010). Changes in zooplankton community included the declines in cladocerans and the increased importance of calanoids (Barbiero et al., 2012, 2018).
Somewhat similar changes in water quality variables were recorded in Lake Ontario after the introduction and expansion of dreissenids in the 1990s, including the increase in Secchi depth (Dobiesz & Negel, 2009; Dove & Chapra, 2015), a decline in spring total phosphorous (Dove, 2009; Dove & Chapra, 2015), and an increase in soluble reactive silica that is happening across the Great Lakes excluding Lake Superior (Dove, 2009; Dove & Chapra, 2015). Although there was no strong evidence of the decline in spring diatom bloom or chlorophyll concentration in Lake Ontario associated with Dreissena activity (Watkins et al., 2013; Dove & Chapra, 2015), increase in silica concentration may suggest at least some decline in diatoms, or transport of released silica from Lake Erie. In the eastern basin of Lake Erie, dreissenid expansion was associated with the increase in Secchi depth and dissolved silica concentration and declines in turbidity, total phosphorus, and total dissolved phosphorus concentrations (Makarewicz et al., 2000; Barbiero & Tuchman, 2004; Barbiero et al., 2006; Dobiesz & Negel, 2009; Karatayev et al., 2018d). The increase in spring dissolved silica in the eastern basin of Lake Erie coincided with dramatic declines in total phytoplankton biomass and a shift towards a limited number of centric diatoms (Barbiero et al., 2006).
In Oneida Lake, New York, the secondary invasion and dominance of quagga mussels corresponded to significantly greater visibility and lower chlorophyll concentrations during early spring and late fall but not in warmer months, compared to years of zebra mussel dominance (Karatayev et al., 2021d). This helps explain findings in lakes Michigan and Huron, where the spring diatom blooms disappeared only after quagga mussel invasion (Nalepa, 2010; Vanderploeg et al., 2002; Barbiero et al., 2018). These patterns likely arise from higher physiological activity of quagga mussels compared to zebra mussels at colder temperatures (Baldwin et al., 2002; Stoeckmann, 2003) and explain the higher per-biomass impact of the species on phytoplankton.
The significant decline in total phosphorus concentrations associated with the dreissenid activity resulted in oligotrophication of lakes Michigan and Huron (Evans et al., 2011; Barbiero et al., 2011a, 2012, 2018; Dove & Chapra, 2015), Lake Ontario (Dove, 2009; Chapra & Dolan, 2012; Dove & Chapra, 2015) and the eastern basin of Lake Erie (Dove & Chapra, 2015; Karatayev et al., 2018d). The quagga mussel is now the primary regulator of phosphorus cycling in the lower four Great Lakes, representing a dramatic example of large-scale reorganization of a geochemical cycle by a single invader (Li et al., 2021). In dreissenid-free lakes total phosphorus concentrations mainly reflect the balance between external inputs and phosphorus removal to outflow and net sedimentation, but in invaded lakes the post invasion phosphorus concentrations respond strongly to changes in mussel biomass, transferring the control of phosphorus dynamics away from external inputs (Li et al., 2021). Thus, dreissenid filter feeding now exceeds > tenfold the passive sedimentation as a sink for phosphorus in Lake Michigan; mussel-colonized sediments exchange phosphorus with the water column orders of magnitude faster than mussel-free sediments, and mussel excretion and egestion resupply phosphorus from sediments into the water column an order of magnitude faster than the preinvasion transport. At ∼8 times the rate at which phosphorus enters the lake from watershed, these fluxes now dominate not only the sediment–water exchanges of phosphorus, but also the phosphorus balance for the entire lake (Li et al., 2021). A growing quagga mussel population can rapidly deplete particulate phosphorus from the water column: thus, sequestration into mussel tissues and shells accounted for 20 to 40% of the total benthic phosphorus sink in Lake Michigan since around 2010, and if the growth is unimpeded, would continue depleting total phosphorus from the water column even if the external phosphorus inputs returned to their high 1970s levels. In contrast, when Dreissena populations decline, phosphorus from decaying biomass could quickly increase the total phosphorus concentration in the water column. Thus, the substantial decline in Dreissena density in the central basin of Lake Erie in the late 1990s was concomitant with the increase in the total phosphorus in the water (Karatayev et al., 2018d).
Impact on benthos
Lake Ontario, due to intensive surveys and the longest history of dreissenid invasion among other deep Great Lakes, provides one of the best examples of the effect of quagga mussel on benthic community (reviewed in Burlakova et al., 2022a). The short period of zebra mussel dominance in Lake Ontario in the early 1990s coincided with the increase in density of amphipod Diporeia, other Amphipoda, Oligochaeta, Hirudinea, Gastropoda, and Trichoptera (Haynes et al., 1999; Dermott & Geminiuc, 2003; Lozano & Nalepa, 2003; Burlakova et al., 2022a). These quantitative changes were not accompanied by changes in taxonomic structure of the community which was not significantly different between the pre- and early Dreissena period (1964–1990) and the period of zebra mussel dominance in the 1990s. The same dominant species (Diporeia and Oligochaeta) were still present throughout the community until 1999 when zebra mussels were almost completely replaced with quagga mussels (Burlakova et al., 2022a).
The replacement of zebra by quagga mussels in nearshore regions was first observed in 1995 (Mills et al., 1999) and was complete at depths < 30 m by 1998–1999. The dramatic expansion of quagga mussels coincided with declines in Diporeia, Sphaeriidae, and Oligochaeta (Watkins et al., 2007; Burlakova et al., 2022a). In the shallow zone Diporeia started to decline in the 1990s and was no longer recorded after 1999 (Dermott, 2001; Watkins et al., 2007); in the intermediate depths Diporeia densities sharply declined from 1995–1997 (Lozano et al., 2001; Dermott & Geminiuc, 2003; Watkins et al., 2007); and in the deepest zone density declined to < 1/m2 by 2013–2018 (Burlakova et al., 2022a). Diporeia was historically the dominant macrozoobenthic species in the Great Lakes largely feeding on spring diatom bloom (reviewed in Edlund et al., 2021). Food competition with Dreissena is one of the leading hypotheses of Diporeia decline across all deep Great Lakes colonized with quagga mussels (Nalepa et al., 2009a; Barbiero et al., 2011b, 2018; Ryan et al., 2012; Watkins et al., 2012), as both species feed on the settling spring diatoms (Vanderploeg et al., 2010). Pre-dreissenid Diporeia in Lake Michigan fed selectively and exclusively on more nutritious and highly preferred large and filamentous-centric diatoms but has shifted to less nutritious small-centric and araphid diatoms after offshore expansion of quagga mussels (Edlund et al., 2021). The decline in Diporeia likely started after the arrival of zebra mussels but was exacerbated by the arrival of quagga mussels in both Lake Ontario (Dermott, 2001; Watkins, 2007, 2013) and in Lake Michigan (Nalepa et al., 2006, 2009a). An alternative explanation could be the impact of a pathogen (Hewson et al., 2013), however, searches for viral or bacterial pathogens have not yet identified a likely candidate (Winters et al., 2014, 2015; Bistolas et al., 2017) and the exact mechanism of Diporeia decline is still unclear (reviewed in Watkins et al., 2012, 2013). Sphaeriidae, previously found in abundance in Lake Ontario at all depths, followed the same trend as Diporeia and are currently found at low densities only at depths > 90 m (Burlakova et al., 2022a). A strong decline in Sphaeriids was also recorded in the eastern basin of Lake Erie along with the complete extirpation of Diporeia after the proliferation of Dreissena in the mid-1990s, but in the central basin where Dreissena density is very low due to periodic hypoxia, sphaeriid densities were always high and in 2019 reached the highest level ever recorded in the basin (1229/m2).
Dramatic decline of Diporeia in the Great Lakes contradicts with the inland stratified Finger Lakes (New York). Watkins et al. (2012) reported abundant Diporeia populations in all six lakes sampled, including Owasco Lake that has only zebra mussels and in Cayuga and Seneca lakes that have had zebra and quagga mussels since 1994, as well as in lakes Skaneateles, Canandaigua, and Keuka where quagga mussels have recently expanded. The authors suggest that Diporeia may coexist with abundant quagga mussels by using food resources associated with terrestrial detritus that cannot be intercepted by dreissenids.
To address the impact of Dreissena on major groups of native benthic invertebrates we summarized data collected during lake-wide benthic surveys in 1990–2018 in lakes Ontario, Michigan main basin, and Huron main basin (summarized in Karatayev et al., , 2020, 2021b; Nalepa et al., 2020; Burlakova et al., 2018, 2022a). As expected, there was a distinctive and highly significant decline in the lake-wide (basin-wide) densities of Diporeia and Sphaeriidae with the progression of quagga mussel invasion, while oligochaetes and chironomids did not exhibit any substantial trends (Fig. 6). These substantial declines and even extirpation of Diporeia and, to a lesser extent, of Sphaeriidae were associated with, and likely driven by, quagga mussel proliferation in the Great Lakes (Dermott, 2001; Lozano et al., 2001; Dermott & Geminiuc, 2003; Watkins et al., 2007; Nalepa et al., 2006, 2009a, b, 2014; Vanderploeg et al., 2010; Karatayev et al., 2015a; Burlakova et al., 2018, 2022a; Barbiero et al., 2018). However, although quagga mussels population in the Great Lakes varied by three orders of magnitude, from the highest in lakes Michigan (4,347/m2, average for 2000–2015) and Ontario (2,196/m2, average for 1995–2018) to only 177/m2 in Georgian Bay and 2.7/m2 in North Channel (both averaged for 2002–2017), the scale of the decline in Diporeia was not proportional to Dreissena population densities and was second largest (547-fold) in Georgian Bay with very low mussel abundance. Both decline in Diporeia and Sphaeriidae exhibit higher correlation with time since quagga rather than zebra mussel invasion (r2 = 0.52–0.78 vs. 0.35–0.57, correspondingly), suggesting stronger system-wide impacts of quagga mussels on Great Lakes benthos.
