Abstract
Dreissena polymorpha (zebra mussel) and D. rostriformis bugensis (quagga mussel) continue to spread in Europe and in North America, and have large ecological and economic impacts where they invade. Today many more waterbodies are invaded by zebra mussels, and therefore the extent of their impact is greater than that of quagga mussels. Both species provide additional space and food for invertebrates in the littoral zone, increasing their diversity and density. In contrast, in the profundal zone, quagga mussels may compete for space and food resources with benthic invertebrates, decreasing their diversity and density. The system-wide effect of dreissenids depends on water mixing rates, lake morphology, and turnover rates. Because quagga mussels are found in all regions of a lake, and form larger populations, they may filter larger volumes of water and may have greater system-wide effects, especially in deep lakes, than zebra mussels, which are restricted to shallower portions of lakes. Shortly after initial invasion, as populations increase, both dreissenids will have their largest effects on communities, and most of them will be direct effects. After the initial stage of invasion, impacts are less predictable, and more likely to be caused by indirect effects through changes in the ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dreissena polymorpha (Pallas), the zebra mussel, and D. rostriformis bugensis (Andrusov), the quagga mussel, based on their ecological and economic impacts, are considered the most aggressive freshwater invaders in the Northern hemisphere (reviewed in Nalepa & Schloesser, 1993, 2014; Karatayev et al., 2002, 2007a). Both species continue to spread in Europe and in North America at virtually all spatial scales (Karatayev et al., 2007a, 2011; Pollux et al., 2010; Benson, 2014), and can have large ecological and economic impacts where they invade (reviewed in Karatayev et al., 1997, 2002, 2007a; Vanderploeg et al., 2002; Hecky et al., 2004; O’Neill, 2008). These two congeners have similar life history characteristics including high reproductive potential, planktonic free-swimming larvae, and an attached benthic adult stage, and they are highly efficient suspension feeders. These features are typical of marine mussels, but represent a novel ecological type in freshwaters (Karatayev et al., 2007b). This life history facilitates their success as invaders, allowed them to spread rapidly across landscapes, and become extremely abundant when introduced into a new waterbody. The ecological impacts of both species are likely associated with their role as ecosystem engineers (reviewed in Karatayev et al., 2002, 2007a; Vanderploeg et al., 2002; Gutierrez et al., 2003; Sousa et al., 2009) as they can “directly or indirectly control the availability of resources to other organisms by causing physical state changes in biotic or abiotic materials” (Jones et al., 1994, 1997).
Recent approaches using functional groups or functional traits to predict or assess community or ecosystems impacts of species, especially invaders, have gained a lot of attention (Flynn et al., 2011). Using these approaches, these two congeners are often considered to be interchangeable in terms of the impacts they may have on communities or ecosystems (Karatayev et al., 2007a; Keller et al., 2007; Ward & Ricciardi, 2007; Higgins & Vander Zanden, 2010; Kelley et al., 2010). Although these two species are similar, they are not identical, and to understand and predict their ecological and ecosystem impacts on systems they invade, it is essential to understand not only their species specific characteristics, but also their population dynamics and within-lake distributions, which are different, and change as a function of time since invasion. Because of this, the local and lake-wide impacts of invasion by one or both of these species are dynamic, and difficult to predict.
Across a landscape, the overall ecological impact of zebra and quagga mussels will depend on the numbers of waterbodies colonized, their total population density in a given waterbody, their population dynamics, and the distribution within a waterbody (Karatayev et al., 2010a, 2011). Although D. polymorpha is among the best studied freshwater invertebrates, comparable information for D. r. bugensis is generally lacking (Nalepa, 2010; Karatayev et al., 2014a, Table 1), limiting our ability to predict the spread and ecological impacts of this important freshwater invader. Since 1989, when quagga mussels were found in North America and they expanded in Europe, research efforts on D. r. bugensis have increased; however, 87% of all Dreissena-related papers published since 1989 are on D. polymorpha (Table 1).
The goal of this study is to review key similarities and differences between zebra mussels and quagga mussels in their rates of spread across the landscape, their population dynamics and distributions in waterbodies they invade, and their impacts on invaded ecosystems. We also identify the essential information that needs to be determined to understand the impacts and spread of these invaders.
Spread across the landscape
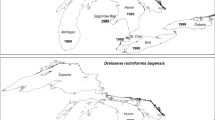
In the early 1800s, zebra mussels began to spread from their native range in the Ponto-Caspian basin through canals connecting the Baltic and Black See basins for commerce and international trade (Zhadin, 1946; Mordukhai-Boltovskoi, 1960; Kinzelbach, 1992; Starobogatov & Andreeva, 1994; Pollux et al., 2010; van der Velde et al., 2010). Zebra mussels then rapidly spread across the central and western Europe (reviewed in Kerney & Morton, 1970; Kinzelbach, 1992; Starobogatov & Andreeva, 1994; Karatayev et al., 2007a, 2011; Pollux et al., 2010; van der Velde et al., 2010) in the nineteenth century at an exponential rate colonizing ~3.9 regions (i.e., countries, or geographical provinces within large countries) per decade (Karatayev et al., 2011). This was followed by the industrial revolution and increased water pollution, which essentially stopped the spread of D. polymorpha in Europe for almost a century (reviewed in Kinzelbach, 1992; Karatayev et al., 2007a, 2011). Then, starting in the 1960s, there was a second period of exponential spread of D. polymorpha on a global scale, including spread in Europe, and in North America (Karatayev et al., 2007a, 2011), where this species was introduced in the mid-1980s (Carlton, 2008). The spread of D. polymorpha at the global scale (including the spread among countries or major regions within countries in Europe and spread among North American states and provinces) from 1962 to 2008 was much faster than the initial spread across Europe, and averaged ~6.6 regions per decade (Karatayev et al., 2011).
In contrast to D. polymorpha, D. r. bugensis remained restricted to its native range: the Dnieper-Bug Liman (a large shallow productive estuary with variable salinity), the Dnieper River delta, and lower reaches of the South Bug and Ingulets rivers, until the 1940s (reviewed in Zhulidov et al., 2004, 2010; Karatayev et al., 2007a, 2011; Van der Velde et al., 2010). The delay in the spread of quagga mussels was likely due to their inability to use mechanisms and vectors responsible for spread as efficiently as zebra mussels. Due to differences in byssal production rates and attachment strength, and a flattened ventral portion of their valves, D. polymorpha appears to be more resistant to dislodgment than D. r. bugensis (Mackie, 1991; Dermott & Munawar, 1993; Claxton & Mackie, 1998; Peyer et al., 2009, 2010). Therefore, zebra mussels were more likely to remain attached to boat hulls and rafts than quagga mussels, facilitating their transport to new habitats. In addition, during the initial spread of zebra mussels in Europe, most introductions were in the shallow areas of lakes, rivers, and canals, habitats that may be less suitable for quagga mussels, which prefer quiet areas of deep lakes and reservoirs (Orlova et al., 2005; Nalepa et al., 2010; Karatayev et al., 2014a).
When quagga mussels spread across Europe, most lakes they invaded were already colonized by zebra mussels, making it difficult to compare their rates of spread. Because North America was colonized by both species approximately at the same time (1980s) and in the same area, their rates of spread in North America are directly comparable. D. polymorpha was first found in the New World in Lake Erie in 1986, and D. r. bugensis was first documented, also in Lake Erie, in 1989 (Mills et al., 1993; Carlton, 2008). In addition, detailed information is available on the year of invasion of states (in USA) and provinces (in Canada), as well as for counties within states, and for individual lakes, reservoirs, and rivers within the United States (Benson et al., 2013a, b).
By 2008, zebra mussels had colonized twice as many US states as quagga mussels, almost eight times more counties, and over 15 times more water bodies (Karatayev et al., 2011). By 2010, both species of Dreissena had colonized a total of 772 inland lakes, reservoirs, impoundments, and quarries in USA and Canada in addition to the Great Lakes. Of these, 729 waterbodies were colonized by zebra mussels alone, 33 by quagga mussels alone, and only 10 by both species (Benson, 2014). Therefore, 25 years after their introduction to North America, D. polymorpha had colonized 17 times more waterbodies than D. r. bugensis.
Although dreissenids can spread over large geographical areas relatively quickly, their rate of colonization of inland lakes was very slow, even for zebra mussels. For example in Belarus, after >200 years of invasion, only 33% of all colonizable (based on pH, calcium and total mineralization limits) lakes were invaded by 2008 (Karatayev et al., 2010b). Similarly in Wisconsin, USA after >20 years of invasion, only 120 of >15,000 inland lakes (<1%) were invaded by 2013 (Wisconsin, 2013). None of the inland lakes in Belarus or in Wisconsin are colonized by quagga mussels thus far. Therefore, the extent of ecological impacts of zebra mussels is much higher than that of quagga mussels, as many more waterbodies are invaded by D. polymorpha than by D. r. bugensis.
