Abstract
Invasive zebra (ZM) and quagga (QM) mussels continue to spread within and across inland waters worldwide. Oneida Lake, NY, USA, is a large (207 km2), mesotrophic, polymictic lake surveyed annually for dreissenids across substrates since 1992. We estimated abundance and distribution of ZMs and QMs across substrates, calculated lakewide density and biomass, analyzed seasonal and annual differences in veliger density, and explored dynamics of species replacement. ZMs and QMs were detected in 1991 and 2005, respectively. ZM density peaked at almost 30,000 ind/m2 in 1992, declined to between 2600 and 7600 ind/m2 until 2008, and further declined to 370–560 ind/m2 in 2010–2013 concurrent with increasing QM abundance. ZM biomass remained stable from 1992 to 2008 (140–530 g shell-on dry wt/m2) but declined to < 10 g from 2010 to 2013. QMs increased from 38% of the total biomass in 2008 to ≥ 90% from 2010 to 2013 which was accompanied by a decrease in ZM lengths and increase in QM lengths. In shallow (< 9 m) waters, both mussels were more abundant on rock than sand and silt substrate. Only QMs were abundant in deep silt substrate. The shift from ZM to QM dominance increased total biomass, but not density, due to larger QMs. Veliger densities were higher in May and August–November after arrival of QMs; however, there was no correlation between number of veligers and new recruits in fall or adult mussel biomass. The replacement of ZM occurred over about 4 years even though published mechanisms for QM dominance are not operating in summer in Oneida Lake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zebra and quagga mussels are closely related bivalves from the Ponto-Caspian region of Eastern Europe which have invaded extensive areas of Europe and North America (Mills et al. 1996; Zhulidov et al. 2006). Approximately 200 years ago, zebra mussels began to spread throughout Eurasia and have since colonized most of Western Europe; however, quagga mussels have only begun to spread outside their native range within the last 30 years (Zhulidov et al. 2006). Both species were reported in the Great Lakes region in the late 1980s and are rapidly spreading throughout North America and Europe (Hebert et al. 1989; May and Marsden 1992; Kraft and Johnson 2000; Matthews et al. 2014).
Dreissenids have caused ecological and economic impacts in both North America and Europe (reviews in Karatayev et al. 1997, 2015; Higgins and Vander Zanden 2010; Mayer et al. 2014). Mussels are ecosystem engineers (sensu Jones et al. 1994) altering both ecosystem structure and function by increasing water clarity and modifying bottom structure (Strayer et al. 1999; Karatayev et al. 2002). Reported effects of dreissenids include declines in zooplankton through competition for phytoplankton resources (Kissman et al. 2010), decreases in young fish survival (Irwin et al. 2009), changes in fish feeding patterns (Mayer et al. 2001), increases in benthic invertebrates (Stewart and Haynes 1994; Mayer et al. 2002), and increases in submerged aquatic vegetation (Chu et al. 2004; Zhu et al. 2006), resulting in a more benthic-oriented food web structure (Mills et al. 2003; Zhu et al. 2006; Miehls et al. 2009). Additionally, mussels biofoul any solid substrate, such as docks, piers, intake pipes, and boat hulls, costing hundreds of millions of dollars annually (O’Neill Jr. 1997; Nalepa and Schloesser 2014). Nakano and Strayer (2014) conservatively estimate the potential global cost of freshwater biofouling to be ~ $277 million per year.
Impacts of dreissenid invasions may depend on which of the two species dominates (Karatayev et al. 2015). The two species can have different spatial distributions; zebra mussels prefer hard substrate for attachment and are usually more abundant in littoral zones than in deeper water, whereas quagga mussels can colonize soft substrates in the profundal zone (Karatayev et al. 2011, 2015). Thus, the magnitude of mussel impacts is likely to vary among different lake types and depend on which of the two species is dominant. In addition, populations of dreissenids often vary widely over time, resulting in temporal variability in potential impacts (Strayer and Malcom 2006; Nalepa et al. 2010; Karatayev et al. 2014).
Despite the variability of dreissenid populations in space and time, Karatayev et al. (2015) predict that in shallow lakes with zebra mussels alone, maximum abundance and impact would be expected within 3–5 years after invasion; in shallow lakes with both species, maximum combined impact would be expected later, within 5–10 years after invasion. In deep lakes with both species, quagga mussel abundance and impact will exceed that of zebra mussels. Furthermore, when both species colonize the same waterbody, quagga mussels usually establish high densities and outcompete zebra mussels in deep lakes with large profundal zones while zebra mussels remain abundant and coexist with quagga mussels in shallow lakes and rivers (Karatayev et al. 2011, 2014; Zhulidov et al. 2010).
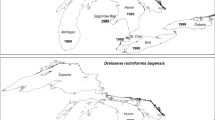
To test such predictions, time series data covering the periods following the initial invasions of both species are needed. Such data are available from Oneida Lake, New York, USA. Oneida Lake is a relatively large, shallow lake that has been surveyed for dreissenid mussels beginning in 1992, the year after zebra mussels were first found in the lake (Mellina et al. 1995; Mills et al. 2016). The first record of quagga mussels in Oneida Lake is from 2005 (JEC, unpubl. data). This is one of the longest continuous data sets on dreissenids available for any lake and is important for understanding the dynamics and ecosystem effects of both species in shallow, polymictic lakes—an important lake type worldwide. Here, we estimate abundance and distribution of zebra and quagga mussels across bottom types in Oneida Lake from 1992 to 2013 and calculate lakewide density and biomass. We are particularly interested in comparing the time series of abundance and biomass of both mussel species with the invasion cycle of these two species postulated by Karatayev et al. (2015) and in exploring the dynamics of the species replacement process from zebra to quagga mussel dominance. We also analyzed seasonal and annual differences in veliger density and tested if veliger density is related to number of newly settled mussel recruits. Since zebra and quagga mussels continue to spread within and across freshwater systems in North America and Europe with expected significant ecological and economic impacts, it is important to understand dreissenid population dynamics to assist in guiding future management strategies worldwide.