Changes in lake-wide weighted average densities of Diporeia, Sphaeriidae, Oligochaeta, and Chironomidae, whole benthos density, and wet biomass with and without Dreissena spp. in lakes Ontario, Michigan, Huron main basin, Georgian Bay, and North Channel with the time since quagga mussel invasion (significant regressions are indicated). Note: benthos biomass with Dreissena is given without North Channel, where mussels population was low
Observed system-wide impacts of quagga mussels on benthic community of deep Great Lakes contradict with the previously reported increase in diversity and density of non-dreissenid benthos in inland lakes (Karatayev et al., 1983, 1997, 2002, 2007a, 2007b; Mayer et al., 2002; Gutierrez et al., 2003; Beekey et al., 2004; Ozersky et al., 2011; Burlakova et al., 2012), but most of these studies were done on lakes invaded by zebra mussels. Quagga mussels may have a different impact, especially in sublittoral and profundal areas of deep lakes where epifaunal invertebrates that may take advantages of Dreissena habitat modifications (e.g. amphipods other than Diporeia sp.) are scarce, while food resources for filter-feeders are much more limited than in the littoral zone (reviewed in Karatayev et al., 2015a). While the introduction of zebra mussels in lakes Ontario and Erie was followed by an increase in amphipods, gastropods, chironomids, and oligochaetes, the sharp decline of many non-dreissenid benthic taxa in both lakes was associated with the replacement of zebra with quagga mussels (Barrett et al., 2017; Burlakova et al., 2022a; Karatayev et al., in review) and potentially with some other factors. We hypothesize that the decline in non-dreissenid benthos in the wave-zone of the eastern basin of Lake Erie (Ratti & Barton, 2003) could be due to the predation by round goby (Neogobius melanostomus) whose densities dramatically increased in the basin during 1999–2002 (Barton et al., 2005; Johnson et al., 2005), and in Lake Ontario in 1998–2005 (Weidel et al., 2019) coinciding with the decline in benthos and proliferation of quagga mussels. Gobies actively prey on benthic invertebrates and were reported to reduce abundance of small (3–14 mm) dreissenids, amphipods, chironomids, gastropods, mayflies, trichopterans, and leaches (Barton et al., 2005; Johnson et al., 2005; Brush et al., 2012; Barrett et al., 2017; Foley et al., 2017), and often prefer non-dreissenid benthic invertebrates over Dreissena spp. (Walsh et al., 2007; Brush et al., 2012; Foley et al., 2017).
Due to the ongoing colonization of deep-water Great Lakes habitat by thriving quagga mussel communities (Karatayev et al., 2021a), it is possible that local positive interactions, typically observed nearshore, may be now occurring between Dreissena and some benthic taxa in offshore communities. Comparing recent benthic communities of lakes Michigan and Huron across depths, Bayba et al. (2022) found that the total benthic abundance and species richness increased consistently with quagga mussel density in the nearshore, while Oligochaeta densities and biomass increased in dreissenid aggregations across all depth gradients, patterns that are consistent with facilitative effects of quagga mussels. Comparison with historical pre-dreissenid data indicated that, while Diporeia and Sphaeriidae biomass in both lakes have declined over the last decades, the biomass of Oligochaeta increased. As a result, non-dreissenid benthos in Lake Michigan is now higher than it was in the 1930s, and in Lake Huron the combined biomass of Oligochaeta, Chironomidae, and Sphaeriidae is now higher than in the 1970s. Including dreissenids, the total benthos biomass increased at least two orders of magnitude in both lakes. Therefore, a deep-water benthic community once characterized by Diporeia and Sphaeriidae, has shifted to a community dominated by quagga mussels and Oligochaeta.
Impact on fish
As dreissenids are very recent invaders in North America, fishes, including benthivorous species, were likely at first “naïve” to the new potential prey, and due to limited predation (Johnson et al., 2005) Dreissena were considered as a “dead end” in Great Lakes food webs (reviewed in Madenjian et al., 2010). Therefore, the proliferation of dreissenid mussels was anticipated to be a major loss of energy and potential production, as food resources were withdrawn from the pelagia and incorporated into benthos (Johnson et al., 2005). Moreover, the introduction and expansion of zebra, and especially quagga mussels, into the lakes caused the decline in the abundance of commercially important whitefish (Coregonus clupeaformis) through the dramatic decrease in their main food, Diporeia (Dermott & Kerec, 1997; Lozano et al., 2001; Pothoven et al., 2001; Nalepa et al., 2009a; Nalepa, 2010), likely outcompeted by dreissenids (see above). This outcome cannot be predicted from the European experience as Dreissena introduction was always associated with an increase in amphipod abundance (Karatayev & Lyakhnovich 1990; Karatayev et al., 1997, 2002, 2007a, 2007b; 1996; Stewart et al., 1998a, 1998b, 1999; Burlakova et al., 2012), and Monoporeia affinis, a European species closely related to Diporeia, has coexisted with zebra mussels in Lake Malaren, Sweden since the 1920s (reviewed in Nalepa et al., 2005). Diporeia decline in the Great Lakes resulted in a shift in diet of whitefish from the amphipod to quagga mussels (Nalepa et al., 2009b; Madenjian et al., 2010). However, again in contrast to European studies where the shift in the diet of roach to zebra mussels resulted in faster growth and higher lipid content, the shift from Diporeia to quagga mussel resulted in the decline of lake whitefish condition, growth, and abundance (Pothoven et al., 2001; Hoyle et al., 2008; Nalepa et al., 2009b; Rennie et al., 2009). The decline in Diporeia was also associated with the decline of alewife (Alosa pseudoharengus), sculpin (Cottus cognatus), bloater (Coregonus hoyi), and other fish that are prey for larger piscivores, including salmon and trout (reviewed in Nalepa, 2010).
One unexpected effect of the loss of Diporeia in the Great Lakes can be a potential decline in fish contamination with polybrominated diphenyl ethers (PBDEs). The highest concentration of BDE-209 among phyto- and zooplankton, lake whitefish, lake trout, and Chinook salmon was detected in Diporeia (Perez-Fuentetaja et al., 2015), which was possibly one of the main dietary sources of BDE-209 for fish in Lake Michigan. Recent declines in PBDEs were found in the bloater chub and the slimy sculpin in Lake Michigan compared to increasing PBDEs in other fish and biota (Hahm et al., 2009), likely because the decline in their major food, Diporeia, may be causing the fish to shift to different benthic food sources lower in PBDEs.
The introduction of another Ponto-Caspian invader, round goby, into the Great Lakes in the 1990s (Jude et al., 1992; Charlebois et al., 1997) added a very important missing link between dreissenids and commercially and recreationally valuable fish species (Johnson et al., 2005; Madenjian et al., 2011). Round goby actively feeds on dreissenids both in their native range (Nekrasova & Kovtun, 1975; Borcherding et al., 2013) and in North America (Bunnell et al., 2005; Kornis et al., 2012; Naddafi & Rudstam, 2014) and, in turn, is actively consumed by some North American fish including lake trout (Salvelinus namaycush) (Dietrich et al., 2006), burbot (Lota lota) (Madenjian et al., 2011), yellow perch (Perca flavescens) (Weber et al., 2011), smallmouth bass (Micropterus dolomieu) (Crane & Einhouse, 2016), lake sturgeon (Acipenser fulvescens) (Jacobs et al., 2017), and walleye (Sander vitreus) (Pothoven et al., 2017). In the Niagara River, the round goby was found to be the primary contributor to the long-term average diet of lake sturgeon, a species of conservation concern in many U.S. states and Canadian provinces (Bruestle et al., 2019). In contrast to other systems, adult lake sturgeon in the Niagara River are now primarily piscivorous, and the high availability of energetically rich but non-native food resources potentially support sturgeon recovery. All 6 of these fish species, along with 9 others, are known to consume dreissenids in North America (reviewed in Molloy et al., 1997; Burlakova et al., 2022b), however their consumption of round gobies substantially increased the passage of energy accumulated by dreissenids in benthic environment back to the pelagic, and eventually increased fish productivity, including commercially important species.
It is debatable to what extent goby predation can control Dreissena population density in the Great Lakes. There are data suggesting that round goby invasion caused changes in quagga mussels length frequency distributions as well as declines in nearshore Dreissena populations (Barton et al., 2005; Patterson et al., 2005; Wilson et al., 2006). Lake-wide decline in dreissenid populations were also reported in inland North American lakes following round goby invasion (Rudstam & Gandino, 2020; Jackson et al., 2020). We found a large decline in dreissenid populations and other benthos in recent years in Lake Erie, potentially induced by round goby predation (Karatayev et al., 2021b; Karatayev et al., in review). However, even if predation may have notable effects on the mussels in shallow areas/lakes, it is unlikely that round goby may have substantial lake-wide effect in the deep Great Lakes (Bunnell et al., 2005; Johnson et al., 2005; Foley et al., 2017). In spite of almost two decades of round gobies and mussels coexisting in Lake Huron, quagga mussel density was still growing as of 2017 across all depth zones > 50 m, as well as lake-wide (Karatayev et al., 2020, 2021a). In Lake Michigan, although quagga mussel populations started declining after 2010 at depths < 90 m (Nalepa et al., 2020; Mehler et al., 2020), it is likely that factors other than round goby predation (e.g. food limitation) have caused this decline.
Conclusions
The introduction and rapid spread of zebra and quagga mussels across the Northern Hemisphere that caused significant ecological impacts and damage to industry triggered a strong spike in their research that produced valuable information on spread, population dynamics, biology, and ecological impacts of both species. Especially intriguing, however, are data on quagga mussels from the Great Lakes. Thirty years of research conducted on various aspects of quagga mussel biology, population dynamics, and distribution in lakes Michigan, Huron, Erie, and Ontario resulted in a large body of new information for this species. These data were not previously available from Europe, as North American Great Lakes are the only large fresh waterbodies in the world not only colonized by both dreissenids, but also a subject of long-term systematic research and monitoring.
It became clear that lake morphometry determines competition outcome, distribution, growth rate, and reproduction of both dreissenid species as well as the magnitude of their ecological impacts. In shallow embayments and basins of the Great Lakes, both species can colonize the whole bottom and form more or less similar population density. In the deep Great Lakes, zebra mussels are largely limited to a narrow nearshore zone, while quagga mussels colonize the whole lake bottom with the maximum abundance between 50–100 m, and form lake-wide population densities by an order of magnitude higher than that of zebra mussels. These patterns of quagga mussel distribution in the deep Great Lakes were not possible to predict based on European experience as no similar research was conducted in deep waterbodies.
