Abstract
We examined trends in expansion patterns and relative abundances of Dreissena bugensis in reservoirs and major river systems in eastern Europe. Based on our own data and data from the literature, it is apparent that trends were variable across river basins and not easily related to environmental conditions. In some cases these did not conform to the patterns typically found for dreissenids. In the early period of expansion beyond its native range in the Dnieper-Bug delta and estuary, D. bugensis rapidly replaced Dreissena polymorpha in the upper Dnieper River system, but increased only gradually and over time became less abundant relative to D. polymorpha in the Don-Manych River system. Contrary to the Dnieper and Don River systems, in the Volga River system considerable spatial variability in relative abundances was apparent, particularly in northern reservoirs. Moreover, even though D. bugensis usually displaces D. polymorpha as the dominant dreissenid, the latter can remain dominant in certain types of habitats where conditions are less favourable for the former. Suggested factors that may be responsible for differences in invasion patterns in the river systems may include differential responses to temperature, or to some other factor(s) associated with geographical latitude, the level of water mineralization, and selective predation by molluscivorous fish. In particular, the northward expansion of D. bugensis seems to be limited by temperature. The lack of long-term data on appropriate scales precludes linking these differences to specific features within the environment, but our comparisons indicate that the expansion of D. bugensis relative to D. polymorpha is more complex than previously believed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most studies of biological invasions focus, first of all, on interactions between the invading species and the whole recipient ecosystem, or on interactions between the invading species and individual native species. However, the growing number and proportion of nonindigenous species in both terrestrial and aquatic ecosystems make interactions between these species themselves the subject of increased interest. Among well-known interactions are those that are facilitative in nature, including the “invasional meltdown” effect when early invaders alter ecosystems and facilitate further colonization by species from similar donor ecosystems (Simberloff and Von Holle 1999; Ricciardi 2001). Less information is available on interactions that are competitive, such as when one invasive species appears to prevail over a former dominant invader, or even completely replaces it.
The quagga mussel, Dreissena bugensis (Andrusov, 1897) [note: Therriault et al. (2004) considered it a subspecies of Dreissena rostriformis and hence changed its status to Dreissena rostriformis bugensis] and the zebra mussel, Dreissena polymorpha (Pallas, 1771) are closely related species of Ponto-Caspian origin that have invaded extensive areas in both Europe and North America (Mills et al. 1993; Kharchenko 1995; Nalepa et al. 2001; Orlova and Scherbina 2002; Orlova et al. 2004; Therriault et al. 2004; Van der Velde and Platvoet 2007). These dreissenids have attracted great attention not merely because of their outstanding ability to colonize new habitats and water bodies, but also because of their great impact on aquatic communities, especially in the New World (Ludyanskiy et al. 1993; Vanderploeg et al. 2002; Orlova et al. 2004). The recent and asynchronous range expansion of these two ecologically similar species places them in direct competition for available resources. In areas where they are sympatric, D. bugensis seems to be competitively superior to D. polymorpha, having quickly displaced D. polymorpha as the dominant dreissenid in North American lakes and rivers (Mills et al. 1999; Ricciardi and Whoriskey 2004), and in rivers and man-made lakes of Ukraine and Russian Federation (Kharchenko 1995; Mills et al. 1996; Orlova and Scherbina 2002). In some areas, however, a partitioning of available substrate has allowed D. polymorpha to remain co-dominant with D. bugensis (Diggins et al. 2004).
In our previous studies, we unexpectedly found low abundances of D. bugensis relative to D. polymorpha in the lower Don River basin despite the former species having colonized the region for over a decade (Zhulidov et al. 2004). A further and more thorough investigation of relative trends in D. bugensis and D. polymorpha abundances over a 27-year period in the same lower Don River basin has revealed a complex temporal pattern. Prior to 1999, D. bugensis increased in abundance relative to D. polymorpha and eventually became the dominant species; however, after 1999 this species declined relative to D. polymorpha (Zhulidov et al. 2006). This unexpected recent tendency of D. bugensis to decline in numbers (in relation to total dreissenid abundance) in at least part of its non-native range suggests a closer scrutiny of all earlier data dealing with the relative abundance and expansion of D. bugensis. In this paper, we re-examine the invasion history of D. bugensis in all the main river basins in the former USSR with the following objectives: (1) summarize available data on the relative number of dreissenids throughout Europe; (2) determine whether the relative abundance of D. bugensis, after a period of initial increase, tends to decrease in a general and temporally consistent pattern over its range in Europe; (3) compare shifts in dreissenid relative abundances across different river systems and between dreissenid populations found in the old and new worlds; (4) compare invasion patterns of D. polymorpha and D. bugensis relative to their ecophysiological characteristics and to environmental factors among and within the main river basins.
Materials and methods
To reconstruct relative abundance trends of D. bugensis in the largest river systems of Eastern Europe, we gathered and organized all available data derived from our own sampling and from that of others, both published and unpublished. From our own data set, a total of 219 samples from 59 localities in the Dnieper, Danube, Don, and Volga River basins were examined. These data were collected in most years between 1977 and 2007 by several independently working research groups. Additional literature-derived data from 51 samples at 23 localities over the period 1975–2007 within these river systems were also examined. In our studies, samples of dreissenids were collected from the upper, middle and lower courses of the Volga River, lower Don River (downstream from the Tsimlyansk Reservoir dam to the delta), Manych River, different portions of the Dnieper River basin, and from the Danube River delta (Fig. 1). The initial year of sampling and the length of time series varied between the sites. Samples were usually taken once a year during the ice-free season (April to October). Dreissenids were collected in the framework of biomonitoring programs under the supervision of the Hydrochemical Institute of the Federal Service of Russia for Hydrometeorology and Environmental Monitoring (Rostov-on-Don, Russian Federation), Institute for Biology of Inland Waters Russian Academy of Sciences (IBIW RAS), (Borok, Russian Federation) and the Institute of Hydrobiology (IHB NASU), (Kiev, Ukraine).
Since our purpose was to document general trends in relative abundances of the two species over a wide area, we focused, when possible, on collecting specimens from a variety of substrates and water depths. In particular, in the lower Don River and lower Volga River, mussels at each site were collected from two depths (Zhulidov et al. 2006). In shallow water (<1 m), they were collected by hand from any accessible substrate along the shore (concrete piles, twigs, macrophytes, etc.). In deeper water (1–6 m) mussels were collected with an Eckman-type box corer (area = 0.01 m2). These methods were followed throughout the entire study period. In the upper and middle Volga River basin samples were collected only with the Eckman box corer.
Besides collecting samples in the major river systems, samples were also collected in some of the major reservoirs. In the cooling pond of the Chernobyl nuclear power plant mussels were collected from stones by hand, at depths of 0.5 and 2 m. In deeper water, an Eckman-type box corer was used (area = 0.01 m²). Mussel samples in the Kiev Reservoir and Kanev Reservoir were collected using SCUBA at depths up to 3 m.