Population dynamics
Dreissena polymorpha and D. r. bugensis are characterized by different population dynamics when they invade a new waterbody (Fig. 1). The time lag between when each species was first detected in a waterbody and when it reached its maximum population size is much shorter for zebra mussels (2.5 ± 0.2 years, average ± standard error) than for quagga mussels (12.2 ± 1.5 years) (reviewed in Karatayev et al., 2011). Shortly after initial invasion, when populations are greatest, Dreissena spp. have their largest and most direct ecological effects (Karatayev et al., 2002). The longer lag time between the initial invasion and the maximum population size for quagga mussels suggests that their maximum ecological impacts will be delayed compared to that of zebra mussels (Fig. 1). In addition, data suggest that it takes longer for quagga mussels to obtain their maximum density in deeper areas of lakes than in shallow areas (Watkins et al., 2007; French et al., 2009; Nalepa et al., 2010). After 9 years or more of coexistence, the density of quagga mussels in both shallow and deep areas of a lake can far outstrip those of zebra mussels (Fig. 2).
Conceptual illustration of the dynamics of population size of Dreissena polymorpha (gray line) and D. rostriformis bugensis (dashed line) depending on lake morphometry, based on data that zebra mussels reach their maximum density in 2.5 ± 0.2 years and quagga mussels reach their maximum density in 12.2 ± 1.5 years from the time of first detection in a waterbody (Karatayev et al., 2011). Bold black line indicates the cumulative population size of both species
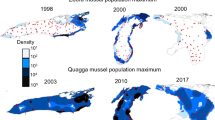
Density of Dreissena polymorpha and D. rostriformis bugensis in different waterbodies. Mean values (±standard error of the mean, sample size above the bars) were calculated using data from Uchinskoe Reservoir, Russia, 1967 (Lvova, 1977), Lake Lukomskoe, Belarus, 1978 (Karatayev, 1983), lakes Naroch, Myastro, Batorino, and Drozdy Reservoir, Belarus, 1995 (Burlakova, 1998), Lake Erie western and eastern basins, USA, 1992 (Jarvis et al., 2000), Lake St. Clair, USA, 2001 (Hunter & Simons, 2004), Lake Erie western and eastern basins, USA, 2002 (Patterson et al., 2005), Lake Ontario, USA, 1995, 2003 (Watkins et al., 2007), Lake Ekoln, Sweden, 2006 (Goedkoop et al., 2011), Lake Huron, 2003 (Nalepa et al., 2009b), Southern Lake Michigan, USA, 1999, 2004, and 2008 (Nalepa et al., 2010), and Lake Oneida, USA, 2012 (Karatayev et al., in review)
The population size of both zebra and quagga mussels can fluctuate widely through time (Ramcharan et al., 1992; Burlakova et al., 2006; Strayer & Malcom, 2006; Nalepa et al., 2010; Karatayev et al., 2011, 2014b), affecting their impacts on ecosystems. Usually, after reaching an initial maximum population size, the sustainable total biomass of D. polymorpha declines due to density-dependent processes, including substrate and food limitation, as well as competition, and the effects of predation (Fig. 1; Lvova, 1977; Karatayev et al., 1997, 2002, 2011; Hunter & Simons, 2004; Hecky et al., 2004; Patterson et al., 2005; Burlakova et al., 2006). When only zebra mussels have invaded a lake, they maintain a relatively large population size, and dominate the benthic community through time. To date, however, there are no data on long-term lake-wide populations of quagga mussels for lakes where zebra mussels have not invaded that can be compared to the patterns for zebra mussels. In contrast, in deep waterbodies with large profundal zones, when both Dreissena species have been introduced, zebra mussels are the first to invade, but quagga mussels replace zebra mussels after 9 or more years of coexistence (Fig. 1; Patterson et al., 2005; Watkins et al., 2007; Nalepa et al., 2010; Karatayev et al., 2011, 2014b). This pattern is likely possible due to their greater energetic efficiency, ability to allocate less energy to metabolic maintenance and more to growth and reproduction, than D. polymorpha, allowing them to outcompete zebra mussels if food is limiting (Mills et al., 1999; Diggins, 2001; Baldwin et al., 2002; Stoeckmann, 2003; Karatayev et al., 2010c; Nalepa et al., 2010). In addition, quagga mussels can reproduce at lower temperatures, including the profundal zone, where zebra mussels will not reproduce, giving them a demographic advantage (Roe & MacIsaac, 1997; Claxton & Mackie, 1998; Nalepa et al., 2010). These demographic and physiological traits of quagga mussels allow them to colonize the large cold profundal zone of deep lakes, which is unsuitable for zebra mussels. Thus, they can occur at high densities, and outcompete zebra mussels by depleting food resources to levels that are too low for zebra mussels, but high enough to support quagga mussels (Nalepa et al., 2010; Karatayev et al., 2011, 2014a). Zebra mussels can still have an advantage in shallow lakes and rivers, where the two species will coexist (Zhulidov et al., 2006, 2010; Grigorovich et al., 2008; Peyer et al., 2009; Karatayev et al., 2011, 2014b).
Distribution within waterbodies
Zebra mussels require hard substrate for attachment and are better adapted to the unstable environment of the littoral zone, where they may experience higher water velocities and more waves. Alternatively, quagga mussels are able to colonize silty sediments, especially those found in the profundal zones of deep large lakes (Fig. 2). Even in shallow, well-mixed lakes, zebra mussels are primarily limited to the littoral zone, and have a maximum density (average per depth ranging from 3,500 to 11,000 m2) between 1 and 6 m deep (Lvova, 1977; Karatayev, 1983; Lyakhnovich et al., 1994; Burlakova et al., 2006; Goedkoop et al., 2011) depending on the availability of hard substrates. In Europe, the largest density of zebra mussels (9,169,000 m2) was found on semi-submerged macrophytes in the South Bug Liman, Ukraine, and the largest density of quagga mussels (up to 130,000 m2) was found in Zaporozhskoe Reservoir, Ukraine (reviewed in Karatayev et al., 1998). Both zebra and quagga mussels have extremely patchy distributions in littoral zones, and may form large multilayer druses that are separated by bare sediment, with few or no mussels (Karatayev et al., 1983, 1997; Nalepa et al., 2010; Burlakova et al., 2012).
In the Great Lakes, zebra mussels may be more dense in deeper areas (e.g., Lake Ontario at 15–25 m, Mills et al., 1999; Lake Michigan at 27–46 m, Fleischer et al., 2001). Zebra mussels are present only occasionally on soft substrates in the profundal zone, where they can initially attach to plant fragments, wood, shells, stones, or artificial substrates (e.g., discarded debris) and subsequently attach to each other forming druses (Mordukhai-Boltovskoi, 1960; Hunter & Bailey, 1992, Dermott & Munawar, 1993; Lyakhnovich et al., 1994; Karatayev et al., 1998). In deep dimictic lakes, zebra mussels are even more limited to shallow areas, and are not found in the profundal zone below the thermocline. In contrast, quagga mussels are found at much higher densities in the profundal zone (Fig. 2). In the soft sediments of the profundal zone, D. r. bugensis usually has a more even distribution across the bottom, and rarely forms large druses (Nalepa et al., 2010).
When both species are present in a lake, their abundances frequently depend on the interval since invasion for each species, and the size and depth of the lake (Figs. 1, 2). In shallow lakes with a small or no profundal zone, and in rivers, both species co-exist (Zhulidov et al., 2006, 2010; Grigorovich et al., 2008; Peyer et al., 2009; Karatayev et al., 2011, 2014b) with zebra mussels generally restricted to the littoral zone, while quagga mussels can be abundant in both littoral and profundal areas (Fig. 2). For example, although zebra mussels were replaced by quagga mussels in the deep central and eastern basin of Lake Erie after 10 years of coexistence (Patterson et al., 2005), after 20 years of coexistence in the shallow western basin of Lake Erie D. polymorpha represent >30% of the mussels (Karatayev et al., 2014b). Both species are also co-dominant within their native range, the Dnieper River Delta and in the Dnieper-Bug Liman, Ukraine, which has no pronounced profundal zone (reviewed in Zhulidov et al., 2010; Karatayev et al., 2011).
In deep lakes, after quagga mussels invade and reach their population maximum, they can sustain high densities across the whole lake and these densities are much higher than in shallow lakes colonized by either zebra mussels alone or by both Dreissena species (Figs. 1, 2). Zebra mussels are then usually restricted to the mouths of inflowing rivers, bays, and upper littoral zone of open shores where water motion gives them an advantage over quagga mussels (Karatayev et al., 2013). Because many marinas in lakes Ontario and Erie are located in the river mouths, more zebra mussels are attached to resident boats than quagga mussels. Thus, although these lakes are dominated by quagga mussels, they continue to be a potential source for the spread of zebra mussels (Karatayev et al., 2013).
Impacts of invasion
Because both species are ecosystem engineers and sessile suspension feeders that attach to substrate with byssal threads and form druses, they can increase habitat complexity for other benthic invertebrates and affect the planktonic community, trophic relationships, and nutrient cycling via their feeding activities (Karatayev et al., 1997, 2002, 2007a, b; Beekey et al., 2004; Burlakova et al., 2012). In all cases, the ecological effects of dreissenids in the invaded waterbody depend on interval since the initial invasion, which species are present, lake morphometry, and the total abundance of mussels of both species (Figs. 1, 2). We predict that in shallow lakes with zebra mussels alone, their maximum abundance and impact would be expected within 3–5 years after the invasion. In shallow lakes with both species, the maximum combined impact would be expected later, in 5–10 years, following the peak in total density, but then will decline (Figs. 1, 2). In deep lakes with both species, the quagga mussels will be much more abundant and will impact the ecosystem much stronger, eventually out competing zebra mussels.