Lake morphometry also determines quagga mussels’ population dynamics. In shallow regions (< 30 m) of the deep Great Lakes, quagga mussels overshoot their carrying capacity and begin to decline within 13–15 years after first detection. However, at the intermediate zone of deep lakes (> 30–90 m) quagga mussels densities increase more slowly, peak later, and subsequently decline from their maximum levels, but to a lesser extent. In the deepest zone (> 90 m) quagga mussel populations continue to increase in all deep lakes even after 35 years of initial invasion. This increase, resulting in an ongoing shift of dreissenid populations toward deeper areas, suggests that a peak at the deepest depths has yet to occur, and that the shift will likely become more pronounced over time. Again, these patterns were unexpected due to recent quagga mussel invasion and the lack of similar studies in deep European waterbodies. We would like to emphasize that different depth-wise dynamics make predictions of mussel population density and biomass as well as their ecosystem impacts extremely difficult and require regular Dreissena monitoring.
In the profundal zone of the Great Lakes, quagga mussels have been found to spawn at colder water temperatures, their larvae exhibit different seasonal dynamics, and are present in plankton year-round. Growth rate of quagga mussels declines with increasing depth due to lower food availability and temperature resulting in increased mussel longevity that may exceed 15 years. It is also likely that quagga mussels’ growth and longevity depend on the time since invasion which is especially evident in the deep profundal zone. Early in the invasion when food resources are abundant, quagga mussels may grow faster and live less long than later in the invasion when food resources are depleted.
After the establishment of quagga mussel populations, benthic biomass in the Great Lakes increased about two orders of magnitude and is currently greatly exceeding zooplankton biomass. This decline in the ratio of planktonic to benthic biomass is a result of strong benthic/pelagic coupling by massive benthic population of exotic powerful filter-feeders redirecting food and energy resources from the water column to the bottom. This “benthification” (Mayer et al., 2014) caused pronounced and continuous impacts on the ecosystems of all deep Great Lakes, including increase in Secchi depth, and a decline in total phosphorus, chlorophyll, phytoplankton primary production, phytoplankton and zooplankton biomass. The nearly complete loss of winter and spring diatom blooms in lakes Michigan and Huron caused an increase in silica concentrations. The decline in total phosphorus concentrations associated with the dreissenid activity resulted in oligotrophication of all deep Great Lakes. The quagga mussel is now the primary regulator of phosphorus cycling in the lower four Great Lakes, representing a dramatic example of large-scale reorganization of geochemical cycle by a single invader. Future changes in quagga mussel populations will thus strongly affect phosphorus dynamics in the Great Lakes, making changes in this large ecosystem less predictable.
The dramatic expansion of quagga mussels in the Great Lakes coincided with declines and local extirpations of Diporeia, most likely due to the food competition as both species feed on the settling spring diatoms. Sphaeriidae, previously found in abundance in the lower Great Lakes, followed the same trend as Diporeia and are currently found at very low densities. With exception of selected taxa (e.g. Oligochaeta), observed lake-wide impacts of quagga mussels on the profundal benthic community of deep Great Lakes contradict the previously reported increase in diversity and density of non-dreissenid benthos in other lakes.
The introduction and proliferation of dreissenids into the Great Lakes caused declines in the abundance of commercially important fishes as a result of dramatic decrease in their main food, Diporeia—an outcome that could not be predicted from European experience as Dreissena introduction was always associated with increase in amphipod abundance. However, the introduction of another Ponto-Caspian invader, the round goby, into the Great Lakes in the 1990s, provided an important missing link between dreissenids and commercially and recreationally valuable fish species, increasing their productivity.
References
Ackerman, J. D., M. R. Loewen & P. F. Hamblin, 2001. Benthic-pelagic coupling over a zebra mussel reef in western Lake Erie. Limnology and Oceanography 46: 892–904.
Alimov, A. F., 1974. Patterns of the growth of freshwater bivalve molluscs. Zhurnal Obschei Biologii 35: 576–589 (in Russian).
Allen, Y. C., B. A. Thompson & C. W. Ramcharan, 1999. Growth and mortality rates of the zebra mussel, Dreissena polymorpha, in the Lower Mississippi River. Canadian Journal of Fisheries and Aquatic Sciences 56: 748–759.
Baldwin, B. S., M. S. Mayer, J. Dayton, N. Pau, J. Mendilla, M. Sullivan, A. Moore, A. Ma & E. L. Mills, 2002. Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 680–694.
Balogh, C., A. Vláčilová & L. G.-Tóth & Z. Serfőző, 2018. Dreissenid colonization during the initial invasion of the quagga mussel in the largest Central European shallow lake, Lake Balaton, Hungary. Journal of Great Lakes Research 44: 114–125.
Barbiero, R. P. & M. L. Tuchman, 2004. Long-term dreissenid impacts on water clarity in Lake Erie. Journal of Great Lakes Research 30: 557–565.
Barbiero, R. P., D. C. Rockwell, G. J. Warren & M. L. Tuchman, 2006. Changes in spring phytoplankton communities and nutrient dynamics in the eastern basin of Lake Erie since the invasion of Dreissena spp. Canadian Journal of Fisheries and Aquatic Sciences 63: 1549–1563.
Barbiero, R. P., B. M. Lesht & G. J. Warren, 2011a. Evidence for bottom–up control of recent shifts in the pelagic food web of Lake Huron. Journal of Great Lakes Research 37: 78–85.
Barbiero, R. P., K. Schmude, B. M. Lesht, C. M. Riseng, G. J. Warren & M. L. Tuchman, 2011b. Trends in Diporeia populations across the Laurentian Great Lakes, 1997–2009. Journal of Great Lakes Research 37: 9–17.
Barbiero, R. P., B. M. Lesht & G. J. Warren, 2012. Convergence of trophic state and the lower food web in Lakes Huron, Michigan and Superior. Journal of Great Lakes Research 38: 368–380.
Barbiero, R. P., B. M. Lesht, G. J. Warren, L. G. Rudstam, J. M. Watkins, E. D. Reavie, K. E. Kovalenko & A. Y. Karatayev, 2018. A comparative examination of recent changes in nutrients and lower food web structure in Lake Michigan and Lake Huron. Journal of Great Lakes Research 44: 573–589.
Barbiero, R. P., L. G. Rudstam, J. M. Watkins & B. M. Lesht, 2019. A cross-lake comparison of crustacean zooplankton communities in the Laurentian Great Lakes, 1997–2016. Journal of Great Lakes Research 45: 672–690.
Barrett, K. B., J. M. Haynes & D. I. Warton, 2017. Thirty years of change in a benthic macroinvertebrate community of southwestern Lake Ontario after invasion by four Ponto-Caspian species. Freshwater Science 36(1): 90–102.
Barton, D. R., R. A. Johnson, L. Campbell, J. Petruniak & M. Patterson, 2005. Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. Journal of Great Lakes Research 31: 252–261.
Bayba, S., L. E. Burlakova, A. Y. Karatayev & R. J. Warren II., 2022. Non-native Dreissena associated with increased native benthic community abundance with greater lake depth. Journal of Great Lakes Research 48: 734–745.
Beekey, M. A., D. J. McCabe & J. E. Marsden, 2004. Zebra mussels affect benthic predator foraging success and habitat choice on soft sediments. Oecologia 141: 164–170.
Benson, A. J., 2014. Chronological history of zebra and quagga mussels (Dreissenidae) in North America, 1988–2010. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton, Florida, USA: 9–31.
bij de Vaate, A., G. Van der Velde, R. S. E. W. Leuven & K. C. M. Heiler, 2014. Spread of the quagga mussel, Dreissena rostriformis bugensis, in Western Europe. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton, Florida, USA: 83–92.
Bistolas, K. S. I., E. W. Jackson, J. M. Watkins, L. G. Rudstam & I. Hewson, 2017. Distribution of circular-stranded DNA viruses associated with benthic amphipods of genus Diporeia in the Laurentian Great Lakes. Freshwater Biology 62: 1220–1231.
Bitterman, A. M., R. D. Hunter & R. C. Haas, 1994. Allometry of shell growth of caged and uncaged zebra mussels (Dreissena polymorpha) in Lake St. Clair. American Malacological Bulletin 11(1): 41–49.
Borcherding, J., M. Dolina, L. Heermann, P. Knutzen, S. Krüger, S. Matern, R. van Treeck & S. Gertzen, 2013. Feeding and niche differentiation in three invasive gobies in the Lower Rhine, Germany. Limnologica 43: 49–58.
Botts, P. S. & B. A. Patterson, 1996. Zebra mussel effects on benthic invertebrates: physical or biotic? Journal of the North American Benthological Society 15: 179–184.
Bruestle, E. L., C. Karboski, A. Hussey, A. T. Fisk, K. Mehler, C. Pennuto & D. Gorsky, 2019. Novel trophic interaction between lake sturgeon (Acipenser fulvescens) and non-native species in an altered food web. Canadian Journal of Fisheries and Aquatic Sciences 76(1): 6–14.
Brush, J. M., A. T. Fisk, N. E. Hussey & T. B. Johnson, 2012. Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): stable isotopes indicate that stomach contents overestimate the importance of dreissenids. Canadian Journal of Fisheries and Aquatic Sciences 69: 573–586.
Bunnell, D. B., T. B. Johnson & C. T. Knight, 2005. The impact of introduced round gobies (Neogobius melanostomus) on phosphorus cycling in central Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 62: 15–29.
Bunnell, D. B., R. P. Barbiero, S. A. Ludsin, C. P. Madenjian, G. J. Warren, D. M. Dolan, T. O. Brenden, R. Briland, O. T. Gorman, Ji. X. He, T. H. Johengen, B. F. Lantry, B. M. Lesht, T. F. Nalepa, S. C. Riley, C. M. Riseng, T. J. Treska, I. Tsehaye, M. G. Walsh, D. M. Warner & B. C. Weidel, 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: bottom-up and top-down regulation. BioScience 64: 26–39.
Burlakova, L. E., 1998. Ecology of Dreissena polymorpha (Pallas) and its role in the structure and function of aquatic ecosystems. Candidate Dissertation, Zoology Institute of the Academy of Science Republic Belarus, Minsk (Belarus): 167 pp. (in Russian).
Burlakova, L. E., A. Y. Karatayev & D. K. Padilla, 2006. Changes in the distribution and abundance of Dreissena polymorpha within lakes through time. Hydrobiologia 517: 133–146.
Burlakova, L. E., A. Y. Karatayev & V. A. Karatayev, 2012. Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 685: 121–134.
Burlakova, L. E., B. L. Tulumello, A. Y. Karatayev, R. A. Krebs, D. W. Schloesser, D. T. Zanatta, W. L. Patterson, T. A. Griffith, M. W. Scott & T. Crail, 2014. Competitive replacement of invasive congeners may relax impact on native species: interactions among zebra, quagga, and native unionid mussels. PLoS ONE 9(12): E114926. https://doi.org/10.1371/journal.pone.0114926.
