Abstract
Objective
This study was conducted to characterize recombinant α-l-rhamnosidase from Chloroflexus aurantiacus and apply the enzyme in the production of isoquercitrin from rutin.
Results
The α-l-rhamnosidase from C. aurantiacus was cloned and expressed in Escherichia coli BL21 and purified as a soluble enzyme. α-l-rhamnosidase purified from C. aurantiacus has a molecular mass of approximately 105 kDa and is predicted to exist as a homodimer with a native enzyme of 200 kDa. The purified enzyme exhibited the highest specific activity for rutin among the reported isoquercitrin producing α-l-rhamnosidases and was applied in the production of isoquercitrin from rutin. Under the optimised conditions of pH 6.0, 50 °C, 0.6 U mL−1 α-l-rhamnosidase, and 30 mM rutin, α-l-rhamnosidase from C. aurantiacus produced 30 mM isoquercitrin after 2 h with a 100% conversion yield and productivity of 15 mM h−1.
Conclusions
We achieved a high productivity of isoquercitrin from rutin. Moreover, these results suggest that α-l-rhamnosidase from C. aurantiacus is an effective isoquercitrin producer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-l-Rhamnosidase hydrolyze the terminal α-l-rhamnose of natural glycosides such as rutin, naringin, quercitrin, and hesperidin. α-l-Rhamnosidase has been used in the industrial field for the debittering of citrus fruit juices, aroma enhancement in wine, and preparation of many drugs and drug precursors (Vila-Real et al. 2011). The α-l-rhamnosidases from diverse microorganisms including Bacteroides thetaiotaomicron (Wu et al. 2018), Bacteroides JY-6 (Jang and Kim 1996), Lactobacillus plantarum NCC245 (Avila et al. 2009), and Sphingomonas paucimobilis (Miyata et al. 2005) have been purified and characterised. The various α-l-rhamnosidases have been applied to derhamnosylation of natural products (Monti et al. 2004; Yadav et al. 2010).
Isoquercitrin, a plant flavonoid and the 3-O-glucoside of quercetin, has antioxidant, anti-inflammatory, anti-carcinogenic, antidiabetic, and anti-allergic activities (Makino et al. 2013). Isoquercitrin displays higher biological activity than quercetin or rutin because of its higher water solubility and better intestinal absorption. However, isoquercitrin is found at very low levels in nature and therefore it is traded at a high price. Isoquercitrin can be produced by derhamnosylation of rutin (quercetin-3-O-rutinoside), which is present in relatively large amounts in a wide variety of plants including buckwheat and fava d’anta.
Thus, in recent years, the conversion of rutin to isoquercitrin has been widely studied using heat and acid treatments, microbial transformation, and enzymatic hydrolysis (Valentova et al. 2014). Studies using rhamnosidases have mainly been carried out to produce isoquercitrin at high conversion rates without by-products because of their high specificity. The production of isoquercitrin through the derhamnosylation of rutin by α-l-rhamnosidases from Aspergillus terreus (Gerstorferova et al. 2012), Penicillium griseoroseum (Yadav et al. 2017), Bacillus litoralis strain C44 (Lu et al. 2012), Bifidobacterium breve (Zhang et al. 2015), and Aspergillus niger (You et al. 2010) has been reported. However, a commercial α-l-rhamnosidase that produced isoquercitrin from rutin showed higher productivity than the above enzymes (Kim et al. 2016). Recently, a paper correcting the inaccurate results obtained for the production of isoquercitrin by α-l-rhamnosidase has been reported (Kren and Valentova 2018).
Enzymes derived from thermophiles have been used for production studies because they generally possess higher stability and activity. Therefore, in the present study, we cloned α-l-rhamnosidase from Chloroflexus aurantiacus, one of the bacteria isolated from hot springs. The enzyme displayed higher hydrolytic activity than the commercial α-l-rhamnosidase and was applied to produce isoquercitrin from rutin.