On each sampling date regardless of water body, care was taken to collect a representative sample (90–300 individuals) from each site. All specimens were dried whole (shell and soft tissue) and stored in doubled polyethylene bags. Subsequently, all dreissenids collected were identified to species. Identifications were based on shell morphology as described by May and Marsden (1992). Specimens identified as D. polymorpha had a well-developed carina between the ventral and dorsal surfaces, while specimens identified as D. bugensis did not have a carina. In the lower Don River and lower Volga River only large individuals of similar size (15–22 mm) were identified, following protocols of the monitoring program of the Hydrochemical Institute (Rostov-on-Don). The percentage of individuals of both species was calculated from our own collections and from data obtained from the literature. In some cases, literature values were given not as abundances but as biomass. These values were also included in our analysis since, in spite of possible size differences between both dreissenid species, relative trends would still be valid and proportionately comparable.
Results
Dnieper River delta and Dnieper-Bug Liman (estuary) (native range), Ukraine
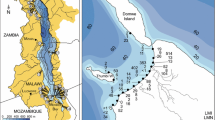
Dreissena bugensis was first described as Dreissena rostriformis from the lowermost, estuarine portion of the Southern Bug River (also known as Southern Bug River Liman), near the city of Nikolaev (Andrusov, 1890; Figs. 1, 2). Documentation of its early occurrence and abundance are rather fragmentary, giving rise to conflicting accounts of initial distributions. For instance, it was stated that the historical range of D. bugensis was restricted to the Southern Bug River estuary and the lower Ingulets River until 1940s, being absent both in the Dnieper portion of the Dnieper-Bug estuary and in the lower Dnieper River (Mills et al. 1996). However, the Ingulets River is a tributary to the lower Dnieper River, joining the main river just upstream from the city of Kherson and is not connected to the Southern Bug River. This river may have been confused with the Ingul River since the mouth of the Ingul River was the type location of D. bugensis (Andrusov, 1890). On the other hand, Zhuravel (1951) first recorded a locally abundant population of D. bugensis in the Ingulets River from the mouth to about 100 km upstream, and had no doubt it was native there. While D. bugensis was not found between these two river systems, it is unlikely that D. bugensis maintained such a disrupted distribution until its recent expansion. Moreover, according to Son (2007a) D. bugensis was collected from the Dnieper River delta near Kherson City in the late 1890s, which is virtually the same time it was found and described. For these reasons, we here recognize that the whole Dnieper-Bug estuary, Dnieper River delta, and lower Ingulets River constitute the native range of D. bugensis.
Location of sampling sites in the Dnieper River system. Site enumeration as in Table 1
A rapid expansion outside this small area into the newly constructed Dnieper River reservoirs and to other parts of the Ponto (Black Sea)-Azov Sea region began in the 1940s (Zhuravel 1951, 1967; Tseeb et al. 1966; Zhulidov et al. 2005). As its range expanded, it replaced D. polymorpha as the dominant dreissenid in these water bodies (Kharchenko 1995; Mills et al. 1996). Despite the dominance of D. bugensis in this expanded range, its present status within its native range is less clear. Interestingly, several recent surveys in the 1990s indicated that D. bugensis was now rare in the Southern Bug River. For instance, Alimov and Bogutskaya (2004) did not find D. bugensis in the Bug River, although the authors did not specify which river section was examined. Another survey in the early 1990s reported that D. bugensis was rare in the Southern Bug River estuary (Zhulidov et al. 2006), and a survey in the late 1990s found that D. polymorpha was 10 times more abundant than D. bugensis (I.A. Grigorovich, personnel communication). The situation appears quite different in the Dnieper River portion of the native range. Moroz (1993) reported that in the Dnieper River near Kherson City the contribution of D. bugensis increased from 59.0 to 99.8% between 1975 and 1978. In the same area, Rosenberg and Ludyanskiy (1994) found that D. bugensis comprised 99% of dreissenid biomass in 1993, and Orlova et al. (2004) estimated the relative number of D. bugensis was 86% for the riverbed slope and 62% for the littoral zone in 2001. Our data on dreissenid relative abundance within the native range of D. bugensis (Fig. 2) are presented in Table 1. It is evident that in the Dnieper River basin the proportion of D. bugensis relative to D. polymorpha increases upstream, from the lowermost site (Valyzhin Les) where only single individuals of D. bugensis were collected to the Dnieper River at Kherson City where it was highly dominant. This finding is generally consistent with the literature cited above. However, in the shallow water periphyton community near Kherson City it comprised only a small fraction of the total dreissenid abundance. In the Dnieper-Bug Liman both species contributed to total dreissenid abundance in almost equal proportions.
Dnieper River reservoirs, Ukraine
The general trend noted in all parts of the Dnieper River basin was that D. bugensis tended to replace D. polymorpha and dominate the dreissenid population in about 5–10 years after becoming established within the invasion area (Tseeb et al. 1966; Kharchenko 1995; Mills et al. 1996; Alimov and Bogutskaya 2004). Unfortunately, there were extensive periods when no data was collected in some parts of the Dnieper River cascade (Mills et al. 1996) so a detailed record of this process is not available, especially in the last 15 years. In 1993 the contribution of D. bugensis to the total dreissenid biomass in different parts of the Dnieper River system was as follows: 77% (Desna River mouth), 91% (Dnieper River at Kiev City) and 0.6% (Dnieper River at Dnepropetrovsk City) (Rosenberg and Ludyanskiy 1994; see also the data for the Dnieper River at Kherson City for comparison, Fig. 2). The proportion of D. bugensis at one of three locations was therefore unexpectedly low and not consistent with common observations. In the Kanev Reservoir, the proportion of D. bugensis was highly variable. In the river portion of the reservoir near Kiev City the number of D. bugensis was 27 times greater than the number of D. polymorpha within periphyton, while in the lake portion its number on the same substrate was 1.5 greater (Silaeva and Proasov 2005). In the Kiev Reservoir, D. polymorpha comprised 87% of the dreissenid population in numbers and 94% of biomass on shells of Unio sp., but D. bugensis comprised 80% of numbers on sand bottom (Silaeva and Proasov 2005). Alimov and Bogutskaya (2004) indicated that in the lower Dnieper River reservoirs (Dneprodzerzhinsk Reservoir and Kakhovka Reservoir) the species ratio in dreissenid assemblages was about 1:1. However, earlier investigations (in 1995) in the Kakhovka Reservoir near the Zaporozhskaya nuclear power plant cooling pond reported that all dreissenids were D. bugensis on the stone substrates examined (Protasov, unpublished data). In the water cooling reservoir of the Krivoy Rog town thermoelectric power station, which is a 30 km channel and receives water supplies from Kakhovka Reservoir, contributions of D. polymorpha and D. bugensis to total dreissenid biomass in benthos was, according to Alimov and Bogutskaya (2004), 51.6 and 48.4%, respectively (no sampling year is provided, but authors apparently mean recent data). Relative values within the shallow, periphyton habitat were much different: D. polymorpha accounted for 90% of total dreissenid biomass and D. bugensis accounted for 10%.