Burlakova, L. E., R. P. Barbiero, A. Y. Karatayev, S. E. Daniel, E. K. Hinchey & G. J. Warren, 2018. The benthic community of the Laurentian Great Lakes: analysis of spatial gradients and temporal trends from 1998–2014. Journal of Great Lakes Research 44: 600–617.
Burlakova, L. E., A. Y. Karatayev, A. R. Hrycik, S. E. Daniel, K. Mehler, L. G. Rudstam, J. M. Watkins, R. Dermott, J. Scharold, A. K. Elgin & T. F. Nalepa, 2022a. Six decades of Lake Ontario ecological history according to benthos. Journal of Great Lakes Research 48: 274–288.
Burlakova, L. E., A. Y. Karatayev, D. Boltovskoy & N. M. Correa. 2022b. Ecosystem services provided by the exotic bivalves Dreissena polymorpha, D. rostriformis bugensis, and Limnoperna fortunei. Hydrobiologia. https://doi.org/10.1007/s10750-022-04935-4
Casper, A. F., L. E. Johnson & H. Glémet, 2014. In situ reciprocal transplants reveal species-specific growth pattern and geographic population differentiation among zebra and quagga mussels. Journal of Great Lakes Research 40: 705–711.
Chapra, S. C. & D. M. Dolan, 2012. Great Lakes total phosphorus revisited: 2. Mass balance modeling. Journal of Great Lakes Research 38: 741–754.
Charlebois, P. M., J. E. Marsden, R. G. Goettel, R. K. Wolfe, D. J. Jude & S. Rudnicka, 1997. The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature, Illinois Natural History Survey Special Publications, No:, 20.
Chase, M. E. & R. C. Bailey, 1999. The ecology of the zebra mussels (Dreissena polymorpha) in the lower Great Lakes of North America: I. Population dynamics and growth. Journal of Great Lakes Research 25: 107–124.
Claxton, W. T. & G. L. Mackie, 1998. Seasonal and depth variations in gametogenesis and spawning of Dreissena polymorpha and Dreissena bugensis in eastern Lake Erie. Canadian Journal of Zoology 76: 2010–2019.
Crane, D. P. & D. W. Einhouse, 2016. Changes in growth and diet of smallmouth bass following invasion of Lake Erie by the round goby. Journal of Great Lakes Research 42: 405–412.
Dall, P. C. & K. Hamburger, 1996. Recruitment and growth of Dreissena polymorpha in Lake Esrom, Denmark. Limnologica 26(1): 27–37.
Dermott, R., 1994. Benthic invertebrate fauna of Lake Erie 1979: distribution, abundance and biomass. Canadian Technical Report of Fisheries and Aquatic Sciences 2018: 82 pp.
Dermott, R., 2001. Sudden disappearance of the amphipod Diporeia from eastern Lake Ontario. Journal of Great Lakes Research 27: 423–433.
Dermott, R. & M. Munawar, 1993. Invasion of Lake Erie offshore sediments by Dreissena, and its ecological implications. Canadian Journal of Fisheries and Aquatic Sciences 50: 2298–2304.
Dermott, R. & D. Kerec, 1997. Changes to the deep water benthos of eastern Lake Erie since the invasion of Dreissena: 1979–1993. Canadian Journal of Fisheries and Aquatic Sciences 54: 922–930.
Dermott, R. & M. Geminiuc, 2003. Changes in the benthic fauna of Lake Ontario 1990–1995, with local trends after 1981. In Munawar, M. (ed), State of Lake Ontario (SOLO)-Past, present, and future Ecovision World Monograph Series, Backhaus Publishers, Leiden, The Netherlands: 323–345.
Dietrich, J. P., B. J. Morrison & J. A. Hoyle, 2006. Alternative ecological pathways in the eastern Lake Ontario food web—round goby in the diet of lake trout. Journal of Great Lakes Research 32: 395–400.
Diggins, T. P., 2001. A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. Journal of Great Lakes Research 27: 457–466.
Dobiesz, N. E. & P. L. Negel, 2009. Changes in mid-summer water temperature and clarity across the Great Lakes between 1968 and 2002. Journal of Great Lakes Research 35: 371–384.
Dorgelo, J., 1993. Growth and population structure of the zebra mussel (Dreissena polymorpha) in Dutch lakes different in trophic state. In Nalepa, T. F. & D. W. Schloesser (ed), Zebra Mussels. Biology, Impacts, and Control. Lewis Publishers, Ann Arbor, Michigan, USA: 79–94.
Dove, A., 2009. Long-term trends in major ions and nutrients in Lake Ontario. Aquatic Ecosystem Health & Management 12: 281–295.
Dove, A. & S. C. Chapra, 2015. Long-term trends of nutrients and trophic response variables for the Great Lakes. Limnology and Oceanography 60: 696–721.
Edlund, M. B., D. J. Jude & T. F. Nalepa, 2021. Diets of the benthic amphipod Diporeia in southern Lake Michigan before and after the dreissenid invasion. Journal of Great Lakes Research 47: 447–462.
Edwards, W. J., C. R. Rehmann, E. McDonald & D. A. Culver, 2005. The impact of a benthic filter feeder: limitations imposed by physical transport of algae to the benthos. Canadian Journal of Fisheries and Aquatic Sciences 62: 205–214.
Elgin, A. K., P. W. Glyshaw & B. C. Weidel, 2022. Depth drives growth dynamics of dreissenid mussels in Lake Ontario. Journal of Great Lakes Research 48: 289-299.
Evans, M. A., G. Fahnenstiel & D. Scavia, 2011. Incidental oligotrophication of North American Great Lakes. Environmental Science and Technology 45: 3297–3303.
Fahnenstiel, G. L., G. A. Lang, T. F. Nalepa & T. H., Johengen, 1995. Effects of zebra mussel (Dreissena polymorpha) colonization on water quality parameters in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 435–448.
Fahnenstiel, G. L., S. A. Pothoven, T. F. Nalepa, H. A. Vanderploeg, D. M. Klarer & D. Scavia, 2010. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. Journal of Great Lakes Research 36: 20–29.
Fleischer, G. W., T. J. Desorcie & J. D. Holuszko, 2001. Lake-wide distribution of Dreissena in Lake Michigan, 1999. Journal of Great Lakes Research 27: 252–257.
Foley, C. J., S. R. Andree, S. A. Pothoven, T. F. Nalepa & T. O. Höök, 2017. Quantifying the predatory effect of round goby on Saginaw Bay dreissenids. Journal of Great Lakes Research 43: 121–131.
Franchini, D. A., 1978. Vertical distribution of Dreissena polymorpha (Pallas) in Garda Lake. Bollettino Di Zoologia 45: 257–260.
French, J. R. P., III., J. S. Schaeffer, E. F. Roseman, C. S. Kiley & A. Fouilleroux, 2009. Abundance and distribution of benthic invertebrates in offshore soft sediments in Western Lake Huron, 2001–2007. Journal of Great Lakes Research 35: 120–127.
Garton, D. V. & L. E. Johnson, 2000. Variation in growth rates of the zebra mussel, Dreissena polymorpha, within Lake Wawasee. Freshwater Biology 45: 443–451.
Gerstenberger, S. L., A. A. Mueting & W. H. Wong, 2011. Veligers of invasive quagga mussels (Dreissena rostriformis bugensis, Andrusov 1897) in Lake Mead, Nevada-Arizona. Journal of Shellfish Research 30: 933–938.
Ginn, B. K., R. Bolton, D. Coulombe, T. Fleischaker & G. Yerex, 2018. Quantifying a shift in benthic dominance from zebra (Dreissena polymorpha) to quagga (Dreissena rostriformis bugensis) mussels in a large, inland lake. Journal of Great Lakes Research 44: 271–282.
Glyshaw, P. W., C. M. Riseng, T. F. Nalepa & S. A. Pothoven, 2015. Temporal trends in condition and reproduction of quagga mussels (Dreissena rostriformis bugensis) in southern Lake Michigan. Journal of Great Lakes Research 41: 16–26.
Goedkoop, W., R. Naddafi & U. Grandin, 2011. Retention of N and P by zebra mussels (Dreissena polymorpha Pallas) and its quantitative role in the nutrient budget of eutrophic Lake Ekoln, Sweden. Biological Invasions 13: 1077–1086.
Griffiths, R. W., D. W. Schloesser, J. H. Leach & W. P. Kovalak, 1991. Distribution and dispersal of the zebra mussel (Dreissena polymorpha) in the Great Lakes region. Canadian Journal of Fisheries and Aquatic Sciences 48: 1381–1388.
Grim, J., 1971. Tiefenverteilung der Dreikantmuschel Dreissena polymorpha (Pallas) im Bodensee. Gas Wasserfach Wasser Abwasser 112: 437–441 (in German).
Gutierrez, J. L., C. G. Jones, D. L. Strayer & O. O. Iribarne, 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101: 79–90.
Hahm, J., J. B. Manchester-Neesvig, D. DeBord & W. C. Sonzogni, 2009. Polybrominated diphenyl ethers (PBDEs) in Lake Michigan forage fish. Journal of Great Lakes Research 35: 154–158.
Haynes, J. M., T. W. Stewart & G. E. Cook, 1999. Benthic macroinvertebrate communities in southwestern Lake Ontario: continuing change. Journal of Great Lakes Research 25: 828–838.
Hecky, R. E., R. E. H. Smith, D. R. Barton, S. J. Guildford, W. D. Taylor, M. N. Charlton & E. T. Howell, 2004. The near shore phosphorus shunt: A consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1285–1293.
Heiler, K. & A. bij de Vaate, K. Ekschmitt, P. von Oheimb, C. Albrecht & T. Wilke, 2013. Reconstruction of the early invasion history of the quagga mussel (Dreissena rostriformis bugensis) in Western Europe. Aquatic Invasions 8: 53–57.
Hetherington, A., L. G. Rudstam, R. L. Schneider, K. T. Holeck, C. W. Hotaling, J. E. Cooper & J. R. Jackson, 2019. Invader invaded: population dynamics of zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena rostriformis bugensis) in polymictic Oneida Lake, NY, USA (1992–2013). Biological Invasions 21: 1529–1544.