Although we have a great deal of information about the ecosystem impacts of zebra mussels, fewer data exist for the impacts of quagga mussels (Karatayev et al., 2007a; Fahnenstiel et al., 2010). In addition, because most of the waterbodies invaded by quagga mussels were first invaded by zebra mussels, it is difficult to separate the impacts of these two species and determine the role of quagga mussels alone. Moreover, most studies of the ecological impacts of quagga mussels are from research conducted on the Laurentian Great Lakes, while research on zebra mussels is mostly from small inland lakes, making comparisons challenging.
Local benthic effects
Littoral zone
In the littoral zone, druses of Dreissena change the physical habitat and provide shelter and food for other benthic invertebrates, affecting community stability, diversity, and interspecies interactions. By creating reef-like three-dimensional structure, Dreissena provides refuges from predation and from other stressors (waves, currents, desiccation) for benthic organisms that would otherwise be absent from this environment (Botts & Patterson, 1996, Karatayev et al., 1997, 2002, 2007a, b; Stewart et al., 1998a; Gutierrez et al., 2003, Beekey et al., 2004; Burlakova et al., 2012).
Dreissena polymorpha has positive effects on native epifaunal invertebrates occurring in the littoral zone (e.g., amphipods, isopods, leeches, turbellarians, hydrozoans, and some oligochaetes and chironomids), which take advantage of both the structural complexity and food resources provided by zebra mussels (Karatayev et al., 1983, 1997, 2002, 2007a, b, Botts & Patterson, 1996; Stewart et al. 1998a, b, 1999; Mayer et al., 2001; Cobb & Watzin, 2002; Gutierrez et al., 2003; Beekey et al., 2004; Burlakova et al., 2012). Burrowing organisms (e.g., oligochaetes and chironomids) may be negatively affected by zebra mussels due to oxygen depletion caused by high concentrations of organic material from feces and pseudofeces (Effler & Siegfried, 1994; Caraco et al., 2000). In general, the overall density and biomass of native invertebrates are always higher in D. polymorpha druses compared to nearby bare sediments (reviewed in Karatayev et al., 1983, 1997, 2002, 2007b; Burlakova et al., 2012), resulting in a significant increase in the biomass of the majority of native invertebrates in the littoral zone (reviewed in Karatayev et al., 1997, 2002; Higgins & Vander Zanden, 2010). To date, we do not have parallel data for the quagga mussel, but the limited information available (Bially & MacIsaac, 2000) suggests that their impacts in the littoral zone may be similar to those of the zebra mussel.
Profundal zone
In the soft sediments of the profundal zone, quagga mussels usually do not create large druses attached to hard substrates. They live individually or form small aggregations that float on the surface of soft silt (rather than sink), often spaced apart by the length of their siphons (Dermott & Kerec, 1997; Karatayev & Burlakova, personal observations). In contrast to littoral zone, there are few highly mobile epifaunal invertebrates in the deep profundal that can take advantage of the shelter provided by quagga mussels (Mörtl & Rothhaupt, 2003). Most profundal zone benthic invertebrates are less mobile (Merritt et al., 1984), including burrowing chironomids and oligochaetes, which are then negatively affected through spatial competition with quagga mussels (Burlakova et al., 2014).
While in the well-mixed littoral zone there is an abundant food supply for dreissenids that is transferred from the water column to the bottom in the form of feces and pseudofeces, in the deep profundal zone, especially during summer stratification, quagga mussels may be food limited. They may feed on the benthic nepheloid layer, which is rich in organic matter that originates from small epilimnetic phytoplankton and fine sediment (Watkins et al., 2007) and develops each summer in Great Lakes just above profundal sediments during stratified conditions (Mudroch & Mudroch, 1992; Hawley & Muzzi, 2003). Quagga mussels may also benefit from the deep chlorophyll layer, which develops in the Great Lakes just below the thermocline (Barbiero & Tuchman, 2001; Pothoven & Fahnenstiel, 2013). Since dreissenids invaded the Great Lakes, the deep chlorophyll maximum and spring phytoplankton bloom have disappeared, which has been linked to the loss of Diporeia spp., although other factors may have also been important in the decline of this group (Nalepa et al., 2009a; Barbiero et al., 2011; Ryan et al., 2012; Watkins et al., 2012). The establishment of quagga mussels in the profundal zone of Great Lakes has not only been linked to the dramatic decline of native large bodied amphipods like Diporeia spp. (Dermott & Kerec, 1997; Nalepa et al., 1998, 2007, 2009a, b; Lozano et al., 2001; Watkins et al., 2007; Nalepa, 2010), but also to molluscs (Sphaeriidae, Lozano et al., 2001; Watkins et al., 2007; Nalepa et al., 2007, 2009a, b), oligochaetes (Lozano et al., 2001; Watkins et al., 2007; Soster et al., 2011; Burlakova et al., 2014), and chironomids (Nalepa et al., 2009a, b; Burlakova et al., 2014).
We hypothesize that by having different spatial distributions in a waterbody, and different druse-forming habits, these two species of Dreissena can have very different effects on benthic invertebrate communities. While in the littoral zone, zebra and quagga mussels provide additional space and food for many invertebrates, and thus have overall positive impacts on the benthic community by increasing diversity, density, and biomass of invertebrates; in the profundal zone, quagga mussels compete for space and food resources with most of native invertebrates decreasing their overall diversity, density, and biomass.
Impact on unionids
Zebra mussels have had pronounced negative impacts on unionid bivalves in both Europe and North America (Sebestyen, 1937; Mackie, 1991; Hunter & Bailey, 1992; Haag et al., 1993; Gillis & Mackie, 1994; Tucker, 1994; Ricciardi et al., 1996; Schloesser et al., 1996; Karatayev et al., 1997; Burlakova et al., 2000; Lucy et al., 2014). Although there are no comparable data on the impacts of quagga mussels on unionids (Lucy et al., 2014), the limited information that does exist suggests that D. r. bugensis may have smaller impacts on unionids than D. polymorpha (Conn & Conn, 1993; Ricciardi et al., 1996; Schloesser & Masteller, 1999; Sherman et al., 2013). In the Kiev Reservoir, Ukraine, quagga mussels are 4 times more abundant than zebra mussels, yet zebra mussels compose 87% of the mussels found on unionid shells (reviewed in Zhulidov et al., 2010). A recent study by Burlakova et al. (in review) showed that D. polymorpha is much more common on unionid shells than D. r. bugensis in the lower Great Lakes even though quagga mussels represent over 95% of dreissenids in these lakes. They also found that the proportion of unionids colonized by dreissenids, and the number and weight of mussels attached to each unionid, were lower in lakes dominated by D. r. bugensis. These data suggest that the ongoing replacement of zebra mussels by quagga mussels in the Great Lakes may reduce the negative impacts on unionids and facilitate their recovery.
System-wide effects
The system-wide effects of dreissenids are associated with their role as suspension feeders. Because both zebra and quagga mussels usually attain high population densities, they filter large volumes of water in a short period of time. Dreissenids transfer materials from the water column to the benthos, providing a direct link between planktonic and benthic components of the ecosystem (benthic-pelagic coupling). This can trigger a suite of connected changes that increase the relative importance of the benthic community—a process sometimes referred to as benthification (Mayer et al., 2014).
The system-wide effect of zebra mussels depends on water mixing rates, lake morphology, and turnover rates (Reed-Andersen et al., 2000), and may be very local in deep water lakes (Ackerman et al., 2001). Because quagga mussels are found throughout the entire waterbody, rather than being restricted shallow areas, or areas with hard substrates as are the zebra mussels, they have larger total population sizes (Fig. 2). The larger populations of quagga mussels will filter larger volumes of water, and may have greater system-wide effects than zebra mussels, especially in deep lakes and reservoirs with large profundal zones. However in the profundal zone, isolated from the epilimnion by the thermocline, the impact of quagga mussels on the water column may be lower than that of mussels in the well-mixed littoral zone. In addition, because the clearance rate is temperature dependent (Fanslow et al., 1995), we hypothesize that the filtering activity in the cold profundal zone should be much less than that in the littoral zone.
Increases in water clarity and light penetration, and decreases in turbidity, seston, and organic matter in the water column are among the most common and well-documented impacts of zebra mussels on invaded waterbodies (reviewed in Karatayev et al., 1997, 2002, 2007a, b; Vanderploeg et al., 2002; Higgins & Vander Zanden, 2010; Kelley et al., 2010; Mayer et al., 2014). Although fewer data are available, the effect of quagga mussels appears to be similar (Barbiero & Tuchman, 2004; Nalepa, 2010; Pothoven & Fahnenstiel, 2013; Bunnell et al., 2014). In addition, because D. r. bugensis can colonize the profundal zone, their impacts may be even greater in deep lakes. Zebra and quagga mussels also alter nutrient cycling, increasing concentrations of ammonia, nitrates, and phosphates in the water (reviewed in Vanderploeg et al., 2002; Karatayev et al., 2002, 2007a, b; Higgins & Vander Zanden, 2010; Kelley et al., 2010; Nalepa, 2010). Feeding by dreissenids results in the deposition of large amounts of seston on the bottom, which could increase siltation of otherwise available substrates for zebra mussels (reviewed in Karatayev et al., 1997, 2007b; Zhukova, 2001), thereby decreasing their population density through time (Lvova, 1977). Quagga mussels may colonize soft substrates, and may therefore not experience this negative feedback.