Methods
Study site
Oneida Lake (43°10′N, 75°52′W) is a 207 km2, shallow, polymictic lake with a mean depth of 6.8 m and a maximum depth of 16.8 m (Rudstam et al. 2016a). Since 1992, the year when zebra mussels became abundant in Oneida Lake, high water clarity and low chlorophyll-a concentrations have been typical of the lake except during summer cyanobacteria blooms (Zhu et al. 2006; Rudstam et al. 2016a). Since the mid-1990s, the lake has been considered mesotrophic during much of the year with reduced chlorophyll concentrations and increased water clarity compared to earlier decades when the lake was classified as eutrophic.
Field sampling
Dreissenid surveys were conducted in late summer to late fall (28 August–16 November) of 1992–2013 (Rudstam 2017a). The fall was initially selected for these surveys to obtain information on annual production of both adults and newly settled mussels (Mellina et al. 1995), and fall surveys then became the standard for comparisons across time in Oneida Lake. From 1992 to 2002, up to 10 sites (10 sites in 1993 and 1994; 9 sites in 1992, 1995–2000, and 2002; and 8 sites in 2001) were sampled in triplicate (duplicate at 2 sites in 1995) by SCUBA using quadrants placed on the lake bottom. Area sampled (0.06–1 m2) was determined by divers depending on mussel density at the site. From 2003 to 2013, up to 16 additional sites were sampled using SCUBA. Depth and substrate (rock, sand, silt) of each site were noted at the time of collection by the divers and the onboard personnel (KTH in most years). Samples were sieved and all mussels returned to the laboratory in plastic bags and frozen until processing. Further details are available in the online supplemental material (Rudstam 2017a).
We grouped all samples based on depth (shallower and deeper than 9 m) and substrate into four bottom type categories: shallow-sand, shallow-rock, shallow-silt and deep-silt bottoms. Most samples collected prior to 2010 during the surveys were from water depths shallower than 9 m, as few zebra mussels were found in deeper water. Therefore, we included data from two deeper, silt sites (11–12 m) at Buoy 125 and Shackelton Point that were sampled bi-weekly with Ekman grabs (0.0225 m2) as part of the long-term, limnology sampling (Mayer et al. 2016). Here, we use the data from these two sites from September through November for 1992–2013. After 2010, we also sampled additional sites (7.6–16.8 m) with Ekman grabs to better estimate deep water mussel populations.
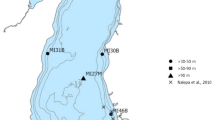
Oneida Lake veligers were sampled weekly at 4–6 sites from May through October 1992–2013 (Fig. 1). Samples were collected using a 50-cm diameter conical plankton net with a mesh size of 53 µm. The net was hauled vertically through the water column from 1 m off the bottom to the surface. A calibrated flow meter was used to measure the volume of water filtered in each tow and calculate densities. For years when the flowmeter was not available or malfunctioning, we applied the average efficiency of 77% calculated from all tows with functioning flowmeter. Flowmeter data were available for 64% of the 2342 veliger samples. Net samples were preserved with 8% sugar formalin.
Laboratory
Mussel sample processing was conducted by Cornell Biological Field Station (CBFS) technicians (1992–1999) and by JEC (2000–2013). Thawed samples were sorted and rocks, plants, and unionid mussel shells removed. Subsamples were taken as necessary. Dreissenids measuring ≥ 2 mm were identified to species. Quagga mussels were identified and separated beginning in 2008. In each sample, mussels were measured along the longest axis to the nearest mm using an ocular micrometer (≤ 10 mm) or digital caliper (> 10 mm). Zebra and quagga mussels were counted into 1-mm size classes by species starting at 2 mm (3 mm in 1992 and 1995–1997; 5 mm in 1993 and 1994) with those measuring < 2 mm (< 3 mm in 1992 and 1995–1997; < 5 mm in 1993 and 1994) counted and classified as Dreissena sp. All mussel size classes by species for subsamples were dried separately in a drying oven at 30 °C until all moisture was eliminated. Subsample proportions were calculated by dividing total subsample dry weight by total sample dry weight. Mussels classified as Dreissena sp. were apportioned to the two species based on the proportion of mussels identified by species at each site. All biomass and density values in this paper are based on mussels 2 mm and larger.
Veliger samples were analyzed by CBFS technicians from 1992 to 2013. Aliquots of 1 ml were counted using a compound scope with cross-polarized light filters at 40 × magnification (Johnson 1995). From 1992 to 2008, dreissenid larvae within a sample were separated from other constituents by allowing 10 mL of the sample to settle through a column of sugar solution (Schaner 1990). A settling efficiency rate of 60% was included in the calculations (Schaner 1990).
Data analysis
Shell-on dry weight was calculated from the size distribution and equations relating shell-on dry weight to shell length. These equations were obtained from measured dry weights of each 1-mm size class of each mussel species collected between 2008 and 2015. The equations were:
-
Zebra mussels: ln(SODW) = 2.864 * ln(SL) − 9.622, N = 2963; Range 2–26 mm; R2 = 0.982
-
Quagga mussels: ln(SODW) = 2.766 * ln(SL) − 9.472, N = 6892; Range 2–33 mm; R2 = 0.977
where SODW is shell-on dry weight (g) and SL is shell length of the major axis (mm). Biomass was calculated by multiplying the density in each 1-mm size group with the individual SODW calculated for the length at the midpoint of the size group (e.g., 2.5 mm for the 2–3 mm size group). For mussels < 5 mm, broader length groups were used in 1993 and 1994. For these groups, we used SODW calculated for the midpoint of the size group (e.g., 3.5 mm for group 2— < 5 mm). Because mussel lengths were not measured from the deep silt sites before 2010, we assigned those mussels the average species-specific weight of mussels from that year when estimating lakewide biomass.