Hewson, I., J. B. Eaglesham, T. O. Hook, B. A. LaBarre, M. S. Sepulveda, P. D. Thompson, J. M. Watkins & L. G. Rudstam, 2013. Investigation of viruses in Diporeia spp. from the Laurentian Great Lakes and Owasco Lake as potential stressors of declining populations. Journal of Great Lakes Research 39: 499–506.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: A meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Horvath, T. G. & G. A. Lamberti, 1999. Recruitment and growth of zebra mussels (Dreissena polymorpha) in a coupled lake-stream system. Archiv Für Hydrobiologie 145: 197–217.
Hoyle, J. A., J. N. Bowlby & B. J. Morrison, 2008. Lake whitefish and walleye population responses to dreissenid mussel invasion in eastern Lake Ontario. Aquatic Ecosystem Health and Management 11: 403–411.
Hunter, R. G. & K. A. Simons, 2004. Dreissenids in Lake St. Clair in 2001: Evidence for population regulation. Journal of Great Lakes Research 30: 528–537.
Ibelings, B. W., R. Portielje, E. H. R. R. Lammens, R. Noordhuis, M. S. van den Berg, W. Joosse & M. L. Meijer, 2007. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10: 4–16.
Jackson, J. R., A. J. VanDeValk, T. E. Brooking, K. T. Holeck, C. Hotaling & L. G. Rudstam, 2020. The fisheries and limnology of Oneida Lake 2019, New York State Department of Environmental Conservation, Albany, NY, USA:, 125.
Jacobs, G. R., E. L. Bruestle, A. Hussey, D. Gorsky & A. T. Fisk, 2017. Invasive species alter ontogenetic shifts in the trophic ecology of Lake Sturgeon (Acipenser fulvescens) in the Niagara River and Lake Ontario. Biological Invasions 19: 1533–1546.
Jantz, B. & D. Neumann, 1992. Shell growth and population dynamics of Dreissena polymorpha in the River Rhine. In Neumann, D. & H. A. Jenner (eds), The Zebra Mussel Dreissena polymorpha: Ecology, Biological Monitoring and First Applications in the Water Quality Management Gustav Fisher, Stuttgart: 49–66.
Jarvis, P., J. Dow, R. Dermott, & R. Bonnell, 2000. Zebra (Dreissena polymorpha) and quagga mussel (Dreissena bugensis) distribution and density in Lake Erie, 1992–1998. Fisheries and Oceans Canada. Canadian Technical Report of Fisheries and Aquatic Sciences 2304: 45 pp.
Johengen, T. H., T. F. Nalepa, G. L. Fahnenstiel & G. Goudy, 1995. Nutrient changes in Saginaw Bay, Lake Huron after the establishment of the zebra mussel Dreissena polymorpha. Journal of Great Lakes Research 21: 449–464.
Johnson, L. E. & J. T. Carlton, 1996. Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 77: 1686–1690.
Johnson, T. B., D. B. Bunnell & C. T. Knight, 2005. A potential new energy pathway in Central Lake Erie: the round goby connection. Journal of Great Lakes Research 31(Suppl. 2): 238–251.
Jude, D. J., R. H. Reider & G. R. Smith, 1992. Establishment of Gobiidae in the Great Lakes basin. Great Lakes Research Review 3: 27–34.
Karatayev, A. Y., 1983. Ecology of Dreissena polymorpha Pallas and its effects on macrozoobenthos of the thermal power plant’s cooling reservoir. Candidate Dissertation, Zoology Institute of Academy of Science Belorussian SSR, Minsk, Belarus: 153 pp. (in Russian).
Karatayev, A. Y. & V. P. Lyakhnovich, 1990. Effect of Dreissena polymorpha Pallas on benthic crustaceans (Gammarus lacustris Sars, Pallasea quadrispinosa Sars and Asellus aquaticus L.) in Lukomskoe lake. In Khmeleva, N. N., et al., (eds), Species within their range: biology, ecology, and productivity of aquatic invertebrates Navuka i Tekhnika Press, Minsk, Belarus: 123–125. (in Russian).
Karatayev, A. Y. & L. E. Burlakova, 1995. Present and further patterns in Dreissena polymorpha (Pallas) population development in the Narochanskaya lakes system. Vestsi Akademii Navuk Belarusi. Seriya Biyalagichnikh Navuk 3: 95–99 (in Belarusian).
Karatayev, A. Y., G. M. Tishchikov & I. V. Karatayeva, 1983. Dreissena polymorpha Pallas as a specific community of benthic animals. Biologiya Vnutrennikh Vod. Informatsionnyi Byulleten 61: 18–21 (in Russian).
Karatayev, A. Y., V. P. Lyakhnovich, S. A. Afanasiev, L. E. Burlakova, V. P. Zakutskiy, S. M. Lyakhov, M. P. Miroshnichenko, T. G. Moroz, M. Y. Nekrasova, S. P. Nechvalenko, I. A. Skalskaya, T. G. Kharchenko, & A. A. Protasov, 1994. The place of species in ecosystem. In Starobogatov, J. I. (ed), Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, USSR: 180–195 (in Russian).
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1997. The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in eastern Europe. Journal of Shellfish Research 16: 187–203.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1998. Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). Journal of Shellfish Research 17: 1219–1235.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2002. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In Leppakoski, E., S. Gollach & S. Olenin (eds), Invasive Aquatic Species of Europe: Distribution, Impacts and Management Kluwer Academic Publishers, Dordreicht, The Netherlands: 433–446.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2003. Patterns of spread of the zebra mussel (Dreissena polymorpha (Pallas)): the continuing invasion of Belarussian lakes. Biological Invasions 5: 213–221.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2006. Growth rate and longevity of Dreissena polymorpha (Pallas): a review and recommendations for future study. Journal of Shellfish Research 25: 23–32.
Karatayev, A. Y., D. Boltovskoy, D. K. Padilla & L. E. Burlakova, 2007a. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. Journal of Shellfish Research 26: 205–213.
Karatayev, A. Y., D. K. Padilla, D. Minchin, D. Boltovskoy & L. E. Burlakova, 2007b. Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biological Invasions 9: 161–180.
Karatayev, A. Y., L. E. Burlakova, S. E. Mastitsky & S. Olenin, 2008. Past, current, and future of the Central European Corridor for aquatic invasions in Belarus. Biological Invasions 10: 215–232.
Karatayev, A. Y., L. E. Burlakova, S. E. Mastitsky, D. K. Padilla & E. L. Mills, 2011a. Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. Journal of Shellfish Research 30: 923–931.
Karatayev, A. Y., S. E. Mastitsky, D. K. Padilla, L. E. Burlakova & M. M. Hajduk, 2011b. Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia 668: 183–194.
Karatayev, V. A., A. Y. Karatayev, L. E. Burlakova & D. K. Padilla, 2013. Lakewide dominance does not predict the potential for spread of dreissenids. Journal of Great Lakes Research 39: 622–629.
Karatayev, A. Y., L. E. Burlakova, C. Pennuto, J. Ciborowski, V. A. Karatayev, P. Juette & M. Clapsadl, 2014a. Twenty five years of changes in Dreissena spp. populations in Lake Erie. Journal of Great Lakes Research 40: 550–559.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2014b. General overview of zebra and quagga mussels what we do and do not know. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton, Florida, USA: 695–703.
Karatayev, V. A., A. Y. Karatayev, L. E. Burlakova & L. D. Rudstam, 2014c. Eutrophication and Dreissena invasion as drivers of biodiversity: a century of change in the mollusc community of Oneida Lake. PLoS One 9(7): e101388. https://doi.org/10.1371/journal.pone.0101388.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2015a. Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746: 97–112.
Karatayev, A., D. Boltovskoy, L. Burlakova, D. Padilla, 2015b. Parallels and contrasts between Limnoperna fortunei and Dreissena species. In: Boltovskoy, D. (ed) Limnoperna fortunei, Invading Nature – Springer Series in Invasion Ecology: 261–297.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, E. K. Hinchey & G. Warren, 2018a. Benthic video image analysis facilitates monitoring of Dreissena populations across spatial scales. Journal of Great Lakes Research 44: 629–638.
Karatayev, A. Y., V. A. Karatayev, L. E. Burlakova, M. D. Rowe, K. Mehler & M. D. Clapsadl, 2018b. Food depletion regulates the demography of invasive dreissenid mussels in a stratified lake. Limnology and Oceanography 63: 2065–2079.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, S. A. Bocaniov, P. D. Collingsworth, G. Warren, R. T. Kraus & E. K. Hinchey, 2018c. Biomonitoring using invasive species in a large lake: Dreissena distribution maps hypoxic zones. Journal of Great Lakes Research 44: 639–649.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, R. P. Barbiero, E. K. Hinchey & P. D. Collingsworth K. E. Kovalenko & G. Warren, 2018d. Life after Dreissena: The decline of exotic suspension feeder may have significant impacts on lake ecosystems. Journal of Great Lakes Research 44: 650–659.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, S. E. Daniel, A. K. Elgin, & T. F. Nalepa, 2020. Lake Huron Benthos Survey Cooperative Science and Monitoring Initiative 2017. Technical Report. USEPA-GLRI GL00E02254. Great Lakes Center, SUNY Buffalo State, Buffalo, New York, USA: 59 pp. Available at: http://greatlakescenter.buffalostate.edu/sites/greatlakescenter.buffalostate.edu/files/uploads/Documents/Publications/LakeHuronBenthosSurveyCSMI2017FinalReport.pdf.
Karatayev, A. Y., V. A. Karatayev, L. E. Burlakova, K. Mehler, M. D. Rowe, A. K. Elgin & T. F. Nalepa, 2021a. Lake morphometry determines Dreissena invasion dynamics. Biological Invasions 23: 2489–2514.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, S. E. Daniel & A. R. Hrycik, 2021b. Lake Ontario Benthos Survey Cooperative Science and Monitoring Initiative 2018. Technical Report. USEPA-GLRI GL00E02254. Great Lakes Center, SUNY Buffalo State, Buffalo, New York, USA: 37 pp. Available at: https://greatlakescenter.buffalostate.edu/sites/greatlakescenter.buffalostate.edu/files/uploads/Documents/Publications/LakeOntarioBenthosSurveyCSMI2018FinalReport.pdf .
Karatayev, A. Y., L. E. Burlakova, K. Mehler, E. K. Hinchey, M. Wick, M. Bakowska & N. Mrozinska, 2021c. Rapid assessment of Dreissena population in Lake Erie using underwater videography. Hydrobiologia 848: 2421–2436. https://doi.org/10.1007/s10750-020-04481-x.