We do not have regular monitoring data on relative dreissenid abundances or biomass for all the Dnieper River reservoirs over the last decade. Perhaps the most extensive temporal data set available is from the cooling reservoir of the Chernobyl nuclear power plant in the lower part of the Pripyat River. It is not clear when D. bugensis became established in this reservoir. However, it was found in the vicinity of the reservoir in 1990 when it was recorded in the lower Pripyat River, a tributary to the Dnieper River connected to the Chernobyl cooling reservoir by a channel (Lukashev 2001). A survey in 1998 showed that the proportion of D. bugensis was 32–63% on stones throughout the reservoir except in the area nearest the warm-water discharge where the percentage was only 2%. Thus, apparently D. bugensis does not do as well as D. polymorpha in areas with elevated water temperatures, although Alimov and Bogutskaya (2004) suggest temperature was not the only factor in this case. In 2002, which was the second year after the power plant shutdown, the proportion of D. bugensis on stone or concrete bank facing ranged from 84 to 100% (10 sites, mean 94%) at depth 0,5 m, and from 85 to 98% (7 sites, mean 93%) at depth 2.5 m. The proportion within littoral periphyton communities at depth 3 m was 100% for all sites examined.
In July, 2007 D. bugensis was unexpectedly found in the Sluch River, a second-order tributary of the Pripyat River, at the town of Novogorod-Volynskiy, which is upstream from the Pripyat River mouth. The dreissenid community consisted mostly of D. polymorpha with only single individuals of D. bugensis being collected. No evidence of this species presence was found in other Pripyat River tributaries (Styr, Goryn, Ubort, Stviga, Slovechna rivers) nor in the upper Pripyat River and associated floodplain lakes.
Lower Don and the Manych River basins, Russian Federation
A likely pathway by which D. bugensis entered the Don River system was via the Dnieper-Donbass canal, which was built in the early 1980s (Kharchenko 1987; Alimov and Bogutskaya 2004). The subsequent expansion of D. bugensis in the Don River basin has not been well documented, with data being scarce and contradictory (Zhulidov et al. 2004, 2005). Orlova et al. (1999) indicated that D. bugensis invaded the Don River basin in the late 1980s and Kharchenko (1987) first recorded it in the Severskiy Donets River in 1987. The Severskiy Donets River is a main tributary to the lower Don River and is considered an invasion corridor.
Samples of Dreissena were collected from 15 monitoring sites in the lower Don River system, in the lower part of the Tsimlyansk Reservoir, and also in the Manych River system within the western part of the Kuma-Manych Depression (Fig. 3; see also Zhulidov et al. 1997) during the period 1977–2004 (five sites), 1999–2004 (eight sites), 2001–2004 (one site) and 2002–2004 (one site) (Zhulidov et al. 2006). D. bugensis was recorded at these sites in 1980, and was first found at sites in the lower portion of the Don River and then at sites in the upper portion. The relative proportion of D. bugensis gradually increased at each of these sites, reaching a peak of 30–50% of the dreissenid population in 1999. However, beginning in 1999 through 2004, the relative proportion of D. bugensis declined at 14 of the 15 sites sampled. The only location where D. bugensis did not decline was at the site in Tsimlyansk Reservoir (see Station 1, Fig. 3) where it comprised only a small fraction of the dreissenid population (≤5%). For the 14 remaining sites, the mean decline between 1999 (13 sites) or 2001 (1 site) and 2004 was 24% (range 14–36%), and this difference was significant (Wilcoxon signed rank test; P < 0.01). Proportional declines of D. bugensis in the lower Don River and the Manych River basins were similar despite greater initial abundances of this species in the latter system. Over the 6-year period, the association in trends between the stations was highly significant (Kendall’s coefficient of concordance = 0.81; P < 0.001; excluding Station 1). In 1999, D. bugensis accounted for 25–50% of total dreissenid numbers at sites in the Don River downstream from the Tsimlyansk dam and 65–75% in the Manych River. By 2004, relative proportions decreased to 10–18 and 33–43% in the two rivers, respectively.
According to Antonov and Khokhlova (2004), the proportion of D. bugensis in the total dreissenid population in the Severskiy Donets River in 2003 was 40%. This value, while higher than that in the main Don River in 2003–2004, is consistent with the lower relative abundance of D. bugensis found in the Don-Manych River system as compared to the Dnieper River system.
The Volga River basin, Russian Federation
According to Antonov (1993), D. bugensis was first found in the middle stretch of the Volga River (Saratov and Kuybyshev reservoirs) in 1992. Since this first record, it has invaded most reservoirs in the upper, middle, and lower reaches of this river system, ranging upstream as far as Uglich Reservoir, and downstream as far as the river delta and transitional zone of the Caspian Sea (Orlova et al. 2004; see Fig. 1). Between 1992 and 2001, Orlova et al. (2004) repeatedly collected dreissenid samples throughout all the Volga River cascade, and described population dynamics and invasion chronology of D. bugensis in this river system. Their data provided a basis of comparison to our own data set.
In the Volga River system, we collected dreissenid samples from 24 sites, 8 of which were located on the upper Volga River section (Rybinsk Reservoir, sampled in 1997–2005), 5 on the middle Volga River section (Gorky Reservoir, sampled in 2000–2005) and 11 on the lower Volga River section (Volgograd Reservoir, sampled in 1981–2003). Trends in the proportion of D. bugensis in the total dreissenid population in the lower Volga River are shown in Fig. 4. According to our records, D. bugensis was present in the lower stretch of the Volga River already in 1981, which is much earlier than previously believed. Samples taken in 1981 and 1983 were not quantitative so relative abundances cannot be assessed. Samples were quantitative in subsequent years, and the data showed only minor contributions of D. bugensis to total dreissenid abundance, with percent contribution ranging between 2 and 15% in 1986–1995, and between 18 and 22% in 1999–2003. This slight increase contrasts with temporal trends in abundances reported by Orlova et al. (2004; Fig. 4). These authors report proportions of 1–4% in 1992 but up to 92–100% in 1998 at the settlement of Mordovo (Saratov Reservoir), and 13% in 1994 up to 98% in 2000 in the region of Damchik (Volga River delta).
The percentage of Dreissena bugensis within the total dreissenid population at different sampling sites in the lower Volga River. Open: our data collected at a site near Volgograd City; Dots: data from Orlova et al. (2004) collected at the Damchik area of the Astrakhan biosphere nature reserve (Volga River delta); Grid: data from Orlova et al. (2004), Volga-Caspian canal (Volga River avandelta); Diagonal hatching: data from Orlova et al. (2004), a site in Gandurino Canal (Volga River avandelta); Horizontal hatching: data from Orlova et al. (2004), Chistaya banka Island (Northern Caspian Sea)
In 2007, D. bugensis was discovered in Sharony Lake, which is located at the foot of the Yergheni Hills (Zhulidov et al. 1997) south of Volgograd City (Nikitenko 2007). This lake is fed from the lower Volga River through an irrigation canal. The proportion of D. bugensis averaged 35% and only individual yearlings were present in the population.