In most waterbodies, dreissenids decrease phytoplankton density (chlorophyll concentration) and primary production (reviewed in Karatayev et al., 1997, 2007a, b; Barbiero & Tuchman, 2004; Higgins & Vander Zanden, 2010; Kelley et al., 2010; Fahnenstiel et al., 2010; Pothoven & Fahnenstiel, 2013). Dreissenid impacts on phytoplankton may be direct, top-down control through feeding on planktonic algae and indirect, bottom-up control by sequestering phosphorous that otherwise will be available to phytoplankton (Bunnell et al., 2014). Declines in phytoplankton abundance often correlate with changes in phytoplankton community composition (reviewed in Kelley et al., 2010). Several studies attribute one of the most important environmental issues in North America and Europe, toxic blooms of the cyanobacteria Microcystis (Pires et al., 2010; Michalak et al., 2013), to selective grazing and rejection of toxic strains of blue-green algae or the release of soluble nutrients to the invasion of zebra mussels and then later by quagga mussels (Makarewicz et al., 1999; Vanderploeg et al., 2001, 2009; Conroy et al., 2005a, b; Bykova et al., 2006). In contrast, other studies (both in North America and Europe) have found that zebra mussels may actively consume and reduce the density of Microcystis (Strayer et al., 1999; Baker & Levinton, 2003; Pires et al., 2005, 2010).
We suggest that the impact of profundal quagga mussels on phytoplankton, especially diatoms, could be largely limited to the spring, when the whole water column is well-mixed. The recent colonization of the profundal zone of Lake Michigan by quagga mussels resulted in a fourfold decline of spring chlorophyll concentrations and a diatom bloom that was not evident when only zebra mussels were present in the lake (reviewed in Nalepa, 2010).
Increased light penetration and phosphorus availability due to suspension feeding by zebra mussels in the littoral zone allow macrophytes, periphyton, and benthic algae to grow deeper and cover larger portions of the bottom of invaded waterbodies (reviewed in Karatayev et al., 1997, 2007a, b; Nalepa et al., 1999; Vanderploeg et al., 2002; Hunter & Simons, 2004; Higgins & Vander Zanden, 2010; Mayer et al., 2014). While in many inland lakes vascular plants and Chara benefit from these changes in the littoral environment (Lyakhnovich et al., 1988; Hunter & Simons, 2004; Reeders et al., 1990; Ibelings et al., 2007; Mayer et al., 2014), in most of the Great Lakes increased water clarity and phosphorus concentrations led to the re-establishment of the nuisance filamentous algae Cladophora and Lyngbya (Higgins et al., 2008; Bridgeman & Penamon, 2010). Increases in macrophyte abundance associated with the introduction of zebra and quagga mussels may cause, or contribute, to the shift from turbid to clear water phase in eutrophic lakes (Ibelings et al., 2007; Mayer et al., 2014) and have different consequences for zebra and quagga mussels. Zebra mussels, which produce stronger attachment to hard substrates, may take greater advantage of the additional substrates provided by macrophytes than quagga mussels. Even in areas with >90% quagga mussels on the bottom, zebra mussels dominate on macrophytes (Diggins et al., 2004).
The impact of dreissenids on zooplankton is less evident, but most data suggest that zooplankton density and biomass decline after the introduction of zebra mussels, along with the changes in plankton community structure (reviewed in Karatayev et al., 1997, 2007a, b; Vanderploeg et al., 2002; Higgins & Vander Zanden, 2010; Kelley et al., 2010). This decline could be due to the direct consumption of small zooplankton by zebra mussels, reduction of their food resources (phytoplankton and detritus), or due to increased consumption by planktivorous fish, facilitated by the increase in visibility, or by increases in benthivorous fishes, whose larvae feed on zooplankton (reviewed in Karatayev et al., 1997, 2002, 2007a, b; Kelley et al., 2010; Mayer et al., 2014). At present, limited data on the impact of quagga mussels on zooplankton suggest that their effect is similar to that of zebra mussels (reviewed in Nalepa, 2010; Pothoven & Fahnenstiel, 2013).
In both European and North America waters, dreissenids provide an abundant food resource for fishes. At least 27 fish species in Europe and 14 species in North America feed on Dreissena spp. including three species that occur on both continents: common carp (Cyprinus carpio, native to Eurasia), pumpkinseed (Lepomis gibbosus, native to North America), and round goby (Neogobius melanostomus, native to Eurasia), as well as lake sturgeon in North America (Acipenser fulvescens) (Molloy et al., 1997 and references therein). In their native range, the Northern Caspian Sea, fish consume up to 90% of the annual production of Dreissena spp. (130,000 tonnes) (reviewed in Molloy et al., 1997). In North America, both species of Dreissena are actively consumed by the invasive round goby (Lederer et al., 2008; Kornis et al., 2012) to such an extent that several studies have suggested that gobies may control Dreissena spp. populations in the Great Lakes (Barton et al., 2005; Patterson et al., 2005; Wilson et al., 2006; Lederer et al., 2008).
The overall direct and indirect impacts of zebra mussels on fish vary depending on the feeding mode of the fishes and the morphology of the invaded waterbody (Karatayev et al., 1997, 2002; Strayer et al., 2004), and are likely to be similar for quagga mussels. Impact may be time-dependent as native predators may need time to adapt to effectively consume an exotic resource (Carlsson et al., 2011). In most cases, there has been an increase in benthivorous fishes in the littoral zone, even those that do not feed on dreissenids, because invasion by dreissenids usually results in the increase in biomass of native benthic invertebrates in this zone (reviewed in Karatayev et al., 1997, 2002, 2007a, b; Higgins & Vander Zanden, 2010; Kelley et al., 2010). Data suggest that planktivorous fishes can be negatively affected by dreissenids because of the decrease in phytoplankton and zooplankton abundance due to suspension feeding by the mussels, or because an increase in water transparency results in increased predation on larval fish (Francis et al., 1996). At the same time, increased water clarity and light penetration may positively affect visual fish predators (Mayer et al., 2001, 2014; Mills et al., 2003). Strayer et al. (2004) found a decline in abundance and growth rate of pelagic fishes in the Hudson River after invasion by zebra mussels, and an increase in the abundance and growth rate of littoral species.
In the profundal zone of the Great Lakes, the introduction of zebra, and especially quagga mussels is linked to the decline in the abundance of Coregonus clupeaformis (whitefish) through the dramatic decrease in their main food, the amphipod Diporeia spp. (Dermott & Kerec, 1997; Hoyle et al., 1999; Lozano et al., 2001; Pothoven et al., 2001; Nalepa et al., 2009a; Nalepa, 2010). The decline in Diporeia spp. resulted in a shift in diet of medium and large whitefish from Diporeia spp. to D. r. bugensis (Nalepa et al., 2009b; Madenjian et al., 2010). However, because of the lower energy content of quagga mussels, this shift resulted in the decline of lake whitefish condition, growth, and abundance (Pothoven et al., 2001; Hoyle et al., 2008; Nalepa et al., 2009b; Rennie et al., 2009). The decline in Diporeia spp. is also associated with the decline of alewife, sculpin, bloater, and other fish that are prey for larger piscivores, including salmon and trout (reviewed in Nalepa, 2010). While most North American studies report that a shift to dreissenid-based diets has resulted in decreased growth rates and condition in fish, several European studies have shown that this shift resulted in increased growth, average and maximum size, and condition for some species of fish (Lyagina & Spanowskaya, 1963; Poddubnyi, 1966).
Conclusions and future needs
Although we have a great deal of information about the ecosystem impacts of the zebra mussel, fewer data exist for impacts of the quagga mussel. In addition, because most of the waterbodies invaded by quagga mussels were first invaded by zebra mussels, it is difficult to separate the impacts of these two species and determine the role of quagga mussels alone. Moreover, most of the information on the ecological impact of quagga mussels was obtained from the research conducted in the Laurentian Great Lakes, while research on the effects of zebra mussels has been primarily in small inland lakes. The overall ecological impacts of both zebra and quagga mussels will depend on the number of waterbodies colonized in a region and their total population density and spatial distribution in a given waterbody. Today many more waterbodies both in Europe and in North America have been invaded by zebra mussels, and therefore the extent of their ecological impact is much greater than that of quagga mussels. Because the time lag between when a species was first detected in a waterbody and reaches its maximum population size is much shorter for zebra mussels than for quagga mussels, their greatest ecological impacts will be also delayed compared to that of zebra mussels. By having different spatial distributions in the waterbody, these two species of Dreissena have very different effects on benthic invertebrates. In the well-mixed littoral zone, zebra and quagga mussels provide additional space and food for many invertebrates, and can have a net positive impact on the rest of the benthic community by increasing diversity, density, and biomass of other taxa. In contrast, in the profundal zone, quagga mussels compete for space and food recourses with most of native invertebrates, decreasing their overall diversity, density, and biomass.
The system-wide effects of dreissenids on the water column largely depend on water mixing rates, lake morphology, and turnover rates. Because quagga mussels are found in all regions of a lake, they may filter larger volumes of water and may have greater system-wide effects than zebra mussels, especially in deep lakes with large profundal zones. However, during much of the growing season, quagga mussels in the deep profundal zone are isolated from the epilimnion by the thermocline, and their impact on the water column may be lower than that of quagga or zebra mussels in the well-mixed littoral zone.