Karatayev, V. A., L. G Rudstam, A. Y. Karatayev, L. E. Burlakova, B. V. Adamovich, H. A Zhukava, K. T. Holeck, A. L. Hetherington, J. R. Jackson, C. W. Hotaling, T. V. Zhukova, T. M. Mikheyeva, R. Z. Kovalevskaya, O. A. Makarevich & D. V. Kruk, 2021d. Serial invasions can disrupt the time course of ecosystem recovery. bioRxiv preprint doi: https://doi.org/10.1101/2021.10.29.466526
Karatayev, A. Y., L. E. Burlakova, K. Mehler, L. G. Rudstam, J. M. Watkins & M. Wick, 2022. Dreissena in Lake Ontario 30 years post-invasion. Journal of Great Lakes Research. 48: 264–273.
Karatayev, A. Y., L. E. Burlakova, A. R. Hrycik, S. E. Daniel, K. Mehler, E. K. Hinchey, R. Dermott, G. W. Kennedy & R. Griffiths, In review. Long-term dynamics of Lake Erie benthos: One lake, three distinct communities. Journal of Great Lakes Research.
Kelly, D. W., L.-M. Herborg & H. J. MacIsaak, 2010. Ecosystem changes associated with Dreissena invasions: recent developments and emerging issues. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden, The Netherlands: 199–209.
Kerfoot, W. C., F. Yousef, S. A. Green, J. W. Budd, D. J. Schwab & H. A. Vanderploeg, 2010. Approaching storm: disappearing winter bloom in Lake Michigan. Journal of Great Lakes Research 36(Suppl. 3): 30–41.
Kerney, M. P. & B. S. Morton, 1970. The distribution of Dreissena polymorpha (Pallas) in Britain. Journal of Conchology 27: 97–100.
Kinzelbach, R., 1992. The main features of the phylogeny and dispersal of the zebra mussel Dreissena polymorpha. In Neumann, D. & H. A. Jenner (eds), The Zebra Mussel Dreissena polymorpha: Ecology, Biological Monitoring and First Applications in the Water Quality Management. Gustav Fisher, Stuttgart, Germany: 5–17.
Kornis, M. S., N. Mercado-Silva & M. J. Vander Zanden, 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80: 235–285. https://doi.org/10.1111/j.1095-8649.2011.03157.x.
Li, J., V. Ianaiev, A. Huff, J. Zalusky, T. Ozersky & S. Katsev, 2021. Benthic invaders control the phosphorus cycle in the world’s largest freshwater ecosystem. Proceedings of the National Academy of Sciences of the United States of America 118(6): e2008223118. https://doi.org/10.1073/pnas.2008223118.
Lozano, S. J. & T. F. Nalepa, 2003. Disruption of the benthic community in Lake Ontario. In Munawar, M. (ed), State of Lake Ontario. Michigan State University Press: 305–322.
Lozano, S. J., J. V. Scharold & T. F. Nalepa, 2001. Recent declines in benthic macroinvertebrate densities in Lake Ontario. Canadian Journal of Fisheries and Aquatic Sciences 58: 518–529.
Lvova, A. A., 1980. Ecology of Dreissena (Dreissena polymorpha polymorpha (Pall.)). Trudy Vsesouznogo Gidrobiologicheskogo Obschestva 23: 101–119 (in Russian).
Lyakhnovich, V. P., A. Y. Karatayev, N. I. Andreev, S. I. Andreeva, S. A. Afanasiev, A. K. Dyga, V. P. Zakutskiy, V. I. Zolotareva, A. A. Lvova, M. Y. Nekrasova, V. F. Osadchikh, Y. V. Pligin, A. A. Protasov & G. M. Tischikov, 1994. Living conditions. In Starobogatov, J. I. (ed), Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, USSR: 109–119 (in Russian).
MacIsaac, H. J., 1994. Comparative growth and survival of Dreissena polymorpha and Dreissena bugensis, exotic molluscs introduced to the Great Lakes. Journal of Great Lakes Research 20(4): 783–790.
MacIsaac, H. J., W. G. Sprules, O. E. Johannsson & J. H. Leach, 1992. Filtering impacts of larval and sessile zebra mussels (Dreissena polymorpha) in western Lake Erie. Oecologia 92: 30–39.
Mackie, G. L., 1991. Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219: 251–268.
Madenjian, C. P., S. A. Pothoven, P. J. Schneeberger, M. P. Ebener, L. C. Mohr, T. F. Nalepa & J. R. Bence, 2010. Dreissenid mussels are not a ‘“dead end”’ in Great Lakes food webs. Journal of Great Lakes Research 36: 73–77.
Madenjian, C. P., M. A. Stapanian, L. D. Witzel, S. A. Pothoven, D. W. Einhouse & H. L. Whitford, 2011. Evidence for predatory control of the invasive Round Goby. Biological Invasions 13: 987–1002.
Madenjian, C. P., D. B. Bunnell, D. M. Warner, S. A. Pothoven, G. L. Fahnenstiel, T. F. Nalepa, H. A. Vanderploeg, I. Tsehaye, R. M. Claramunt & R. D. Clark Jr., 2015. Changes in the Lake Michigan food web following dreissenid mussel invasions: a synthesis. Journal of Great Lakes Research 41(Supplement 3): 217–231.
Makarewicz, J. C., 1993. A lakewide comparison of zooplankton biomass and its species composition in Lake Erie, 1983–87. Journal of Great Lakes Research 19: 275–290.
Makarewicz. J. C., T. W. Lewis & P. Bertram, 1995a. Epilimnetic phytoplankton and zooplankton biomass and species composition in Lake Ontario, 1986 to 1992. Technical Reports. 12. https://digitalcommons.brockport.edu/tech_rep/12.
Makarewicz, J. C., P. Bertram, T. Lewis & E. H. Brown Jr., 1995b. A decade of predatory control of zooplankton species composition of Lake Michigan. Journal of Great Lakes Research 21: 620–640.
Makarewicz, J. C., P. Bertram & T. D. Lewis, 2000. Chemistry of the offshore surface waters of Lake Erie: pre- and post-Dreissena introduction (1983–1993). Journal of Great Lakes Research 26: 82–93.
Marescaux, J., P. Boets, J. Lorquet, R. Sablon, K. Van Doninck & J.-N. Beise, 2015. Sympatric Dreissena species in the Meuse River: towards a dominance shift from zebra to quagga mussels. Aquatic Invasions 10(3): 287–298.
Marsden, J. E., N. Trudeau & T. Keniry, 1993. Zebra mussel study of Lake Michigan. Illinois Natural History Survey, Aquatic Ecology Technical Report 93/14: 35 pp.
Mayer, C. M., R. A. Keats, L. G. Rudstam & E. L. Mills, 2002. Scale-dependent effects of zebra mussels on benthic invertebrates in a large eutrophic lake. Journal of the North American Benthological Society 21: 616–633.
Mayer, C. M., L. E. Burlakova, P. Eklöv, D. Fitzgerald, A. Y. Karatayev, S. A. Ludsin, S. Millard, E. L. Mills, A. P. Ostapenya, L. G. Rudstam, B. Zhu & T. V. Zhukova, 2014. The benthification of freshwater lakes: exotic mussels turning ecosystems upside down. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton, Florida, USA: 575–586.
Mehler, K., L. E. Burlakova, A. Y. Karatayev, A. K. Elgin, T. F. Nalepa, C. P. Madenjian & E. Hinchey, 2020. Long-term trends of Lake Michigan benthos with emphasis on the southern basin. Journal of Great Lakes Research 46: 528–537.
Metz, O., A. Temmen, K. C. M. von Oheimb, C. Albrecht, P. Schubert & T. Wilke, 2018. Invader vs. invader: intra- and interspecific competition mechanisms in zebra and quagga mussels. Aquatic Invasions 13(4): 473–480.
Mida, J. L., D. Scavia, G. L. Fahnenstiel, S. A. Pothoven, H. A. Vanderploeg & D. M. Dolan, 2010. Long–term and recent changes in southern Lake Michigan water quality with implications for present trophic status. Journal of Great Lakes Research 36(S3): 42–49.
Mills, E. L., R. M. Dermott, E. F. Roseman, D. Dustin, E. Mellina, D. B. Corm & A. P. Spidle, 1993. Colonization, ecology, and population structure of the “quagga” mussel (Bivalvia: Dreissenidae) in the lower Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 50: 2305–2314.
Mills, E. L., J. R. Chrisman, B. Baldwin, R. W. Owens, R. O’Gorman, T. Howell, E. Roseman & M. K. Raths, 1999. Changes in the dressenid community in the lower Great Lakes with emphasis on southern Lake Ontario. Journal of Great Lakes Research 25: 187–197.
Molloy, D. P., A. Y. Karatayev, L. E. Burlakova, D. P. Kurandina & F. Laruelle, 1997. Natural enemies of zebra mussels: predators, parasites and ecological competitors. Reviews in Fisheries Science 5: 27–97.
Morton, B., 1969a. Studies on the biology of Dreissena polymorpha Pall. III. Population dynamics. Proceedings of the Malacological Society of London 38: 471–481.
Morton, B., 1969b. Studies on the biology of Dreissena polymorpha Pall. II. Correlation of the rhythms of adductor activity, feeding, digestion and excretion. Proceedings of the Malacological Society of London 38: 401–414.
Naddafi, R. & L. G. Rudstam, 2014. Predator-induced morphological defences in two invasive dreissenid mussels: implications for species replacement. Freshwater Biology 59: 703–713.
Nalepa, T. F., 2010. An overview of the spread, distribution, and ecological impacts of the quagga mussel, Dreissena rostriformis bugensis, with possible implications to the Colorado River system. Proceedings, Colorado River Basin Science and Resource Management Symposium. Publications, Agencies and Staff of the U.S. Department of Commerce, 412: 121 pp. Available at https://digitalcommons.unl.edu/usdeptcommercepub/412.
Nalepa, T. F. & D. W. Schloesser, 1993. Zebra Mussels Biology, Impacts, and Control, Lewis Publishers, Ann Arbor, Michigan, USA: 810 pp.
Nalepa, T. F. & D. W. Schloesser, 2014. Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton, Florida, USA: 775 pp.
Nalepa, T. F., J. A. Wojcik, D. L. Fanslow & G. A. Lang, 1995. Initial colonization of the zebra mussel (Dreissena polymorpha) in Saginaw Bay, Lake Huron: population recruitment, density and size structure. Journal of Great Lakes Research 21: 417–434.
Nalepa, T. F., D. J. Hartson, G. W. Gostenik, D. L. Fanslow & G. A. Lang, 1996. Changes in the freshwater mussel community of Lake St. Clair: from Unionidae to Dreissena polymorpha in eight years. Journal of Great Lakes Research 22: 354–369.