In the upper and middle stretches of the Volga River, spatial patterns in relative abundances of dreissenid species appear even more complex. D. bugensis was first discovered in the upper river section in 1997, and, based on our own data and that of others collected in 1997, 2000, 2001, and 2005, there is no evidence that D. bugensis increased relative to D. polymorpha over this period, even though densities indicated the population was well established (Orlova et al. 2004). Moreover, our data suggest a striking spatial heterogeneity in relative abundances that is manifested on various scales (Table 2). For example, on a local scale, sites No. 3 and No. 4 (Fig. 5) are merely 1 km apart yet differ strongly in relative dreissenid species numbers, with relative abundances of D. bugensis 20 and 91%, respectively. In the southern part of this reservoir, D. bugensis attained maximum abundance 3–4 years after its first record in the Volga River reach in 1997 (Orlova et al. 2004). Four years after that, in 2005, a more detailed survey revealed a complex spatial distribution pattern, with the proportion D. bugensis ranging from 18 to 99.4% at individual sites.
Location of sampling sites and relative abundances of D. bugensis and D. polymorpha in the upper Volga River system (Rybinsk Reservoir). Site enumeration as in Table 2; D. bugensis = black shading, D. polymorpha = grey shading
On a wider scale, the proportion of D. bugensis dramatically decreased from the southern to the central and northern parts of the Rybinsk Reservoir. In particular, in 2000 and 2001 at most sites in Mologa River reach of the reservoir and near the Central Cape (located between Mologa reach and Sheksna reach), D. bugensis was not found or was rare (Orlova and Scherbina 2001; Orlova et al. 2004). Further, data collected in 2001 and 2005 indicated that the D. bugensis population did not expand relative to D. polymorpha over this period. In the Gorky Reservoir, the proportion of D. bugensis was less variable, ranging in 2005 from 5 to 45% at various stations. Nonetheless, this spatial heterogeneity complicates any conclusions concerning relative abundance trends in dreissenid communities. Between 1996 and 1997, the relative abundance of D. bugensis in the Gandurino Canal (Volga River avandelta) increased from 4 up to 78% (Orlova et al. 2004). If we compare this increase with the population dynamics of the quagga mussel in the lower Volga River at Volgograd City (see Fig. 4), it gives rise to the question whether these values were representative of the whole canal stretch or simply habitats with highly different proportions of D. bugensis were sampled in two subsequent years.
Therefore, at least in the Volga River basin, especially in the northern portion of the invasion range, temporal patterns in the relative dreissenid distribution, even if they exist, seem to be masked by spatial heterogeneity of the data.
There seem to be very few data on D. bugensis occurrence in tributaries of the Volga River. The only known record of this species is in the Izh River where single individuals were found in 2003–2004 about 30 km upstream from the Nizhnekamskoye Reservoir (Antonov and Khokhlova 2004). This reservoir is the lowermost in the Kama River cascade. The Kama River is the largest Volga River tributary flowing from the east and forms a large bay of the Kuybyshev Reservoir (Fig. 1). Thus, the distribution of D. bugensis is rather disjointed in this region.
In October, 2003 D. bugensis was found in the Moskva River within the boundaries of the City of Moscow (Lvova 2004). At the time it comprised 12% of total dreissenid abundance and all individuals were <2 years old. By 2004, its proportion in the dreissenid community at the same site had increased to 71%.
Water bodies west of the Dnieper River basin
In 1992–1993, D. bugensis was found in the Dniester Reservoir located in the middle part of the Dniester River (Kharchenko 1995). By 2001 it was recorded also in the lower stretch of this river basin including the Dniester River Estuary (Alimov and Bogutskaya 2004). Recently, D. bugensis was found in the lower Danube River, Romania (Micu and Telembici 2004; Popa and Popa 2006). The latter authors made a prediction that this species will be able to reach Western Europe very quickly owing to well-developed artificial waterway system. Indeed, in April, 2006, D. bugensis was discovered in the Rhine River delta near the town of Willemstad in the Netherlands (Molloy et al. 2007) and in May, 2007 in the Main River near Hörblach (Germany), close to the Danube-Main canal (Van der Velde and Platvoet 2007). Based on the size of individuals found, the latter authors concluded that D. bugensis became established several years before their finding. The proportion of D. bugensis in the dreissenid communities at these new sites was variable; in the Hollands Diep there were about 1% of D. bugensis while in the Main River 13 of 39 (33%) collected dreissenid specimens were D. bugensis.
In August, 2007 we sampled dreissenids from shallow-water periphyton communities in the Danube Delta at Vilkovo settlement (Ukraine) and in the Romanian part of the delta and found D. bugensis to comprise at different sites from 7 to 39% of total dreissenid number. Lyashenko et al. (2009) also sampled not far from Vilkovo settlement and found both dreissenid species to contribute to total dreissenid abundance in equal proportions, that is, 50%.
Discussion
This review of D. bugensis expansion in Eastern Europe revealed a generally complex pattern of expansion that seems to vary greatly between different river basins, and was at times inconsistent with current views of invasion patterns as related to rates and temporal trends. This complex pattern was also noted when expansion was examined within its native range, in the fresh and brackish water bodies of the northern Black Sea region (Son 2007b). The commonly accepted scenario assumes a time delay of several years to several decades in the expansion of D. bugensis relative to D. polymorpha, a rapid population increase in water bodies and biotopes previously occupied by the latter species, and a subsequent competitive displacement of D. polymorpha as the dominant species up to its almost complete elimination from dreissenid assemblages.
This temporal sequence seems to be the case in the region of earliest expansion, i.e., in the Dnieper River reservoirs, although the extent of replacement is seemingly not as uniform as first believed. It is noteworthy that all cases where it was evident D. bugensis declined after a period of strong dominance (if such a period truly took place on a consistent basis) occurred in the lower part of the Dnieper River cascade, that is, within the region of the initial range extension. Within the native range, prevalence of its competitor, D. polymorpha, was also frequently observed. On the contrary, in the cooling reservoir of Chernobyl nuclear power plant located in the upper portion of the Dnieper River cascade, which was only recently invaded, the dreissenid species ratio is distributed very evenly despite a fairly heterogeneous environment (Protasov and Silaeva 2005). However, it should be noted that evidence of D. bugensis declining in the Dnieper River system as a whole is insufficient.
A substantially different pattern was observed in the lower Don River system. All sites within the lower Don River and Manych River basins displayed a similar relative decline in D. bugensis after a period of gradual and relatively slow increases as compared to the Dnieper River basin. The maximum attained contribution of D. bugensis to total dreissenid number (30–50%) is considerably less than in the Dnieper River (99%; Rosenberg and Ludyanskiy 1994). This consistent decline occurred over a waterway system of more than 600 km, and across sites that were likely subjected to a variety of local influences. To cause such an extensive and synchronous decline, either a broad, fundamental change in river conditions would have to occur beginning in 1999, or conditions were unfavourable for D. bugensis to expand in the new habitat after successful initial colonization.