Methods
Bacterial strains, plasmid, gene cloning, and expression
Chloroflexus aurantiacus DSM636 (DSMZ, Brauschweig, Germany), Escherichia coli BL21 (New England Biolabs, Ipswich, MA, USA), and pET-28a (+) (Novagen, Darmstadt, Germany) were used as sources of the α-l-rhamnosidase gene, host cells, and expression vector, respectively. A putative α-l-rhamnosidase gene was amplified by PCR using C. aurantiacus genomic DNA. The oligonucleotide primer sequences used for gene cloning were based on the DNA sequence of putative α-l-rhamnosidase from C. aurantiacus (GenBank accession number, A9WDK5). Forward (5′-TTGCTAGCATGTCATATGAACAGTTGTCTACT-3′) and reverse primers (5′-AACTCGAGTTATATGCCATCCTTTTTAGGAAT-3′) were designed to introduce the NheI and XhoI restriction sites (underlined), respectively. The PCR product was ligated with pET-28a (+) and then transformed into E. coli BL21.
Instrument and reagent for NMR
One-and two-dimensional nuclear magnetic resonance (NMR) spectra were measure on a Bruker Avance III UltraStabilized 800 (1H-NMR at 800 MHz, 13C-NMR at 200 MHz) NMR spectrometer (Billerica, MA, USA), using deuterium oxide (D2O) and methanol-d4 (CD3OD), as solvent and tetramethylsilane (TMS) as an internal standard. Chemical shifts were expressed in terms of a values (Bruker, Karlsruhe, Germany).
Culture conditions
The recombinant E. coli for α-l-rhamnosidase expression was cultured in a 2000 mL Erlenmeyer flask containing 500 mL of LB (Luria–Bertani) medium and 20 µg kanamycin mL−1 at 37 °C with agitation at 200 rpm on a shacking incubator (Jeio Tech. CO.LTD. Daejeon, Korea). When the OD at 600 reached 0.6, 100 µM IPTG was added to induce recombinant α-l-rhamnosidase expression, and the culture was further incubated at 16 °C for 16 h with agitation at 150 rpm.
Enzyme purification
The cultured cells were harvested from the medium and disrupted in 50 mM sodium/phosphate buffer (pH 7.4) containing 5 mM imidazole and 300 mM NaCl with 1 mg lysozyme mL−1 using a sonicator on ice. Cell debris and unbroken cells were discarded by centrifugation, and the sample was applied to a His-trap HP affinity column equilibrated with 50 mM sodium/phosphate buffer (pH 7.4) on a fast protein liquid chromatography system. The bound recombinant enzyme was eluted with the same buffer containing a linear gradient of imidazole (10–250 mM) at 1 mL min−1. The collected active fractions were dialysed against 50 mM citrate/phosphate buffer (pH 6.0) for 16 h at 4 °C. The resulting solution of dialysis was used as a purified enzyme.
Hydrolytic activity
One unit (U) of α-l-rhamnosidase activity using aryl-glycoside as a substrate was defined as the amount of enzyme required to release 1 µmol p-nitrophenol (pNP) from p-nitrophenyl-α-l-rhamnopyranoside per min at 50 °C and pH 6.0. The hydrolytic reactions were performed at 30–70 °C and pH 4–8 for 10 min using 1 mM aryl-glycoside or flavonoid with 5% dimethyl sulfoxide (DMSO) as a substrate. Activity for aryl-glycosides was determined from the increase in OD 405 nm due to the release of NP. Flavonoid activity was measured as the increase of the formed product.
Analytical methods
Rutin, isoquercitrin, hesperidin, hesperetin 7-glucose, naringin, and prunin were evaluated by high-performance liquid chromatography (Agilent 1260, Santa Clara, CA, USA) at 350 nm with a C18 column (ZORBAX Eclipse XDB-C18, 4.6 × 250 mm, 5 µm).
Results and discussion
Gene cloning, enzyme purification, and molecular mass determination
A gene encoding putative α-l-rhamnosidase from C. aurantiacus (GenBank Accession Number, A9WDK5) was cloned and expressed in E. coli BL21. The expressed recombinant α-l-rhamnosidase was purified as a single soluble protein with a specific activity of 304.3 U mg−1 using His-Trap HP affinity chromatography. The molecular mass of recombinant α-l-rhamnosidase from C. aurantiacus was determined using SDS-PAGE. Molecular mass of the purified recombinant enzyme was approximately 105 kDa. The calculated value of the molecular mass of α-l-rhamnosidase from C. aurantiacus was 107,942 Da based on 957 amino acids with six histidine residues (Fig. 1a). The reported molecular masses of α-l-rhamnosidase from Bacteroides JY-6 (Jang and Kim 1996), Lactobacillus plantarum NCC 245 (Avila et al. 2009), and B. breve (Zhang et al. 2015) were 120, 73, and 87 kDa, respectively. The native α-l-rhamnosidase from C. aurantiacus was suggested to exist as a homodimer with a native enzyme of 200 kDa as determined by gel-filtration chromatography (Fig. 1b). α-l-Rhamnosidases from Bacteroides JY-6 (Jang and Kim 1996) and Lactobacillus plantarum NCC 245 (Avila et al. 2009) were reported as dimers with total molecular masses of 240 and 155 kDa, respectively.