Average mussel densities were calculated for each of four substrate category and weighted by the relative proportion of those substrates in the lake to obtain lakewide densities. We used ArcGIS 10.2.2 (ESRI Redlands, CA) to calculate proportions of Oneida Lake substrate type (rock, sand, and silt) at 1-m depth intervals from substrate (Greeson 1971) and bathymetry (Fitzgerald et al. 2016) layers. Depths > 9 m were considered deep and classified as deep-silt (95% of the bottom greater than 9 m is silt; remainder is sand). Shallower depths were classified according to bottom type. The proportions of the four bottom types were: shallow-rock (13.0% of the bottom), shallow-sand (16.4%), shallow-silt (34.2%), and deep-silt (36.5%). In 2000, the shallow-silt bottom type was not sampled, so we used an average of the values of the shallow silt sites from 1999 and 2001. All other bottom types had at least one site sampled each year.
Veliger volumetric densities were calculated for each site sampled and multiplied by site depth to calculate veliger areal density by site. The water column was sampled 1m from the bottom to the surface at each site. Monthly average veliger volumetric density was calculated from all weekly samples collected that month. Veliger volumetric densities in #/m3 are approximately 10% of areal densities given average depth of sampling sites is approximately 10 m.
Statistics
Each site-year combination was considered one sampling unit. Average mussel density and biomass for each site were first calculated from replicates from that site and specific year. These values were loge(x + minimum value) transformed to reduce heteroscedasticity. Each site-year was then classified into one of the four bottom types (i.e., rock, sand, shallow-silt, deep-silt). We used a mixed effects analysis of variance (ANOVA) with post hoc Tukey’s honestly significant difference (HSD) tests (JMP® Pro 11.0.0, SAS, Cary, NC) to determine significant differences among years for zebra and quagga mussel density and biomass across bottom type categories. The model included fixed effects of year and bottom type and a random effect of site. Relationships were considered significant at P < 0.05. Change point analysis on zebra and quagga mussel density and biomass and on veliger density was performed using Change-Point Analyzer Version 2.3 (Taylor Enterprises, Libertyville, IL). This nonparametric analysis uses iterative application of cumulative sum and bootstrapping (1000 times) to detect change points in the data series (Taylor 2015). The number of veligers (May–September) was compared to the number of settled recruits in the fall defined as mussels between 2 and 9 mm at that time. We chose 2–9 mm for recruits as this size range represented 88% of zebra mussel recruits and 90% of quagga mussel recruits settled on new substrate in Oneida Lake in experiments conducted during the summer and fall of 2015 (LGR, unpublished data). The number of veligers was also compared to the adult mussel biomass (mussels > 9 mm) present the same year.
Results
Zebra mussel density was highest on all shallow substrates in 1992 (Fig. 2), the year after the species was first recorded in Oneida Lake (Mills et al. 2016). These mussels were small and mainly from the 1992 year class. Overall, there were significant differences in density and biomass among bottom types (analysis using data from 1992 to 2007, Table 1). Rock and sand bottoms showed the highest zebra mussel densities, shallow-silt had lower densities, and deep-silt had very low densities. Sand and shallow-silt were not significantly different (Figs. 2, 3; Table 1). This pattern was the same for biomass (Fig. 2; Table 1).
Quagga mussels were not separated from zebra mussels in these samples until 2008, as they were rare in previous years (JEC, personal observations). Analysis of data from 2008 to 2013 show that quagga mussel density and biomass were highest on rock and lowest on the deep-silt bottom types which is similar to zebra mussel density and biomass (Fig. 2; Table 1). Although, in contrast to zebra mussels, both density and biomass of quagga mussels were higher on shallow-silt than on sand bottoms. There were no significant differences between rock and shallow-silt for quagga mussel density and among all three shallow substrates for quagga mussel biomass. Quagga mussels had higher density and biomass than zebra mussels on all substrates from 2009 to 2013 (Figs. 2, 3). The proportion of the lakewide mussel biomass in different substrate types were 50% (rock), 27% (sand), 22% (silt) and < 0.1% (deep silt) for the years 1992–2007 when zebra mussel dominated, and 42% (rock), 18% (sand), 33% (silt) and 7% (deep silt) for the quagga mussel dominated years (2009–2013).
Zebra mussel lakewide density was highest from 1992 to 1994, then declined but remained relatively stable from 1995 to 2008 (range 2600–7600 ind/m2), declined further in 2009 and remained low through 2013 (range 370–560 ind/m2 from 2010 to 2013, Fig. 4). Peak zebra mussel biomass occurred in 1994 (Fig. 4) as densities remained high and the average size of mussels increased from 1992 to 1994. Similar to zebra mussel density, biomass did not decline significantly until 2008 when quagga mussels were increasing (Fig. 4; Table 2). From 1992 through 2008, biomass ranged from 140 to 530 g/m2 (average 288 g/m2) with no time trend. After an intermediate year in 2009 (54 g/m2), zebra mussel biomass remained below 10 g/m2 from 2010 through 2013 (Fig. 4). Quagga mussel lakewide density increased from 2008 to 2009 and then stabilized through 2013 with biomass increasing from 2008 to 2010 (Fig. 4; Table 2). Thus, quagga mussels increased from an assumed minor component of the mussel population in 2006 to 80% of the mussel density and biomass in 2009. Quagga mussels continued to represent ≥ 90% of total mussel density and biomass from 2010 to 2013. The combined mussel biomass increased after quagga mussels became dominant with the highest average lakewide biomass recorded in 2012 and the third highest in 2013 (Fig. 4). Overall lakewide densities did not increase with quagga mussels and remained between 2600 and 7600 ind/m2 (average 5350 ind/m2) from 1995 (after the initial decline in density) to 2013 (Fig. 4); however, mussel densities increased on sand and silt bottoms (Figs. 2, 3). Quagga mussel average length increased from 2009 to 2013 (Fig. 5) which explains the increase in lakewide mussel biomass (Fig. 4).
Change point analysis (Table 2) identified a decrease in zebra mussel density in 1995 and a further decrease in 2008, reflecting the initial decline in density after the invasion and the response to quagga mussels after 2008. A negative change point in zebra mussel biomass occurred in 2008. Quagga mussel density and biomass exhibited positive change points in 2009 and 2010, respectively. Total mussel biomass showed a positive change point in 2012 as a result of higher mussel biomass in 2012 and 2013; however, total mussel density only changed in 1995 with higher mussel densities from 1992 to 1994 than in subsequent years.