The third type of expansion pattern of D. bugensis in areas already inhabited by D. polymorpha is manifested in the Volga River basin and features marked spatial heterogeneity. Most publications documenting the invasion history of D. bugensis in this river system have paid special attention to temporal rather than spatial patterns of dreissenid species ratio (Orlova et al. 2004). Extensive field studies shortly after the first records of D. bugensis in this river system (Orlova et al. 2004) seemingly suggest the same expansion pattern and species replacement as in the Dnieper River basin. However, this preliminary conclusion needs to be reconsidered given more recent data collected in the Rybinsk Reservoir, Gorky Reservoir, and the Gandurino Canal of the Volga River Delta that suggests extensive spatial heterogeneity. It is still difficult to determine whether differences in relative dreissenid abundance trends discovered between the large river basins reflect real specificity of invasion history in these basins and/or habitats being colonized, or whether differences are merely a result of comparing incomplete data series, often with inconsistent sampling methods and sampling schemes. The longest and most regular series of observations made at fixed monitoring sites occurred within the Don River basin, and these observations show a very uniform pattern of dreissenid distribution. Despite far shorter time series obtained from the Volga River basin, it is evident that pronounced spatial heterogeneity of the data would interfere with any temporal trend assessment. The situation in the Dnieper River basin appears to be more complex and comprises both areas with relatively stable and uniform dreissenid population ratio (Chernobyl cooling reservoir) and areas for which inconsistent and somewhat conflicting data were obtained (the lowermost stretch of the Dnieper River).
In examining invasion patterns and relative abundance of D. bugensis in these river systems, it is difficult to delineate the most important biological or environmental characteristics that can explain observed differences. The geographical scale and diversity of water bodies and habitats examined suggests multiple factors may regulate and control relative abundances of dreissenid species. Both D. polymorpha and D. bugensis have biological and ecological adaptations ensuring their successful dispersal far beyond their native ranges. Both have pelagic larvae allowing them to spread by passive migration downstream in rivers and canals, and the adult-attached stage enables both species to spread by shipping, boating, etc. as components of biofouling. Both species have high ecological plasticity enabling them to survive in a broad range of environmental factors (Kharchenko 1995; Mills et al. 1996; Alimov and Bogutskaya 2004; Orlova et al. 2005).
Most all previous studies in both Eurasia and North America have shown that D. bugensis, over time, displaces D. polymorpha. Displacement has occurred in a variety of different habitat types, ranging from warm-shallow and deep-cold lakes, to rivers and reservoirs (Kharchenko 1995; Mills et al. 1996, 1999; Orlova and Scherbina 2002; Ricciardi and Whoriskey 2004). Reasons for the competitive advantage of D. bugensis seem to lie in species-specific differences in physiological characteristics. D. bugensis has higher assimilation efficiency than D. polymorpha so that at low food levels it maintains higher growth and fecundity rates (Baldwin et al. 2002). Also, D. bugensis has a lower respiration rate under different seasonal temperature regimes (Stoeckmann 2003). Lower respiration rates decrease metabolic costs, allowing D. bugensis to have greater growth and greater allocations of energy to soft body mass than D. polymorpha at similar food conditions (Roe and MacIsaac 1997). These attributes give D. bugensis a strong competitive advantage during periods of low food and high temperatures. Also, because of higher growth rates, D. bugensis larvae settle at a larger size than D. polymorpha larvae, giving them a competitive advantage (Martel et al. 2001). While some laboratory studies found D. bugensis to be less tolerant of higher temperatures than D. polymorpha (Domm et al. 1993), controlled studies under more natural conditions showed that growth and survival of D. bugensis was similar to, or greater than, D. polymorpha at typical summer-warm temperatures (MacIsaac 1994; Thorp et al. 2002).
There is a somewhat paradoxical situation noted by Alimov and Bogutskaya (2004) in which an ultimately more successful species that gains dominance over its precursor in benthic communities appears to be less aggressive in terms of range extension. According to Orlova et al. (2005), this contradiction may be explained by certain characteristics of D. bugensis, a representative of the ‘liman opportunists’ faunistic group preferring more lentic lacustrine environments. These authors conceive the process of reservoir impoundment as, in a way, river ‘estuarization’ facilitating further expansion of such animals into river systems. Another important feature of this species is a higher tolerance to oligotrophic, cold-water conditions compared to D. polymorpha, probably resulting from its close phylogenetic relationships with the Caspian deep-water form D. rostriformis distincta (Orlova et al. 2005). These characteristics likely prove limiting for its expansion into riverine habitats as compared to D. polymorpha. After construction of large reservoirs on the Dnieper River and stabilization of their hydrological regime, in particular, forming a cold-water hypolimnion, the appropriate conditions were created for D. bugensis to start expanding upstream and subsequent rapid expansion to a number of large river basins via both larval drift and shipping.
Our data suggests that this scenario is probably too simplified. Both literature and our data suggest that D. bugensis can also colonize riverine and other lotic habitats. Alimov and Bogutskaya (2004) found D. bugensis in the canals connecting the lower Dnieper River reservoirs with Crimea Peninsula, and in the Donbass region, although in proportions less than 1:1. The Dnieper-Donbass canal is considered the pathway by which D. bugensis expanded into the Don River system. But the canal, as well as the Severskiy Donets River and the lower Don River, have constant water flow with no impoundment dams. D. bugensis started to successfully colonize this river system right after canal construction. If D. bugensis was able to survive and establish populations in these typically riverine conditions, the question arises, what prevented its human-mediated migration upstream in the Dnieper River basin before construction of the reservoir cascade? The vulnerability of the lower Don River as a target ecosystem for D. bugensis invasion looks equally high as for “estuary-like” artificial reservoirs. We currently have no appropriate answer to this question.
Despite being considered more of a cold-water species compared to D. polymorpha, D. bugensis seems to be limited in its dispersal in the northernmost part of its invasion range in the Volga River basin by temperature, or by some other factor associated with geographical latitude. The whole northern part of the Rybinsk Reservoir appears to be a real “dead zone” for this species while D. polymorpha is commonly found. This seemingly atypical pattern needs more thorough examination as it can elucidate possible causes for the absence of this otherwise extremely successful invader in the reservoirs north of the Volga River and Dnieper River basins, in particular, in water bodies of the Volga-Baltic traffic system. In this respect, lack of D. bugensis virtually in the whole Kama River and the presence of D. polymorpha is also of interest. This largest Volga River tributary has a developed system of artificial reservoirs that were constructed several decades ago and, to all appearances, there is nothing to preclude the range extension of D. bugensis into this river system. In any case, it is noteworthy that, as the latest findings in Central and Western Europe suggest, westward expansion of D. bugensis seems to be much faster than to the north or north-east.
Another important factor controlling the D. bugensis expansion into inland waters is probably the mineral content of the water, especially in arid zones. In a previous paper, we speculated that the greater dominance of D. bugensis in the Manych River compared to the lower Don River might be due to differences in total mineral/calcium content (Zhulidov et al. 2004). Total mineral content was 1780–2360 mg/l in the Manych River, but only 450–810 mg/l in the Don River system; calcium content was 119 mg/l and 45–78 mg/l, respectively. We suggested that higher calcium levels in the Manych River favoured D. bugensis prevalence over D. polymorpha since these levels exceeded the optimum for D. polymorpha (70 mg/l; Ludyanskiy et al. 1993). Orlova et al. (2005) have generally agreed with such a suggestion. It is noteworthy, however, that more limnic conditions in the Manych River (a slowly flowing steppe river with dams) as compared to Don River could contribute to this pattern, too.