Analysis of SDS-PAGE and determination of molecular mass for α-l-rhamnosidase from C. aurantiacus.a SDS-PAGE analysis of α-l-rhamnosidase from C. aurantiacus. All protein bands at each step of purification were dyed with Coomassie blue for visualization. Lane 1, size marker; lane 2, cell debris; lane 3, crude extract; and lane 4, purified enzyme. b Determination of molecular mass of α-l-rhamnosidase from C. aurantiacus by gel filtration chromatography. The purified α-l-rhamnosidase was transported through a HiPrep 16/60 Sephacryl S-300 HR column (GE Healthcare) and eluted with 150 mM NaCl in 50 mM citrate/phosphate buffer (pH 6.0) at a flow rate of 0.5 ml min−1. Molecular masses of the reference proteins (filled circle) such as conalbumin, aldolase, catalase, and ferritin are 75, 158, 232, and 440 kDa. The retention time of purified α-l-rhamnosidase corresponded to 200 kDa (open circle), which was calculated by comparing with the migration length of the reference proteins
Effects of metal ions, pH, and temperature on the activity of α-l-rhamnosidase from C. aurantiacus
The effects of metal ions (Ca2+, Co2+, Cu2+, Cs2+, Fe2+, Mn2+, Mg2+, Zn2+, and EDTA) on the enzyme activity were investigated. However, the metal ions did not affect enzyme activity with pNP-α-l-rhamnopyranoside as a substrate (data not shown).
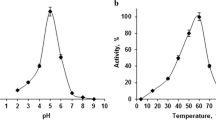
The effects of pH and temperature on α-l-rhamnosidase hydrolytic activity were investigated. The maximum hydrolytic activity of α-l-rhamnosidase from C. aurantiacus was observed at pH 6.0 and 50 °C (Fig. 2a, b). The optimum pH and temperature of α-l-rhamnosidases from P. griseoroseum (Yadav et al. 2017), A. terreus (Gerstorferova et al. 2012), B. brever (Zhang et al. 2015), A. niger (You et al. 2010), and B. litoralis (Lu et al. 2012) were 6.5 and 57 °C; 8.0 and 70 °C; 6.5 and 55 °C; 6.0 and 45 °C; and 7.5 and 37 °C, respectively. The optimum pH and temperature of commercial α-l-rhamnosidase were equal to those of α-l-rhamnosidase from C. aurantiacus (Kim et al. 2016). α-l-Rhamnosidase from C. aurantiacus displayed first-order kinetics for thermal inactivation and the half-lives of the enzyme were 138, 78, 50, 13, and 7 h at 30, 40, 50, 60, and 70 °C, respectively (Fig. 3). The half-lives of commercial α-l-rhamnosidase at 50 °C (Kim et al. 2016) and microbial α-l-rhamnosidases from P. griseoroseum at 50 °C (Yadav et al. 2017), A. terreus at 70 °C (Gerstorferova et al. 2012), B. breve at 55 °C (Zhang et al. 2015), and A. niger at 70 °C (You et al. 2010) were 7, < 1, < 0.5, 96, and 0.5 h, respectively. Although these enzymes were incubated under different conditions, the thermostability of α-l-rhamnosidases based on only the reported information was in the source strain order of: B. breve > C. aurantiacus > commercial enzyme > A. terreus and A. niger > P. griseoroseum.