Average length of both zebra and quagga mussels varied across years and bottom types (Table 1). Since mussels were not measured for deep-silt bottom samples prior to 2010, this bottom type is not included in the analysis of average lengths for zebra mussels. Rock bottoms had larger mussels of both species, but there was no difference in lengths of quagga mussels on rock, shallow-silt or deep-silt bottom types. Furthermore, there was no difference in lengths of both species between sand and shallow-silt bottoms. In years when both species were present, zebra mussels were larger in 2008–2009 and quagga mussels were larger in 2010–2013 (Fig. 5). Zebra mussel lengths decreased after quagga mussels became dominant. Average length of zebra mussels from 1995 to 2008 was 8.8 mm (range between years 8.0 and 12.3 mm), while average length of zebra mussels from 2009 to 2013, when quaggas dominated, was 6.3 mm (range 5.3–7.8 mm), a significant difference (P < 0.01). Average length of quagga mussels from 2009 to 2013 was 9.6 mm (range 7.0–11.7 mm). Average lengths of zebra and quagga mussels were not different between the two periods when each dominated (P = 0.139).
Mean May–September veliger density increased over time (R2 = 0.320, N = 22, P = 0.006, Fig. 6), but the pattern varied among months. Veliger density increased with time (1992–2013) for the months of May and August through November, but not for the months of June and July (Fig. 7). Veliger density was higher in the years when quagga mussels dominated in the months of May and August through November, higher when zebra mussels dominated in July, and not different in June (Table 3). Annual (May–September) averages were marginally higher for the quagga mussel years (Table 3). Change point analysis showed positive change points in 2008 for May, August, September and October, associated with the arrival of quagga mussels (Fig. 7). There was one poorly defined positive change point for the overall average May–September veliger numbers in 2007 (96% CL 2003–2010) consistent with the increase in the months of May, August and September at that time. Veligers and < 9 mm settled recruits were not correlated (Fig. 6, R2 = 0.026). Furthermore, there was no correlation between veliger density and the total biomass of mussels > 9 mm (R2 = 0.12, P = 0.113).
Oneida Lake average veliger density (top panel) and average settled recruits (< 9 mm) mussel density (lower panel) (1992–2013). Average veliger density calculated from May through September based on averages of monthly average densities. Missing months (May and Aug–Sep 2000 and 2002) were imputed with the average of those months in surrounding years to calculate average veliger densities for May–September in 2000–2002
Oneida Lake average veliger density by month (1992–2013). Average veliger density calculated based on all samples collected in a given month. Samples not available marked with “N”. Year + indicates a positive time trend, Year NS a non-signficant time trend, R2 is the adjusted value for the regression over time, P the signficance level of the time trend, CP indicates the year with a signficant change point and the years in brackets indicate the 95% confidence limits around that change point. All change points indicated had a likelihood of over 95%
Discussion
The Oneida Lake data set is unique in having annual surveys of dreissenid mussels since the year after zebra mussels were first detected, including measurements of mussel size and veliger density. We do not know of any other North American lake that has as long a data series of annual assessments, although long-term data do exist from the Great Lakes (Karatayev et al. 2014; Birkett et al. 2015), Hudson River (Strayer and Malcom 2006) and some European lakes (Zhukova et al. 2017). Here, we analyzed data from Oneida Lake up to 2013 because of the changes expected with the invasion of round goby (Neogobius melanostomus) to Oneida Lake in 2014 (Rudstam et al. 2016b). Methods used have been similar over these 22 years with some exceptions. Intital sampling was done only with SCUBA divers. With divers, sampling a large number of sites can be prohibitive and samples were therefore collected in triplicate at each of 10 standard sites. Additional sites were added in 2003 and a random survey design on silt and sand bottom stratified by depth region added in 2013 and sampled with Ekman grabs. These changes increased the precision of the estimates but did not change time trends. Hetherington (2016) calculated time trends as presented here with only standard sites and found similar results; therefore, we only presented estimations based on all sites in this paper.
After an early peak shortly after establishment, Oneida Lake zebra mussel density decreased in 1995 and again in 2008; biomass decreased only in 2008. The initial decline in zebra mussel density is likely associated with intraspecific density-dependent processes, including competition for substrate and/or food (Hunter and Simons 2004; Burlakova et al. 2006; Karatayev et al. 2011). After the initial decline in density and before the large increase of quagga mussels (years 1995–2007), lakewide zebra mussel density varied between 2600 and 7600 ind/m2 and biomass varied between 160 and 390 g/m2 SODW. As the confidence limits on these estimates are typically ± 50% (Hetherington 2016), variations among years could be largely due to sampling. The high densities of zebra mussels on sand and silt bottoms in the early years could have been due to the presence of unionid clams whose shells were heavily colonized by dreissenids. Unionid clams were absent in later years as fouling by zebra mussels caused their extirpation. There were not any detectable time trends in zebra mussel density from 1995 to 2007 (R2 = 0.06) or in biomass from 1992 to 2007 (R2 = 0.05), and these relatively stable density and biomass levels are likely the result of limited substrate availability and perhaps food supply in Oneida Lake. Summer average chlorophyll-a decreased and water clarity increased from 1992 to 1995 after the arrival of zebra mussels in Oneida Lake, but they did not change dramatically through 2008 (Zhu et al. 2006; Rudstam et al. 2016a). Further declines in zebra mussels occurred after the invasion of quagga mussels. Density and biomass of zebra mussels after 2010 was 9% (density) and 2% (biomass) of the values observed between 1995 and 2007. Zebra mussels were on average smaller after quagga mussels became established.
Quagga mussels largely replaced zebra mussels on shallow substrates, dominating in terms of both density (90–95% quagga) and biomass (97–99% quagga) on all bottom types from 2009 to 2013. This is a common observation after the arrival of quagga mussels to lakes occupied by zebra mussels (e.g., Patterson et al. 2005; Wilson et al. 2006; Nalepa et al. 2009; Ginn et al. 2018). Both dreissenids exhibit a preference for hard substrates (Wilson et al. 2006), and the shallow rock bottom type had the highest densities of both species in Oneida Lake. In addition, quagga mussels expanded to the deep, silt bottoms (36% of total lake bottom of Oneida Lake), a bottom type with few mussels during the period of zebra mussel dominance. Total average mussel biomass increased in 2012 as a result of the expansion to deeper bottoms and higher abundance of quagga mussels on shallow, sand, and silt bottoms. However, this increase was not of the same magnitude as observed in quagga mussel expansions in deeper, colder lakes (Orlova et al. 2005; Nalepa et al. 2009; Birkett et al. 2015). Oneida Lake lakewide average mussel density from 1992 to 2013 was within the range of mussel densities for other lakes in North America (1000–100,000/m2) and Europe (100–8000/m2) (Fig. 4) (Ramcharan et al. 1992; Naddafi et al. 2011).