Dreissena populations are extremely variable in space and time. Densities can vary by several orders of magnitude from their initial invasion to when population size is at a maximum, and subsequently decline as density-dependent factors become important. Therefore, the ecological impacts of dreissenids are also density-dependent. Shortly after initial invasion, as populations increase, both zebra and quagga mussels will have their largest and most obvious effects on communities, and most of the impacts will be direct effects. After the initial stage of invasion, impacts are less predictable, and more likely to be caused by indirect effects through changes in the ecosystem.
References
Ackerman, J. D., M. R. Loewen & P. F. Hamblin, 2001. Benthic-pelagic coupling over a zebra mussel reef in western Lake Erie. Limnology and Oceanography 46: 892–904.
Baker, S. M. & J. S. Levinton, 2003. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia 505: 97–105.
Baldwin, B. S., M. S. Mayer, J. Dayton, N. Pau, J. Mendilla, M. Sullivan, A. Moore, A. Ma & E. L. Mills, 2002. Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 680–694.
Barbiero, R. P. & M. L. Tuchman, 2001. Results from the U.S. EPA’s Biological Open Water Surveillance program of the Laurentian Great Lakes: II. Deep chlorophyll maxima. Journal of Great Lakes Research 27: 155–166.
Barbiero, R. P. & M. L. Tuchman, 2004. Long-term dreissenid impacts on water clarity in Lake Erie. Journal of Great Lakes Research 30: 557–565.
Barbiero, R. P., K. Schmude, B. M. Lesht, C. M. Riseng, G. J. Warren & M. L. Tuchman, 2011. Trends in Diporeia populations across the Laurentian Great Lakes, 1997–2009. Journal of Great Lakes Research 37: 9–17.
Barton, D. R., R. A. Johnson, L. Campbell, J. Petruniak & M. Patterson, 2005. Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. Journal of Great Lakes Research 31: 252–261.
Beekey, M. A., D. J. McCabe & J. E. Marsden, 2004. Zebra mussels affect benthic predator foraging success and habitat choice on soft sediments. Oecologia 141: 164–170.
Benson, A. J., 2014. Chronological history of zebra and quagga mussels (Dreissenidae) in North America, 1988–2010. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd edn. CRC Press, Boca Raton: 9–31.
Benson, A. J., D. Raikow, J. Larson & A. Fusaro, 2013a. Dreissena polymorpha. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. http://nas.er.usgs.gov/queries/collectioninfo.aspx?speciesID=5 Revision Date: 6/6/2012. Accessed on 24 August 2013.
Benson, A. J., M. M. Richerson, E. Maynard, J. Larson, & A. Fusaro, 2013b. Dreissena rostriformis bugensis. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=95 Revision Date: 6/28/2012. Accessed on 24 August 2013.
Bially, A. & H. J. MacIsaac, 2000. Fouling mussels (Dreissena spp.) colonize soft sediments in Lake Erie and facilitate benthic invertebrates. Freshwater Biology 43: 85–97.
Bridgeman, T. B. & W. A. Penamon, 2010. Lyngbya wollei in western Lake Erie. Journal of Great Lakes Research 36: 167–171.
Botts, P. S. & B. A. Patterson, 1996. Zebra mussel effects on benthic invertebrates: physical or biotic? Journal of the North American Benthological Society 15: 179–184.
Bunnell, D. B., R. P. Barbiero, S. A. Ludsin, C. P. Madenjian, G. J. Warren, D. M. Dolan, T. O. Brenden, R. Briland, O. T. Gorman, J. X. He, T. H. Johengen, B. F. Lantry, B. M. Lesht, T. F. Nalepa, S. C. Riley, C. M. Riseng, T. J. Treska, I. Tsehaye, M. G. Walsh, D. M. Warner & B. C. Weidel, 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: bottom-up and top-down regulation. BioScience 64: 26–39.
Burlakova, L. E., 1998. Ecology of Dreissena polymorpha (Pallas) and Its Role in the Structure and Function of Aquatic Ecosystems. Candidate Dissertation, Zoology Institute of the Academy of Science Republic Belarus, Minsk (Belarus). 167 pp. (in Russian).
Burlakova, L. E., A. Y. Karatayev & D. K. Padilla, 2000. The impact of Dreissena polymorpha (Pallas) invasion on unionid bivalves. International Review of Hydrobiology 85: 529–541.
Burlakova, L. E., A. Y. Karatayev & D. K. Padilla, 2006. Changes in the distribution and abundance of Dreissena polymorpha within lakes through time. Hydrobiologia 517: 133–146.
Burlakova, L. E., A. Y. Karatayev & V. A. Karatayev, 2012. Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 685: 121–134.
Burlakova, L. E., A. Y. Karatayev, C. Pennuto & C. Mayer, 2014. Changes in Lake Erie benthos over the last 50 years: historical perspectives, current status, and main drivers. Journal of Great Lakes Research. doi:10.1016/j.jglr.2014.02.008.
Burlakova, L. E., B. L. Tulumello, A. Y. Karatayev, R. A. Krebs, D. W. Schloesser, W. L. Paterson, T. A. Griffith, M. W. Scott, T. Crail & D. T. Zanatta, in review. Twenty five years of Dreissena spp. impacts on native Unionidae in the lower Great Lakes: dreissenid species matters. Biological Conservation.
Bykova, O., A. Laursen, V. Bostan, J. Bautista & L. McCarthy, 2006. Do zebra mussels (Dreissena polymorpha) alter lake water chemistry in a way that favors Microcystis growth? Science of the Total Environment 371: 362–372.
Caraco, N. F., J. J. Cole, S. E. G. Findlay, D. T. Fischer, G. G. Lampman, M. L. Pace & D. L. Strayer, 2000. Dissolved oxygen declines in the Hudson River associated with the invasion of the zebra mussel (Dreissena polymorpha). Environmental Science and Technology 34: 1204–1210.
Carlsson, N. O. L., H. Bustamante, D. L. Strayer & M. L. Pace, 2011. Biotic resistance on the increase: native predators structure invasive zebra mussel populations. Freshwater Biology 56: 1630–1637.
Carlton, J. T., 2008. The zebra mussel Dreissena polymorpha found in North America in 1986 and 1987. Journal of Great Lakes Research 34: 770–773.
Claxton, W. T. & G. L. Mackie, 1998. Seasonal and depth variations in gametogenesis and spawning of Dreissena polymorpha and Dreissena bugensis in eastern Lake Erie. Canadian Journal of Zoology 76: 2010–2019.
Cobb, S. E. & M. C. Watzin, 2002. Zebra mussel colonies and yellow perch foraging: spatial complexity, refuges, and resource enhancement. Journal of Great Lakes Research 28: 256–263.
Conn, D. B. & D. A. Conn, 1993. Parasitism, predation and other biotic associations between dreissenid mussels and native animals in the St. Lawrence River. In Tsou, J. L. (ed.), Third International Zebra Mussel Conference. Electric Power Research Institute, Paolo Alto, CA, Toronto, ON: 223–234.
Conroy, J. D., D. D. Kane, D. M. Dolan, W. J. Edwards, M. N. Charlton & D. A. Culver, 2005a. Temporal trends in Lake Erie plankton biomass: roles of external phosphorus loading and dreissenid mussels. Journal of Great Lakes Research 31(Suppl. 2): 89–110.
Conroy, J. D., W. J. Edwards, R. A. Pontius, D. D. Kane, H. Zhang, J. F. Shea, J. N. Richey & D. A. Culver, 2005b. Soluble nitrogen and phosphorus excretion of exotic freshwater mussels (Dreissena spp.): potential impacts for nutrient remineralization in western Lake Erie. Freshwater Biology 50: 1146–1162.
Dermott, R. & M. Munawar, 1993. Invasion of Lake Erie offshore sediments by Dreissena, and its ecological implications. Canadian Journal of Fisheries and Aquatic Sciences 50: 2298–2304.
Dermott, R. & D. Kerec, 1997. Changes to the deep water benthos of eastern Lake Erie since the invasion of Dreissena: 1979–1993. Canadian Journal of Fisheries and Aquatic Sciences 54: 922–930.
Diggins, T. P., 2001. A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. Journal of Great Lakes Research 27: 457–466.
Diggins, T. P., M. Weimer, K. M. Stewart, R. E. Baier, A. E. Meyer, R. F. Forsberg & M. A. Goehle, 2004. Epiphytic refugium: are two species of invading freshwater bivalves portioning spatial resources? Biological Invasions 6: 83–88.
Effler, S. W. & C. Siegfried, 1994. Zebra mussel (Dreissena polymorpha) populations in the Seneca River, New York: impact on oxygen resources. Environmental Science and Technology 28: 2216–2221.
Fahnenstiel, G. L., S. A. Pothoven, T. F. Nalepa, H. A. Vanderploeg, D. M. Klarer & D. Scavia, 2010. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. Journal of Great Lakes Research 36: 20–29.
Fanslow, D. L., T. F. Nalepa & G. A. Lang, 1995. Filtration rates of the zebra mussel (Dreissena polymorpha) on natural seston from Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 489–500.
Fleischer, G. W., T. J. Desorcie & J. D. Holuszko, 2001. Lake-wide distribution of Dreissena in Lake Michigan, 1999. Journal of Great Lakes Research 27: 252–257.