The activity of α-l-rhamnosidase from C. aurantiacus with changes in pH and temperature. a Effects of pH. The reactions were carried out with 1 mM rutin and 5% DMSO in 50 mM citrate/phosphate buffer (pH 4–8) at 50 °C for 30 min. b Effects of temperature. The reactions were carried out with 1 mM rutin and 5% DMSO in 50 mM citrate/phosphate buffer (pH 6.0) at 30–70 °C for 30 min. Data are represented in the means of triplicate experiments, and error bars are shown by the standard deviation
Thermal inactivation of α-l-rhamnosidase from C. aurantiacus. The enzyme was incubated at 30 (filled triangle), 40 (open square), 50 (filled square), 60 (open circle), and 70 °C (filled circle) in 50 mM citrate/phosphate buffer (pH 6.0) for various periods of time. The incubation of a sample was stopped at each time point and assay was performed with 1 mM rutin and 5% DMSO in 50 mM citrate/phosphate buffer (pH 6.0) at 50 °C for 30 min. The experimental data were applied to a first-order curve fitting, and the half-lives were calculated using Sigma Plot 10.0 Software. Data are represented in the means of triplicate experiments, and error bars are shown by the standard deviation
Substrate specificity of α-l-rhamnosidase from C. aurantiacus
The substrate specificity of α-l-rhamnosidase from C. aurantiacus was investigated using aryl-glycosides (Table 1). The specific activity followed the order pNP-α-l-rhamnopyranoside > pNP-α-d-glucopyranoside > pNP-α-d-galactopyranoside > pNP-β-d-galactopyranoside > oNP-α-d-glucopyranoside. However, no hydrolytic activity was detected for pNP-β-d-glucopyranoside, pNP-β-d-mannopyranoside, pNP-α-l-arabinopyranoside, pNP-α-l-arabinofuranoside, pNP-β-d-xylopyranoside, and oNP-β-d-xylopyranoside. Commercial α-l-rhamnosidase exhibited hydrolytic activity only for pNP-α-l-rhamnopyranoside (Kim et al. 2016), while α-l-rhamnosidase from C. aurantiacus exhibited activity for pNP-α-d-glucopyranoside, pNP-β-d-galactopyranoside, pNP-α-d-galactopyranoside, and oNP-α-d-glucopyranoside. α-l-Rhamnosidase from C. aurantiacus displayed the highest specific activity for pNP-α-l-rhamnopyranoside among the reported α-l-rhamnosidases. α-l-Rhamnosidases from A. terreus (Gerstorferova et al. 2012), P. griseoroseum (Yadav et al. 2017), B. breve (Zhang et al. 2015), and commercial α-l-rhamnosidase (Kim et al. 2016) showed 2.5-, 11.27-, 5.43-, and 1.73-fold lower activities for pNP-α-l-rhamnopyranoside than for α-l-rhamnosidase from C. aurantiacus.
The specific activities of α-l-rhamnosidase from C. aurantiacus for rhamnose-containing flavonoids, such as rutin, hesperidin, and naringin, and rhamnose-hydrolysed flavonoids such as isoquercitrin, hesperetin 7-glucose, and pruning were investigated (Table 1). α-l-Rhamnosidase from C. aurantiacus was active only towards rhamnose-containing flavonoids, indicating that the enzyme hydrolyses only the rhamnose moiety. The enzyme exhibited higher activity for α-1,6 linkage (rutin and hesperidin) than for α-1,2 linkage (naringin), similar to the commercial α-l-rhamnosidase (Kim et al. 2016). The hydrolytic activity towards rutin by α-l-rhamnosidase from C. aurantiacus was higher than that of commercial α-l-rhamnosidase under a high hydrostatic pressure condition, representing the highest activity for rutin reported to date. These results indicate that the α-l-rhamnosidase from C. aurantiacus is suitable for producing isoquercitrin from rutin. The exact chemical structures of these compound were determined by comparison of their spectroscopic data with those of previously reported reference compounds (Supplementary Figs. 1–3).
Isoquercitrin
1H-NMR (800 MHz, D2O and CD3OD) δ 3.26 (1H, m, H-5″), 3.41 (1H, t, H-4″), 3.48 (1H, t, H-3″), 3.54 (1H, dd, J = 7.79 and 9.28 Hz, H-2″), 3.59 (1H, dd, J = 5.07 and 12.16 Hz, H-6″), 3.70 (1H, dd, J = 2.25 and 12.08 Hz, H-6″), 5.14 (1H, d, J = 7.68 Hz, H-1″), 6.25 (1H, d, J = 2.06 Hz, H-6), 6.44 (1H, d, J = 2.00 Hz, H-8), 6.94 (1H, d, J = 8.41 Hz, H-5′), 7.57 (1H, dd, J = 2.07, 8.39 Hz, H-6′), 7.69 (1H, d, J = 2.13 Hz, H-2′). 13C-NMR (200 MHz, D2O and CD3OD) δ 60.83 (C-6″), 69.48 (C-4″) 74.06 (C-2″), 76.20 (C-3″), 76.64 (C-5″), 93.91 (C-8), 98.83 (C-6), 102.53 (C-1″), 104.40 (C-10), 115.09 (C-5′), 116.32 (C-2′), 121.71 (C-1′), 122.22 (C-6′), 134.09 (C-3), 144.22 (C-3′), 148.20 (C-4′), 156.94 (C-9), 157.91 (C-2), 160.97 (C-5), 164.10 (C-7), 178.05 (C-4).