Nalepa, T. F., D. J. Hartson, D. L. Fanslow & G. L. Lang, 2001. Recent population changes in freshwater mussels (Dreissena polymorpha) in Lake St. Clair, U.S.A. American Malacological Bulletin 16: 141–145.
Nalepa, T. F., D. L. Fanslow, M. B. Lansing, G. A. Lang, M. Ford, G. Gostenick & D. J. Hartson, 2002. Abundance, biomass, and species composition of benthic macroinvertebrate populations in Saginaw Bay, Lake Huron, 1987–96. NOAA Technical Memorandum GLERL-122: 31 pp.
Nalepa, T. F., D. L. Fanslow, M. B. Lansing & G. A. Lang, 2003. Trends in the benthic macroinvertebrate community of Saginaw Bay, Lake Huron, 1987 to 1996: Responses to phosphorus abatement and the zebra mussel, Dreissena polymorpha. Journal of Great Lakes Research 29: 14–33.
Nalepa, T. F., D. L. Fanslow & G. Messick, 2005. Characteristics and potential causes of declining Diporeia spp. populations in southern Lake Michigan and Saginaw Bay, Lake Huron. In Proceedings of a Workshop on the Dynamics of Lake Whitefish (Coregonus clupeaformis) and the amphipod Diporeia spp. in the Great Lakes. In Mohr, L. C. & T. F. Nalepa (eds), Great Lakes Fishery Commission, Technical Report 66 Ann Arbor, Michigan, USA: 157–188.
Nalepa, T. F., D. L. Fanslow, A. J. Foley III., G. A. Lang, B. J. Eadie & M. A. Quigley, 2006. Continued disappearance of the benthic amphipod Diporeia spp. in Lake Michigan: is there evidence for food limitation? Canadian Journal of Fisheries and Aquatic Sciences 63: 872–890.
Nalepa, T. F., D. L. Fanslow, S. A. Pothoven, A. J. Foiley III. & G. A. Lang, 2007. Long-term trends in benthic macroinvertebrate populations in Lake Huron over the past four decades. Journal of Great Lakes Research 33: 42–436.
Nalepa, T. F., D. L. Fanslow & G. A. Lang, 2009a. Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to invasive mussel Dreissena rostriformis bugensis. Freshwater Biology 54: 466–479.
Nalepa, T. F., S. A. Pothoven & D. L. Fanslow, 2009b. Recent changes in benthic macroinvertebrate populations in Lake Huron and impact on the diet of lake whitefish (Coregonus clupeaformus). Aquatic Ecosystem Health and Management 12: 2–10.
Nalepa, T. F., D. L. Fanslow & S. A. Pothoven, 2010. Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. Journal of Great Lakes Research 36: 5–19.
Nalepa, T. F., D. L. Fanslow, G. A. Lang, K. Mabrey & M. Rowe, 2014. Lake-wide benthic surveys in Lake Michigan in 1994–95, 2000, 2005, and 2010: Abundances of the amphipod Diporea spp. and abundances and biomass of the mussels Dreissena polymorpha and Dreissena rostriformis bugensis. NOAA Technical Memorandum GLERL-164: 20 pp.
Nalepa, T. F., L. E. Burlakova, A. K. Elgin, A. Y. Karatayev, G. A. Lang & K. Mehler, 2020. NOAA Tech Memo GLERL-175. Abundance and Biomass of Benthic Macroinvertebrates in Lake Michigan in 2015, With a Summary of Temporal Trends. Tech. Memo, NOAA Great Lakes Environmental Research Laboratory, NOAA Great Lakes environmental Research Laboratory, Ann Arbor Michigan, USA: 41 pp.
Nekrasova, M. Y. & I. F. Kovtun, 1975. Annual fluctuations in the abundance of the round goby, Gobius melanostomus, in relation to natural variations in its food supply in the Azov Sea. Journal of Ichthyology 15: 331–336.
Noordhuis, R., B. G. van Zuidam, E. T. H. M. Peeters & G. J. van Geestet, 2016. Further improvements in water quality of the Dutch Border lakes: two types of clear states at different nutrient Levels. Aquatic Ecology 50: 521–539.
Orlova, M. I., J. R. Muirhead, P. I. Antonov, G. K. Shcherbina, Y. I. Starobogatov, G. I. Biochino, T. W. Therriault & H. J. MacIsaac, 2004. Range expansion of quagga mussels Dreissena rostriformis bugensis in the Volga River and Caspian Sea basin. Aquatic Ecology 38: 561–573.
Orlova, M. I., T. W. Therriault, P. I. Antonov & G. K. Shcherbina, 2005. Invasion ecology of quagga mussels (Dreissena rostriformis bugensis): a review of evolutionary and phylogenetic impacts. Aquatic Ecology 39: 401–418.
Ozersky, T., D. R. Barton & D. O. Evans, 2011. Fourteen years of dreissenid presence in the rocky littoral zone of a large lake: effects on macroinvertebrate abundance and diversity. Journal of the North American Benthological Society 30: 913–922.
Patterson, M. W. R., J. J. H. Ciborowski & D. R. Barton, 2005. The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie, 2002. Journal of Great Lakes Research 31(Supplement 2): 223–237.
Perez-Fuentetaja, A., S. A. Mackintosh, L. R. Zimmerman, M. D. Clapsadl, M. Alaee & D. S. Aga, 2015. Trophic transfer of flame retardants (PBDEs) in the food web of Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 72: 1886–1896.
Peterson, C. H. & B. F. Beal, 1989. Bivalve growth and higher order interactions: importance of density, site, and time. Ecology 70(5): 1390–1404.
Peyer, S. M., A. J. McCarthy & C. E. Lee, 2009. Zebra mussels anchor byssal threads faster and tighter than quagga mussels in flow. Journal of Experimental Biology 212: 2026–2035.
Peyer, S. M., J. C. Hermanson & C. E. Lee, 2010. Developmental plasticity of shell morphology of quagga mussels from shallow and deep-water habitats of the Great Lakes. Journal of Experimental Biology 213: 2602–2609.
Pollux, B. J. A., G. van der Velde & A. bij de Vaate, 2010. A perspective on global spread of Dreissena polymorpha: a review on possibilities and limitations. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden, The Netherlands: 45–58.
Pothoven, S. A. & G. L. Fahnenstiel, 2013. Recent change in summer chlorophyll a dynamics of southeastern Lake Michigan. Journal of Great Lakes Research 39: 287–294.
Pothoven, S. A. & G. L. Fahnenstiel, 2014. Lake Michigan after dreissenid mussel invasion. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton, Florida, USA: 545–553.
Pothoven, S. A. & H. A. Vanderploeg, 2020. Seasonal patterns for Secchi depth, chlorophyll a, total phosphorus, and nutrient limitation differ between nearshore and offshore in Lake Michigan. Journal of Great Lakes Research 46: 519–527.
Pothoven, S. A., T. Nalepa, P. Schneeberger & S. Brandt, 2001. Changes in diet and body condition of lake whitefish in southern Lake Michigan associated with changes in benthos. North American Journal of Fisheries Management 21: 876–883.
Pothoven, S. A., C. P. Madenjian & T. O. Hook, 2017. Feeding ecology of the walleye (Percidae, Sander vitreus), a resurgent piscivore in Lake Huron (Laurentian Great Lakes) after shifts in the prey community. Ecology of Freshwater Fish 26: 676–685.
Pryanichnikova, E. G., 2012. Structure and function of dreissenid populations in Rybinsk Reservoir. Abstract of the candidate dissertation, Papanin Institute for Biology of Inland Waters. Russian Academy of Sciences, Russia: 20 pp. (in Russian).
Ramcharan, C. W., D. K. Padilla & S. I. Dodson, 1992. Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Canadian Journal of Fisheries and Aquatic Sciences 49: 2611–2620.
Ratti, C. & D. R. Barton, 2003. Decline in diversity of benthic invertebrates in the wave-zone of eastern Lake Erie, 1974–2001. Journal of Great Lakes Research 29: 608–615.
Reed-Andersen, T., S. R. Carpenter, D. K. Padilla & R. C. Lathrop, 2000. Predicted impact of zebra mussel (Dreissena polymorpha) invasion on water clarity in Lake Mendota. Canadian Journal of Fisheries and Aquatic Sciences 57: 1617–1626.
Reid, N. J., M. A. Anderson & W. D. Taylor, 2010. Distribution of quagga mussel veligers, Dreissena bugensis, in the reservoirs of the Colorado River Aqueduct. Lake and Reservoir Management 26: 328–335.
Rennie, M. D., W. G. Sprules & T. B. Johnson, 2009. Resource switching in fish following a major food web disruption. Oecologia 159: 789–802.
Roe, S. L. & H. J. MacIsaac, 1997. Deepwater population structure and reproductive state of quagga mussels (Dreissena bugensis) in Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 54: 2428–2433.
Rowe, M. D., D. R. Obenour, T. F. Nalepa, H. A. Vanderploeg, F. Yousef & W. C. Kerfoot, 2015. Mapping the spatial distribution of the biomass and filter-feeding effect of invasive dreissenid mussels on the winter-spring phytoplankton bloom in Lake Michigan. Freshwater Biology 60: 2270–2285.
Rudstam, L. G. & C. J. Gandino, 2020. Zebra or quagga mussel dominance depends on trade-offs between growth and defense - Field support from Onondaga Lake. NY. Plos One 15: e0235387.
Ryan, D. J., M. S. Sepulveda, T. F. Nalepa & T. O. Hook, 2012. Spatial variation in RNA:DNA ratios of Diporeia spp. in the Great Lakes region. Journal of Great Lakes Research 38: 187–195.
Schloesser, D. W. & C. Schmuckal, 2012. Bibliography of Dreissena polymorpha (zebra mussels) and Dreissena rostriformis bugensis (quagga mussels): 1989 to 2011. Journal of Shellfish Research 31: 1205–1263.
Skubinna, J. P., T. G. Coon & T. R. Batters, 1995. Increased abundance and depth of submersed macrophytes in response to decreased turbidity in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 476–478.
Smit, H. & A. bij de Vaate & A. Fioole, 1992. Shell growth of the zebra mussel Dreissena polymorpha Pallas in relation to selected physiochemical parameters in the Lower Rhine and some associated lakes. Archiv Für Hydrobiologie 124(3): 257–280.