Species replacement did change the seasonal patterns of veliger densities with more veligers produced in May and from August through November during years after quagga mussels became dominant. This shift in the seasonality of veliger density is partly due to the documented higher activity of quagga mussels in colder temperatures (Baldwin et al. 2002; Karatayev et al. 2015). As with other studies (Nalepa et al. 1995; Jones and Ricciardi 2014), yearly trends in densities of veligers, adults, and settled recruits were poorly correlated in Oneida Lake. Larval survival and recruitment to the adult population are affected by predation by adults (MacIsaac et al. 1991) and other species, as well as inter-annual differences in how currents affect dispersal. Additionally, high mortality at the settling stage can result from food (Sprung 1989) or substrate (Sprung 1989; Stanczykowska and Lewandowski 1993) limitations. Thus, veliger density was not a good indicator of mussel abundance or the number of recruits in Oneida Lake.
According to Karatayev et al. (2015), in shallow lakes with only zebra mussels, maximum abundance and impact is expected within 3–5 years after invasion; however, in shallow lakes with both species, maximum combined impact is expected in 5–10 years. These expectations apply to systems where quagga mussels invade a zebra mussel-dominated water body as there are few data sets from quagga mussel only lakes (see also Strayer et al. in review). Mills et al. (2016) reported that zebra mussels arrived in Oneida Lake in 1991; the first documented quagga mussel was collected by JEC in 2005 (up to 25 mm at Lake Shore Yacht Club, Cicero, NY). Based on the maximum zebra mussel size of 28 mm in 1992 and quagga mussel size of 25 mm in 2005, and assuming a fast growth rate of 15 mm per year to be conservative (Karatayev et al. 2006), we conclude that zebra mussels were likely present in 1990 and quagga mussels in 2003. Quagga mussels could have been in the lake earlier, as quagga mussels were identified from the inlet to nearby Onondaga Lake in 1992 (Mills et al. 1993). Unfortunately, samples were not available to further verify the assumed invasion years. We did have samples from seven sites collected in 2004 by another study on sturgeon diets using a bottom sled and a ponar grab. All of the 1060 individual mussels in those samples were zebra mussels indicating that quagga mussels were indeed rare at that time. Assuming an arrival of zebra mussels in 1990 and quagga mussels in 2003, zebra mussels increased to peak density in 2 years (by 1992) and quagga mussels in 6 years (by 2009). Maximum zebra mussel biomass occurred in 1994, 4 years after their assumed arrival; whereas, the maximum quagga mussel biomass occurred in 2012, 9 years after assumed arrival. Quagga mussels did not become abundant on deep silt bottoms until 2011, and the slower spread to this bottom type may be part of the reason for the longer time period between quagga mussel arrival to a lake and maximum impact of this species compared to zebra mussels.
Quagga mussel replacement of zebra mussels has been documented in several studies reviewed by Karatayev et al. (2015; see also Ginn et al. 2018; Strayer et al. in review). Authors have attributed dominance of quagga mussels to greater energetic efficiency, resulting in higher growth rates at lower temperature and lower food concentrations (Mills et al. 1999; Diggins 2001; Baldwin et al. 2002; Stoeckmann 2003; Nalepa et al. 2010; Ram et al. 2012) and greater quagga mussel reproduction at lower temperatures (Roe and MacIsaac 1997; Claxton and Mackie 1998; Nalepa et al. 2010; Marescaux et al. 2015). Quagga mussels did grow larger than zebra mussels when quagga mussels dominated (2009–2013), but quagga mussels were not larger than the zebra mussels present when zebra mussel dominated. Thus, our field observations suggest that quagga mussels do depress zebra mussel growth rates, perhaps through local competition for food. But experimental data on growth rates of Oneida Lake mussels are inconsistent. Quagga mussels did grow faster than zebra mussels in aquarium experiments using Oneida Lake water when predators were present but not when predators were absent (Nadaffi and Rudstam 2014a). Zebra mussels grew faster than quagga mussels in mesocosms with summer temperatures (Mei et al. 2016) and at similar rates or faster than quagga mussels in in situ enclosures (LGR, unpubl. data). A quagga mussel growth advantage may be limited to colder seasons with quagga mussels growing later into the fall and earlier in the spring, which is consistent with the colonization of quagga mussels to deeper, colder depths in deep lakes. It is possible that the larger size of quagga mussels is not related to a growth advantage but instead related to higher mortality of larger zebra mussels, perhaps over-winter, and to poor recruitment. Our observations of extended reproductive period for quagga mussels would give that species an advantage in colonizing substrates opened up by predators or senescence of older mussels. However, the proportion of newly settled mussels (< 9 mm) that were quagga mussels in 2009–2013 (76–89%) was not larger than the proportion of the adult dreissenid biomass (> 9 mm) that were quagga mussels in the same years (80–99%).
The replacement of zebra mussels and the colonization of deeper areas of the lake by quagga mussels can have implications for the ecosystem. An increase in total dreissenid biomass due to quagga mussels in deeper waters is likely a major cause for increased mussel effects in Lake Michigan (Vanderploeg et al. 2010; Barbiero et al. 2018). Dominance of quagga mussels can also extend mussel effects earlier and later in the season as quagga mussels are active at colder temperatures (Vanderploeg et al. 2010; Karatayev et al. 2015). Further changes to the Oneida Lake dreissenid population may occur in the future due to the invasion of round gobies in 2014 (Rudstam et al. 2016b). Round gobies may shift dreissenid mussel length-frequency distributions by preferentially consuming smaller mussels (Ray and Corkum 1997; Wilson et al. 2006; Naddafi and Rudstam 2014b). Since the quagga mussel has a thinner shell, weaker attachment strength, and less antipredatory behavior than the zebra mussel (Naddafi and Rudstam 2013, 2014a, b), selective predation by fish, such as the round goby, could potentially result in declines in quagga mussel populations (as suggested by Zhulidov et al. 2006, 2010) and perhaps a return of zebra mussels with further changes to the Oneida Lake ecosystem.