Production of isoquercitrin from rutin by α-l-rhamnosidase from C. aurantiacus
The effect of recombinant α-l-rhamnosidase from C. aurantiacus concentration on the production of isoquercitrin was investigated using 20 mM rutin as the substrate with varying enzyme concentrations from 0.1 to 1 U mL−1 for 90 min (Fig. 4a). Isoquercitrin production increased with increasing enzyme concentration of up to 0.6 U mL−1. However, at concentrations greater than 0.6 U mL−1, the increase in isoquercitrin production was significantly reduced, indicating that the enzyme concentration was optimal at 0.6 U mL−1. The production of isoquercitrin from rutin was assessed with 0.6 U enzyme mL−1 for 90 min by varying the concentration of rutin from 5 to 40 mM (Fig. 4b). Conversion yield decreased with increasing concentrations of rutin. However, up to 30 mM rutin, isoquercitrin production constantly increased. At greater than 30 mM rutin, the increase in isoquercitrin production was considerably reduced. Thus, 30 mM rutin was selected as the substrate concentration for producing isoquercitrin.
The production of isoquercitrin from rutin by α-l-rhamnosidase from C. aurantiacus with changes in enzyme and substrate concentrations. a Effect of change in enzyme concentration. The reactions were carried out with 20 mM rutin and 5% DMSO in 50 mM citrate/phosphate buffer (pH 6.0) at 50 °C for 90 min. b Effect of change in substrate concentration. The reactions were carried out with 0.6 U enzyme ml−1 and 5% DMSO in 50 mM citrate/phosphate buffer (pH 6.0) at 50 °C for 90 min. Isoquercitrin production and conversion yield were represented with filled circle and open square, respectively. Data are represented in the means of triplicate experiments, and error bars are shown by the standard deviation
The optimal reaction conditions for producing isoquercitrin from rutin were pH 6.0, 50 °C, 0.6 U enzyme mL−1, and 30 mM rutin. Under the optimised conditions, α-l-rhamnosidase from C. aurantiacus completely converted rutin to 30 mM isoquercitrin after 2 h with a productivity of 15 mM h−1. After 2 h, isoquercitrin was not further hydrolysed by the enzyme. α-l-Rhamnosidase from C. aurantiacus showed 1.88-fold higher productivity than commercial α-l-rhamnosidase, which showed the highest productivity reported to date (Kim et al. 2016). The results suggest that C. aurantiacus α-l-rhamnosidase is an effective producer of isoquercitrin (Fig. 5).
Production of isoquercitrin (filled circle) from rutin (open circle) by α-l-rhamnosidase from C. aurantiacus. The reactions were carried out with 0.6 U enzyme ml−1, 30 mM rutin, and 5% DMSO in 50 mM citrate/phosphate buffer (pH 6.0) at 50 °C. Data are represented in the means of triplicate experiments, and error bars are shown by the standard deviation
In summary, α-l-rhamnosidase from C. aurantiacus was cloned, characterised, and applied to produce isoquercitrin from rutin. Enzyme activity for rutin as a substrate represented the highest value reported to date. Under the optimal conditions, 30 mM isoquercitrin was completely converted from rutin by α-l-rhamnosidase from C. aurantiacus after 2 h with a productivity of 15 mM h−1, which is the highest reported value. Thus, α-l-rhamnosidase from C. aurantiacus is an effective enzyme for producing isoquercitrin from rutin.