Variation in relative dreissenid abundance between synchronously taken samples as evident in the Volga River and, to some extent, in the Dnieper River basin, may reflect habitat partitioning between D. bugensis and D. polymorpha. Although D. bugensis usually displaces D. polymorpha as the dominant dreissenid, the latter can remain dominant in certain types of habitats. Recent studies found that D. polymorpha was more prevalent on macrophytes and in other periphyton communities than on adjacent benthic substrates (Diggins et al. 2004; Protasov 1994). On macrophytes, D. polymorpha comprised 30–61% of all dreissenids, while on benthic substrates, D. bugensis comprised 92–100%. This observation is consistent with our data (Table 1), although only one site with periphyton was sampled in the Dnieper-Bug estuary. Habitat partitioning was also observed vertically along a canal wall (Ricciardi and Whoriskey 2004). D. polymorpha dominated biomass at an upper wall location (1.5–2.0 m), but D. bugensis dominated at lower locations (>2.5 m). While a comparison of changes on such a small scale to changes in a large river system may not be justifiable, it does illustrate that abrupt shifts in relative abundances do occur.
We still cannot provide a satisfactory explanation for the abrupt and widespread decline of D. bugensis in the lower Don River and Manych River basins beginning in 1999. A fundamental change in river conditions seems unlikely. Based on long term monitoring data, broad changes in hydrochemical conditions such as flow regimes or chemical composition have not occurred in these river systems over the past decade (Zhulidov et al. 2006). Moreover, any changes on a broader scale should have impacted adjacent river basins as well. Given the above data on substrate specificity, a simultaneous increase in macrophytes in the Don River and Manych River, while unlikely, could have occurred during our study period, leading to a proportional increase in D. polymorpha. However, we sampled a variety of different substrates and all displayed a similar decrease in D. bugensis.
In a recent paper we suggested that one of the possible causes for the abrupt decline may be selective predation by the fish community (Zhulidov et al. 2006). D. bugensis has a thinner, more fragile shell than D. polymorpha allowing even large individuals to be more efficiently crushed and digested by fish (Diggins et al. 2004; Protasov 1994). As noted, D. polymorpha is more closely associated with macrophytes and periphyton communities while D. bugensis prefers benthic habitats. This distribution pattern may make the latter species more susceptible to fish predation. In Eurasia these bivalves are important food items for a variety of fish, both cyprinids, such as roach Rutilus rutilus (L.), common bream Abramis brama (L.), ide (Leuciscus idus (L.), common carp Cyprinus carpio L., and black carp Mylopharingodon piceus Richardson and non-cyprinids, especially round goby Neogobius melanostomus (Pallas) (Starobogatov 1994; Reshetnikov 2003). We have indicated that the recent expansion of D. bugensis into areas previously inhabited only by D. polymorpha can lead to shifts in food preferences of some common Eurasian fish species that previously did not feed intensively on dreissenids. Kasyanov and Izyumov (1995) pointed out that the dreissenid invasion into Volga River reservoirs resulted in a divergence of roach populations into molluscivorous and herbivorous forms, with different growth rates and different morphological and ecological characteristics. It is possible that after an initial colonization period and abundances of D. bugensis peak, the fish community adapts to this new food source, contributing to subsequent declines. D. bugensis has only recently invaded the Rybinsk Reservoir, but it has now become the dominant prey item of ide and bream that normally do not consume D. polymorpha (Scherbina 2008).
Despite some attractiveness, this hypothesis cannot be considered the only possible explanation. Based on the evidence collected, a pronounced decline of D. bugensis did not occur everywhere in other river systems while the composition of the fish community is virtually the same in all the main river basins of Eastern Europe, and there seems to be no basis for notable differences in the degree of selective pressure. However, the intriguing uniformity of post-colonization declines of D. bugensis in the lower Don River basin suggests that the cause can be attributed to very few or perhaps even a single key factor. For the Volga and Dnieper River basins a multifactor model of D. bugensis abundance determination seems more appropriate. Consequently, predicting the dynamics of dreissenid communities within these river systems is likely more difficult.
It is commonly accepted (Antonov 1993; Alimov and Bogutskaya 2004; Orlova et al. 2004, 2005) that D. bugensis first became established in the Volga River basin in 1992. However, re-examination of historic samples revealed the presence of D. bugensis (a single specimen) as early as 1981 in the lower Volga River near the town of Akhtubinsk (Zhulidov et al. 2004). Most likely, D. bugensis penetrated the Volga from the Don River (maybe with ships, either ballast waters, or as a part of biofouling species). Then this species spread over the lower Volga stretches. It seems logical then that the first occurrence of this species in the Volga River system would be close to the Volga-Don canal, which is the most likely pathway of introduction. The suggestion of Orlova et al. (2004) that the first colonization event took place in the upper or middle portion of the Kuybyshev Reservoir, with subsequent establishment downstream in the lower Volga River via veliger drift is less likely. The timing of our first record in the Volga River corresponds to the year of first record of D. bugensis in the Don River system. According to our data, D. bugensis appeared first in the lowermost portion of the Don River gradually moving upstream. In August, 1980 it had been found near the town of Azov (Fig. 3) in May, 1981 near the settlement of Aksay and village of Bagaevskaya, in August, 1984 at the village of Razdorskaya and only in May, 1991 in the lower reach of the Tsimlyansk Reservoir. It is important that all sites where the quagga mussel was found from 1980 to 1984 lie below the Severskiy Donets River mouth and were likely colonized via drifting whilst its appearance in the Tsimlyansk Reservoir is apparently explained by a human-mediated vector. It follows that D. bugensis would be introduced into the Volga River immediately from the Don River delta (where it was already established) via the Don-Volga canal.
Conclusions
The purpose of this study was to clarify relative abundance trends and invasion patterns of D. bugensis over large territories in Ukraine and Russian river systems. Expansion patterns were complex, varied considerably between river basins, and in some cases were contradictory to patterns typically found for dreissenids. In most cases, there is a time delay of several years to several decades between first colonization and expansion of D. bugensis relative to D. polymorpha. Once the expansion process proceeds, D. bugensis increases rapidly and subsequently displaces D. polymorpha as the dominant species and almost completely eliminates the latter from dreissenid communities. We found that such a general pattern occurred during the initial expansion of D. bugensis outside of its native range and into Dnieper River reservoirs. However, the expansion pattern in the lower Don River system (the rivers Don and Manych) was quite different. That is, the proportion of D. bugensis declined after a period of a slow, gradual increase as compared to the Dnieper River basin. Possible reasons for such an extensive and synchronous decline might include drastic changes in the hydrological and/or hydrochemical conditions in the river system after 1999 that were unfavourable to D. bugensis. However, at least some data indicates that such environmental changes did not occur, and thus the exact nature and causes of the proportional decline in D. bugensis remain unknown.