Supporting information
Additional Supporting Information is available at The Knowledge Network for Biocomplexity (https://knb.ecoinformatics.org/) (Rudstam 2017a, b). The mussel data package (https://knb.ecoinformatics.org/view/kgordon.23.54) includes tables with details on the survey design for each year, site-specific averages of biomass, density and size used for statistical analyses, whole-lake annual averages by bottom type used for Fig. 2 and whole lake values used for Fig. 4. The benthic invertebrates package (https://knb.ecoinformatics.org/view/kgordon.4.65) includes data for the benthic grabs used in Fig. 3.
References
Baldwin BS, Mayer MS, Dayton J, Pau N, Mendilla J, Sullivan M et al (2002) Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Can J Fish Aquat Sci 59:680–694
Barbiero RP, Lesht BM, Warren GJ, Rudstam LG, Watkins JM, Reavie ED et al (2018) A comparative examination of recent changes in nutrients and lower food web structure in Lake Michigan and Lake Huron. J Great Lakes Res 44:573–589
Birkett K, Lozano S, Rudstam LG (2015) Long-term trends in Lake Ontario’s benthic macroinvertebrate community from 1994–2008. Aquat Ecosyst Health Manag 18:76–85
Burlakova LE, Karatayev AY, Padilla DK (2006) Changes in the distribution and abundance of Dreissena polymorpha within lakes through time. Hydrobiologia 517:133–146
Chu C, Minns CK, Moore JE, Millard ES (2004) Impact of oligotrophication, temperature, and water levels on walleye habitat in the Bay of Quinte, Lake Ontario. Trans Am Fish Soc 133:868–879
Claxton WT, Mackie GL (1998) Seasonal and depth variations in gametogenesis and spawning of Dreissena polymorpha and Dreissena bugensis in eastern Lake Erie. Can J Zool 76:2010–2019
Diggins TP (2001) A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. J Great Lakes Res 27:457–466
Fitzgerald D, Zhu B, Mills E, Rudstam L, Hoskins S, Haddad D et al (2016) Dynamics of aquatic vegetation in Oneida Lake, 1915–2005: a response to ecosystem changes. In: Rudstam L, Mills E, Jackson J, Stewart D (eds) Oneida Lake: long-term dynamics of a managed ecosystem and its fisheries. American Fisheries Society, Bethesda, pp 181–200
Ginn BK, Bolton R, Coulombe D, Fleischaker T, Yerex G (2018) Quantifying a shift in benthic dominance from zebra (Dreissena polymorpha) to quagga (Dreissena rostriformis bugensis) mussels in a large, inland lake. J Great Lakes Res 44:271–282
Greeson P (1971) Limnology of Oneida Lake with emphasis on factors contributing to algae blooms. United States Department of the Interior Geological Survey, Albany
Hebert PDN, Muncaster BW, Mackie GL (1989) Ecological and genetic studies on Dreissena polymorpha (Pallas): a new mollusk in the Great Lakes. Can J Fish Aquat Sci 46:1587–1591
Hetherington AL (2016) Ecological forecasting for Oneida Lake: impacts of climate change and invasive mussels on lake dynamics. Dissertation, Cornell University
Higgins SN, Vander Zanden MJ (2010) What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol Monogr 80:179–196
Hunter RG, Simons KA (2004) Dreissenids in Lake St. Claire in 2001: evidence for population regulation. J Great Lakes Res 30:528–537
Irwin BJ, Rudstam LG, Jackson JR, VanDeValk AJ, Forney JL, Fitzgerald DG (2009) Depensatory mortality, density-dependent growth, and delayed compensation: disentangling the interplay of mortality, growth, and density during early life stages of yellow perch. Trans Am Fish Soc 138:99–110
Johnson LE (1995) Enhanced early detection and enumeration of zebra mussel (Dreissena spp.) veligers using cross-polarized light microscopy. Hydrobiologia 312:139–146
Jones LA, Ricciardi A (2014) The influence of pre-settlement and early post-settlement processes on the adult distribution and relative dominance of two invasive mussel species. Freshw Biol 59:1086–1100
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Karatayev AY, Burlakova LE, Padilla DK (1997) The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in Eastern Europe. J Shellfish Res 16:187–203
Karatayev AY, Burlakova LE, Padilla DK (2002) Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe: distribution, impacts and management. Springer, Dordrecht, pp 433–446
Karatayev AY, Burlakova LE, Padilla DK (2006) Growth rate and longevity of Dreissena polymorpha (Pallas): a review and recommendation for future study. J Shellfish Res 25:23–32
Karatayev AY, Burlakova LE, Mastitsky SE, Padilla DK, Mills EL (2011) Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. J Shellfish Res 30:923–931
Karatayev AY, Burlakova LE, Pennuto C, Ciborowski J, Karatayev VA, Juette P et al (2014) Twenty five years of change in Dreissena spp. populations in Lake Erie. J Great Lakes Res 40:550–559
Karatayev AY, Burlakova LE, Padilla DK (2015) Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746:97–112
Kissman CEH, Knoll LB, Sarnelle O (2010) Dreissenid mussels (Dreissena polymorpha and Dreissena bugensis) reduce microzooplankton and macrozooplankton biomass in thermally stratified lakes. Limnol Oceanogr 55:1851–1859
Kraft CE, Johnson LE (2000) Regional differences in rates and patterns of North American inland lake invasions by zebra mussels (Dreissena polymorpha). Can J Fish Aquat Sci 57:993–1001
MacIsaac HJ, Sprules WG, Leach JH (1991) Ingestion of small-bodied zooplankton by zebra mussels: can cannibalism on larvae influence population dynamics? Can J Fish Aquat Sci 48:2151–2160
Marescaux J, Boets P, Lorquet J, Sablon R, Van Doninck K, Beisel J-N (2015) Sympatric Dreissena species in the Meuse River: towards a dominance shift from zebra to quagga mussels. Aquat Invasion 10:287–298