References
Avila M, Jaquet M, Moine D, Requena T, Pelaez C, Arigoni F, Jankovic I (2009) Physiological and biochemical characterization of the two α-l-rhamnosidases of Lactobacillus plantarum NCC245. Microbiology 155:2739–2749. https://doi.org/10.1099/mic.0.027789-0
Gerstorferova D, Fliedrova B, Halada P, Marhol P, Kren V, Weignerova L (2012) Recombinant α-l-rhamnosidase from Aspergillus terreus in selective trimming of rutin. Process Biochem 47:828–835. https://doi.org/10.1016/j.procbio.2012.02.014
Jang IS, Kim DH (1996) Purification and characterization of α-l-rhamnosidase from Bacteroides JY-6, a human intestinal bacterium. Biol Pharm Bull 19:1546–1549
Kim DY, Yeom SJ, Park CS, Kim YS (2016) Effect of high hydrostatic pressure treatment on isoquercetin production from rutin by commercial α-l-rhamnosidase. Biotechnol Lett 38:1775–1780. https://doi.org/10.1007/s10529-016-2157-5
Kren V, Valentova K (2018) Isoquercetin enzymatic production: a true story response to the paper “Zhua et al. [1]”. Mol Catal 458:112–114. https://doi.org/10.1016/j.mcat.2018.04.033
Lu Z, Wang J, Lin S, Zhang Y (2012) Degradation of rutin into isoquercitrin by Bacillus litoralis strain C44. IOSR J Eng 2:1154–1161
Makino T, Kanemaru M, Okuyama S, Shimizu R, Tanaka H, Mizukami H (2013) Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. J Nat Med 67:881–886. https://doi.org/10.1007/s11418-013-0760-5
Miyata T, Kashige N, Satho T, Yamaguchi T, Aso Y, Miake F (2005) Cloning, sequence analysis, and expression of the gene encoding Sphingomonas paucimobilis FP2001 α-l-rhamnosidase. Curr Microbiol 51:105–109. https://doi.org/10.1007/s00284-005-4487-8
Monti D, Pisvejcova A, Kren V, Lama M, Riva S (2004) Generation of an α-l-rhamnosidase library and its application for the selective derhamnosylation of natural products. Biotechnol Bioeng 87:763–771. https://doi.org/10.1002/bit.20187
Valentova K, Vrba J, Bancirova M, Ulrichova J, Kren V (2014) Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol 68:267–282. https://doi.org/10.1016/j.fct.2014.03.018
Vila-Real H, Alfaia AJ, Bronze MR, Calado AR, Ribeiro MH (2011) Enzymatic synthesis of the flavone glucosides, prunin and isoquercetin, and the aglycones, naringenin and quercetin, with selective α-l-rhamnosidase and β-D-glucosidase activities of naringinase. Enzyme Res 2011:692618. https://doi.org/10.4061/2011/692618
Wu T, Pei J, Ge L, Wang Z, Ding G, Xiao W, Zhao L (2018) Characterization of a α-l-rhamnosidase from Bacteroides thetaiotaomicron with high catalytic efficiency of epimedin C. Bioorg Chem 81:461–467. https://doi.org/10.1016/j.bioorg.2018.08.004
Yadav V, Yadav PK, Yadav S, Yadav KDS (2010) α-l-Rhamnosidase: a review. Process Biochem 45:1226–1235. https://doi.org/10.1016/j.procbio.2010.05.025
Yadav S, Yadava S, Yadav KD (2017) α-l-Rhamnosidase selective for rutin to isoquercitrin transformation from Penicillium griseoroseum MTCC-9224. Bioorg Chem 70:222–228. https://doi.org/10.1016/j.bioorg.2017.01.002
You HJ, Ahn HJ, Ji GE (2010) Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J Agric Food Chem 58:10886–10892. https://doi.org/10.1021/jf102871g
Zhang R, Zhang BL, Xie T, Li GC, Tuo Y, Xiang YT (2015) Biotransformation of rutin to isoquercitrin using recombinant α-l-rhamnosidase from Bifidobacterium breve. Biotechnol Lett 37:1257–1264. https://doi.org/10.1007/s10529-015-1792-6
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant Number 2015R1D1A1A01059570).
Supporting information
Supplementary Figure 1—Structure of isoquercitrin.
Supplementary Figure 2—1H-NMR spectrum of isoquercitrin using NMR spectroscopy (800 MHz NMR).
Supplementary Figure 3—13C-NMR spectrum of isoquercitrin using NMR spectroscopy (800 MHz NMR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shin, KC., Seo, MJ., Oh, DK. et al. Cloning and characterization of α-l-rhamnosidase from Chloroflexus aurantiacus and its application in the production of isoquercitrin from rutin. Biotechnol Lett 41, 419–426 (2019). https://doi.org/10.1007/s10529-019-02648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02648-8