In the Volga River reservoirs’ cascade a third type of D. bugensis invasion pattern was apparent. This pattern is characterized by considerable spatial heterogeneity. During initial stages of the expansion of D. bugensis through the Volga River system, temporal pattern and propensity to replace D. polymorpha as the dominant dreissenid was similar to the pattern observed in the Dnieper River. However, later observations in Rybinsk Reservoir, Gorky Reservoir, and the Volga River Delta suggest extensive spatial heterogeneity, and this spatial heterogeneity together with the lack of consistent long-term data sets hinders any meaningful assessment of relative trends over time.
Studies of invasion patterns and relative abundance trends in populations of D. bugensis within these different river systems do not provide needed biological or environmental parameters to explain observed differences between systems. The geographical scale and diversity of studied water bodies and habitats suggest that multiple factors may be responsible for differing trends in relative abundances of the two dreissenid species. Both D. polymorpha and D. bugensis are very adaptive to a broad range of environmental conditions and subtle differences in these conditions may provide the impetus for a competitive advantage. Previous studies in Eurasia and North America have shown that in most cases D. bugensis, over time, displaces D. polymorpha. However, as the present summary shows, this is not necessarily true for all studied river systems in Ukraine and Russia. Some laboratory-demonstrated advantages of the former species over the latter include higher assimilation efficiency, lower respiration rate, decreasing metabolic costs of growth and greater allocations of energy to mantle tissues, and a comparatively higher growth rate. However, the present study suggests that considering only such physiological advantages of D. bugensis over D. polymorpha likely oversimplifies the competitive process. Given our present state of knowledge, we can not ascertain reasons for the differing patterns between the various river systems. Suggested factors may include differential response to temperature, or some other factor(s) associated with geographical latitude, the level of water mineralization, and selective predation by the molluscivorous fish. Even though D. bugensis usually displaces D. polymorpha as the dominant dreissenid, the latter can remain dominant in certain types of habitats where conditions are less favourable for the former. At any rate, we believe that defining reasons for different invasion patterns in these two closely related species is worth further investigation.
References
Alimov AF, Bogutskaya NG (eds) (2004) Biological invasions in aquatic and terrestrial ecosystems. KMK Scientific Press, Ltd., St. Petersburg (in Russian)
Antonov PI (1993) On the invasion of bivalve Dreissena bugensis (Andr.) into the Volga River reservoirs. In: Ecological problems of large river basins. Book of abstracts of intern. Conference, Togliatti, 6–10 September, 1993. Publishers of IEVB RAS, Togliatti, Russia, pp 52–53 (in Russian)
Antonov PI, Khokhlova SV (2004) The mollusks of g. Dreissena of small rivers of the Volga and Don basins: biodiversity, biology, conservation. In: Abstracts, All-Russian Sci. Conf., 16-19 November, 2004, Borok, Russia, pp 10–11
Baldwin BS, Mayer MS, Dayton J, Pau N, Mendilla J, Sullivan M, Moore A, Mills EL (2002) Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Can J Fish Aquat Sci 59:680–694
Diggins TP, Weimer M, Stewart KM et al (2004) Epiphytic refugium: are two species of invading freshwater bivalves partitioning spatial resources? Biol Invasions 6:83–88
Domm SR, McCauley W, Kott E (1993) Physiological and taxonomic separation of two dreissenid mussels in the Laurentian Great Lakes. Can J Fish Aquat Sci 50:2294–2297
Kasyanov AN, Izyumov YUG (1995) Growth and morphology of the roach Rutilus rutilus in the Pleshcheevo Lake in connection with Dreissena establishment. Vopr Ikhtiologii 35:546–548 (in Russian)
Kharchenko TA (1987) Dreissena—good or evil? Nauka i Zhizn 2:147–148 (in Russian)
Kharchenko TA (1995) Dreissena: range, ecology, fouling impacts. Gidrobiol Zhurn 31:3–20 (in Russian)
Ludyanskiy ML, McDonald D, MacNeill D (1993) Impact of the zebra mussel, a bivalve invader. Bioscience 43:533–544
Lukashev DV (2001) Modern state of the Dreissena populations in the cooling pond of chernobyl nuclear power plant. Gidrobiol Zhurn 37:40–45 (in Russian)
Lvova AA (2004) On penetration of Dreissena bugensis (Bivalvia, Dreissenidae) into the Ucha reservoir (Moscow province) and the Moskva River. Zool Zhurn 83:766–768 (in Russian)
Lyashenko AV, Zorina-Sakharova KE, Makovsky VV (2009) Finding of the quagga mussel Dreissena bugensis in the Kiliya part of the Danube River delta (Ukraine). Vestnik Zool 43:92
MacIsaac HJ (1994) Comparative growth and survival of Dreissena polymorpha and Dreissena bugensis, exotic molluscs introduced to the Great Lakes. J Great Lakes Res 20:783–790
Martel AL, Baldwin BS, Dermott RM, Lutz RA (2001) Species and epilimnion/hypolimnion-related differences in size at larval settlement and metamorphosis in Dreissena (Bivalvia). Limnol Oceangr 46:707–713
May B, Marsden JE (1992) Genetic identification and implications of another invasive species of dreissenid mussel in the Great Lakes. Can J Fish Aquat Sci 49:1501–1506
Micu D, Telembici A (2004) First record of Dreissena bugensis (Andrusov 1897) from the Romanian stretch of River Danube. In: Abstracts of the International Symposium of Malacology, August 19–22, 2004, Sibiu, Romania
Mills EL, Dermott R, Roseman EF et al (1993) Colonization, ecology, and population structure of the quagga mussel in the Lower Great Lakes. Can J Fish Aquat Sci 50:2305–2314
Mills EL, Rosenberg G, Spidle AP et al (1996) A review of the biology and ecology of the quagga mussel (Dreissena bugensis), a second species of freshwater dreissenid introduced to North America. Amer Zool 36:271–286
Mills EL, Chrisman JR, Baldwin B et al (1999) Changes in the dreissenid community in the lower Great Lakes with emphasis on southern Lake Ontario. J Great Lakes Res 25:187–197
Molloy DP, Bij de Vaate A, Wilke T, Giamberini L (2007) Discovery of Dreissena rostriformis bugensis (Andrusov 1897) in western Europe. Biol Invasions 9:871–874
Moroz TG (1993) Macrozoobenthos of estuaries and lower sections of rivers in the Northern-Western Black Sea. Naukova Dumka Press, Kiev (in Russian)
Nalepa TF, Schloesser DW, Pothoven SA et al (2001) First finding of the amphipod Echinogammarus icshnus and the mussel Dreissena bugensis in Lake Michigan. J Great Lakes Res 27:384–391
Nikitenko EV (2007) Distribution of the dreissenids Dreissena polymorpha (Pallas, 1771) and Dreissena bugensis (Andrusov, 1847) in the water bodies of Kalmykia. In: Proceedings of the 13th international youth school-conference, Borok, October 23–26, 2007, pp 143–147 (in Russian)