Smit, H., A. bij de Vaate, E. H. Van Nes & R. Noordhuis, 1993. Colonization, ecology, and positive aspects of zebra mussels (Dreissena polymorpha) in the Netherlands. In Nalepa, T. F. & D. W. Schloesser (ed), Zebra Mussels. Biology, Impacts, and Control. Lewis Publishers, Ann Arbor, Michigan, USA: 55–77.
Sprung, M., 1991. Costs of reproduction: a study on metabolic requirements of the gonads and fecundity of the bivalve Dreissena polymorpha. Malacologia 33: 63–70.
Sprung, M., 1992. Observation on shell growth and mortality of Dreissena polymorpha in lakes. In Neumann, D. & H. A. Jenner (ed), The Zebra Mussel Dreissena polymorpha: Ecology, Biological Monitoring and First Applications in the Water Quality Management. Gustav Fisher, Stuttgart, Germany: 19–28.
Sprung, M., 1995. Physiological energetics of the zebra mussel Dreissena polymorpha in lakes. I. Growth and Reproductive Effort. Hydrobiologia 304: 117–132.
Stewart, T. W. & J. M. Haynes, 1994. Benthic macroinvertebrate communities of southwestern Lake Ontario following invasion of Dreissena. Journal of Great Lakes Research 20: 479–493.
Stewart, T. W., J. G. Miner & R. L. Lowe, 1998a. Quantifying mechanisms for zebra mussel effects on benthic macroinvertebrates: Organic matter production and shell-generated habitat. Journal of the North American Benthological Society 17: 81–94.
Stewart, T. W., J. G. Miner & R. L. Lowe, 1998b. An experimental analysis of crayfish (Orconectes rusticus) effects on a Dreissena-dominated benthic macroinvertebrate community in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 55: 1043–1050.
Stewart, T. W., J. C. Gafford, J. G. Miner & R. L. Lowe, 1999. Dreissena-shell habitat and antipredator behavior: combined effects on survivorship of snails co-occurring with molluscivorous fish. Journal of the North American Benthological Society 18: 274–283.
Stoeckmann, A., 2003. Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Canadian Journal of Fisheries and Aquatic Sciences 60: 126–134.
Stoeckman, A. & D. Garton, 1997. A seasonal energy budget for zebra mussels (Dreissena polymorpha) in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 54: 2743–2751.
Strayer, D. L., 1991. Projected distribution of zebra mussel, Dreissena polymorpha in North America. Canadian Journal of Fisheries and Aquatic Sciences 48: 1389–1395.
Strayer, D. L. & L. C. Smith, 1993. Distribution of zebra mussel (Dreissena polymorpha) in estuaries and brackish waters. In Nalepa, T. F. & D. W. Schloesser (ed), Zebra Mussels. Biology, Impacts, and Control. Lewis Publishers, Ann Arbor, Michigan, USA: 715–727.
Strayer, D. L., B. V. Adamovich, R. Adrian, D. C. Aldridge, C. Balogh, L. E. Burlakova, H. Fried-Petersen, L. G.-Tóth, A. L. Hetherington, T. S. Jones, A. Y. Karatayev, J. B. Madill, O. A. Makarevich, J. E. Marsden, A. L. Martel, D. Minchin, T. F. Nalepa, R. Noordhuis, T. J. Robinson, L. G. Rudstam, A. N. Schwalb, D. R. Smith, A. D. Steinman & J. M. Jeschke, 2019. Long-term population dynamics of dreissenid mussels (Dreissena polymorpha and D. rostriformis): a cross-system analysis. Ecosphere, https://doi.org/10.1002/ecs2.2701.
van der Velde, G., S. Rajagopal & A. bij de Vaate, 2010. The Zebra Mussel in Europe, Backhuys Publishers, Leiden, The Netherlands: 490 pp.
van der Velde, G., S. Rajagopal & A. bij de Vaate, 2010b. From zebra mussels to quagga mussels: an introduction to the Dreissenidae. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds). The Zebra Mussel in Europe. Backhuys Publishers, Leiden, The Netherlands: 1–10.
Vanderploeg, H. A., T. F. Nalepa, D. J. Jude, E. L. Mills, K. T. Holeck, J. R. Liebig, I. A. Grigorovich & H. Ojaveer, 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 1209–1228.
Vanderploeg, H. A., J. R. Liebig, T. F. Nalepa, G. L. Fahnenstiel & A. Pothoven, 2010. Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. Journal of Great Lakes Research 36: 50–59.
Walsh, G., D. E. Dittman & R. O’Gorman, 2007. Occurrence and food habits of the round goby in the profundal zone of southwestern Lake Ontario. Journal of Great Lakes Research 33: 83–92.
Walz, N., 1973. Untersuchungen zur Biologie von Dreissena polymorpha Pallas im Bodensee. Archiv für Hydrobiologie – Supplements 42: 452–482.
Walz, N., 1978a. The energy balance of the freshwater mussel Dreissena polymorpha Pallas in laboratory experiments and in Lake Constance. II Reproduction. Archiv für Hydrobiologie – Supplements 55: 106–119.
Walz, N., 1978b. The energy balance of the freshwater mussel Dreissena polymopha Pallas in laboratory experiments and in Lake Constance. IV. Growth in Lake Constance. Archiv für Hydrobiologie –Supplements 55: 142–156.
Watkins, J. M., R. Dermott, S. J. Lozano, E. L. Mills, L. G. Rudstam & J. V. Scharold, 2007. Evidence for remote effects of dreissenids mussels on the amphipod Diporeia: analysis of Lake Ontario benthic surveys, 1997–2003. Journal of Great Lakes Research 33: 642–657.
Watkins, J. M., L. G. Rudstam, E. L. Mills & M. A. Teece, 2012. Coexistence of the native benthic amphipod Diporeia spp. and exotic dreissenid mussels in the New York Finger Lakes. Journal of Great Lakes Research 38: 226–235.
Watkins, J. M., L. G. Rudstam, D. L. Crabtree & M. G. Walsh, 2013. Is reduced benthic flux related to the Diporeia decline? Analysis of spring blooms and whiting events in Lake Ontario. Journal of Great Lakes Research 39: 395–403.
Weber, M. J., J. M. Dettmers, D. H. Wahl & S. J. Czesny, 2011. Size preferences and behaviors of native yellow perch foraging on invasive round gobies. Journal of Great Lakes Research 37: 584–587.
Wegner, B., A. L. Kronsbein, M. Gillefalk, K. van de Weyer, J. Köhler, E. Funke, M. T. Monaghan & S. Hilt, 2019. Mutual facilitation among invading nuttall’s waterweed and quagga mussels. Frontiers in Plant Science 10. https://doi.org/10.3389/fpls.2019.00789.
Weidel, B. C., M. J. Connerton & J. P. Holden, 2019. Bottom trawl assessment of Lake Ontario prey fishes. New York State Department of Environmental Conservation Lake Ontario Unit 2018 Annual Report Section 12: 24 pp.
Wilson, K. A., E. T. Howell & D. A. Jackson, 2006. Replacement of zebra mussels by quagga mussels in the Canadian nearshore of Lake Ontario: the importance of substrate, round goby abundance, and upwelling frequency. Journal of Great Lakes Research 32: 11–28.
Winters, A. D., S. Fitzgerald, T. O. Brenden, T. Nalepa & M. Faisal, 2014. Spatio-temporal dynamics of parasites infecting Diporeia spp. (Amphipoda, Gammaridae) in southern Lake Michigan (USA). Journal of Invertebrate Pathology 121: 37–45.
Winters, A. D., T. L. Marsh, T. O. Brenden & M. Faisal, 2015. Analysis of bacterial communities associated with the benthic amphipod Diporeia in the Laurentian Great Lakes Basin. Canadian Journal of Microbiology 61: 72–81.
Wong, W. H., S. Gerstenberger, W. Baldwin & B. Moore, 2012. Settlement and growth of quagga mussels (Dreissena rostriformis bugensis Andrusov, 1897) in Lake Mead, Nevada-Arizona, USA. Aquatic Invasions 7(1): 7–19.
Yu, N. & D. A. Culver, 1999. In situ survival and growth of zebra mussels (Dreissena polymorpha) under chronic hypoxia in a stratified lake. Hydrobiologia 392: 205–215.
Zhu, B., D. G. Fitzgerald, C. M. Mayer, L. G. Rudstam & E. L. Mills, 2006. Alteration of ecosystem function by zebra mussels in Oneida Lake: Impacts on submerged macrophytes. Ecosystems 9: 1017–1028.
Zhulidov, A. V., D. F. Pavlov, T. F. Nalepa, G. H. Scherbina, D. A. Zhulidov & T. Yu. Gurtovaya, 2004. Relative distributions of Dreissena bugensis and Dreissena polymorpha in the lower Don River system, Russia. International Review of Hydrobiology 89: 326–333.
Zhulidov, A. V., A. V. Kozhara, G. H. Scherbina, T. F. Nalepa, A. Protasov, S. A. Afanasiev, E. G. Pryanichnikova, D. A. Zhulidov, T. Yu. Gurtovaya & D. F. Pavlov, 2010. Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biological Invasions 12: 1923–1940.
Acknowledgements
This research was supported by an agreement with Cornell University, under Prime Agreement Award GL 00E02259-2 from the U.S. EPA (PI L. Rudstam, Co-PIs Burlakova & Karatayev). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of Cornell University nor those of the U.S. EPA. The authors would like to thank V. Karatayev (The University of Kansas, Lawrence) for help with data analysis, Administrative Assistant S. Dickinson (GLC) for proofreading the manuscript, and two anonymous reviewers for helpful comments.
Funding
This research supported by a cooperative agreement with Cornell University, Department of Natural Resources under Prime Agreement Award GL 00E02259-2 from the U.S. EPA “Great Lakes Long-Term Biological Monitoring Program 2017–2022” (PI L. Rudstam, Co-PIs Burlakova & Karatayev). The authors declare that there is no conflict of interest and no competing interests to declare regarding the publication of this article, as well as no relevant financial or non-financial interests to disclose. The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Sidinei Magela Thomaz
Guest editors: Manuel P. M. Lopes-Lima, Lyubov E. Burlakova, Ting Hui Ng, Alexandra Zieritz & Ronaldo G. Sousa / Biology and Impact of Invasive Freshwater Molluscs
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karatayev, A.Y., Burlakova, L.E. Dreissena in the Great Lakes: what have we learned in 30 years of invasion. Hydrobiologia (2022). https://doi.org/10.1007/s10750-022-04990-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-022-04990-x