Flynn, D., N. Mirotchnick, M. Jain, M. I. Palmer & S. Naeem, 2011. Functional and phylogenetic diversity as predictors of biodiversity-ecosystem function relationships. Ecology 92: 1573–1581.
Francis, J. T., S. R. Robillard & J. E. Marsden, 1996. Yellow perch management in Lake Michigan: a multi-jurisdictional challenge. Fisheries (Bethesda) 21: 18–20.
French III, J. R. P., J. S. Schaeffer, E. F. Roseman, C. S. Kiley & A. Fouilleroux, 2009. Abundance and distribution of benthic invertebrates in offshore soft sediments in Western Lake Huron, 2001–2007. Journal of Great Lakes Research 35: 120–127.
Goedkoop, W., R. Naddafi & U. Grandin, 2011. Retention of N and P by zebra mussels (Dreissena polymorpha Pallas) and its quantitative role in the nutrient budget of eutrophic Lake Ekoln, Sweden. Biological Invasions 13: 1077–1086.
Gillis, P. L. & G. L. Mackie, 1994. Impact of the zebra mussel, Dreissena polymorpha, on populations of Unionidae (Bivalvia) in Lake St. Clair. Canadian Journal of Zoology 72: 1260–1271.
Grigorovich, I. A., T. R. Angradi & C. A. Stepien, 2008. Occurrence of the quagga mussel (Dreissena bugensis) and the zebra mussel (Dreissena polymorpha) in the upper Mississippi River system. Journal of Freshwater Ecology 23: 429–435.
Gutierrez, J. L., C. G. Jones, D. L. Strayer & O. O. Iribarne, 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101: 79–90.
Haag, W. R., D. R. Berg, D. W. Garton & J. L. Farris, 1993. Reduced survival and fitness in native bivalves in response to fouling by the introduced zebra mussel (Dreissena polymorpha) in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 50: 13–19.
Hawley, N. & R. W. Muzzi, 2003. Observations of nepheloid layers made with an autonomous vertical profiler. Journal of Great Lakes Research 29: 124–133.
Hecky, R. E., R. E. H. Smith, D. R. Barton, S. J. Guildford, W. D. Taylor, M. N. Charlton & E. T. Howell, 2004. The near shore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1285–1293.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Higgins, S. N., S. Y. Malkin, E. T. Howell, S. J. Guildford, L. Campbell, V. Hiriart-Baer & R. E. Hecky, 2008. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. Journal of Phycology 44: 839–854.
Hoyle, J. A., T. Schaner, J. M. Casselman & R. Dermott, 1999. Changes in lake whitefish (Coregonus clupeaformis) stocks in eastern Lake Ontario following Dreissena mussel invasion. Great Lakes Research Review 4: 5–10.
Hoyle, J. A., J. N. Bowlby & B. J. Morrison, 2008. Lake whitefish and walleye population responses to dreissenid mussel invasion in eastern Lake Ontario. Aquatic Ecosystem Health and Management 11: 403–411.
Hunter, R. D. & J. F. Bailey, 1992. Dreissena polymorpha (zebra mussel): colonization of soft substrate and some effects on unionid bivalves. The Nautilus 106: 60–67.
Hunter, R. G. & K. A. Simons, 2004. Dreissenids in Lake St. Clair in 2001: evidence for population regulation. Journal of Great Lakes Research 30: 528–537.
Ibelings, B. W., R. Portielje, E. H. R. R. Lammens, R. Noordhuis, M. S. van den Berg, W. Joosse & M. L. Meijer, 2007. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: lake Veluwe as a case study. Ecosystems 10: 4–16.
Jarvis, P., J. Dow, R. Dermott & R. Bonnell, 2000. Zebra (Dreissena polymorpha) and quagga mussel (Dreissena bugensis) distribution and density in Lake Erie, 1992–1998. Canadian Technical Report of Fisheries and Aquatic Sciences: 2304.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Jones, C. G., J. H. Lawton & M. Shachak, 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78: 1946–1957.
Karatayev, A. Y., 1983. Ecology of Dreissena polymorpha Pallas and its effects on macrozoobenthos of the thermal power plant’s cooling reservoir. Candidate Dissertation, Zoology Institute of Academy of Science Belorussian SSR, Minsk, Belarus. 153 pp. (in Russian).
Karatayev, A. Y., G. M. Tishchikov & I. V. Karatayeva, 1983. Dreissena polymorpha Pallas as a specific community of benthic animals. Informatsionnyi Byulleten Biologiya Vnutrennikh Vod 61: 18–21. (in Russian).
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1997. The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in eastern Europe. Journal of Shellfish Research 16: 187–203.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1998. Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). Journal of Shellfish Research 17: 1219–1235.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2002. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In Leppakoski, E., S. Gollach & S. Olenin (eds), Invasive Aquatic Species of Europe: Distribution, Impacts and Management. Kluwer Academic Publishers, Dordreicht.
Karatayev, A. Y., D. K. Padilla, D. Minchin, D. Boltovskoy & L. E. Burlakova, 2007a. Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biological Invasions 9: 161–180.
Karatayev, A. Y., D. Boltovskoy, D. K. Padilla & L. E. Burlakova, 2007b. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. Journal of Shellfish Research 26: 205–213.
Karatayev, A. Y., L. E. Burlakova, V. A. Karatayev & D. Boltovskoy, 2010a. Limnoperna fortunei vs. Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves. Journal of Shellfish Research 29: 975–985.
Karatayev, A.Y., L. E. Burlakova & D. K. Padilla, 2010b. Dreissena polymorpha in Belarus: history of spread, population biology, and ecosystem impacts. In van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden: 101–112.
Karatayev, A. Y., S. E. Mastitsky, D. K. Padilla, L. E. Burlakova & M. M. Hajduk, 2010c. Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia 668: 183–194.
Karatayev, A. Y., L. E. Burlakova, S. E. Mastitsky, D. K. Padilla & E. L. Mills, 2011. Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. Journal of Shellfish Research 30: 923–931.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2014a. General overview of zebra and quagga mussels what we do and do not know. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton: 695–703.
Karatayev, A. Y., L. E. Burlakova, C. Pennuto, J. Ciborowski, V. A. Karatayev, P. Juette & M. Clapsadl, 2014b. Twenty five years of changes in Dreissena spp. populations in Lake Erie. Journal of Great Lakes Research. doi:10.1016/j.jglr.2014.04.010.
Karatayev, V. A., A. Y. Karatayev, L. E. Burlakova & D. K. Padilla, 2013. Lakewide dominance does not predict the potential for spread of dreissenids. Journal of Great Lakes Research 39: 622–629.
Karatayev, V. A., A. Y. Karatayev, L. E. Burlakova & L. D. Rudstam, in review. Eutrophication and Dreissena invasion as drivers of biodiversity: a century of change in the mollusc community of Lake Oneida. PLoS ONE.
Keller, R. P., J. M. Drake & D. M. Lodge, 2007. Fecundity as a basis for risk assessment of nonindigenous freshwater molluscs. Conservation Biology 21: 191–200.
Kelley, D. W., L.-M. Herborg & H. J. MacIsaak, 2010. Ecosystem changes associated with Dreissena invasions: recent developments and emerging issues. In van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden: 199–209.
Kerney, M. P. & B. S. Morton, 1970. The distribution of Dreissena polymorpha (Pallas) in Britain. Journal of Conchology 27: 97–100.
Kinzelbach, R., 1992. The main features of the phylogeny and dispersal of the zebra mussel Dreissena polymorpha. In Neumann, D. & H. A. Jenner (eds), The Zebra Mussel Dreissena polymorpha: Ecology, Biological Monitoring and First Applications in the Water Quality Management. Gustav Fisher, Stuttgart: 5–17.
Kornis, M. S., N. Mercado-Silva & M. J. Vander Zanden, 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80: 235–285.
Lederer, A. M., J. Janssen, T. Reed & A. Wolf, 2008. Impacts of the introduced round goby (Apollonia melanostoma) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and on macroinvertebrate community between 2003 and 2006 in the littoral zone of Green Bay, Lake Michigan. Journal of Great Lakes Research 34: 690–697.
Limanova, N. A., 1964. Dreissena: bibliography. Biology of Dreissena and its control. Proceedings of Institute of Biology of Inland Waters of Academy of Science of the USSA 7: 83–136. (in Russian).
Limanova, N. A., 1978. Dreissena: Bibliographic Guide. Moscow: 115 pp (in Russian).
Lozano, S. J., J. V. Scharold & T. F. Nalepa, 2001. Recent declines in benthic macroinvertebrate densities in Lake Ontario. Canadian Journal of Fisheries and Aquatic Sciences 58: 518–529.
Lucy, F. E., L. E. Burlakova, A. Y. Karatayev, S. E. Mastitsky & D. T. Zanatta, 2014. Zebra mussel impacts on unionids: A synthesis of trends in North America and Europe. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton: 623–646.
Lvova, A. A., 1977. The ecology of Dreissena polymorpha (Pall.) in Uchinskoe Reservoir. Candidate Dissertation, Moscow State University, Moscow, USSR (in Russian).
Lyagina, T. N. & V. D. Spanowskaya, 1963. Morphological peculiarities of some fish in the Uchinskoe water storage. In: Uchinskoe and Mozhaiskoe Resrvoirs: Hydrobiological and Ichthyological Studies. Moscow State University, Moscow: 269–310 (in Russian).