Orlova MI, Scherbina GKH (2001) Dreissena bugensis (Andr.) (Dreissenidae, Bivalvia) range expansion in Europe, invasion history, patterns and future invasion perspectives. In: US-Russia Invasive Species Workshop, Borok, Russia, 27–31 August, 2001. Book of Abstracts, pp 150–152 (in Russian)
Orlova MI, Scherbina GKH (2002) On distribution of Dreissena bugensis (Dreissenidae, Bivalvia) in reservoirs of the Upper Volga River basin. Zool Zhurn 81:515–520 (in Russian)
Orlova MI, Arakelova EA, Komendantov AYU (1999) Co-occurrence of Dreissena bugensis Andr. and Dreissena polymorpha Pall. in the Volga delta and shallow waters of the Northern Caspian Sea. In: Jubilee Conference ‘State, research and conservation of landscapes of Astrakhan’ biosphere reserve in conditions of Caspian Sea level rise and increasing anthropogenic load’ Book of Abstracts, 23–28 August, 1999, Astrakhan, Russia, pp 67–69 (in Russian)
Orlova MI, Muirhead JR, Antonov PI et al (2004) Range expansion of quagga mussels Dreissena rostriformis bugensis in the Volga River and Caspian Sea basin. Aquatic Ecol 38:561–573
Orlova MI, Therriault THW, Antonov PI et al (2005) Invasion ecology of quagga mussels (Dreissena rostriformis bugensis) a review of evolutionary and phylogenetic impacts. Aquatic Ecol 39:401–418
Popa OP, Popa LO (2006) The most westward European occurrence point for Dreissena bugensis (Andrusov, 1897). Malacol Bohemoslov 5:3–5
Protasov AA (1994) Freshwater periphyton. Naukova Dumka Publication, Kiev, p 307 (in Russian)
Protasov AA, Silaeva AA (2005) Invertebrate communities of the cooling pond of Chernobyl nuclear power plant. 2. Zooperiphyton communities, their composition and structure. Gidrobiol Zhurn 41:13–32 (in Russian)
Reshetnikov YUS (2003) Atlas of Russian freshwater fishes, 2nd edn. Nauka Publishing House, Moscow (in Russian)
Ricciardi A (2001) Facilitative interactions among aquatic invaders: is an ‘invasional meltdown’ occurring in the Great Lakes? Can J Fish Aquat Sci 58:2513–2525
Ricciardi A, Whoriskey FG (2004) Exotic species replacement: shifting dominance of dreissenid mussels in the Soulanges canal, upper St. Lawrence River, Canada. J N Am Benthol Soc 23:507–514
Roe SL, MacIsaac HJ (1997) Deepwater population structure and reproductive state of quagga mussels (Dreissena bugensis) in Lake Erie. Can J Fish Aquat Sci 54:2428–2433
Rosenberg G, Ludyanskiy M (1994) A nomenclatural review of Dreissena (Bivalvia; Dreissenidae) with identification of the quagga mussel as Dreissena bugensis. Can J Fish Aquat Sci 51:1474–1484
Scherbina GKH (2008) Modern distribution, structure and environment-forming role of dreissenids of some waterbodies in the Russia North-West and value of mollusks as a food of benthivorous fish. In: Dreissenids: evolution, systematics, ecology. Proceedings of the 1st international school-conference, Borok, October 28–November 1, 2008, pp 23–43 (in Russian)
Silaeva AA, Proasov AA (2005) The structure of Dreissena communities in the littoral zone of the Kanev reservoir. Vestn Tiumen State Univ 5:112–115
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasion meltdown? Biol Invas 1:21–32
Son MO (2007a) Native range of the zebra mussel and quagga mussel and new data on their invasions within the Ponto-Caspian region. Aquat Invasions 2:174–184
Son MO (2007b) Invasive molluscs in fresh and brackish waters of the Northern Black Sea Region. Druk, Odessa, p 132
Starobogatov JI (1994) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Publishing House, Mosco (in Russian)
Stoeckmann A (2003) Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Can J Fish Aquat Sci 60:126–134
Therriault TW, Docker MF, Orlova MI et al (2004) Molecular resolution of the family Dreissenidae (Mollusca: Bivalvia) with emphasis on Ponto-Caspian species, including first report of Mytilopsis leucophaeata in the Black Sea basin. Mole Phylogenet Evol 30:479–489
Thorp JH, Alexander JE Jr, Cobbs GA (2002) Coping with warmer, large rivers: a field experiment on potential range expansion of northern quagga mussels (Dreissena bugensis). Freshw Biol 47:1779–1790
Tseeb YAYA, Almazov AM, Vladimirov VI (1966) Laws of change of hydrological, hydrochemical and hydrobiological modes in connection with management a drain of Dniepr and their impact on a biological and sanitary condition of water basins. Gidrobiol Zhurn 2:3–18 (in Russian)
Van der Velde G, Platvoet D (2007) Quagga mussels Dreissena rostriformis bugensis (Andrusov, 1897) in the main river (Germany). Aquat Invasions 2:261–264
Vanderploeg HA, Nalepa TF, Jude DJ et al (2002) Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can J Fish Aquat Sci 59:1209–1228
Zhulidov AV, Headley JV, Robarts RD et al (1997) Atlas of Russian wetlands: biogeography and metal concentrations. National Hydrology Research Institute, Canada 309 p
Zhulidov AV, Pavlov DF, Nalepa TF, Scherbina GH et al (2004) Relative Distributions of Dreissena bugensis and Dreissena polymorpha in the Lower Don River System, Russia. Int Rev Hybrobiol 89:326–333
Zhulidov AV, Zhulidov DA, Pavlov DF et al (2005) Expansion of the invasive bivalve mollusk Dreissena bugensis (quagga mussel) in the Don and Volga river basins: revisions based on archived specimens. Ecohydrol Hydrobiol 5:127–133
Zhulidov AV, Nalepa TF, Kozhara AV et al (2006) Recent trends in relative abundance of two dreissenid species, Dreissena polymorpha and Dreissena bugensis in the lower Don river system, Russia. Arch Hydrobiol 165:209–220
Zhuravel PA (1967) On settling of the Bug Dreissena in artificial reservoirs. Gidrobiol Zhurn 3:87-90 (in Russian)
Zhuravel PA (1951) On the Dreissena bugensis (Mollusca) from the system of the Dnepr river and on its recent appearance in the Dneprovskoye Reservoir. Zool Zhurn 39:186–188 (in Russian)
Acknowledgments
This work was carried out under the Russian Federation Environmental Management Project (North Caucasus water management and protection sub-component under the World Bank loan to the Russian Federation, 1996–2000) and the Rostov Strategic Plan for Sewerage and Water Supply, financed by the British Department for International Development, 2001–2002. The South Russian Regional Centre for Preparation and Implementation of International Projects (CPPI-S), Rostov-on-Don, Russia provided partial funding, 2003–2008. As from 2006 the work was carried out under the EU project R20; Water Scenarios for Europe and for Neighbouring States R21; (“SCENES”) (PRIORITY 6.3 GLOBAL CHANGE AND ECOSYSTEMS). We thank Dr. G. Podtopta, Rostov-on-Don, Russia for help in preparing the maps.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhulidov, A.V., Kozhara, A.V., Scherbina, G.H. et al. Invasion history, distribution, and relative abundances of Dreissena bugensis in the old world: a synthesis of data. Biol Invasions 12, 1923–1940 (2010). https://doi.org/10.1007/s10530-009-9641-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9641-y