Matthews J, Van der Velde G, Bij de Vaate A, Collas FPL, Koopman KR, Leuven RSEW (2014) Rapid range expansion of the invasive quagga mussel in relation to zebra mussel presence in the Netherlands and Western Europe. Biol Invasions 16:23–42
May B, Marsden JE (1992) Genetic identification and implications of another invasive species of dreissenid mussel in the Great Lakes. Can J Fish Aquat Sci 49:1501–1506
Mayer CM, Rudstam LG, Mills EL, Cardiff SG, Bloom CA (2001) Zebra mussels (Dreissena polymorpha), habitat alteration, and yellow perch (Perca flavescens) foraging: system-wide effects and behavioural mechanisms. Can J Fish Aquat Sci 58:2459–2467
Mayer CM, Keats RA, Rudstam LG, Mills EL (2002) Scale-dependent effects of zebra mussels on benthic invertebrates in a large eutrophic lake. J the N Am Benthol Soc 21:616–633
Mayer CM, Burlakova LE, Eklöv P, Fitzgerald D, Karatayev AY, Ludsin SA et al (2014) Benthification of freshwater lakes: exotic mussels turning ecosystems upside down. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 575–585
Mayer CM, Zhu B, Cecala R (2016) The zebra mussel invasion of Oneida Lake: benthification of a eutrophic lake. In: Rudstam L, Mills E, Jackson J, Stewart D (eds) Oneida Lake: long-term dynamics of a managed ecosystem and its fisheries. American Fisheries Society, Bethesda, pp 161–180
Mei X, Zhang X, Kassam SS, Rudstam LG (2016) Will the displacement of zebra mussels by quagga mussels increase water clarity in shallow lakes during summer? Results from a mesocosm experiment. PLoS ONE 11:e0168494
Mellina E, Rasmussen JB, Mills EL (1995) Impact of the zebra mussel (Dreissena polymorpha) on phosphorus cycling and chlorophyll in lakes. Can J Fish Aquat Sci 52:2553–2573
Miehls ALJ, Mason DM, Frank KA, Krause AE, Peacor SD, Taylor WW (2009) Invasive species impacts on ecosystem structure and function: a comparison of Oneida Lake, New York, USA, before and after zebra mussel invasion. Ecol Model 220:3194–3209
Mills EL, Dermott RM, Roseman EF, Dustin D, Mellina E, Conn DB, Spidle AP (1993) Colonization, ecology, and population structure of the quagga mussel (Bivalvia, Dreissenidae) in the lower Great Lakes. Can J Fish Aquat Sci 50:2305–2314
Mills EL, Rosenberg G, Spidle AP, Ludyanskiy M, Pligin Y, May B (1996) A review of the biology and ecology of the quagga mussel (Dreissena bugensis), a second species of freshwater dreissenid introduced to North America. Am Zool 36:271–286
Mills EL, Chrisman JR, Baldwin B, Owens RW, O’Gorman R, Howell T et al (1999) Changes in the dreissenid community in the lower Great Lakes with emphasis on southern Lake Ontario. J Great Lakes Res 25:187–197
Mills EL, Casselman JM, Dermott R, Fitzsimons JD, Gal G, Holeck KT et al (2003) Lake Ontario: food web dynamics in a changing ecosystem (1970–2000). Can J Fish Aquat Sci 60:471–490
Mills E, Forney J, Holeck K (2016) Oneida Lake: A century of biotic introductions and ecosystem change. In: Rudstam L, Mills E, Jackson J, Stewart D (eds) Oneida Lake: long-term dynamics of a managed ecosystem and its fisheries. American Fisheries Society, Bethesda, pp 87–110
Naddafi R, Rudstam LG (2013) Predator-induced behavioural defences in two competitive invasive species. Anim Behav 86:1275–1284
Naddafi R, Rudstam LG (2014a) Does differential predation explain the replacement of zebra by quagga mussels? Freshw Sci 33:895–903
Naddafi R, Rudstam LG (2014b) Predator-induced morphological defences in two invasive dreissenid mussels: implications for species replacement. Freshw Biol 59:703–713
Naddafi R, Blenckner P, Eklöv P, Pettersson K (2011) Physical and chemical properties determine zebra mussel invasion success in lakes. Hydrobiologia 669:227–236
Nakano D, Strayer DL (2014) Biofouling animals in fresh water: biology, impacts, and ecosystem engineering. Front Ecol Environ 12:167–175
Nalepa TF, Schloesser DW (eds) (2014) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton
Nalepa TF, Wojcik JA, Fanslow DL, Lang GA (1995) Initial colonization of the zebra mussel (Dreissena polymorpha) in Saginaw Bay, Lake Huron: population recruitment, density and size structure. J Great Lakes Res 21:417–434
Nalepa TF, Fanslow DL, Lang GA (2009) Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to the invasive mussel Dreissena rostriformis bugensis. Freshw Biol 54:466–479
Nalepa TF, Fanslow DL, Pothoven SA (2010) Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. J Great Lakes Res 36:5–19
O’Neill CR Jr (1997) Economic impact of zebra mussels: results of the 1995 National Zebra Mussel Information Clearinghouse study. Great Lakes Res Rev 3:35–42
Orlova MI, Muirhead JR, Antonov PI, Shcherbina GK, Starobogatov YI, Biochino GI et al (2005) Range expansion of quagga mussels (Dreissena rostriformis bugensis) in the Volga River and Caspian Sea basin. Aquat Ecol 38:561–573
Patterson MW, Ciborowski JJ, Barton DR (2005) The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie, 2002. J Great Lakes Res 31:223–237
Ram JL, Karim AS, Banno F, Kashian DR (2012) Invading the invaders: reproductive and other mechanisms mediating the displacement of zebra mussels by quagga mussels. Invertebr Reprod Dev 56:21–32
Ramcharan CW, Padilla DK, Dodson SI (1992) Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Can J Fish Aquat Sci 49:2611–2620
Ray WJ, Corkum LD (1997) Predation of zebra mussels by round gobies, Neogobius melanostomus. Environ Biol Fishes 50:267–273