Lyakhnovich, V. P., A. Y. Karatayev, P. A. Mitrakhovich, L. V. Guryanova & G. G. Vezhnovets, 1988. Productivity and prospects for utilizing the ecosystem of the Lake Lukoml thermoelectric station cooling reservoir. Soviet Journal of Ecology 18: 255–259.
Lyakhnovich, V. P., A. Y. Karatayev, N. I. Andreev, S. I. Andreeva, S. A. Afanasiev, A. K. Dyga, V. P. Zakutskiy, V. I. Zolotareva, A. A. Lvova, M. Y. Nekrasova, V. F. Osadchikh, Y. V. Pligin, A. A. Protasov & G. M. Tischikov, 1994. Living conditions. In Starobogatov, J. I. (ed), Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, Ecology, Practical Meaning. Nauka Press, Moscow: 109–119 (in Russian).
Mackie, G. L., 1991. Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219: 251–268.
Madenjian, C. P., S. A. Pothoven, P. J. Schneeberger, M. P. Ebener, L. C. Mohr, T. F. Nalepa & J. R. Bence, 2010. Dreissenid mussels are not a "dead end" in Great Lakes food webs. Journal of Great Lakes Research 36: 73–77.
Makarewicz, J. C., T. W. Lewis & P. Bertram, 1999. Phytoplankton composition and biomass in the offshore waters of Lake Erie: pre- and post-Dreissena introduction (1983–1993). Journal of Great Lakes Research 25: 135–148.
Mayer, C. M., L. G. Rudstam, E. L. Mills, S. G. Cardiff & C. A. Bloom, 2001. Zebra mussels (Dreissena polymorpha), habitat alteration, and yellow perch (Perca flavescens) foraging: system-wide effects and behavioural mechanisms. Canadian Journal of Fisheries and Aquatic Sciences 58: 2459–2467.
Mayer, C. M., L. E. Burlakova, P. Eklöv, D. Fitzgerald, A. Y. Karatayev, S. A. Ludsin, S. Millard, E. L. Mills, A. P. Ostapenya, L. G. Rudstam, B. Zhu & T. V. Zhukova, 2014. The benthification of freshwater lakes: exotic mussels turning ecosystems upside down. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton: 575–586.
Merritt, R. W., K. W. Cummins & T. M. Burton, 1984. The role of aquatic insects in the processing and cycling of nutrients. In Resh, V. H. & D. M. Rosenberg (eds), The Ecology of Aquatic Insects. Praeger Scientific, New York: 134–163.
Michalak, A. M., E. J. Anderson, D. Beletsky, S. Boland, N. S. Bosch, T. B. Bridgeman, J. D. Chaf, K. Cho, R. Confesor, I. Daloglu, J. V. DePinto, M. A. Evans, G. L. Fahnenstiel, L. He, J. C. Ho, L. Jenkins, T. H. Johengen, K. C. Kuo, E. LaPorte, X. Liu, M. R. McWilliams, M. R. Moore, D. J. Posselt, R. P. Richards, D. Scavia, A. L. Steiner, E. Verhamme, D. M. Wright & M. A. Zagorski, 2013. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proceeding of National Academy of Science (Online First).
Mills, E. L., R. M. Dermott, E. F. Roseman, D. Dustin, E. Mellina, D. B. Corm & A. P. Spidle, 1993. Colonization, ecology, and population structure of the “quagga” mussel (Bivalvia: Dreissenidae) in the lower Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 50: 2305–2314.
Mills, E. L., J. R. Chrisman, B. Baldwin, R. W. Owens, R. O’Gorman, T. Howell, E. Roseman & M. K. Raths, 1999. Changes in the dressenid community in the lower Great Lakes with emphasis on southern Lake Ontario. Journal of Great Lakes Research 25: 187–197.
Mills, E. L., J. M. Casselman, R. Dermott, J. D. Fitzsimons, G. Gal, K. T. Holeck, J. A. Hoyle, O. E. Johannsson, B. F. Lantry, J. C. Makarewicz, E. S. Millard, I. F. Munawar, M. Munawar, R. O’Gorman, R. W. Owens, L. G. Rudstam, T. Schaner & T. J. Stewart, 2003. Lake Ontario: food web dynamics in a changing ecosystem (1970–2000). Canadian Journal of Fisheries and Aquatic Sciences 60: 471–490.
Molloy, D. P., A. Y. Karatayev, L. E. Burlakova, D. P. Kurandina & F. Laruelle, 1997. Natural enemies of zebra mussels: predators, parasites and ecological competitors. Reviews in Fisheries Science 5: 27–97.
Mordukhai-Boltovskoi, F. D., 1960. Caspian Fauna in the Azov and Black Sea Basins. Moscow, Academia Nauk Press: 286 pp (in Russian).
Mörtl, M. & K.-O. Rothhaupt, 2003. Effects of adult Dreissena polymorpha on settling juveniles and associated macroinvertebrates. International Review of Hydrobiology 88: 561–569.
Mudroch, A. & P. Mudroch, 1992. Geochemical composition of the nepheloid layer in Lake Ontario. Journal of Great Lakes Research 189: 132–153.
Nalepa, T. F., 2010. An overview of the spread, distribution, and ecological impacts of the quagga mussel, Dreissena rostriformis bugensis, with possible implications to the Colorado River system. Proceedings, Colorado River Basin Science and Resource Management Symposium. Coming Together, Coordination of Science and Restoration Activities for the Colorado River Ecosystem, Scottsdale, AZ, November 18–20, 2008. U.S. Geological Survey Scientific Investigations Report: 2010–5135.
Nalepa, T. F. & D. W. Schloesser, 1993. Zebra Mussels Biology, Impacts, and Control. Lewis Publishers, Boca Raton: 810.
Nalepa, T. F. & D. W. Schloesser, 2014. Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd edn. CRC Press, Boca Raton: 775
Nalepa, T. F., D. J. Hartson, D. L. Fanslow, G. A. Lang & S. J. Lozano, 1998. Decline in benthic macroinvertebrate populations in southern Lake Michigan, 1980-1993. Canadian Journal of Fisheries and Aquatic Sciences 55: 2402–2413.
Nalepa, T. F., G. L. Fahnenstiel & T. H. Johengen, 1999. Impacts of the zebra mussel (Dreissena polymorpha) on water quality: a case study in Saginaw Bay, Lake Huron. In Claudi, R. & J. H. Leach (eds), Non-Indigenous Freshwater Organisms in North America: Their Biology and Impact. FL, CRC Press, Lewis Publishers, Boca Raton: 255–271.
Nalepa, T. F., D. L. Fanslow, S. A. Pothoven, A. J. Foiley III & G. A. Lang, 2007. Long-term trends in benthic macroinvertebrate populations in Lake Huron over the past four decades. Journal of Great Lakes Research 33: 42–436.
Nalepa, T. F., D. L. Fanslow & G. A. Lang, 2009a. Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to invasive mussel Dreissena rostriformis bugensis. Freshwater Biology 54: 466–479.
Nalepa, T. F., S. A. Pothoven & D. L. Fanslow, 2009b. Recent changes in benthic macroinvertebrate populations in Lake Huron and impact on the diet of lake whitefish (Coregonus clupeaformus). Aquatic Ecosystem Health and Management 12: 2–10.
Nalepa, T. F., D. L. Fanslow & S. A. Pothoven, 2010. Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. Journal of Great Lakes Research 36: 5–19.
O’Neill, C.R. Jr., 2008. The Silent Invasion: Finding Solutions to Minimize the Impacts of Invasive Quagga Mussels on Water Rates, Water Infrastructure and the Environment. Hearing of the U.S. House of Representatives Committee on Natural Resources—Subcommittee on Water and Power, Washington, D.C. Available at: http://naturalresources.house.gov/uploadedfiles/oneilltestimony06.24.08.pdf.
Orlova, M. I., T. W. Therriault, P. I. Antonov & G. K. Shcherbina, 2005. Invasion ecology of quagga mussels (Dreissena rostriformis bugensis): a review of evolutionary and phylogenetic impacts. Aquatic Ecology 39: 401–418.
Patterson, M. W. R., J. J. H. Ciborowski & D. R. Barton, 2005. The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie, 2002. Journal of Great Lakes Research 31(Supplement 2): 223–237.
Peyer, S. M., A. J. McCarthy & C. E. Lee, 2009. Zebra mussels anchor byssal threads faster and tighter than quagga mussels in flow. Journal of Experimental Biology 212: 2026–2035.
Peyer, S. M., J. C. Hermanson & C. E. Lee, 2010. Developmental plasticity of shell morphology of quagga mussels from shallow and deep-water habitats of the Great Lakes. Journal of Experimental Biology 213: 2602–2609.
Pires, D. L. M., B. M. Bontes, E. Van Donk & B. W. Ibelings, 2005. Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. Journal of Plankton Research 27: 331–339.
Pires, D. L. M., B. W. Ibelings & E. Van Donk, 2010. Zebra mussels as a potential tool in the restoration of eutrophic shallow lakes dominated by toxic cyanobacteria. The Zebra Mussel in Europe. Backhuys Publishers, Leiden: 331–342.
Poddubnyi, A. G., 1966. Adaptive response of Rutilus rutilus to variable environmental conditions. Proceedings of Institute of Biology of Inland Waters of Academy of Science of the USSA 10(13): 131–138. (in Russian).