Roe SL, MacIsaac HJ (1997) Deepwater population structure and reproductive state of quagga mussels (Dreissena bugensis) in Lake Erie. Can J Fish Aquat Sci 54:2428–2433
Rudstam LG (2017a) Lakewide zebra and quagga mussel summary, Oneida Lake, New York, 1992 to present. Web data on Knowledge Network for Biocomplexity. Original data set published in 2008. Updated in 2017. https://knb.ecoinformatics.org/view/kgordon.23.54. Accessed 15 Jan 2019
Rudstam LG (2017b) Benthic invertebrates in Oneida Lake, New York, 1964–present. Web data on Knowledge Network for Biocomplexity. Original data published in 2008. Updated in 2017. https://knb.ecoinformatics.org/view/kgordon.4.65. Accessed 15 Jan 2019
Rudstam LG, Jackson JR, Hetherington AL (2016a) Concluding remarks: Forecasting the future of Oneida Lake and its fishery in an era of climate change and biological invasions. In: Rudstam L, Mills E, Jackson J, Stewart D (eds) Oneida Lake: long-term dynamics of a managed ecosystem and its fisheries. American Fisheries Society, Bethesda, pp 525–540
Rudstam LG, Mills EL, Jackson JR, Stewart DJ (eds) (2016b) Oneida Lake: Long-term dynamics of a managed ecosystem and its fishery. American Fisheries Society, Bethesda
Schaner T (1990) Detection of zebra mussel veliger in plankton samples using sugar solution. In: Ontario Ministry of Natural Resources Lake Ontario Fisheries Unit (ed) Lake Ontario Fisheries Unit 1990 annual report, LOA 91.1.. Ontario Ministry of Natural Resources, Picton, pp 6.1–6.3
Sprung M (1989) Field and laboratory observations of Dreissena polymorpha larvae: abundance, growth, mortality, and food demands. Arch Hydrobiol 115:537–561
Stanczykowska A, Lewandowski K (1993) Thirty years of Dreissena polymorpha ecology in Mazurian Lakes of northeastern Poland. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts, and control. Lewis Publishers, Boca Raton, pp 3–33
Stewart TW, Haynes JM (1994) Benthic macroinvertebrate communities of Southwestern Lake Ontario following invasion of Dreissena. J Great Lakes Res 20:479–493
Stoeckmann A (2003) Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Can J Fish Aquat Sci 60:126–134
Strayer DL, Malcom HM (2006) Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshw Biol 51:117–130
Strayer DL, Adamovich BV, Adrian R, Aldridge DC, Balogh CS, Burlakova LE et al (in review) Long-term population dynamics of zebra and quagga mussels (Dreissena polymorpha and D. rostriformis): a cross-system analysis. Ecosphere
Strayer DL, Caraco NF, Cole JJ, Findlay S, Pace ML (1999) Transformation of freshwater ecosystems by bivalves—a case study of zebra mussels in the Hudson River. Bioscience 49:19–27
Taylor WA (2015) Change-point analysis: a powerful new tool for detecting changes. http://www.variation.com/files/articles/changepoint.pdf. Accessed 27 Aug 2015
Vanderploeg HA, Liebig JR, Nalepa TF, Fahnenstiel GL, Pothoven SA (2010) Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. J Great Lakes Res 36:50–59
Wilson KA, Howell ET, Jackson DA (2006) Replacement of zebra mussels by quagga mussels in the Canadian nearshore of Lake Ontario: the importance of substrate, round goby abundance, and upwelling frequency. J Great Lakes Res 32:11–28
Zhu B, Fitzgerald DG, Mayer CM, Rudstam LG, Mills EL (2006) Alteration of ecosystem function by zebra mussels in Oneida Lake: impacts on submerged macrophytes. Ecosystems 9:1017–1028
Zhukova TV, Radchikova NP, Mikheyeva TM, Veres JK, Medvinsky AB, Lukyanova EV (2017) Long-term dynamics of suspended matter in Naroch Lakes: trend or intervention. Inland Water Biol 10:250–257
Zhulidov AV, Nalepa TF, Kozhara AV, Zhulidov DA, Gurtovaya TY (2006) Recent trends in relative abundance of two dreissenid species, Dreissena polymorpha and Dreissena bugensis in the Lower Don River system, Russia. Arch Hydrobiol 165:209–220
Zhulidov AV, Kozhara AV, Scherbina GH, Nalepa TF, Protasov A, Afanasiev SA et al (2010) Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biol Invasions 12:1923–1940
Acknowledgments
We thank Edward Mills for the inspiration to study mussel dynamics in Oneida Lake and for leading the annual surveys of mussels in Oneida Lake from 1992 through 2009. Many technicians and graduate students at the Cornell Biological Field Station contributed to these data sets, including Spencer Hall, Fred Henson, Catherine Hoffman, Michael Hoffman, Rachel Keats, Jana Lantry, Eric Pueschel, Travis Spier, Jonathan Swan, and Carrie Wafer. We also wish to thank Lyubov Burlakova, Cayelan Carey, Nasseer Idrisi, Alexander Karatayev, and Christine Mayer for discussions on mussel dynamics and Ladd Johnson and an anonymous reviewer for helpful comments on the manuscript. This research was supported by Cornell University, New York State Department of Environmental Conservation (NYSDEC) grant to JRJ and LGR, and United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA), Hatch Project 0226747 to LGR, RLS and JRJ. Additional support to ALH was provided by Cayelan Carey at Virginia Polytechnic Institute and State University through a National Science Foundation (NSF) Grant 1517823. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the NIFA, NSF, NYSDEC, or USDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hetherington, A.L., Rudstam, L.G., Schneider, R.L. et al. Invader invaded: population dynamics of zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena rostriformis bugensis) in polymictic Oneida Lake, NY, USA (1992–2013). Biol Invasions 21, 1529–1544 (2019). https://doi.org/10.1007/s10530-019-01914-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-01914-0