Pollux, B. J. A., G. van der Velde & A. Bij de Vaate, 2010. A perspective on global spread of Dreissena polymorpha: a review on possibilities and limitations. In van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden: 45–58.
Pothoven, S. A., T. Nalepa, P. Schneeberger & S. Brandt, 2001. Changes in diet and body condition of lake whitefish in southern Lake Michigan associated with changes in benthos. North American Journal of Fisheries Management 21: 876–883.
Pothoven, S. A. & G. L. Fahnenstiel, 2013. Recent change in summer chlorophyll a dynamics of southeastern Lake Michigan. Journal of Great Lakes Research 39: 287–294.
Ramcharan, C. W., D. K. Padilla & S. I. Dodson, 1992. Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Canadian Journal of Fisheries and Aquatic Sciences 49: 2611–2620.
Reed-Andersen, T., S. R. Carpenter, D. K. Padilla & R. C. Lathrop, 2000. Predicted impact of zebra mussel (Dreissena polymorpha) invasion on water clarity in Lake Mendota. Canadian Journal of Fisheries and Aquatic Sciences 57: 1617–1626.
Reeders, H. H. & A. Bij de Vaate A., 1990. Zebra mussels (Dreissena polymorpha): a new perspective for water quality management. Hydrobiologia 200/201: 437–450.
Ricciardi, A., F. G. Whoriskey & J. B. Rasmussen, 1996. Impact of the Dreissena invasion on native unionid bivalves in the upper St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 53: 1434–1444.
Rennie, M. D., W. G. Sprules & T. B. Johnson, 2009. Resource switching in fish following a major food web disruption. Oecologia 159: 789–802.
Roe, S. L. & H. J. MacIsaac, 1997. Deepwater population structure and reproductive state of quagga mussels (Dreissena bugensis) in Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 54: 2428–2433.
Ryan, D. J., M. S. Sepulveda, T. F. Nalepa & T. O. Hook, 2012. Spatial variation in RNA: DNA ratios of Diporeia spp. in the Great Lakes region. Journal of Great Lakes Research 38: 187–195.
Sebestyen, O., 1937. Colonization of two new fauna-elements of Pontus-origin (Dreissena polymorpha Pall. and Corophium curvispinum G. O. Sarsforma devium Wundsch) in Lake Balaton. Verhandlungen InternationaleVereinigung für Theoretische und Angewandte Limnologie 8: 169–182.
Sherman, J. J., B. A. Murray, D. A. Woolnough, D. T. Zanatta & D. G. Uzarski, 2013. Assessment of remnant unionid assemblages in a selection of Great Lakes coastal wetlands. Journal of Great Lakes Research 39: 201–210.
Schloesser, D. W. & E. C. Masteller, 1999. Mortality of unionid bivalves (Mollusca) associated with dreissenid mussels (Dreissena polymorpha and D. bugensis) in Presque Isle Bay Lake Erie. Northeastern Naturalist 6: 341–352.
Schloesser, D. W. & C. Schmuckal, 2012. Bibliography of Dreissena polymorpha (zebra mussels) and Dreissena rostriformis bugensis (quagga mussels): 1989 to 2011. Journal of Shellfish Research 31: 1205–1263.
Schloesser, D. W., A. de Bij Vaate & A. Zimmerman, 1994. A bibliography of “Dreissena polymorpha in European and Russian Waters: 1964–1993”. Journal of Shellfish Research 13: 243–267.
Schloesser, D. W., T. F. Nalepa & G. L. Mackie, 1996. Zebra mussel infestation of unionid bivalves (Unionidae) in North America. American Zoologist 36: 300–310.
Soster, F. M., P. L. McCall & K. A. Herrmann, 2011. Decadal changes in the benthic invertebrate community in western Lake Erie between 1981 and 2004. Journal of Great Lakes Research 37: 226–237.
Sousa, R., J. L. Gutierrez & D. C. Aldridge, 2009. Nonindigenous invasive bivalves as ecosystem engineers. Biological Invasions 11: 2367–2385.
Starobogatov, J. I. & S. I. Andreeva, 1994. Distribution and history. In Starobogatov J. I. (ed.), Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, Ecology, Practical Meaning. Nauka Press, Moscow: 47–55 (in Russian).
Stewart, T. W., J. G. Miner & R. L. Lowe, 1998a. Quantifying mechanisms for zebra mussel effects on benthic macroinvertebrates: organic matter production and shell-generated habitat. Journal of the North American Benthological Society 17: 81–94.
Stewart, T. W., J. G. Miner & R. L. Lowe, 1998b. An experimental analysis of crayfish (Orconectes rusticus) effects on a Dreissena-dominated benthic macroinvertebrate community in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 55: 1043–1050.
Stewart, T. W., J. C. Gafford, J. G. Miner & R. L. Lowe, 1999. Dreissena-shell habitat and antipredator behavior: combined effects on survivorship of snails co-occurring with molluscivorous fish. Journal of the North American Benthological Society 18: 274–283.
Stoeckmann, A., 2003. Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Canadian Journal of Fisheries and Aquatic Sciences 60: 126–134.
Strayer, D. L., N. F. Caraco, J. J. Cole, S. Findlay & M. L. Pace, 1999. Transformation of freshwater ecosystems by bivalves—a case study of zebra mussels in the Hudson River. BioScience 49: 19–28.
Strayer, D. L., K. A. Hattala & A. W. Kahnle, 2004. Effects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Canadian Journal of Fisheries and Aquatic Sciences 61: 924–941.
Strayer, D. L. & H. M. Malcom, 2006. Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshwater Biology 51: 117–130.
Tucker, J. K., 1994. Colonization of unionid bivalves by the zebra mussel, Dreissena polymorpha, in Pool 26 of the Mississippi River. Journal of Freshwater Ecology 9: 29–134.
Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel & T. F. Nalepa, 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 58: 1208–1221.
Vanderploeg, H. A., T. F. Nalepa, D. J. Jude, E. L. Mills, K. T. Holeck, J. R. Liebig, I. A. Grigorovich & H. Ojaveer, 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 1209–1228.
Vanderploeg, H. A., T. H. Johengen & J. R. Liebig, 2009. Feedback between zebra mussel selective feeding and algal composition affects mussel condition: did the regime changer pay a price for its success? Freshwater Biology 54: 47–63.
van der Velde, G., S. Rajagopal & A. Bij de Vaate, 2010. From zebra mussels to quagga musels: an introduction to the Dreissenidae. In van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden: 1–10.
Ward, J. M. & A. Ricciardi, 2007. Impacts of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Diversity and Distributions 13: 155–165.
Watkins, J. M., R. Dermott, S. J. Lozano, E. L. Mills, L. G. Rudstam & J. V. Scharold, 2007. Evidence for remote effects of dreissenids mussels on the amphipod Diporea: analysis of Lake Ontario benthic surveys, 1997–2003. Journal of Great Lakes Research 33: 642–657.
Watkins, J. M., L. G. Rudstam, E. L. Mills & M. A. Teece, 2012. Coexistence of the native benthic amphipod Diporeia spp. and exotic dreissenid mussels in the New York Finger Lakes. Journal of Great Lakes Research 38: 226–235.
Wilson, K. A., E. T. Howell & D. A. Jackson, 2006. Replacement of zebra mussels by quagga mussels in the Canadian nearshore of Lake Ontario: the importance of substrate, round goby abundance, and upwelling frequency. Journal of Great Lakes Research 32: 11–28.
Wisconsin, DNR., 2013. Department of Natural Resources Webpage. Invasive Species. http://dnr.wi.gov/lakes/invasives/AISLists.aspx?species=ZM. Accessed on 6 December 2013.
Zhadin, V. I., 1946. The traveling shellfish Dreissena. Priroda 5: 29–37. (in Russian).
Zhukova, T. V., 2001. The phosphorus and nitrogen flows in a boundary layer “bottom-water” and their role in polymictic lakes functioning (on the example of Naroch Lakes ecosystem). Doctoral Dissertation, Zoology Institute of Academy of Science of the Republic of Belarus, Minsk (Belarus), 313 pp (in Russian with English summary).
Zhulidov, A. V., D. F. Pavlov, T. F. Nalepa, G. H. Scherbina, D. A. Zhulidov & T. Yu. Gurtovaya, 2004. Relative distributions of Dreissena bugensis and Dreissena polymorpha in the lower Don River system, Russia. International Review of Hydrobiology 89: 326–333.
Zhulidov, A. V., T. F. Nalepa, A. V. Kozhara, D. A. Zhulidov & T. Yu. Gurtovaya, 2006. Recent trends in relative abundance of two dreissenid species, Dreissena polymorpha and Dreissena bugensis in the Lower Don River system, Russia. Archiv fur Hydrobiologie 165: 209–220.
Zhulidov, A. V., A. V. Kozhara, G. H. Scherbina, T. F. Nalepa, A. Protasov, S. A. Afanasiev, E. G. Pryanichnikova, D. A. Zhulidov, T. Yu Gurtovaya & D. F. Pavlov, 2010. Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biological Invasions 12: 1923–1940.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Sidinei M. Thomaz, Katya E. Kovalenko, John E. Havel & Lee B. Kats / Aquatic Invasive Species
Rights and permissions
About this article
Cite this article
Karatayev, A.Y., Burlakova, L.E. & Padilla, D.K. Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746, 97–112 (2015). https://doi.org/10.1007/s10750-014-1901-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1901-x